Abstract
Background
The multimodal approach is commonly applied to improve knee swelling after total knee arthroplasty. Although the use of a knee compression pad may improve knee swelling, its impact in the multimodal protocol remains unclear.
Methods
A total of 57 knees were randomly assigned to one of 2 groups receiving treatment with either a compression dressing using a polyethylene foam pad designed to effectively compress the postoperative knee combined with an elastic bandage or a compression dressing with use of an elastic bandage only. All patients were treated with the multimodal approach, including an external cooling system, intravenous tranexamic acid, no pneumonic tourniquet, and periarticular multidrug injection.
Results
In intention-to-treat analysis, the circumferences of the thigh measured on postoperative days 1 and 7 were not significantly different between the 2 groups. In addition, there were no differences between the 2 groups in knee flexion angle, perioperative total blood loss, or complication rate.
Conclusions
The polyethylene foam pad had no significant beneficial effect on knee swelling in patients undergoing total knee arthroplasty using the multimodal approach.
Keywords: Knee, Total knee arthroplasty, Swelling, Wound dressing, Multimodal approach
Introduction
Knee swelling after total knee arthroplasty (TKA) can influence the postoperative results [1], [2]. Compression and cooling are generally applied to the operated knee to ameliorate swelling [1], [3].
Recently, there has been a great deal of interest in the multimodal approach to prevent knee swelling after TKA [4]. In addition to compression and cooling, the multimodal approach includes surgery without a pneumonic tourniquet [5], periarticular multidrug injection [6], [7], and intravenous administration of tranexamic acid [8]. Although the knee pad developed to effectively compress the knee could improve swelling, there have been no reports regarding the effectiveness of such a compression pad following TKA.
This randomized controlled trial was performed to investigate the clinical effectiveness of the knee compression pad in the multimodal approach for amelioration of swelling following TKA. We hypothesized that the thigh circumference would be lower in patients treated with a compression dressing using the knee pad than in those treated with the standard compression dressing.
Material and methods
Study design
This was a single-center, 2-arm, parallel group, open-label, randomized controlled trial with 1:1 treatment allocation.
The study was approved by the institutional review board, and patients provided written informed consent. Before enrollment of subjects, the trial was registered as a randomized controlled trial entitled “Impact of postoperative compression dressing avoiding patella on the swelling after TKA: a randomized controlled trial” (University Hospital Medical Information Network registration number UMIN000018934).
Study population
Participants older than age 20 years and medically fit for TKA were eligible for this randomized controlled trial. Patients for whom there was evidence suggesting that they would be unable to adhere to the trial procedures were excluded.
Recruitment and randomization of participants
Patients were recruited between October 2015 and December 2015 from a single orthopaedic clinic specializing in knee and hip surgery in Niigata, Japan. Eligible patients provided written informed consent to inclusion in the trial.
Patients were randomly assigned on a 1:1 basis to receive either a postoperative compression dressing or a standard dressing. Randomized numbers ranging from 0 to 99 were generated using computer software (R, The R Foundation for Statistical Computing) and placed into sealed opaque envelopes. Just before wound closure, a sealed envelope was selected in the operation room by the allocating staff. Patients with even numbers were allocated to the group receiving the compression dressing using the polyethylene foam pad, whereas those with odd numbers were allocated to the group receiving the standard compression dressing without the polyethylene foam pad.
Interventions
The patients received postoperative compression dressing with or without a polyethylene foam pad. Other perioperative interventions, such as surgical technique, external cooling system, rehabilitation plan, and perioperative medications were the same for all patients.
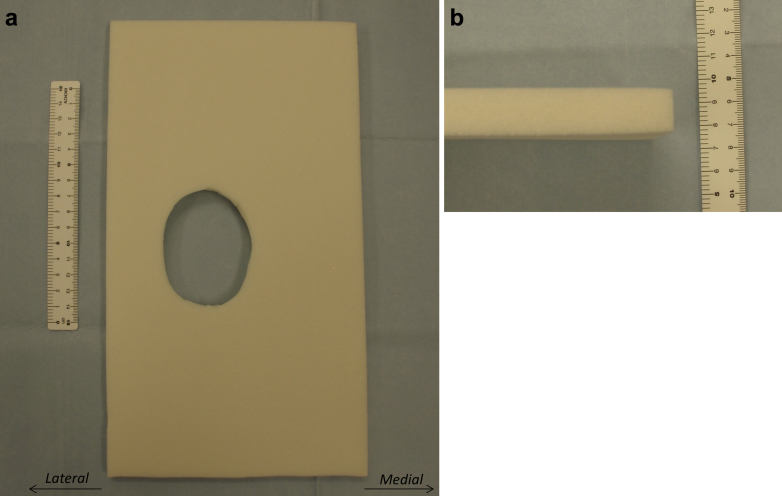
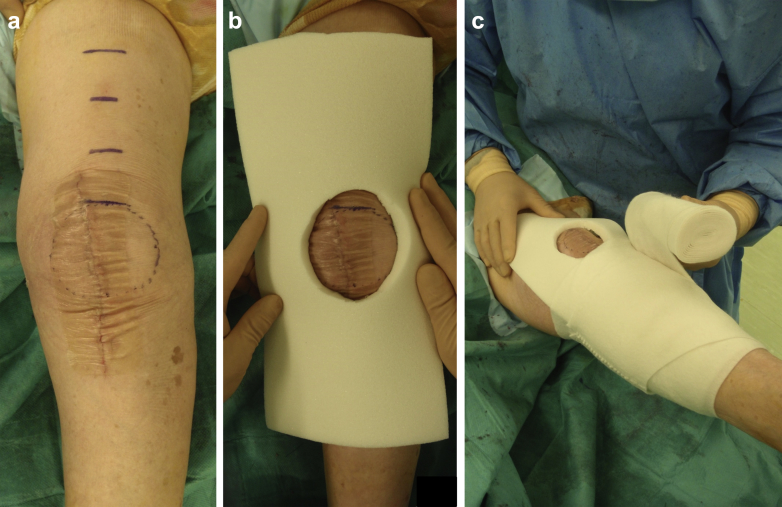
In the postoperative compression dressing with polyethylene foam pad group (compression dressing group), we used a knee pad made of polyethylene foam with an expansion ratio of 30× (Fig. 1). We reused redundant polyethylene foam to package total hip prosthesis after ethylene oxide gas sterilization. To compress the knee effectively, an ellipsoidal hole corresponding in shape and size to the patella was cut out of the polyethylene pad (Fig. 1). The lengths of major and minor axis of the ellipse were 70 and 55 mm, respectively. After tight subcutaneous suture, the incision was closed using a hydrocolloid wound dressing (Karayahesive; Alcare, Tokyo, Japan) (Fig. 2). The pad was placed over the wound, and the knee was wrapped with an elastic bandage (Spandex elastic bandage; Alcare; Fig. 2). The pad was routinely removed at 7 days after surgery. After removal of the pad, we applied standard compression dressing without polyethylene foam pad until 10 days after surgery.
Figure 1.
Polyethylene foam pad developed to obtain effective compression to postoperative knee. (a) Anterior aspect. The pad had a hole cut in the shape of the patella. Note that the width of the medial part from the patella was longer than that of the lateral part. (b) Lateral aspect.
Figure 2.
Compression dressing using the polyethylene foam pad. (a) The operated left knee just before placement of the pad. The surgical wound was closed using hydrocolloid wound dressing. The patella and height at which the thigh circumference were measured was marked using a surgical pen. (b) The pad placed over the wound. (c) An elastic bandage was used to keep the pad over the wound.
In the postoperative standard compression dressing group (standard dressing group), the incision was closed with a hydrocolloid wound dressing, and the operated knee was wrapped with an elastic bandage. No polyethylene pad was used. The standard compression dressing was applied until 10 days after surgery.
Preoperative and postoperative medication
Patients did not receive any preoperative medication during the study period. Periarticular multidrug injection, containing 40 mg of methylprednisolone (Sol Mercort, Fuji, Toyama, Japan) [1 mL], 7.5 mg/mL ropivacaine (Anapeine; AstraZeneca, Osaka, Japan) [40 mL], 10 mg/mL morphine hydrochloride hydrate (Takeda, Osaka, Japan) [0.8 mL], 1.0 mg/mL epinephrine (Bosmin, Daiichi-Sankyo, Tokyo, Japan) [0.3 mL], 50 mg of ketoprofen (Capisten, Kissei, Matsumoto, Japan) [2.5 mL], and normal saline [15.4 mL] was routinely performed during surgery as reported previously [8], [9].
The nonsteroidal antiinflammatory drug, flurbiprofen axetil (50 mg; Ropion, Kaken, Tokyo, Japan) was administered intravenously 4 hours after complete resolution of spinal anesthesia. From the day after surgery, 60 mg of the nonsteroidal antiinflammatory drug, loxoprofen (Surinofen, Aska, Tokyo, Japan) was administered orally 3 times a day.
The intravenous first-generation cephalosporin, cefamezin (Cefazolin; Astellas, Tokyo, Japan) was administered perioperatively and every 8 hours for the first 48 hours after surgery.
Just before skin incision and again at 6 hours after the first administration, 1 g of tranexamic acid was administered intravenously (Transamin; Daiichi-Sankyo). For thromboprophylaxis, 1.5 or 2.5 mg of fondaparinux (Arixtra; GlaxoSmithKline, Tokyo, Japan) was administered subcutaneously once every evening for 10 days, commencing on postoperative day 1.
Surgery and rehabilitation
All patients received spinal anesthesia with 2.0-2.8 mL of 0.5% bupivacaine (Marcaine; AstraZeneca).
All surgeries were performed by one of 2 experienced orthopaedic surgeons (Sachiyuki Tsukada and Motohiro Wakui). Neither a pneumatic tourniquet nor drain was used. An anteromedial straight incision and the subvastus approach were used in all surgeries [10]. All patients received a cemented posterior-stabilized prosthesis (Scorpio NRG, Stryker Orthopaedics, Mahwah, NJ).
An external cooling system was applied (Icing System CF3000; Nippon Sigmax, Tokyo, Japan) for the first 24 hours after surgery.
The postoperative rehabilitation regimens were the same for both groups and were started from the day after surgery.
Outcome measurements
The primary outcome was the circumference of the thigh measured on postoperative days 1 and 7. Measurements were performed in the following order: (1) superior border of the patella, (2) 5 cm above the superior border of the patella, (3) 10 cm above the superior border of the patella, and (4) 15 cm above the superior border of the patella. The thigh circumference was measured separately by 2 investigators.
As a secondary outcome, knee flexion angle was measured by a physical therapist. Data were collected from 1 to 7 days after TKA.
Perioperative total blood loss was also calculated by determining the total blood volume using the blood volume and change in hemoglobin from the preoperative value to postoperative day 4 [11], [12].
Any complications occurring during the course of the trial were recorded with particular emphasis on wound complications.
Incremental cost-effectiveness ratio was calculated based on the difference of the circumference of the thigh 5 cm above the superior border of the patella on postoperative day 7 [13].
The safety of the use of polyethylene foam pad was monitored by 2 orthopaedic surgeons (Sachiyuki Tsukada and Motohiro Wakui) during the study period.
The accuracy of data entry and the adherence of study protocol, especially the process of random allocation were monitored by the in-hospital data monitoring board that did not participate in the recruitment of patients, surgery, and outcome measurements.
We calculated that this trial would require 21 patients per group to illustrate a clinically significant 3.0-cm mean decrease in thigh circumference, with a 2-sided significance level of 5% and power of 80%. For power analysis, we used a standard deviation of 3.2 cm in thigh circumference based on the results of our pilot study.
Statistical analyses
Missing values of the circumference of the thigh were replaced by either linear interpolation in cases where the missing values fell between 2 valid values or by median values from other patients at the same time point in the same treatment group.
All analyses were performed according to the intention-to-treat principle. Comparisons between study groups were performed with Student t test for continuous variables and the chi-square test for categorical variables. All tests were 2 sided, and P < .05 was taken to indicate statistical significance.
To examine interobserver reliability, the intraclass correlation coefficients of the circumference of the thigh were calculated for 2 assessors.
Results
Participants
A total of 59 TKAs during the study period were eligible for inclusion in the study. The trial is outlined in the flow chart presented in Figure 3. Two TKAs were excluded because the patients declined to participate in the trial. The remaining 57 TKAs were randomly assigned into the compression dressing group or standard dressing group. No patients were excluded after randomization.
Figure 3.
Flow chart showing patient recruitment and progression of trial.
Table 1 summarizes the demographic characteristics of the patients in the 2 groups. The baseline characteristics were similar between the 2 groups.
Table 1.
Patient demographic and baseline clinical characteristics.
| Variable | Compression dressing (n = 34) | Standard dressing (n = 23) | P-value |
|---|---|---|---|
| Age, y | 74.3 ± 6.6 | 75.5 ± 7.6 | .52a |
| Sex (female/male) | 25/9 | 17/6 | .97b |
| Side (right/left) | 16/18 | 12/11 | .70b |
| Height, cm | 151.8 ± 7.3 | 152.4 ± 7.1 | .79a |
| Weight, kg | 58.0 ± 9.6 | 60.4 ± 7.7 | .34a |
| Body mass index, kg/m2 | 25.1 ± 3.6 | 26.1 ± 3.5 | .35a |
| History of diabetes mellitus (yes/no) | 7/27 | 7/16 | .40b |
| History of cardiovascular disorder (yes/no) | 1/33 | 4/19 | .059b |
| History of chronic kidney disease (yes/no) | 8/26 | 6/17 | .83b |
| Preoperative circumference of the thigh, cm | |||
| (1) Superior border of the patella | 37.2 ± 3.8 | 37.5 ± 2.5 | .78a |
| (2) 5 cm above the superior border of the patella | 37.8 ± 4.3 | 38.3 ± 3.1 | .73a |
| (3) 10 cm above the superior border of the patella | 40.9 ± 4.7 | 41.4 ± 3.5 | .73a |
| (4) 15 cm above the superior border of the patella | 44.7 ± 5.2 | 44.6 ± 3.8 | .94a |
| Intraoperative blood loss, mL | 214.3 ± 100.9 | 180.4 ± 111.1 | .24a |
| Duration of operation, min | 88.0 ± 12.3 | 88.7 ± 13.8 | .85a |
Results are expressed as means ± standard deviation, unless otherwise stated.
P-values were determined with Student t test.
P-values were determined with the χ2 test.
Primary outcome
Intention-to-treat analysis showed that the thigh circumferences from 0, 5, 10, and 15 cm above the superior border of the patella were not significantly different between the 2 groups at any measurement point (Table 2). The interobserver reliabilities for all measurements are shown in Table 3.
Table 2.
The postoperative measurement of the circumference of the thigh.
| The circumference of the thigh | Compression dressing (n = 34) | Standard dressing (n = 23) | P-value |
|---|---|---|---|
| 1 day after surgery, cm | |||
| (1) Superior border of the patella | 38.4 ± 3.0 | 38.3 ± 2.8 | .86a |
| (2) 5 cm above the superior border of the patella | 39.2 ± 3.8 | 38.7 ± 3.2 | .57a |
| (3) 10 cm above the superior border of the patella | 41.6 ± 4.5 | 41.8 ± 3.6 | .87a |
| (4) 15 cm above the superior border of the patella | 45.0 ± 4.5 | 44.8 ± 3.8 | .91a |
| 7 days after surgery, cm | |||
| (1) Superior border of the patella | 39.4 ± 3.6 | 39.2 ± 2.4 | .77a |
| (2) 5 cm above the superior border of the patella | 40.4 ± 4.1 | 41.1 ± 3.5 | .55a |
| (3) 10 cm above the superior border of the patella | 44.3 ± 4.9 | 45.2 ± 4.1 | .45a |
| (4) 15 cm above the superior border of the patella | 48.4 ± 4.8 | 48.9 ± 4.2 | .74a |
Results are expressed as means ± standard deviation, unless otherwise stated.
P-values were determined with Student t test
Table 3.
Intraclass correlation coefficients of the measured values of the circumferences of the thigh by 2 independent observers.
| Measurement | Estimate | Lower bound 95% | Upper bound 95% |
|---|---|---|---|
| Before surgery, cm | |||
| (1) Superior border of the patella | 0.974 | 0.955 | 0.985 |
| (2) 5 cm above the superior border of the patella | 0.958 | 0.928 | 0.988 |
| (3) 10 cm above the superior border of the patella | 0.948 | 0.912 | 0.970 |
| (4) 15 cm above the superior border of the patella | 0.949 | 0.913 | 0.970 |
| 1 day after surgery, cm | |||
| (1) Superior border of the patella | 0.966 | 0.942 | 0.980 |
| (2) 5 cm above the superior border of the patella | 0.965 | 0.941 | 0.979 |
| (3) 10 cm above the superior border of the patella | 0.964 | 0.922 | 0.973 |
| (4) 15 cm above the superior border of the patella | 0.931 | 0.887 | 0.959 |
| 7 days after surgery, cm | |||
| (1) Superior border of the patella | 0.945 | 0.908 | 0.967 |
| (2) 5 cm above the superior border of the patella | 0.936 | 0.895 | 0.961 |
| (3) 10 cm above the superior border of the patella | 0.927 | 0.879 | 0.956 |
| (4) 15 cm above the superior border of the patella | 0.965 | 0.941 | 0.979 |
Secondary outcomes
There were no significant differences in knee flexion angle between the 2 groups (Table 4).
Table 4.
Knee flexion angle following total knee arthroplasty.
| Flexion angle | Compression dressing (n = 34) | Standard dressing (n = 23) | P-value |
|---|---|---|---|
| Postoperative day 1 | 62.3 ± 14.5 | 60.2 ± 10.0 | .55a |
| Postoperative day 2 | 72.6 ± 12.3 | 76.0 ± 12.6 | .34a |
| Postoperative day 3 | 80.8 ± 11.3 | 82.1 ± 12.7 | .72a |
| Postoperative day 4 | 82.4 ± 12.8 | 88.3 ± 12.9 | .16a |
| Postoperative day 5 | 82.5 ± 7.1 | 88.6 ± 10.8 | .051a |
| Postoperative day 6 | 88.3 ± 9.0 | 86.8 ± 7.6 | .57a |
| Postoperative day 7 | 89.3 ± 8.0 | 90.1 ± 8.8 | .73a |
Results are expressed as means ± standard deviation, unless otherwise stated.
P-values were determined with Student t test.
There were no significant differences in total blood loss between the 2 groups (835.9 ± 380.7 mL in the compression group and 856.3 ± 338.1 mL in the standard dressing group; P = .89).
No complications occurred during the study period.
Cost-effectiveness analysis
The improvement of the circumference of the thigh 5 cm above the superior border of the patella on postoperative day 7 was 0.7 cm when the polyethylene pad was used. The cost for the pad including sterilization was 1497 Japanese Yen (13.3 US Dollar). Incremental cost-effectiveness ratio was 2138 Japanese Yen/cm (19.0 US Dollar/cm).
Discussion
In a randomized controlled trial to investigate the impact of compression dressing using a polyethylene pad on multimodal swelling prevention protocol for TKA, we found no significant improvement in thigh circumference in patients treated using the polyethylene pad. In addition, use of the compression dressing showed no benefits in terms of postoperative knee flexion angle or decrease in hemoglobin level.
Munk et al. [4] conducted a randomized controlled trial to investigate the efficacy of the application of an elastic compression stocking after TKA. The patients in their trial were treated by the multimodal approach for postoperative knee swelling, including a compression bandage from the time of wound closure to the following morning, intravenous tranexamic acid, and periarticular multidrug injection. There were no differences in postoperative knee swelling, range of motion, postoperative pain score, or Oxford knee score between patients treated with and without the elastic compression stocking.
Pinsornsak and Chumchuen [14] compared patients treated with a bulky compression dressing and those treated with a conventional dressing after TKA. The bulky compression dressing was left in place for 24 hours postoperatively and then changed to a conventional dressing. There were no significant differences in knee swelling or bleeding between the 2 groups. The results of these 2 randomized controlled trials support the results of our randomized controlled trial.
The major strength of this study was its randomized controlled design. In addition, this study had high measurement reliability for the primary outcome calculated using the intraclass correlation coefficients.
The most important limitation of this study was its open-label design. Treatment effects may be overestimated when allocation is not blinded [15]. Second, the increased pressure was not directly measured to confirm effective compression. Because the size of ellipsoidal hole was not specific for each patient, the compression might not be always effective.
Conclusions
In the multimodal setting with use of an external cooling system, intravenous tranexamic acid, no pneumonic tourniquet, and periarticular multidrug injection, postoperative application of a compression dressing using a polyethylene foam pad with a hole cut in the shape of the patella showed no significant improvement of thigh circumference compared to use of a compression dressing without the pad.
Acknowledgments
The authors did not receive and will not receive any benefits or funding from any commercial party related directly or indirectly to the subject of this article.
The authors thank Yu Sasazaki, Mayumi Sato, Makiko Takita, Sachika Hara, and Mizuho Yanagisawa for help in data collection. They also thank Minako Ooiwa, Takahiro Sato, and Yasuhiro Funada to organize the in-hospital data monitoring board.
Footnotes
No author associated with this paper has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2016.05.004.
Appendix A. Supplementary data
References
- 1.Brodell J.D., Axon D.L., Evarts C.M. The Robert Jones bandage. J Bone Joint Surg Br. 1986;68:776–779. doi: 10.1302/0301-620X.68B5.3782244. [DOI] [PubMed] [Google Scholar]
- 2.Pua Y.H. The time course of knee swelling post total knee arthroplasty and its associations with quadriceps strength and gait speed. J Arthroplasty. 2015;30:1215–1219. doi: 10.1016/j.arth.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Adie S., Naylor J.M., Harris I.A. Cryotherapy after total knee arthroplasty a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2010;25:709–715. doi: 10.1016/j.arth.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Munk S., Jensen N.J., Andersen I., Kehlet H., Hansen T.B. Effect of compression therapy on knee swelling and pain after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:388–392. doi: 10.1007/s00167-012-1963-0. [DOI] [PubMed] [Google Scholar]
- 5.Wakankar H.M., Nicholl J.E., Koka R., D'Arcy J.C. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 1999;81:30–33. doi: 10.1302/0301-620x.81b1.8971. [DOI] [PubMed] [Google Scholar]
- 6.Kurosaka K., Tsukada S., Seino D., Morooka T., Nakayama H., Yoshiya S. Local infiltration analgesia versus continuous femoral nerve block in pain relief after total knee arthroplasty: a randomized controlled trial. J Arthroplasty. 2016;31:913–917. doi: 10.1016/j.arth.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Tsukada S., Wakui M., Hoshino A. Pain control after simultaneous bilateral total knee arthroplasty: a randomized controlled trial comparing periarticular injection and epidural analgesia. J Bone Joint Surg Am. 2015;97:367–373. doi: 10.2106/JBJS.N.00373. [DOI] [PubMed] [Google Scholar]
- 8.Tsukada S., Wakui M., Hoshino A. The impact of including corticosteroid in a periarticular injection for pain control after total knee arthroplasty: a double-blind randomised controlled trial. Bone Joint J. 2016;98:194–200. doi: 10.1302/0301-620X.98B2.36596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukada S., Wakui M., Hoshino A. Postoperative epidural analgesia compared with intraoperative periarticular injection for pain control following total knee arthroplasty under spinal anesthesia: a randomized controlled trial. J Bone Joint Surg Am. 2014;96:1433–1438. doi: 10.2106/JBJS.M.01098. [DOI] [PubMed] [Google Scholar]
- 10.Tsukada S., Wakui M., Matsueda M. Metal block augmentation for bone defects of the medial tibia during primary total knee arthroplasty. J Orthop Surg Res. 2013;8:36. doi: 10.1186/1749-799X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maniar R.N., Kumar G., Singhi T., Nayak R.M., Maniar P.R. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012;470:2605–2612. doi: 10.1007/s11999-012-2310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadler S.B., Hidalgo J.H., Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 13.Detsky A.S., Naglie I.G. A clinician's guide to cost-effectiveness analysis. Ann Intern Med. 1990;113:147–154. doi: 10.7326/0003-4819-113-2-147. [DOI] [PubMed] [Google Scholar]
- 14.Pinsornsak P., Chumchuen S. Can a modified Robert Jones bandage after knee arthroplasty reduce blood loss? A prospective randomized controlled trial. Clin Orthop Relat Res. 2013;471:1677–1681. doi: 10.1007/s11999-013-2786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood L., Egger M., Gluud L.L., Schulz K.F., Jüni P., Altman D.G. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.