Abstract
Fipronil is a phenylpyrazole insecticide commonly used in residential and agricultural applications. To understand more about the potential risks for human exposure associated with fipronil, urine and serum from dosed Long Evans adult rats (5 and 10 mg/kg bw) were analyzed to identify metabolites as potential biomarkers for use in human biomonitoring studies. Urine from treated rats was found to contain seven unique metabolites, two of which had not been previously reported—M4 and M7 which were putatively identified as a nitroso compound and an imine, respectively. Fipronil sulfone was confirmed to be the primary metabolite in rat serum. The fipronil metabolites identified in the respective matrices were then evaluated in matched human urine (n = 84) and serum (n = 96) samples from volunteers with no known pesticide exposures. Although no fipronil or metabolites were detected in human urine, fipronil sulfone was present in the serum of approximately 25% of the individuals at concentrations ranging from 0.1 to 4 ng/mL. These results indicate that many fipronil metabolites are produced following exposures in rats and that fipronil sulfone is a useful biomarker in human serum. Furthermore, human exposure to fipronil may occur regularly and require more extensive characterization.
Keywords: Fipronil, LC/TOF, Biomarker, Human exposure, Metabolism
1. Introduction
Fipronil (Fig. 1) is a phenylpyrazole broad-spectrum insecticide that is registered for use in residential settings as part of ant and cockroach baits and gels and termite control products; veterinary applications such as spot treatment flea and tick control products for dogs and cats; ornamental turf applications such as fire ant control; and agricultural applications such as pest control on potato crops (Brassard et al., 2011). When initially produced, fipronil was the first insecticide to act by targeting the gamma-aminobutyric acid (GABA) receptor and has favorable selective toxicity towards insects rather than mammals (Hainzl and Casida, 1996; Ikeda et al., 2004; Hainzl et al., 1998).
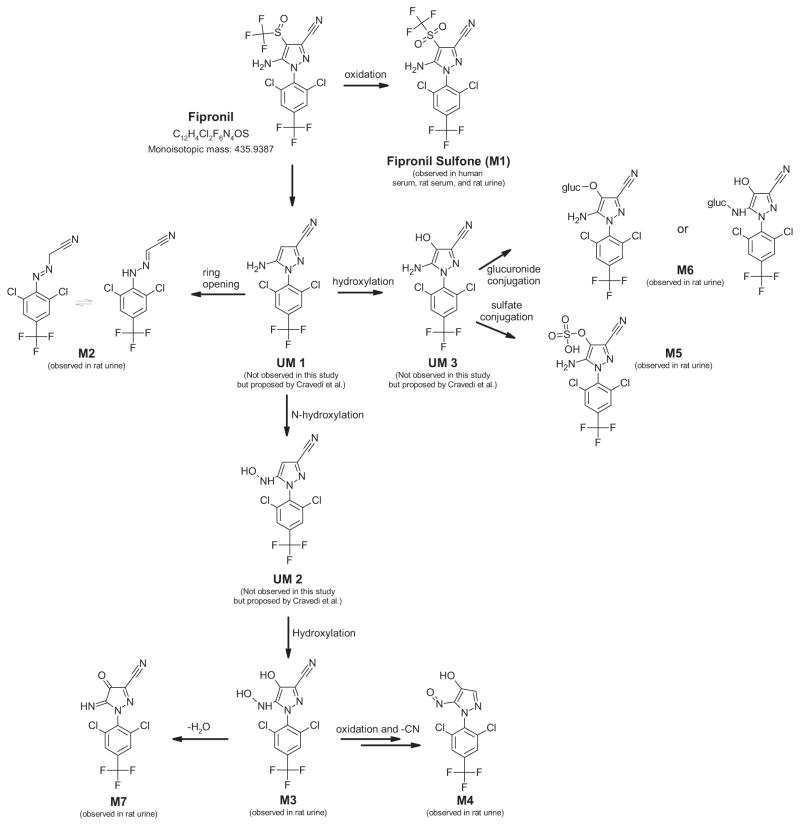
Fig. 1.
Proposed metabolic pathway of fipronil in the rat. M4 and M7 are proposed structures based on MS data, isotope distributions, and exact mass. M1, M2, M3, M5, and M6 were identified in rat urine. Unobserved metabolites labeled (UM) were not identified but are likely intermediates.
A 1997 report indicated that 480-metric tons of fipronil was produced per year by Rhône-Poulenc (1997), and a more recent EPA report indicated that between 1998 and 2008 usage averaged 68,039 kg of active ingredient per 607,028 ha (Brassard et al., 2011). Widespread fipronil use has led to contamination of water and soil (1–158 ng/L of parent or environmental degradate) in several states including, but not limited to Alabama, Georgia, California, Louisiana, and Indiana (Ryberg et al., 2010; Gunasekara et al., 2007). Perhaps as a result of this contamination, fipronil has been implicated as one of the chemicals associated with the bee colony collapse (Erickson, 2013).
Because little was found in the peer-reviewed literature about the disposition of fipronil, Cravedi et al. (2013) performed a thorough study on the metabolism, distribution, and elimination of fipronil in rats and showed that fipronil is primarily converted to fipronil sulfone (M1 Fig. 1), a more persistent metabolite (estimated half-life is 208 h in rodents) (Mohamed et al., 2004) which was stored mainly in adipose tissue and adrenals (Cravedi et al., 2013). In addition, fipronil has been associated with thyroid disruption (Tingle et al., 2003), endocrine disruption (Ohi et al., 2004), and neurotoxic effects (Raquel et al., 2011) in rats which has led to concern about the potential for human health effects.
The effects of acute human exposure to fipronil include headache, dizziness, vomiting, and seizures (Mohamed et al., 2004; Cravedi et al., 2013). Information on the effects of chronic exposure is limited, but the US EPA has classified fipronil as a possible human carcinogen based on data that shows an increase of thyroid follicular cell tumors in both sexes of the rat (Anon, 1996). Vidau et al. (2011) also concluded that fipronil has the potential to cause apoptosis by uncoupling oxidative phosphorylation at relatively low concentrations (5–10 μM) in human cell lines (Vidau et al., 2011), and a case of acute human self-poisoning with fipronil has demonstrated that fipronil levels can remain elevated in serum for days after exposure, and that fipronil sulfone was a primary metabolite (Mohamed et al., 2004). A previous study also showed that fipronil sulfone is the predominant metabolite in human liver microsomes via cytochrome P-450 oxidation (Tang et al., 2004).
Although one occupational exposure study of workers (n = 159) at a fipronil production facility reported a mean fipronil sulfone serum level of 7.8 (+/− 7.7 = SD) ng/mL (Herin et al., 2011), very little is known about human exposure to fipronil in the general population (Mohamed et al., 2004; Vidau et al., 2011; Herin et al., 2011). This may be because human samples can be difficult to obtain and analyze due to high concentrations of endogenous chemicals and significant matrix effects which make the identification of metabolites difficult. Literature on the potential routes of human exposure includes one article by Dyk et al. that describes the potential for non-occupational human exposure through contact with pets that have received fipronil applications in the form of flea and tick treatments (Dyk et al., 2012), and a few other studies that have observed fipronil in various environmental media relevant to human exposure (indoor/outdoor dust Mahler et al., 2009, wastewater Stone et al., 2014, surface water Ryberg et al., 2010; Stone et al., 2014 and residential runoff Gan et al., 2012).
The specific objectives of the study were to characterize human exposure by developing a unique workflow where dosed animal samples were used to identify potential serum/urine biomarkers via time-of-flight mass spectrometry. These biomarkers were subsequently evaluated in serum and urine of a group of volunteers from North Carolina.
2. Materials and methods
2.1. Chemicals
Unlabeled fipronil (5-amino-1-[2,6-dichloro-4-(trifluoromethyl)-phenyl]-4-(trifluoromethylsulfinyl)-1-H-pyrazole-3-carbonitrile, >99%) and its metabolites: fipronil sulfone (5-amino-1-[2,6-dichloro-4-(trifluoromethyl)-phenyl]-4-[(trifluoromethyl)sulfonyl]-1H-pyrazole-3-carbonitrile, >99%), fipronil sulfide (5-amino-1-[2,6-dichloro-4-(trifluoromethyl)-phenyl]-4-[(trifluoromethyl)thio]-1H-pyrazole-3-carbonitrile, 98%), fipronil amide (5-amino-1-[2,6-dichloro-4-(trifluoromethyl)-phenyl]-4-[(trifluoromethyl)thio]-1H-pyrazole-3-carboxamide, >99%), and monochloro fipronil (5-amino-1-[2-chloro-4-(trifluoromethyl)-phenyl]-4-[(trifluoromethyl)sulfinyl]-1H-pyrazole-3-carbonitrile, >97%) were procured as solid analytical standards from the pesticide repository through the US EPA (Fort Meade, MD, USA). These five analytical standards were prepared as a mixture in acetonitrile and used for all subsequent matrix-matched standard curves. The internal standard fipronil des-F3 (see supporting information SI Fig 6 for structure) (5-amino-1-[2,6-dichloro-4-(trifluoromethyl)-phenyl]-4-(methylsulfinyl)-1-H-pyrazole-3-carbonitrile, 99%, 0.1 ng/μL in Acetonitrile) was ordered from Crescent Chemical Company (Islandia, NY, USA).
Acetonitrile and methanol (B&J Brand HighPurity Solvent) were purchased from Honeywell Burdick & Jackson (Muskegon, MI, USA) and ammonium acetate from Sigma Aldrich (St. Louis, MO, USA). Deionized water was generated in house from a Barnsted Easypure UV/UF (Dubuque, IA, USA) coupled with activated charcoal and ion exchange resin canisters.
2.2. Animals
This portion of the study was part of an investigation of the neurotoxic effects of fipronil in rodents (Freeborn et al., 2015; Moser et al., 2015). The animal facility is accredited by the American Association for Accreditation of Laboratory Animal Care International, and all protocols were approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee at the United States Environmental Protection Agency. Male Long Evans rats (60–90 days old) were acquired from Charles River Laboratories (Wilmington, MA). Animal husbandry details are provided in the Supporting Information. Animals were dosed daily by oral gavage at either 5 (lowest dose) or 10 (highest dose) mg/kg with fipronil suspended in corn oil (1 mL/kg) every 24 h for two weeks. Control rats were gavaged with corn oil only. Six hours after the 14th dose, rats were euthanized. Trunk blood (2 mL) was collected in tubes without anticoagulant and stored on ice for 1–1.5 h. The samples were centrifuged at 1300 ×g for 30 min at 4 °C. The serum was collected, frozen on dry ice, and stored at −80 °C until analysis. Urine was collected in a syringe either from voids on a clean table or via bladder puncture and transferred to a micro-centrifuge tube, immediately frozen on dry ice, and stored at −80 °C until analysis.
2.3. Human samples
Matched human urine (n = 84) and serum (n = 96) samples, from individuals with no known fipronil exposure, were collected by the National Institute for Environmental and Health Sciences (NIEHS protocol number 10-E-0063) between April and June 2011. The human samples were simply a sample of convenience and were not meant to be representative of a specific population. The urine collected was a spot sample and was not concentrated or representative of a specific sampling period. Volunteers were anonymous, and no personally identifiable information was provided. The samples were from male and female volunteers of various ethnicities between 19 and 73 years of age who live in the Raleigh–Durham area of North Carolina (Table 1). Although 100 volunteers participated in the study, several urine and serum samples were not included due to an insufficient volume for analysis.
Table 1.
Human demographic data for the 100 volunteers.
| Sex
|
Age
|
Race
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | 19–33 | 34–48 | 48–62 | 62–76 | Asian | Black | White | Other | |
| % | 30 | 70 | 29 | 30 | 33 | 8 | 3 | 32 | 63 | 2 |
2.4. Extraction protocols
Samples were extracted in a manner that optimized recovery and reproducibility while reducing matrix interference. Animal samples were small volumes that did not require solid phase extraction (SPE). However, a protocol involving SPE was performed with the human samples to reduce matrix interference. Sample extraction protocols for biologicals are described below. More information on method development for human samples can be found in the Supporting Information. Rat serum samples were first analyzed by liquid chromatography/time-of-flight mass spectrometer (LC/TOF-MS) in order to identify any metabolites. Human samples were then analyzed by liquid chromatography/triple-quadrupole mass spectrometer (LC/triple-quad) for quantification of metabolites for which analytical standards were possessed. LC/quadrupole/time-of-flight mass spectrometry (LC/Q-TOF) was used for structure elucidation of unknown metabolites.
2.5. Rat serum
Rat serum (25 μL) was denatured with 100 μL of 0.1 M formic acid and precipitated with 1 mL of a cold acetonitrile solution spiked with the internal standard (fipronil des-F3, 25 ng). The sample was then centrifuged for 5 min at 12,500 ×g. An aliquot of the supernatant was mixed 50:50 with 10 mM ammonium acetate buffer, and analyzed via LC/TOF and LC/triple-quad. n = 9 for the highest dose (10 mg/kg/day); n = 10 for low dose (5 mg/kg/day); and n = 11 for control animals, which were treated with vehicle. Quantitation was performed for fipronil and fipronil sulfone. The results of the quantitation are shown in the supporting information.
2.6. Rat urine
Rat urine (100 μL) was precipitated with 900 μL of cold acetonitrile and centrifuged for 8 min at 12,500 ×g. An aliquot of the supernatant was extracted and mixed 50:50 with 10 mM ammonium acetate buffer before LC/MS analysis. n = 3 for the highest dose (10 mg/kg/day); n = 4 for low dose (5 mg/kg/day); and n = 3 for control animals. Quantitation was only performed for the fipronil sulfone metabolite, as standards were not available for other metabolites. Quantitation specifics can be found in the Supporting Information. Fipronil sulfone concentrations in rat urine were used to approximate the relative concentrations of the other observed metabolites.
2.7. Human serum
Human serum (200 μL; n = 96) was denatured with 20 μL of a 0.1 M formic acid solution spiked with internal standard (fipronil des-F3, 5 ng) and precipitated with 2 mL of cold acetonitrile. The sample was centrifuged for 10 min at 12,500 ×g and concentrated using solid phase extraction (SPE) using an Oasis 3 cc HLB cartridge (Waters Corporation, Milford, MA). SPE cartridges were conditioned with 3 mL of methanol and 3 mL of ultrapure water, samples were loaded, washed with 3 mL of 95:5 water/acetonitrile solution, then eluted with 3 mL of acetonitrile. The eluate was evaporated under N2 at 40 °C until approximately 200 μL remained. The concentrated solution was mixed 50:50 with 10 mM ammonium acetate buffer and analyzed via LC/TOF and LC/triple-quad for all compounds listed in Section 2.1. In order to determine the concentration of compounds of interest, a seven-point matrix-matched (blank calf serum-Life Technologies-Gibco®, Grand Island, NY) extracted standard curve from 0.1 to 50 ng/mL, along with a method blank (DI water) and a matrix blank was run with the human serum samples.
2.8. Human urine
Human urine (5–12 mL; n = 84) was precipitated with 1 mL of acetonitrile and concentrated using the SPE method described above with an Oasis 6 cc HLB cartridge with the exception that cartridges were conditioned with 5 mL of methanol and 5 mL of ultrapure water, samples were loaded, washed with 5 mL of 95:5 water/acetonitrile solution, then eluted with 5 mL of acetonitrile. The eluate was evaporated under N2 at 40 °C until approximately 1 mL remained. The concentrated solution was mixed 50:50 with 10 mM ammonium acetate buffer in an LC vial and analyzed by LC-TOF/MS (n = 84) for all compounds listed in Section 2.1, as well as for other unknown metabolites. Note that 16 urine samples were excluded due to insufficient volume.
2.9. Analytical Instrumentation
Targeted analyses (LC/triple-quad) were carried out using an Agilent 1100 HPLC (Agilent Technologies, Palo Alto, CA) interfaced with a Sciex 3000 triple quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA) fitted with an electrospray ionization source (ESI) operated in the negative ionization mode. Compounds contained in the LC/triple-quad method (fipronil, fipronil sulfone, fipronil sulfide, fipronil amide, and monochloro fipronil) were optimized on a compound specific basis. Information regarding transitions are included in the Supporting Information.
The HPLC system consisted of a Phenomenex Luna C18 column (50 × 3 mm, 5 μm; Torrance, CA, USA) with a Security-guard guard column (Phenomenex). The method consisted of the following: 0.4 mL/min flow rate which increased to 0.75 mL/min at time = 2 min; temperature: 30 °C; mobile phases — A: ammonium acetate buffer (0.2 mM) and DI water:methanol (95:5, v/v), and B: ammonium acetate buffer (0.2 mM) and acetonitrile:DI water (95:5, v/v); gradient: 0–2 min 50% A and 50% B; 2.1–4 min, a linear gradient from 50:50 A:B to 10:90 A:B; 4–6 min 10% A and 90% B; 6.1–10 min re-equilibration to 50% A and 50% B.
Non-targeted analyses (LC/TOF) were carried out using an Agilent 1100 HPLC (Agilent Technologies, Palo Alto, CA) interfaced with an Agilent 6210 (TOF) mass spectrometer fitted with an electrospray ionization (ESI) source operated in the negative ionization mode at 120 V. Any drift in the mass accuracy of the TOF was continuously corrected by infusion of two reference compounds (purine [m/z = 119.0363] and hexakis (1H, 1H, 3H-tetrafluoropropoxy) phosphazene [m/z = 966.0007]) via dual-ESI sprayer.
The HPLC method consisted of a Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 3.5 μm; Agilent Technologies, Palo Alto, CA) fitted with a Phenomenex guard column (Torrance, CA). The method consisted of the following: 0.2 mL/min flow rate; at 30 °C; mobile phases: A: ammonium formate buffer (0.4 mM) and DI water:methanol (95:5 v/v), and B: ammonium formate (0.4 mM) and methanol:DI water (95:5 v/v); gradient: 0–5 min a linear gradient from 50:50 A:B to 100% B; 5–15 min, 100% B; 15–18 min re-equilibration to 50% A and 50% B.
2.10. Identification of spectral features
The TOF-MS system has proprietary software that can be used in non-targeted analyses to help identify compounds that are specific to a treatment group or a specific experimental condition. For example, to identify potential biomarkers of fipronil exposure, control and dosed animal samples are analyzed, and molecular features (identifiable peaks) were first extracted according to user specified criteria (e.g., minimum peak height, area count). The two groups of extracted features were then compared using The Mass Profiler software, which singles out only those compounds that are found in the dosed group. This collection of compounds can be thought to represent either the parent compound, metabolites of the parent, or specific biological responses that are attributable to the treatment administered.
The exact monoisotopic mass of each of these “treatment only” features was then used to generate a ranked list of possible chemical formulae for each unknown. The numerical ranking is based on the difference between the calculated and measured mass, the isotopic abundance and the isotope spacing. If authentic standards are available, the identity of a proposed feature can be confirmed using chromatographic retention time, exact monoisotopic mass, and isotopic distribution.
Fipronil is an interesting and somewhat unique compound because it contains six fluorine atoms and two chlorine atoms, that result in a significant negative mass defect (435.93869 Da, with the [M–H]− ion seen in negative ionization mode being 434.9314 m/z) which is preserved in most of its metabolic products to the extent that the F and Cl atoms are retained (Xie et al., 2012). Moreover, the isotopic spacing between the Cl isotopes (Cl-35 [75.77%] and Cl-37 [24.23%]) leads to a distinctive isotopic pattern that aids greatly with identification (SI Fig. 2). Both of these characteristics were useful in identifying fipronil-related metabolites.
Metabolites that were identified using the LC/TOF instrument described above were then investigated further using an Agilent 1200 HPLC system fitted with a 6250 quadrupole time-of-flight mass spectrometer (Q-TOF) (Agilent Technologies, Palo Alto, CA) using the same LC conditions as previously described. The LC/Q-TOF allowed fragmentation at various collision energies of metabolites of interest which helped with structure elucidation.
2.11. Quality assurance/control
For each analysis, method and matrix blanks were evaluated for contamination or background levels of the compounds of interest. The LLOQ was determined as the concentration of the lowest working standard, which back-predicted within 30% of a theoretical value. The LLOQ in the quantitative human serum experiments was validated by calculating signal-to-noise ratios for the fipronil sulfone (451–415 m/z) transition relative to a method blank. R-squared values for all quantitative procedures were monitored to ensure predictability. Three randomly chosen samples were replicated in each quantitative experiment to ensure consistency within the data sets. Parent–daughter ratios should be consistent between unknowns and authentic standards, and ratio monitoring is a robust way to confirm the presence of a specific compound. Therefore, in the targeted screening of samples, the ratio between the primary and secondary parent–daughter transition was monitored to confirm the presence of each compound in the MS method. High and low concentration quality control (QC) samples containing the fipronil mixture of five analytical standards described in Section 2.1 were run with each batch of human serum samples to ensure analytical precision and accuracy.
3. Results
3.1. Quality assurance/control
All lab prepared target and non-target analysis blanks and control samples were below the respective LLOQ for compounds of interest in all experiments. Validation of the LLOQ in the human serum quantitative experiments showed that the lowest curve point differed from the method blank (signal-to-noise ratio for method blank = 3 ± 1; signal-to-noise ratio for 0.1 ng/mL standard = 20 ± 12). All r-squared values were greater than 0.99, which ensured predictability. All replicates for all experiments had a relative standard deviation of <15%. For all targeted analyses, the ion ratios between the primary and secondary parent–daughter transitions were consistent for all standard compounds (mean ± 20%). All QC samples (high and low) were 100% ± 15% of the nominal values.
3.2. Urine from treated rodents
The urine from rodents treated for 14 days with fipronil was analyzed for biomarkers of exposure via non-targeted analysis. As described above, molecular features (significant chromatographic peaks) were extracted from analytical runs of both dosed and control animals, and Mass Profiler software was used to isolate those features that were unique to the dosed animals. The most plausible candidate biomarkers were those compounds with the signature isotope pattern of two chlorine atoms (SI Fig. 2) and/or negative mass defects indicative of fluorine and chlorine atoms. Seven high abundance peaks fitting these criteria were identified, and the exact monoisotopic mass of each was used to generate a ranked list of plausible formulae and corresponding structures. We tentatively assigned compound identity according to known metabolic pathways (e.g., oxidation, sulfation, glucuronidation), the retention of negative mass defect and/or the isotopic pattern associated with chlorine, and consistency with results from previous studies. Information on the seven metabolites can be found in Table 2. Four of the compounds (M1, M2, M3, M5, and M6) were identified in previous studies (Cravedi et al., 2013; Anon, 2001), whereas two more (M4 and M7) are reported for the first time in this study (Fig. 1). It should be noted that the spectral feature observed for the glucuronide conjugate (M6) splits into two chromatographic peaks, most likely meaning that the glucuronide molecule adds to both the oxygen and the nitrogen atom (see Fig. 1). We were unable to differentiate which peak corresponded to which structure, but one was formed preferentially. However, sulfation appears to occur only at one site for M5, as only one chromatographic peak was observed.
Table 2.
LC/TOF characteristics of putative metabolites in rat urine.
| Metabolite | Retention time (min) | Predicted formula of parent | Score of predicted formula | [M–H]− measured mass (m/z) | [M–H]− calculated mass (m/z) | Δ ppm | Monoisotopic mass (m/z) |
|---|---|---|---|---|---|---|---|
| Ml (fipronil sulfone) | 7.57 | C12H4Cl2F6N4O2S | 99.63 | 450.9266 | 450.9263 | 0.67 | 451.9336 |
| M2 | 7.3 | C9H4Cl2F3N3 | 93.50 | 279.9665 | 279.9662 | 1.07 | 280.9734 |
| M3 | 1.62 | C11H4O2N4Cl2F3 | 99.53 | 350.9667 | 350.9669 | 0.43 | 351.9742 |
| M4 | 5.38 | C10H4Cl2F3N3O2 | 98.63 | 323.9565 | 323.9560 | 1.54 | 324.9633 |
| M5 | 1.4 | C11H5Cl2F3N4O4S | 99.38 | 414.9290 | 414.9288 | 0.48 | 415.9361 |
| M6 | 1.39 | C17H13Cl2F3N4O7 | 98.74 | 511.0036 | 511.0041 | 0.98 | 512.0113 |
| M7 | 5.38 | C11H3Cl2F3N4O | 98.93 | 332.9564 | 332.9563 | 0.30 | 333.9563 |
To better characterize the structures of metabolites M4 and M7, the fragmentation patterns of the parent metabolites were analyzed via LC/Q-TOF. No useful information was gained about metabolite M4; however, the fragmentation pattern of metabolite M7 helped to predict a plausible structure. M7 structural information could be gleaned from looking at the exact masses of molecular fragments originating from the parent molecule. For example, if the mass of a CO2 group is observed in the fragmentation pattern, it can be assumed that the molecule likely contained a carboxylic acid. Spectral information regarding this fragmentation pattern can be found in the Supporting Information (SI Fig. 3).
Fipronil sulfone (M1) was confirmed by an authentic standard that had the same retention time, monoisotopic mass, ion fragmentation pattern, and isotope spacing. Rats in the 5 mg/kg/day dose-group had mean concentrations of fipronil sulfone of 24.1 (+/−18.7) ng/mL, while the 10 mg/kg/day group had 31.9 (+/−13.1) ng/mL (SI Fig. 1). If the fipronil sulfone concentrations are used to generate estimated relative response factors for other metabolites that did not have standards (assuming that all respond similarly within the TOF-MS), we estimated the relative concentrations of fipronil metabolites in dosed-rodent urine to be M6 > M4 > M5 > M3 > M7 > M1 > M2. The estimated concentrations of M2 and M6 are 30 and 2000 ng/mL respectively.
3.3. Serum from treated rodents
The serum from treated rats was analyzed for all suspected biological metabolites via LC-TOF to evaluate the presence of possible serum biomarkers. In our analysis we detected no additional metabolites other than small amounts of un-metabolized fipronil and fipronil sulfone which had been previously identified by several groups (Hainzl et al., 1998; Lacroix et al., 2010). Quantitative data for fipronil and fipronil sulfone in rat serum can be found in the Supporting Information.
3.4. Human urine
Urine samples (n = 84) from volunteer North Carolina residents with no known exposures to fipronil or other pesticides were examined for M1–M7 (identified in rodent urine) and for all other plausible fipronil adducts or derivatives using the methods described above. No parent fipronil or any plausible metabolites were found in the human urine samples.
3.5. Human serum
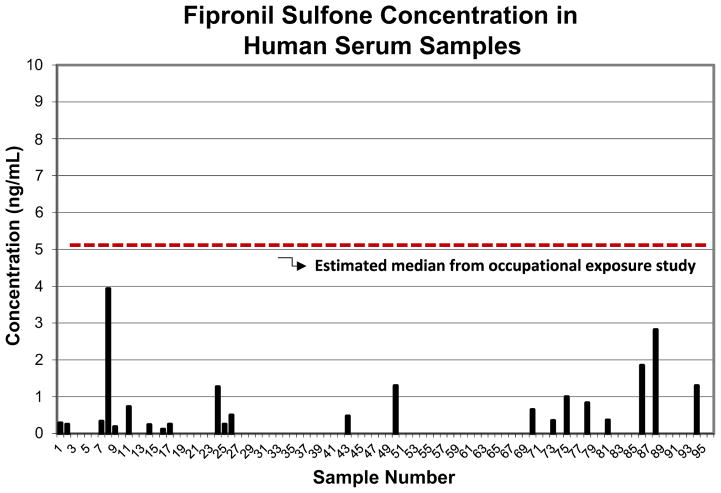
Matched human serum samples (n = 96) were analyzed for the metabolites observed in rat serum (fipronil and fipronil sulfone) by a targeted approach (LC/triple-quad, LOQ = 0.1 ng/mL). Only trace amounts of the parent fipronil were found in the human blood samples. However, fipronil sulfone (the biomarker identified in the rodent study) was detected in approximately 25% of the samples, at levels ranging from 0.1 to 3.9 ng/mL (Fig. 2). Table 3 describes general trends in the data in terms of detects vs. non-detects.
Fig. 2.
Fipronil sulfone concentrations in human serum*. The dotted line represents the median calculated from an occupational exposure study (Herin et al., 2011; Pleil et al., 2014). *n = 96, four samples were excluded due to insufficient volume.
Table 3.
Detects vs. non-detects for each gender and race.
| Gender | Detects | Non-detects | Number of samples |
|---|---|---|---|
| Male | 7 | 12 | 29 |
| Female | 17 | 67 | 67 |
| Race | Detects | Non-detects | Number of samples |
|
| |||
| Caucasian | 22 | 39 | 61 |
| African American | 1 | 29 | 30 |
| Asian | 1 | 2 | 3 |
| Other | 0 | 2 | 2 |
4. Discussion
This study demonstrates how advanced time-of-flight mass spectrometry techniques can be used to more fully describe the metabolism of xenobiotic compounds in treated-animal studies and how this knowledge can be applied in human biomonitoring studies to make relevant conclusions about human exposures to emerging compounds of concern. Our specific goal was to use the biomarkers identified from the dosed rodent work in the analysis of a set of human biological samples to characterize the rate of fipronil exposure in the general population.
In describing the metabolism of fipronil in rodents, our results were largely consistent with previous studies (Cravedi et al., 2013; Anon, 1997, 2001), while also extending what is known about the basic metabolic process. The two novel metabolites observed in rat urine in this study which were not seen by Cravedi et al. (2013) can be attributed to differences in study design. Specifically, our Long Evans rats were dosed (5 or 10 mg/kg/day) for 14 days and then sacrificed 6 h after the last dose. In contrast, Cravedi et al. (2013) dosed acutely at 10 mg/kg and collected urine and serum every 24 h over a 72 h period (Cravedi et al., 2013). Differences between rat strain or length of dosing regimen may have made it possible to identify different products of fipronil metabolism, such as the pyrazole ring opened products or the highly oxidized heteroaromatic amine derivatives.
The proposed metabolic pathway in the rat and compound structures are found in Fig. 1. We propose that a new metabolite (M7) in rat urine, an imine, results from the loss of water from metabolite M3, which is a fipronil metabolite that is hydroxylated at both the carbon and the nitrogen. We also identified what is hypothesized to be nitroso compound (M4). We believe that M3 and M4 are formed from an unobserved hydroxyl amine intermediate (UM 2). The hydroxyl amine (M3) has been identified in this and in previous studies (Cravedi et al., 2013), but to our knowledge this is the first report of a nitroso metabolite of fipronil in rat urine. Although the structure for metabolite M4 is only putative, heterocyclic aromatic amines are known to undergo biological oxidation to form nitroso compounds. This process is mediated by cytochrome P-450 and NADPH (Kim and Guengerich, 2005). Many heterocyclic amines are known carcinogens (Snyderwine et al., 1997; Eisenbrand and Tang, 1993; Pezdirc et al., 2013; Nagao and Sugimura, 1993; Schut and Snyderwine, 1999), due to their ability to be hydroxylated and then form DNA adducts. The observation of N-hydroxylated fipronil metabolites in this and other rodent studies warrants further investigation of fipronil metabolism in humans and the resulting effects.
Noninvasive biomarkers like those present in urine, exhaled breath, hair, fingernails, etc. are optimal for use in human studies, and one intention of this study was to explore whether any of the urinary metabolites found in the rats could be used as biomarkers of exposure in humans. Studies with human liver microsomes have shown that fipronil is metabolized to fipronil sulfone in vitro, and Mohamed et al. (2004) have identified fipronil sulfone as a metabolite in humans acutely exposed to high doses (Mohamed et al., 2004; Tang et al., 2004). Aside from these, no publications comment on the disposition of fipronil in humans. In this study we analyzed human urine samples for any of the metabolites identified as possible biomarkers in rat urine. The absence of fipronil and its metabolites in the human urine samples was undoubtedly related to many factors. To start with, it is possible that most human elimination of these materials occurs via the feces, as is the case with rodents (Anon, 1996, 1997). Secondly, and perhaps more importantly, our study subjects were essentially volunteers from the Raleigh–Durham area of North Carolina with no known exposures to fipronil and/or any other similar pesticides. Identification of small amounts of unknown chemicals in urine from populations with no known exposure can be difficult due to the large amount of endogenous compounds found in the matrix. A more effective strategy would be to work with a group of individuals with higher exposure levels (preferably occupationally) to determine human urinary metabolites. Despite negative findings with the human urine samples, 25% of the serum samples contained measureable amounts of fipronil sulfone (range of 0.1–4 ng/mL), providing clear evidence that humans are regularly exposed to fipronil.
We compared our results to those from a study by Herin et al. where the serum from workers in a fipronil production facility was measured for fipronil and fipronil sulfone. The median from the occupational exposure study was calculated from the mean (μ) and standard deviation (σ) provided via a method by Pleil et al. (2014) where the geometric mean is used to estimate the median which is equal to μ/[1 + 0.5 × (σ/μ)2]. Interestingly, the maximum concentration observed in this study (3.9 ng/mL) was only slightly less than the calculated median of 5.2 (GSD = 2.4) ng/mL for the occupationally exposed workers (Herin et al., 2011) (see Fig. 2), where error is represented in terms of the geometric standard deviation (GSD).
The general population likely shares specific exposure routes. One of the most likely routes of exposure is contact with pets that have received applications of fipronil (i.e. Frontline® Plus) or have had contact with indoor/outdoor applications around the home. Notably, Morgan et al. (2008) concluded that family pets can act as vehicles for human exposure to the organophosphorous insecticides, such as diazinon (Morgan et al., 2008). Specifically, fipronil is widely used to control residential insect pests such as termites and fire ant outdoors where pets are frequent, leading to transport of the material indoors. Furthermore, many flea and tick topical products contain approximately 10% fipronil and are applied directly to the skin and fur of dogs and cats, leading to human exposure through direct contact with pets. Dyk et al. (2012) used a fluorescent indicator to show that these fipronil residues are easily transferred from pets to humans by way of direct contact for one week following application (Dyk et al., 2012). According to estimates from the American Humane Association, up to 46% and 39% of US households keep dogs and cats, respectively. Use of fipronil containing products with these animals could conceivably result in some measurable human exposures. Ongoing efforts in our lab (data not shown) are investigating domestic indoor sources of exposure that may be important, since local WWTP effluent is shown to contain fipronil and metabolites.
Although the study was well-designed, it did have a few limitations. First, the fipronil sulfone metabolite may not be a specific biomarker for fipronil exposure, since it is known that fipronil can undergo photo-chemical oxidation (Hainzl and Casida, 1996) and fipronil sulfone’s presence has been documented in environmental media by several reports (Gunasekara et al., 2007; Ensminger et al., 2013). Therefore, one could be exposed to either fipronil or the degradate. In addition, our sample size was relatively small (n = 100). Furthermore, the number of detects was less than 30% of the total sample; which did not warrant a statistical analysis. More work is needed on a larger and more diverse sample before further conclusions can be drawn. It may be worth mentioning, however, was that approximately 92% of fipronil sulfone detections in human serum were from Caucasians, which represented only 63% of our samples. This result suggests that discrepancies between ethnicities may be present.
While the target of fipronil is insects, the two trifluoromethyl groups of fipronil may increase the compound’s absorption and distribution upon accidental exposure by humans. Approximately 20–25% of drugs produced in the pharmaceutical industry contain at least one strategically incorporated fluorine atom (usually in the form of either one fluorine atom or a trifluoromethyl group) because fluorine can significantly impact lipophilicity and improve the bioavailability of orally administered drugs. Several studies have shown that the addition of fluorine, the most electronegative element, can decrease the pKa and therefore basicity of surrounding functional groups (van Niel et al., 1999; Rowley et al., 2001). Although the effect is not always predictable, this decreased basicity stabilized molecules in the harsh acidic conditions of the stomach and increases bioavailability (Chambers, 2000; Morgenthaler et al., 2007). Another factor that affects the absorption and distribution of a molecule is lipophilicity. Compounds usually enter into cell membranes via passive transport (although active transport is an alternate mechanism). Passive transport requires that the molecule is able to permeate the cell membrane, but also avoid entrapment by the lipid bilayer. The electron withdrawing capabilities of fluorine can, in some cases, be incorporated to tune a compound’s lipophilicity and ease passive transport into cells (Purser et al., 2008; Smart, 2001; Smith et al., 2006). Fipronil’s presence in human serum demonstrated that the chemical is, in fact, absorbed by humans. Further, Hainzl and Casida (1996) found that fipronil lost almost all activity in neurotoxicity studies on mice without the trifluoromethylsulfinyl functional group (Hainzl and Casida, 1996). Metabolites of fipronil have also been found in many rat tissues, including brain cells (Hainzl and Casida, 1996; Hainzl et al., 1998; Cravedi et al., 2013), demonstrating that even highly selective membranes are somewhat permeable to these chemicals. The fluorinated functional groups may increase fipronil’s potency as an insecticide; however, they may also increase absorption and distribution of the potentially toxic compound in non-target organisms, such as humans. Considering that fipronil has been associated with endocrine disruption, neurotoxicity, and carcinogenicity (Ohi et al., 2004; Raquel et al., 2011; Anon, 1996; Vidau et al., 2011), accidental exposure and increased bioavailability may be problematic.
In conclusion, previously reported metabolites in rat urine and serum were confirmed, and two novel urinary metabolites have been proposed. The putative biomarkers determined in the rodent study were used in human serum analysis, where fipronil sulfone was found in approximately 25% of serum samples of convienence from North Carolina residents. Serum fipronil levels in our study suggest that environmental exposures to fipronil may be common, but likely lower than occupational exposures. Matched urine was also analyzed, but no fipronil or any of its metabolites were identified, which suggests that urine may not be an appropriate matrix for biomonitoring populations with no known exposure to fipronil. More extensive characterization of the metabolites produced in humans exposed to higher levels of fipronil, as well as the effects from low but chronic exposure to fipronil is needed. Further investigations are also necessary to describe the sources of fipronil exposure and identify rates of exposure in other populations.
Supplementary Material
Acknowledgments
This research was supported by an appointment to the Research Participation Program at the National Exposure Research Laboratory administered by the Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. Environmental Protection Agency. We thank Agilent Technologies for providing us with the LC/TOF mass spectrometer that was used to investigate metabolic products. We also thank Michael Hays of the US EPA who kindly allowed the use of his LC/Q-TOF mass spectrometer for further structure elucidation, Benny Pyke of Arizona State University for discussions on detection of fipronil in human urine, and Matthew Stiegel, Jon Sobus, and Peter Egeghy for help with data analysis.
Abbreviations
- DI
deionized
- ESI
electrospray ionization
- GABA
gamma-aminobutyric acid
- GSD
geometric standard deviation
- HPLC
high performance liquid chromatography
- LC
liquid chromatography
- LLOQ
lower limit of quantitation
- MS
mass spectrometry
- NIEHS
National Institute for Environmental Health Sciences
- QC
quality control
- Q-TOF
quadrupole time-of-flight
- % RSD
Percent Relative Standard Deviation
- SD
standard deviation
- SPE
solid phase extraction
- TOF
time-of-flight
- UPLC
ultra performance liquid chromatography
- US EPA
United States Environmental Protection Agency
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.envint.2015.01.016.
Footnotes
Author contributions
The manuscript was written through equal contributions of all authors. All authors have given approval to the final version of the manuscript.
Disclaimer
This article will be reviewed in accordance with the policy of the National Exposure Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the view and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- New Pesticide Fact Sheet for Fipronil. US EPA.
- In Pesticide Residues in Food. Fipronil: Residue Evaluation, Joint Meeting on Pesticide Residues in Food, 2001.
- In Pesticide Residues in Food. Fipronil: Residue evaluation, Joint Meeting on Pesticide Residues in Food, 1997.
- Brassard D, Haddad S, Hill E. US EPA BEAD Chemical Profile for Registration Review: Fipronil (PC Code: 129121) 2011. [Google Scholar]
- Chambers RD. Fluorine in Organic Chemistry. Blackwell Publishing; Oxford: 2000. [Google Scholar]
- Cravedi JP, Delous G, Zalko D, Viguie C, Debrauwer L. Disposition of fipronil in rats. Chemosphere. 2013;93:2275–2283. doi: 10.1016/j.chemosphere.2013.07.083. [DOI] [PubMed] [Google Scholar]
- Dyk MB, Liu Y, Chen Z, Vega H, Krieger RI. Fate and distribution of fipronil on companion animals and in their indoor residences following spot-on flea treatments. J Environ Sci Health B. 2012;47:913–924. doi: 10.1080/03601234.2012.706548. [DOI] [PubMed] [Google Scholar]
- Eisenbrand G, Tang W. Food-borne heterocyclic amines. Chemistry, formation, occurrence, and biological activities A literature review. Toxicology. 1993;12:1–82. doi: 10.1016/0300-483x(93)90109-6. [DOI] [PubMed] [Google Scholar]
- Ensminger M, Budd R, Kelley K, Goh K. Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008–2011. Environ Monit Assess. 2013;185(5):3697–3710. doi: 10.1007/s10661-012-2821-8. [DOI] [PubMed] [Google Scholar]
- Erickson BE. Europe to ban fipronil pesticide to protect bees. Chem Eng News Arch. 2013;91(29):21. [Google Scholar]
- Freeborn DL, McDaniel KL, Moser VC, Herr DW. Use of electroencephalography (EEG) to assess CNS changes produced by pesticides with different modes of action: effects of permethrin, deltamethrin, fipronil, imidacloprid, carbaryl, and triadimefon. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2014.11.011. http://dx.doi.org/10.1016/j.taap.2014.11.011. [DOI] [PubMed]
- Gan J, Bondarenko S, Oki L, Haver D, Li JX. Occurrence of fipronil and its biologically active derivatives in urban residential runoff. Environ Sci Technol. 2012;46(3):1489–1495. doi: 10.1021/es202904x. [DOI] [PubMed] [Google Scholar]
- Gunasekara AS, Truong T, Goh KS, Spurlock F, Tjeerderma RS. Environmental fate and toxicology of fipronil. J Pestic Sci. 2007;32:189–199. [Google Scholar]
- Hainzl D, Casida JE. Fipronil insecticide: novel photochemical desulfinylation with retention of neurotoxicity. Proc Natl Acad Sci. 1996;93:12764–12767. doi: 10.1073/pnas.93.23.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainzl D, Cole LM, Casida JE. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem Res Toxicol. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- Herin F, Boutet-Robinet E, Levant A, Dulaurent S, Manika M, Galatry-Bouju F, Caron P, Soulat J-M. Thyroid function tests in persons with occupational exposure to fipronil. Thyroid. 2011:21. doi: 10.1089/thy.2010.0449. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Nagata K, Kono Y, Yeh JZ, Narahashi T. Fipronil modulation of GABAA receptor single-channel currents. Pest Manag Sci. 2004;60(5):487–492. doi: 10.1002/ps.830. [DOI] [PubMed] [Google Scholar]
- Kim D, Guengerich FP. CYTOCHROMEP450 activation of arylamines and heterocyclic amines. Annu Rev Pharmacol Toxicol. 2005;45:27–49. doi: 10.1146/annurev.pharmtox.45.120403.100010. [DOI] [PubMed] [Google Scholar]
- Lacroix MZ, Puel S, Toutain PL, Viguie C. Quantification of fipronil and its metabolite fipronil sulfone in rat plasma over a wide range of concentrations by LC/UV/MS. J Chromatogr B. 2010;878:1934–1938. doi: 10.1016/j.jchromb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Mahler BJ, Van Metre PC, Wilson JT, Musgrove M, Zaugg SD, Burkhardt MR. Fipronil and its degradates in indoor and outdoor dust. Environ Sci Technol. 2009;43(15):5665–5670. doi: 10.1021/es901292a. [DOI] [PubMed] [Google Scholar]
- Mohamed F, Senarathna L, Percy A, Abeyewardene M, Eaglesham G, Cheng R, Azher S, Hittarage A, Dissanayake W, Sheriff MH, Davies W, Buckley NA, ME Acute human self-poisoning with the N-phenylpyrazole insecticide fipronil — a GABAA-gated chloride channel blocker. J Toxicol Clin Toxicol. 2004;42:955–963. doi: 10.1081/clt-200041784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MK, Stout DM, Jones PA, Barr DB. An observational study of the potential for human exposures to pet-borne diazinon residues following lawn applications. Environ Res. 2008;107:336–342. doi: 10.1016/j.envres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Morgenthaler M, Schweizer E, Hoffmann-Ro A, Martin RE, Jaeschke G, Wagner B, Fischer H, Bendels S, Zimmerli D, Schneider J, Diederich F, Mu MKK. Predicting properties and tuning physicochemical in lead optimization: amine basicities. Chem Med Chem. 2007;2:1100. doi: 10.1002/cmdc.200700059. [DOI] [PubMed] [Google Scholar]
- Moser VC, Stewart N, Lyke DF, Crooks J, MacMillan DK, Hedge JM, Wood CE, McMahen RL, Strynar MJ, Herr DW. Assessment of serum biomarkers in rats after exposure to pesticides of different chemical classes. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2014.11.016. http://dx.doi.org/10.1016/j.taap.2014.11.016. [DOI] [PubMed]
- Nagao M, Sugimura T. Carcinogenic factors in food with relevance to colon cancer development. Mutat Res Fundam Mol Mech Mutagen. 1993;290(1):43–51. doi: 10.1016/0027-5107(93)90031-a. [DOI] [PubMed] [Google Scholar]
- Ohi M, Dalsenter PR, Andrade AJM, Nascimento AJ. Reproductive adverse effects of fipronil in Wistar rats. Toxicol Lett. 2004;146(2):121–127. doi: 10.1016/j.toxlet.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Pezdirc M, Zegura B, Filipic M. Genotoxicity and induction of DNA damage responsive genes by food-borne heterocyclic aromatic amines in human hepatoma HepG2 cells. Food Chem Toxicol. 2013;59:386–394. doi: 10.1016/j.fct.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Pleil JD, Sobus JR, Stiegel MA, Hu D, Oliver KD, Olenick C, Strynar MJ, Clark M, Madden MC, Funk WE. Estimating common parameters of lognormally distributed environmental and biomonitoring data: harmonizing disparate statistics from publications. J Toxicol Environ Health B. 2014;17:341–368. doi: 10.1080/10937404.2014.956854. [DOI] [PubMed] [Google Scholar]
- Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev. 2008;37(2):320–330. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]
- Raquel P, Tercariol G, Godinho AF. Behavioral effects of acute exposure to the insecticide fipronil. Pestic Biochem Physiol. 2011;99:221–225. [Google Scholar]
- Rhône-Poulenc Agro to boost fipronil production. Agrow. 1997;294:17. [Google Scholar]
- Rowley M, Hallett DJ, Goodacre S, Moyes C, Crawforth J, Sparey TJ, Patel S, Marwood R, Patel S, Thomas S, Hitzel L, O’Connor D, Szeto N, Castro JL, Hutson PH, MacLeod AM. 3-(4-fluoropiperidin-3-yl)-2-phenylindoles as high affinity, selective, and orally bioavailable h5-HT2A receptor antagonist. J Med Chem. 2001;44(10):1603–1614. doi: 10.1021/jm0004998. [DOI] [PubMed] [Google Scholar]
- Ryberg KR, Vecchia AV, Martin JD, Gilliom RJ. Trends in pesticide concentrations in urban streams in the United States, 1992–2008. Geological Survey Scientific Investigations Report 2010 [Google Scholar]
- Schut HAJ, Snyderwine EG. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 1999;20(3):353–368. doi: 10.1093/carcin/20.3.353. [DOI] [PubMed] [Google Scholar]
- Smart BE. Fluorine substituent effects (on bioactivity) J Fluor Chem. 2001;109(1):3–11. [Google Scholar]
- Smith DA, van de Waterbeemd H, Walker DK. Methods and Principles in Medicinal Chemistry, vol 31: Pharmacokinetics and Metabolism in Drug Design. Wiley-VCH; 2006. p. 31. [Google Scholar]
- Snyderwine EG, Turesky RJ, Turteltaub KW, Davis CD, Sadrieh N, Schut HAJ, Nagao M, Sugimura T, Thorgeirsson UP, Adamson RH, Thorgeirsson SS. Metabolism of food-derived heterocyclic amines in nonhuman primates. Mutat Res Fundam Mol Mech Mutagen. 1997;376(1–2):203–210. doi: 10.1016/s0027-5107(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Stone WW, Gilliom RJ, Ryberg KR. Pesticides in U.S. streams and rivers: occurrence and trends during 1992–2011. Environ Sci Technol. 2014;48(19):11025–11030. doi: 10.1021/es5025367. [DOI] [PubMed] [Google Scholar]
- Tang JA, Usmani K, Hodgson E, Rose RL. In vitro metabolism of fipronil by human and rat cytochrome P450 and its interactions with testosterone and diazepam. Chem Biol Interact. 2004;147(3):319–329. doi: 10.1016/j.cbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Tingle CCD, Rother JA, Dewhurst CF, Lauer S, King WJ. Fipronil: environmental fate, ecotoxicology, and human health concerns. Rev Environ Contam Toxicol. 2003;176:1–66. doi: 10.1007/978-1-4899-7283-5_1. [DOI] [PubMed] [Google Scholar]
- van Niel MB, Collins I, Beer MS, Broughton HB, Cheng SKF, Goodacre SC, Heald A, Locker KL, MacLeod AM, Morrison D, Moyes CR, O’Connor D, Pike A, Rowley M, Russell MGN, Moyes CR, O’Connor D, Pike A, Rowley M, Russell MGN, Sohal B, Stanton JA, Thomas S, Verrier H, Watt AP, Castro JL. Fluorination of 3-(3-(piperidin-1-yl)propyl)indoles and 3-(3-(piperazin-1-yl)propyl)indoles gives selective human 5-HT1D receptor ligands with improved pharmacokinetic profiles. J Med Chem. 1999;42(12):2087–2104. doi: 10.1021/jm981133m. [DOI] [PubMed] [Google Scholar]
- Vidau C, Gonzalez-Polo RA, Niso-Santano M, Gomez-Sanchez R, Bravo-San Pedro JM, Pizarro-Estrella E, Blasco R, Brunet JL, Belzunces LP, Fuentes JM. Fipronil is a powerful uncoupler of oxidative phosphorylation that triggers apoptosis in human neuronal cell line SHSY5Y. NeuroToxicology. 2011;32:935–943. doi: 10.1016/j.neuro.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Xie T, Liang Y, AJ, Hao H, Liu L, Zheng X, Dai C, Zhou Y, Guan T, Liu Y, Xie L, Wang G. Post acquisition data processing techniques for lipid analysis by quadrupole time-of-flight mass spectrometry. J Chromatogr B. 2012;905:43–53. doi: 10.1016/j.jchromb.2012.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.