Abstract
CD4+ CD25+ T cells are a population of regulatory T cells responsible for active suppression of autoimmunity. Specifically, CD4+ CD25+ T cells have been shown to prevent insulin-dependent diabetes mellitus, inflammatory bowel disease, and pancreatitis. Here, we present evidence that CD4+ CD25+ T cells also play a major role in controlling the severity of arthritis detected in Borrelia burgdorferi-vaccinated gamma interferon-deficient (IFN-γ°) C57BL/6 mice challenged with the Lyme spirochete. When B. burgdorferi-vaccinated and challenged IFN-γ° mice were treated with anti-interleukin-17 (IL-17) antibody, the number of CD4+ CD25+ T cells increased in the local lymph nodes. Furthermore, histopathologic examination showed the mice to be free of destructive arthritis. When these anti-IL-17-treated B. burgdorferi-vaccinated and challenged mice were also administered anti-CD25 antibody, the number of CD4+ CD25+ T cells in the local lymph nodes decreased. More importantly, severe destructive arthropathy was induced. In addition, delayed administration of anti-CD25 antibody decreased the severity of the arthritis. These results suggest that CD4+ CD25+ T cells are involved in regulation of a severe destructive arthritis induced with an experimental model of vaccination and challenge with B. burgdorferi.
Our investigators showed previously that Borrelia burgdorferi-vaccinated gamma interferon-deficient (IFN-γ°) mice challenged with B. burgdorferi developed a prominent chronic destructive osteoarthropathy (13). The tibiotarsal joint of vaccinated and challenged mice displayed chronic hypertrophy and hyperplasia characterized by erosion of articular cartilage and focal destruction of bone. Treatment of vaccinated IFN-γ° mice with anti-interleukin-17 (IL-17) antibody followed by challenge with B. burgdorferi prevented the development of arthritis (9). Specifically, animals were free of inflammatory changes of the joints and bones. These findings suggested that IL-17 plays a critical role in the induction of arthritis associated with an experimental model of B. burgdorferi vaccination and challenge of IFN-γ° mice.
The mechanism by which the proinflammatory cytokine IL-17 induces inflammatory arthritis is partially understood (1, 3, 10, 11, 25). IL-17 causes expression of many genes involved in the inflammatory process, including production of nitric oxide (5), IL-6 (17, 49), IL-8 (17, 48), prostaglandin E2 (17), granulocyte colony-stimulating factor (17), intracellular adhesion molecule 1 (2, 48), and complement proteins C3 and factor B (23). Furthermore, treatment with anti-IL-17 antibody suppresses production of IL-1β and tumor necrosis factor alpha, key mediators in the pathogenesis of arthritis (30). Moreover, blockage of IL-17 can prevent its direct effects on destruction of cartilage, even in the absence of inflammatory cells (11, 25).
We hypothesized that blockage of IL-17 may also induce another immune pathway responsible for the inhibition of arthritis in B. burgdorferi-vaccinated and challenged IFN-γ° mice. Neutralization of IL-17 may activate T-regulatory cells for prevention of arthritis. T-regulatory cells, especially CD4+ CD25+ T cells, exist as part of the normal immune repertoire (4, 6, 16, 21, 22, 26, 33, 36, 40) and are responsible for maintaining self-tolerance (21, 36, 41, 42) and controlling the development of autoimmune and inflammatory diseases (4, 34, 36, 37, 39, 41, 42). Specifically, CD4+ CD25+ T cells can prevent insulin-dependent diabetes mellitus (37), inflammatory bowel disease (4, 34, 36, 39), and pancreatitis (36). In addition, CD4+ CD25+ T cells have been shown to suppress or prevent acute graft-versus-host disease after allogeneic transplantation (14, 19, 20, 43). However, the role that CD4+ CD25+ T cells play in preventing arthritis in B. burgdorferi-vaccinated and challenged IFN-γ° mice treated with anti-IL-17 is unknown.
In this report, we provide evidence that anti-IL-17 therapy increases the number of CD4+ CD25+ T cells in arthritis-free B. burgdorferi-vaccinated and challenged IFN-γ° mice. Treatment of these mice concomitantly with anti-CD25 antibody decreased the number of CD4+ CD25+ T cells and, more importantly, induced severe destructive arthritis.
MATERIALS AND METHODS
Mice.
IFN-γ gene-deficient mice (parental strain C57BL/6) were obtained from W. P. Weidanz (University of Wisconsin) with permission from Genentech, Inc. (South San Francisco, Calif.). The mice were bred at the animal facility located at the Wisconsin State Laboratory of Hygiene. Six-to-ten-week-old inbred male and female IFN-γ° mice weighing 20 to 30 g were housed at an ambient temperature of 21°C. Food and acidified water were provided ad libitum during a light and dark cycle of 12 h. Experimental protocols were reviewed and approved by the Animal Care and Use Committee for the University of Wisconsin Medical School.
Organisms and preparation.
Low-passage (<10) virulent B. burgdorferi 297 (human spinal fluid) and C-1-11 (Microtus pennsylvanicus) cells representing two distinct seroprotective groups among isolates of B. burgdorferi were grown at 32°C in modified Barbour-Stoenner-Kelly (BSK) medium until reaching a concentration of approximately 107 spirochetes/ml. Five-hundred-microliter samples were then dispensed into 1.5-ml screw-cap tubes (Sarstedt, Newton, N.C.) containing 500 μl of BSK medium supplemented with 10% glycerol (Sigma Chemical Co., St. Louis, Mo.). The tubes were sealed and stored at −70°C. Six days prior to infection of mice, a frozen suspension of spirochetes was thawed and added to 9 ml of fresh BSK medium and incubated at a temperature of 32°C. On the day of infection, the organisms were visualized by dark-field microscopy and enumerated using a Petroff-Hausser counting chamber.
Vaccine preparation.
B. burgdorferi isolate 297 organisms were grown in 1 liter of BSK medium for 6 days, pelleted by centrifugation (10,000 × g, 15°C, 10 min), and washed three times with phosphate-buffered saline (PBS; pH 7.4). The washed pellet was resuspended in 1% formalin, incubated at 32°C with periodic mixing for 30 min, washed three times by centrifugation with PBS (10,000 × g, 10°C, 15 min), and resuspended in PBS. Subsequently, the formalin-inactivated spirochetes were mixed with a sufficient volume of 1% aluminum hydroxide (Reheis, Berkeley Heights, N.J.) to yield 4 × 106 spirochetes/ml.
Vaccination of mice.
Mice were anesthetized with ether contained in a nose-and-mouth cup and injected subcutaneously in the inguinal regions with 0.25 ml of the formalin-inactivated whole-cell vaccine preparation. Whole cells of B. burgdorferi are not recommended for development of a vaccine for humans, based on past concerns associated with other types of whole-cell vaccines (24). However, the ability of whole cells to consistently induce arthritis in mice allows evaluation of immunological mechanisms responsible for the arthritis (9, 13).
Infection of mice.
Twenty-one days after vaccination with B. burgdorferi isolate 297 in alum, mice were anesthetized with ether contained in a nose-and-mouth cup and injected subcutaneously in the left hind paw with 50 μl of BSK medium containing 106 viable B. burgdorferi isolate C-1-11 cells. It was necessary to infect mice with B. burgdorferi isolate C-1-11 because vaccination with B. burgdorferi isolate 297 induces protective antibodies that prevent the homologous infection from eliciting arthritis (15, 28). Other infectious isolates of B. burgdorferi besides C-1-11 are also effective in eliciting the arthritis (15, 38). Controls included vaccinated mice injected with BSK medium alone.
Administration of anti-IL-17 antibody and anti-CD25 antibody.
Lyophilized goat anti-mouse immunoglobulin G polyclonal IL-17 antibody (100 μg) was obtained from R&D Systems (Minneapolis, Minn.), and purified rat anti-mouse CD25 monoclonal antibody (clone PC61; 0.5 mg) was obtained from BD PharMingen (San Diego, Calif.). The antibodies were resuspended in filter-sterilized (0.2-μm-pore-size filter; Acrodisk; Gelman Sciences, Ann Arbor, Mich.) PBS (pH 7.2) to yield concentrations of 50 μg/ml.
Twenty-one days after vaccination, multiple groups of four mice each were infected with 106 viable B. burgdorferi organisms in the left hind paw (Fig. 1). One hour after challenge, the mice were injected in the left hind paw with 50 μl (2.5 μg) of anti-IL-17 antibody. In addition, multiple groups of vaccinated, nonchallenged mice received 50 μl of anti-IL-17 antibody in the left hind paw 21 days after vaccination. Anti-IL-17 antibody was administered daily thereafter for 7 days.
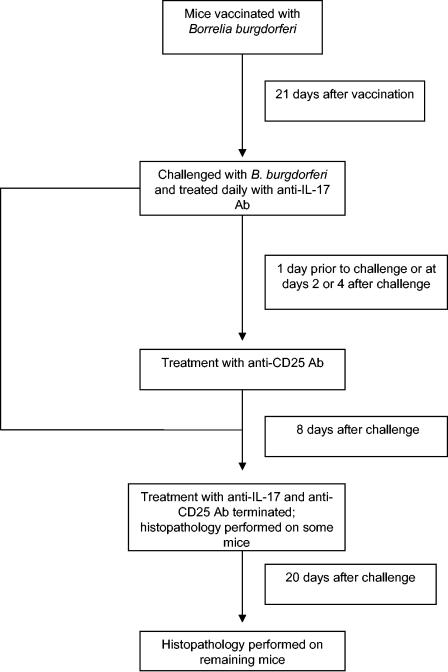
FIG. 1.
Schedule of treatment of B. burgdorferi-vaccinated and challenged mice with anti-IL-17 and anti-CD25 antibodies.
In other studies multiple groups of five anti-IL-17-treated vaccinated and challenged mice were injected in the left hind paw with 50 μl containing 2.5 μg of anti-CD25 antibody 1 day before challenge and daily thereafter for 8 days. In addition, other studies involved delayed administration of anti-CD25 antibody for 48 or 96 h after anti-IL-17-treated vaccinated mice were challenged with B. burgdorferi. Anti-CD25 antibody treatment was discontinued in these latter experiments 5 and 3 days after administration, respectively.
Flow cytometry.
Eight and 20 days after anti-IL-17-treated vaccinated mice with or without challenge with B. burgdorferi were injected with anti-CD25 antibody, the inguinal and popliteal lymph nodes were obtained from the left hind quadrant and left hind leg of the mice. Single-cell suspensions of the lymph node cells were prepared by teasing apart the nodes with forceps and passing them through a sterile nylon mesh screen (Fisher, Hanover Park, Ill.) into cold filter-sterilized PBS. Suspensions of lymph node cells were placed in chilled centrifuge tubes, and the total number of lymphocytes was determined. Cells (5 × 105) were then dispensed into chilled centrifuge tubes, mixed with 2.5 μl each of fluorescein isothiocyanate-conjugated rat anti-mouse CD4 antibody (BD PharMingen), R-phycoerythrin-conjugated rat anti-mouse CD25 antibody (PharMingen), and allophycocyanin-conjugated rat anti-mouse CD8 antibody (PharMingen), and incubated at 4°C for 30 min under dark conditions. Isotype controls for each antibody (BD PharMingen) were also included.
Subsequently, the cells were washed by centrifugation with PBS at 4°C (500 × g, 5 min) and the pellets were resuspended in 300 μl of cold PBS. Propidium iodide (50 μl of a 50-μg/ml solution; Sigma) was added to each tube to discriminate between viable and nonviable cells. Data were acquired using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.) using CellQuest acquisition and analysis (Becton Dickinson). Events were gated to include viable lymphocytes. Ten thousand gated events were collected and analyzed using a quadrant dot blot. Total cell populations in the lymph nodes were calculated by multiplying the percentage of occurrence in a dot plot of a cell population by the total number of cells counted in the node.
Preparation of tissues for histological examination.
At 8 and 20 days after infection, mice were euthanized with ether and their hind paws were amputated at mid-femur. The paws were then fixed in 10% neutral buffered zinc formalin for 24 h. Subsequently, the paws were placed in decalcifying solution (Lerner Laboratories, Pittsburgh, Pa.) for 24 h, followed by addition of fresh decalcifying solution for an additional 48 h. Following decalcification the legs were placed in tissue-embedding cassettes (Fisher Scientific), embedded in paraffin, and cut into 6-μm-thick sections. The sections were placed on glass slides and stained with hematoxylin and eosin. Sections were cryptically coded, and an unbiased histopathological examination was performed by two board-certified pathologists (D.M.E. and J.T.).
Statistical analysis.
The number of lymph node cells among groups was tested by an analysis of variance. Analysis of variance was used to determine whether there were significant differences in the number of lymph node cells found among the various test groups. The alpha level was set at 0.05 before the experiments were started. The standard error for the experiment was then determined.
RESULTS
Anti-IL-17 treatment inhibits development of arthritis.
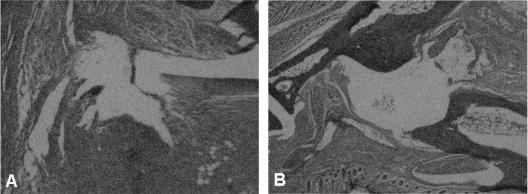
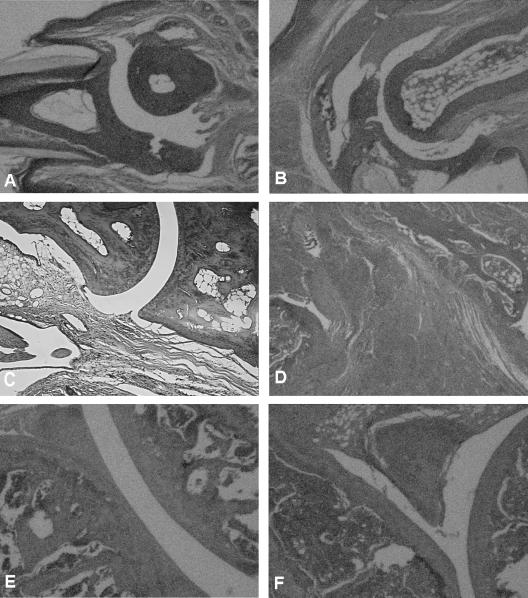
Two groups of four B. burgdorferi-vaccinated IFN-γ-deficient mice each were challenged with 106 viable B. burgdorferi organisms 21 days after vaccination. One of the two groups was treated with anti-IL-17 antibody, while the remaining group was injected with an isotype antibody (goat immunoglobulin G) on the day of challenge and daily thereafter for 8 days. Twenty days after challenge, the non-IL-17-treated vaccinated IFN-γ-deficient mice exhibited histopathologic evidence of synovial hyperplasia, pannus formation, destruction of the articular cartilage, and bone erosion of the ankle joint of the hind paw (Fig. 2A). Treatment of B. burgdorferi-vaccinated and challenged mice with anti-IL-17 antibody prevented synovial inflammation along with cartilage and bone destruction of the ankle (Fig. 2B). Similar findings have been previously reported (9).
FIG. 2.
Histopathologic findings of the ankles of vaccinated IFN-γ-deficient mice challenged with B. burgdorferi after treatment with anti-IL-17 antibody (B) or its isotype antibody (A) at day 20 after infection.
Effects of anti-IL-17 treatment on CD4+ CD25+ T cells.
The purpose of this study was to determine whether the number of CD4+ CD25+ T cells changed in the lymph nodes draining the hind paw of vaccinated and challenged mice with or without treatment with anti-IL-17 antibody. Twenty-one days after vaccination, two groups of 12 vaccinated mice each with or without treatment with anti-IL-17 antibody were challenged with 106 viable B. burgdorferi organisms. A third group of 12 vaccinated mice was also treated with anti-IL-17 antibody but was not infected with B. burgdorferi. Anti-IL-17 antibody was administered to the groups on the day of challenge and daily thereafter for 8 days. Subsequently, inguinal and popliteal lymph nodes were collected from three mice per group at 0, 2, 8, and 20 days after challenge. The number of CD4+ CD25+ T cells in the lymph nodes was determined.
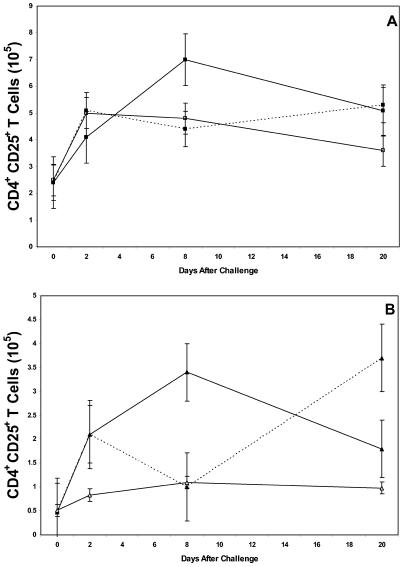
The number of CD4+ CD25+ T cells increased rapidly in the inguinal (Fig. 3A) and popliteal (Fig. 3B) lymph nodes 2 days after vaccinated mice began anti-IL-17 therapy and were challenged with B. burgdorferi. The number of CD4+ CD25+ T cells peaked on day 8 of anti-IL-17 therapy and then gradually decreased. By contrast, the number of CD4+ T cells (range, 7 × 105 to 1 × 106 cells/node) remained relatively constant during this duration. The number of CD4+ CD25+ T cells also increased rapidly (day 2) in non-anti-IL-17 antibody-treated vaccinated and challenged mice. However, the number of CD4+ CD25+ T cells decreased 8 days after challenge before increasing again on day 20 of challenge. Anti-IL-17 therapy of vaccinated, but nonchallenged, mice caused only a slight and sustained increase in the number of CD4+ CD25+ T cells. All these changes were statistically significant (P ≤ 0.05). When these studies were repeated three times, similar results were obtained.
FIG. 3.
Number of CD4+ CD25+ T cells in the inguinal (A) and popliteal (B) lymph nodes of vaccinated IFN-γ° mice with (solid lines) or without (broken lines) anti-IL-17 treatment and with (▪ and ▴) or without (□ and ▵) challenge with the Lyme spirochete. The error bars represent the standard errors of the means.
Histopathologic effects of anti-CD25 antibody treatment of anti-IL-17-treated vaccinated and challenged mice.
Twenty-one days after vaccination, three groups of five mice each were treated with anti-IL-17 antibody and challenged with B. burgdorferi. The anti-IL-17 treatment continued for 8 days after challenge. One of the three groups of anti-IL-17-treated vaccinated and challenged mice also received anti-CD25 antibody 1 day before challenge and daily thereafter for 8 days. The other group was injected with an isotype antibody. In addition, a fourth group of five anti-IL-17-treated vaccinated mice received anti-CD25 antibody but was not challenged with B. burgdorferi.
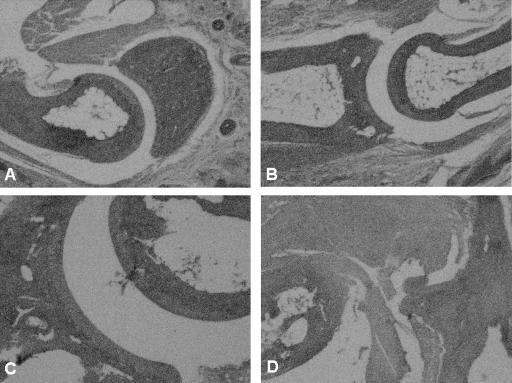
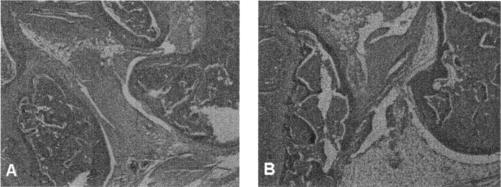
Anti-IL-17-treated vaccinated and challenged mice administered anti-CD25 antibody developed severe swelling of the hind paws 8 days after challenge (data not shown). Histopathologic examination of the small bones of the foot (Fig. 4B) showed moderate inflammation of the periarticular soft tissue. The ankle joint also showed moderate inflammation of the synovial and subsynovial tissues. Synovial hyperplasia (five to six cells thick) and pannus formation were present (Fig. 4D). By contrast, anti-IL-17-treated vaccinated and challenged mice that did not receive anti-CD25 antibody or received the CD25 isotype antibody showed only minimal inflammatory changes of the small bones of the foot (Fig. 4A) and ankle (Fig. 4C). Vaccinated mice injected with both anti-IL-17 and anti-CD25 antibody, but not challenged with B. burgdorferi, were free of inflammation of the small bones of the foot and ankle joint (histopathology not shown). The pathology was similar among all mice within a group.
FIG. 4.
Histopathologic findings of the small bones of the foot (A and B) and ankle joints (C and D) of anti-IL-17-treated vaccinated IFN-γ° mice with (B and D) or without (A and C) treatment with anti-CD25 antibody 8 days after challenge with the Lyme spirochete.
Twenty days after challenge of anti-IL-17-treated vaccinated mice administered anti-CD25 antibody, severe histopathologic changes were present. The small bones of the foot had extensive periarticular inflammation and bone erosion (Fig. 5B). The ankle joint (Fig. 5D) showed pericapsular inflammation and erosion of the periosteum, with pannus formation extending into the marrow space. Moreover, whole areas of the synovial lining were destroyed, and pannus formation was present in the joint. The knee joint (Fig. 5F) showed hypertrophic tenosynovitis and severe inflammation of the surrounding soft tissue. The cartilage of the knee was partially destroyed, and inflammatory cells were present in the joint space. By contrast, anti-IL-17-treated vaccinated and challenged mice showed only minimal changes in the small bone of the foot (Fig. 5A), ankle (Fig. 5C), and knee joint (Fig. 5E). No evidence of inflammation was detected in anti-IL-17-treated vaccinated mice administered anti-CD25 antibody but not challenged (histopathology not shown).
FIG. 5.
Histopathologic findings of the small bones of the foot (A and B), ankle joint (C and D), and knee (E and F) of anti-IL-17-treated vaccinated IFN-γ° mice with (B, D, and F) or without (A, C, and E) treatment with anti-CD25 antibody 20 days after infection with the Lyme spirochete.
Number of CD4+ CD25+ T cells in anti-IL-17-treated vaccinated and challenged mice following therapy with anti-CD25 antibody.
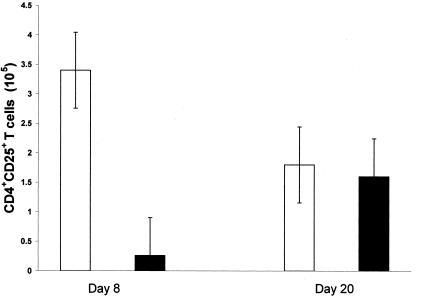
The purpose of this experiment was to determine the effects of treatment with anti-CD25 antibody on the number of CD4+ CD25+ T cells in anti-IL-17-treated vaccinated and challenged mice. Twenty-one days after vaccination, two groups of six mice each were administered anti-IL-17 antibody and challenged with B. burgdorferi with or without concomitant therapy using anti-CD25 antibody. Treatment with anti-CD25 antibody was discontinued 8 days after challenge. At days 8 and 20 after infection, the number of CD4+ CD25+ T cells was determined in the inguinal and popliteal lymph nodes by flow cytometry. Figure 6 shows that the number of CD4+ CD25+ T cells in the popliteal lymph nodes decreased 10-fold or more from 3.4 × 105 to 2.6 × 104 cells following treatment of anti-IL-17-treated vaccinated and challenged mice with anti-CD25 antibody. Twenty days after infection (Fig. 6), the number of CD4+ CD25+ T cells did not differ significantly between anti-IL-17-treated vaccinated and challenged mice with or without treatment with anti-CD25 antibody. Similar results were obtained after determination of the number of CD4+ CD25+ T cells in the inguinal lymph nodes (data not shown) of anti-IL-17-treated vaccinated and challenged mice with or without treatment with anti-CD25 antibody.
FIG. 6.
Number of CD4+ CD25+ T cells in the popliteal lymph nodes of anti-IL-17-treated vaccinated IFN-γ° mice with (▪) or without (□) treatment with anti-CD25 antibody 8 days (A) and 20 days (B) after challenge with the Lyme spirochete. The error bars represent the standard errors of the means.
Histopathologic effects of delayed administration of anti-CD25 antibody.
The purpose of this study was to determine the histopathologic effects of delayed administration of anti-CD25 antibody to anti-IL-17-treated vaccinated and challenged mice. Three groups of four vaccinated (21 days) mice each were administered anti-IL-17 antibody and challenged with B. burgdorferi. The anti-IL-17 antibody was administered to all groups of mice for 8 days after challenge. Subsequently, two groups of anti-IL-17-treated vaccinated and challenged mice were injected with anti-CD25 antibody on day 2 or 4 after challenge. Therapy with anti-CD25 antibody was continued daily thereafter until day 8 after challenge. The third group of four anti-IL-17-treated vaccinated and challenged mice was not treated with anti-CD25 antibody.
Twenty days after challenge, histopathologic changes were detected in the two groups of anti-IL-17-treated vaccinated and challenged mice administered anti-CD25 antibody. Mice injected with anti-CD25 antibody 2 days after challenge had the most severe histopathologic changes (Fig. 7A). The joint showed altered articular cartilage and subchondral bone along with synovial inflammation. Anti-IL-17-treated vaccinated and challenged mice that received anti-CD25 antibody 4 days after challenge showed only synovial hyperplasia (one or two cells thick) with subsynovial inflammation (Fig. 7B). No histopathologic responses, except mild subsynovial inflammation, were detected in the knee joints of anti-IL-17-treated vaccinated and challenged mice without treatment with anti-CD25 antibody (histopathology not shown, but the findings were similar to those presented in Fig. 5E).
FIG. 7.
Histopathologic changes of the knee joints of anti-IL-17-treated B. burgdorferi-vaccinated and challenged mice administered anti-CD25 antibody at day 2 (A) and day 4 (B) after challenge.
DISCUSSION
CD4+ T cells in mice contain a subpopulation of immunoregulatory cells that express the IL-2 receptor α-chain (CD25). These CD4+ CD25+ T cells play a vital role in the induction and maintenance of self-tolerance and prevention of autoimmunity (4, 21, 34, 36, 37, 39, 41, 42). We have presented evidence that CD4+ CD25+ T cells also play a major role in preventing expression of arthritis in an experimental model of B. burgdorferi-vaccinated and challenged mice treated with anti-IL-17 antibody. When anti-IL-17-treated B. burgdorferi-vaccinated and challenged mice were concomitantly administered anti-CD25 antibody, they developed a prominent severe destructive osteoarthropathy. Our results indicate an association between CD4+ CD25+ T cells and inhibition of IL-17 for control of arthritis associated with B. burgdorferi vaccination and challenge in IFN-γ-deficient mice.
In this report, we first showed that severe destructive arthritis could be induced experimentally in B. burgdorferi-vaccinated and challenged mice. When vaccinated and challenged mice were treated with anti-IL-17 antibody, the arthritis abated. These findings confirm the reports of Christopherson et al. (13) and Burchill et al. (9). Furthermore, we showed that treating vaccinated and challenged mice with anti-IL-17 antibody increased the number of CD4+ CD25+ T cells in the regional lymph nodes draining the hind paws of arthritis-free animals. When anti-IL-17-treated vaccinated and challenged mice were also administered anti-CD25 antibody, the number of CD4+ CD25+ T cells decreased in the regional lymph nodes. More importantly, anti-CD25-treated animals developed a prominent osteoarthropathy. Histopathologic examination confirmed that treatment with anti-CD25 antibody caused extensive pathological findings, including destruction of bone and cartilage.
T cells, especially CD4+ T cells, are involved in the induction of arthritis associated with B. burgdorferi vaccination and challenge (27, 29). Vaccinated animals depleted of CD4+ T cells failed to develop severe destructive arthritis after infection with B. burgdorferi (29). The mechanism by which CD4+ T cells induce arthritis has not been elucidated. Most investigations defining the immune arthritic process point toward CD4+ T-cell-driven cytokine regulatory mechanisms (11, 12, 25, 30, 35, 45). Our results also support a cytokine pathway for induction of arthritis. When IL-17 was blocked, the development of arthritis was prevented. Presumably, treatment with anti-IL-17 increased the number of CD4+ CD25+ T cells in the arthritis-free animals. In support, we showed that decreasing the number of CD25+ T cells in anti-IL-17-treated vaccinated and challenged mice induced severe destructive arthritis. These results suggest that CD4+ CD25+ T cells play a major role in the regulation and prevention of arthritis. Conversely, Bardos et al. (7) demonstrated that CD4+ CD25+ T cells are not involved in proteoglycan-induced arthritis. In support of our findings, however, Morgan et al. (32) showed that depletion of CD25+ cells hastens the onset of severe collagen-induced arthritis.
What caused the rapid increase in the number of CD4+ CD25+ T cells in the inguinal and popliteal lymph nodes of anti-IL-17-treated vaccinated and challenged mice? It is known that activated (immune) CD4+ T cells, especially CD4+ memory cells, release IL-17 (1). Blockage of IL-17 may create a situation where activated B. burgdorferi-primed CD4+ T cells cannot effectively promote the development of arthritis. Instead, deprivation of IL-17 may lead to an increased expression of the IL-2 receptor (CD25) on CD4+ T cells that causes the prevention of arthritis. In support of this possibility, the number of CD4+ CD25+ T cells in the lymph nodes of arthritis-free anti-IL-17-treated vaccinated and challenged mice was threefold greater than the number of CD4+ CD25+ T cells found in the lymph nodes of vaccinated and challenged mice without treatment with anti-IL-17 antibody. Furthermore, when arthritis began to resolve in vaccinated and challenged mice, the number of CD4+ CD25+ T cells increased rapidly compared to the number of CD4+ CD25− T cells. Taken together, these results suggest that IL-17 modulates the development of arthritis by influencing the number of effector (CD4+ CD25−) and regulatory (CD4+ CD25+) T cells.
The mechanism by which CD4+ CD25+ T cells prevent arthritis in anti-IL-17-treated B. burgdorferi-vaccinated and challenged mice is unknown. A defining feature of CD4+ CD25+ T cells is their ability to prevent activation and proliferation of CD4+ T cells (4, 42, 46). We hypothesize that vaccination with B. burgdorferi causes the production of both antipathogen T cells and T cells responsible for the expansion of the pathological response. Challenge of vaccinated animals with B. burgdorferi immediately stimulates memory CD4+ CD25− T cells to release the proinflammatory cytokine IL-17. IL-17 then induces the secretion of IL-1β, tumor necrosis factor alpha, and other cytokines or mediators to promote induction and exacerbation of the arthritis. Once the antipathogen CD4+ T cells have successfully promoted the reduction of the spirochete burden, IL-17 production is down-regulated and expression of CD25 on CD4+ T cells is increased. The CD4+ CD25+ T cells then deactivate those CD4+ T cells responsible for the induction of arthritis. Our hypothesis is supported by Thorstenson and Khoruts (47), who showed that low concentrations of antigen can cause increased expression of CD25 on CD4+ T cells. Similarly, Maloy and Powrie (31) proposed that antigen levels also affect the ratio of T regulatory cells to T effector cells. Additional studies are needed to determine if the concentration of spirochetes can affect the number of CD4+ CD25+ T cells in anti-IL-17-treated vaccinated and challenged mice.
It is likely that immune suppression enforced by CD4+ CD25+ T cells is not an all-or-nothing response but rather a quantitative balance between the number of effector CD4+ T cells and the number of CD4+ CD25+ T regulatory cells. In support of this possibility, inflammation or arthritis was induced in anti-IL-17-treated B. burgdorferi-vaccinated and challenged mice, despite an increasing presence of CD4+ CD25+ T regulatory cells. When anti-IL-17-treated B. burgdorferi-vaccinated and challenged mice were also administered anti-CD25 antibody before and after anti-IL-17 treatment, arthritis was detected. The severity of the arthritis, however, was dependent on the duration of anti-CD25 treatment. Administration of anti-CD25 antibody on day 0, 2, or 4 after treatment with anti-IL-17 antibody yielded severe destructive arthritis, moderate inflammation of synovial and subsynovial tissues, or mild synovitis of the ankle joint, respectively. This suggests that sufficient numbers of CD4+ CD25+ T regulatory cells were not present at days 0 or 2 after anti-IL-17 treatment to prevent the arthritis. By day 4 of anti-IL-17 treatment, sufficient numbers of CD4+ CD25+ T regulatory cells were present to limit the inflammatory process.
Our findings were obtained using IFN-γ-deficient mice. These mice lack the gene that encodes IFN-γ and its receptor. Brown and Reiner (8) presented compelling data that IFN-γ does not play an absolute role in the induction or propagation of arthritis after infection of wild-type or IFN-γ-deficient C3H/HeJ mice with B. burgdorferi. Glickstein et al. (18) also showed that IFN-γ receptor-deficient mice and the parental 129/SvEv strain developed mild arthritis of similar severities. Similarly, we showed in this report that B. burgdorferi-vaccinated and challenged IFN-γ-deficient C57BL mice developed severe destructive arthritis. Collectively, these results suggest that other immune mediators, but not IFN-γ, are responsible for the induction of arthritis. There is evidence, however, that IFN-γ can potentiate the proinflammatory effects of IL-17 (44). The dual depletion of IFN-γ and IL-17 may be responsible for the up-regulation of CD25 on CD4+ T cells and prevention of arthritis. Additional studies are needed to confirm that IFN-γ, IL-17, and CD4+ CD25+ T cells have a similar function in wild-type C57BL/6 mice and the C3H mouse, which is recognized as the animal model of choice for Lyme arthritis. These experiments are in progress. However, infection of these mouse strains with B. burgdorferi does not induce the same pathological responses of human Lyme disease, especially Lyme arthritis. More importantly, the roles of IL-17 and CD25 need to be defined in humans with Lyme arthritis.
In conclusion, we have shown that CD4+ CD25+ T cells play a major role in preventing an experimentally induced arthritis in B. burgdorferi-vaccinated and challenged mice. Prevention of arthritis by CD4+ CD25+ T cells was associated with the absence of IL-17. Additional studies are needed to determine if CD4+ CD25+ T cells are also responsible for the resolution of arthritis in vaccinated and challenged mice. The availability of a reproducible mouse model allows development of other approaches for defining the immune mechanisms responsible for the arthritis.
Acknowledgments
This study was supported by the Wisconsin State Laboratory of Hygiene, the public health laboratory for the state of Wisconsin, Madison, Wis., and the Gundersen Medical Foundation, La Crosse, Wis.
We also thank the Flow Cytometry Facility at the University of Wisconsin Hospital (Madison) for their assistance.
REFERENCES
- 1.Aggarwal, S., and A. L. Gurney. 2002. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 71:1-8. [PubMed] [Google Scholar]
- 2.Albanesi, C., A. Cavani, and G. Girolomoni. 1999. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-γ and TNF-α. J. Immunol. 162:494-502. [PubMed] [Google Scholar]
- 3.Antonysamy, M. A., W. C. Fanslow, F. Fu, W. Li, S. Qian, A. B. Troutt, and A. W. Thomson. 1999. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J. Immunol. 162:577-584. [PubMed] [Google Scholar]
- 4.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attur, M. G., R. N. Patel, S. B. Abramson, and A. R. Amin. 1997. Interleukin-17 up-regulation of nitric oxide production in human osteoarthritis cartilage. Arthritis Rheum. 40:1050-1053. [DOI] [PubMed] [Google Scholar]
- 6.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. [DOI] [PubMed] [Google Scholar]
- 7.Bardos, T., M. Czipri, C. Vermes, A. Finnegan, K. Mikecz, and J. Zhang. 2003. CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis. Arthritis Res. Ther. 5:R106-R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burchill, M. A., D. T. Nardelli, D. M. England, D. J. DeCoster, J. A. Christopherson, S. M. Callister, and R. F. Schell. 2003. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect. Immun. 71:3437-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabaud, M., and P. Miossec. 2001. The combination of tumor necrosis factor α blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 44:1293-1303. [DOI] [PubMed] [Google Scholar]
- 11.Chabaud, M., P. Garnero, J.-M. Dayer, P.-A. Guerne, F. Fossiez, and P. Miossec. 2000. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine 12:1092-1099. [DOI] [PubMed] [Google Scholar]
- 12.Choy, E. H. S., and G. S. Panayi. 2001. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 344:907-916. [DOI] [PubMed] [Google Scholar]
- 13.Christopherson, J. A., E. L. Munson, D. M. England, C. L. Croke, M. C. Remington, M. L. Molitor, D. J. DeCoster, S. M. Callister, and R. F. Schell. 2003. Destructive arthritis in vaccinated interferon gamma-deficient mice challenged with Borrelia burgdorferi: modulation by tumor necrosis factor alpha. Clin. Diagn. Lab. Immunol. 10:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, J. L., A. Trenado, D. Vasey, D. Klatzmann, and B. L. Salomon. 2002. CD4+ CD25+ immunoregulatory T cells: new therapeutics for graft-versus-host disease. J. Exp. Med. 196:401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croke, C. L., E. L. Munson, S. D. Lovrich, J. A. Christopherson, M. C. Remington, D. M. England, S. M. Callister, and R. F. Schell. 2000. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect. Immun. 68:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+ CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, J.-J. Pin, P. Garrone, E. Garcia, S. Saeland, D. Blanchard, C. Gaillard, B. D. Mahapatra, E. Rouvier, P. Golstein, J. Bachereau, and S. Lebecque. 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara, M., C. I. Kingsley, M. Niimi, S. Read, S. E. Turvey, A. R. Bushnell, P. J. Morris, F. Powrie, and K. J. Wood. 2001. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166:3789-3796. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, P., J. Ermann, M. Edinger, C. G. Fathman, and S. Strober. 2002. Donor-type CD4+ CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 196:389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317-5326. [PubMed] [Google Scholar]
- 22.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A. H. Enk. 2001. Identification and functional characterization of human CD4+ CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz, Y., O. Nadiv, M. J. Rapoport, and M. Loos. 2000. IL-17 regulates gene expression and protein synthesis of the complement system, C3 and factor B, in skin fibroblasts. Clin. Exp. Immunol. 120:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keitel, W. A. 1999. Cellular and acellular pertussis vaccines in adults. Clin. Infect. Dis. 28(Suppl. 2):S118-S123. [DOI] [PubMed] [Google Scholar]
- 25.Kotake, S., N. Udagawa, N. Takahashi, K. Matsuzaki, K. Itoh, S. Ishiyama, S. Saito, K. Inoue, N. Kamatani, M. T. Gillespie, T. J. Martin, and T. Suda. 1999. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 103:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levings, M. K., R. Sangregorio, and M. Roncarolo. 2001. Human CD25+ CD4+ T regulatory cells suppress naïve and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, and R. F. Schell. 1995. Borrelia burgdorferi-specific T lymphocytes induce severe destructive Lyme arthritis. Infect. Immun. 63:1400-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, J. R. Creson, S. D. Lovrich, S. M. Callister, D. A. Jobe, and R. F. Schell. 1994. Development of destructive arthritis in vaccinated hamsters challenged with Borrelia burgdorferi. Infect. Immun. 62:2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim, L. C. L., D. M. England, N. J. Glowacki, B. K. DuChateau, and R. F. Schell. 1995. Involvement of CD4+ T lymphocytes in induction of severe destructive Lyme arthritis in inbred LSH hamsters. Infect. Immun. 63:4818-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubberts, E., L. A. B. Joosten, B. Oppers, L. van den Bersselaar, C. J. J. Coenen-de Roo, J. K. Kolls, P. Schwarzenberger, F. A. J. van de Loo, and W. B. van den Berg. 2001. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J. Immunol. 167:1004-1013. [DOI] [PubMed] [Google Scholar]
- 31.Maloy, K. J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816-822. [DOI] [PubMed] [Google Scholar]
- 32.Morgan, M. E., R. P. M. Sutmuller, H. J. Witteveen, L. M. van Duivenvoorde, E. Zanelli, C. J. M. Melief, A. Snijders, R. Offringa, R. R. P. de Vries, and R. E. M. Toes. 2003. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 48:1452-1460. [DOI] [PubMed] [Google Scholar]
- 33.Papiernik, M., M. Leite de Moraes, C. Pontoux, F. Vasseur, and C. Pénit. 1998. Regulatory CD4 T cells: expression of IL-2Rα chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10:371-378. [DOI] [PubMed] [Google Scholar]
- 34.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sáez-Llorens, X., H. S. Jafari, K. D. Olsen, H. Nariuchi, E. J. Hansen, and G. H. McCracken, Jr. 1991. Induction of suppurative arthritis in rabbits by Haemophilus endotoxin, tumor necrosis factor-α, and interleukin-1β. J. Infect. Dis. 163:1267-1272. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance by activated T cells expressing IL-2 receptor α-chain (CD25). J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 37.Salomon, B., D. J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J. A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:431-440. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz, J. L., R. F. Schell, A. Hejka, D. M. England, and L. Konick. 1988. Induction of Lyme arthritis in LSH hamsters. Infect. Immun. 56:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suri-Payer, E., A. Z. Amar, A. M. Thornton, and E. M. Shevach. 1998. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212-1218. [PubMed] [Google Scholar]
- 40.Taams, L. S., J. Smith, M. H. Rustin, M. Salmon, L. W. Poulter, and A. N. Akbar. 2001. Human anergic/suppressive CD4+ CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31:1122-1131. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T. W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25+ CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969-1980. [DOI] [PubMed] [Google Scholar]
- 43.Taylor, P. A., R. J. Noelle, and B. R. Blazar. 2001. CD4+ CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 193:1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teunissen, M. B. M., C. W. Koomen, R. de Waal Malefyt, E. A. Wierenga, and J. D. Bos. 1998. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Investig. Dermatol. 111:645-649. [DOI] [PubMed] [Google Scholar]
- 45.Thorbecke, G. J., R. Shah, C. H. Leu, A. P. Kuruvilla, A. M. Hardison, and M. A. Palladino. 1992. Involvement of endogenous tumor necrosis factor α and transforming growth factor β during induction of collagen type II arthritis in mice. Proc. Natl. Acad. Sci. USA 89:7375-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton, A. M., and E. M. Shevach. 2000. Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183-190. [DOI] [PubMed] [Google Scholar]
- 47.Thorstenson, K. M., and A. Khoruts. 2001. Generation of anergic and potentially immunoregulatory CD25+ CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167:188-195. [DOI] [PubMed] [Google Scholar]
- 48.Yao, Z., S. L. Painter, W. C. Fanslow, D. Ulrich, B. M. Macduff, M. K. Spriggs, and R. J. Armitage. 1995. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155:5483-5486. [PubMed] [Google Scholar]
- 49.Yao, Z., W. C. Fanslow, M. F. Seldin, A.-M. Rousseau, S. L. Painter, M. R. Comeau, J. I. Cohen, and M. K. Spriggs. 1995. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3:811-821. [DOI] [PubMed] [Google Scholar]