Abstract
Deficient bioenergetics and diminished redox conservation have been implicated in the development of cerebral ischemia/reperfusion injury. In this study, the mechanisms underlying the neuroprotective effects of cannabidiol (CBD), a nonpsychotropic compound derived from Cannabis sativa with FDA-approved antiepilepsy properties, were studied in vitro using an oxygen–glucose-deprivation/reperfusion (OGD/R) model in a mouse hippocampal neuronal cell line. CBD supplementation during reperfusion rescued OGD/R-induced cell death, attenuated intracellular ROS generation and lipid peroxidation, and simultaneously reversed the abnormal changes in antioxidant biomarkers. Using the Seahorse XFe24 Extracellular Flux Analyzer, we found that CBD significantly improved basal respiration, ATP-linked oxygen consumption rate, and the spare respiratory capacity, and augmented glucose consumption in OGD/R-injured neurons. The activation of glucose 6-phosphate dehydrogenase and the preservation of the NADPH/NADP+ ratio implies that the pentose-phosphate pathway is stimulated by CBD, thus protecting hippocampal neurons from OGD/R injury. This study is the first to document the neuroprotective effects of CBD against OGD/R insult, which depend in part on attenuating oxidative stress, enhancing mitochondrial bioenergetics, and modulating glucose metabolism via the pentose-phosphate pathway, thus preserving both energy and the redox balance.
Keywords: Cannabidiol, Ischemia/reperfusion, Mitochondrial bioenergetics, Pentose-phosphate pathway, Neuroprotection
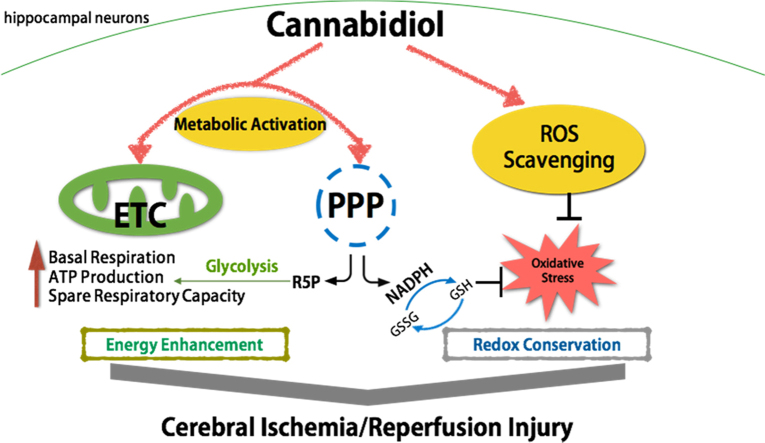
Graphical abstract
Highlights
-
•
Cannabidiol protects hippocampal neurons from OGD/R-induced oxidative stress.
-
•
Cannabidiol enhances mitochondrial bioenergetics.
-
•
Cannabidiol optimizes glucose metabolism via the pentose-phosphate pathway.
1. Introduction
Cerebral ischemia is one of the commonest clinical conditions, and is involved in many serious diseases, including stroke, cardiac arrest, and respiratory arrest. The treatment for this condition usually involves the restoration of blood flow as quickly as possible. However, this can entail secondary injury to the ischemic area, referred to as ‘ischemia/reperfusion injury’ (IRI)[1], [2]. The restoration of the blood circulation causes inflammation and oxidative-stress-induced damage in the area affected by the absence of oxygen and nutrients during the period of cerebral ischemia. Brain ischemia with reperfusion also results in impaired mitochondrial oxidative metabolism and a depletion of the reducing energy of neurons, which contribute to programmed cell death [3]. Appropriate drugs are required to protect neurons from IRI, to mitigate the pathological responses and control the process of neuronal death.
Cannabidiol (CBD) is a nonpsychoactive cannabinoid derived from Cannabis sativa and a weak CB1 and CB2 cannabinoid receptor antagonist, with very low toxicity for humans. It has recently been demonstrated in vivo and in vitro that CBD has a variety of therapeutic properties, exerting antidepressant, anxiolytic, anti-inflammatory, immunomodulatory, and neuroprotective effects [4]. Increasing evidence indicates that CBD is a molecule with potentially neuroprotective properties that can be used to treat neurodegenerative disorders. CBD had been shown to reverse the reduction in neuronal viability and the increased excitoxicity, inflammation, and oxidative stress in newborn piglets with hypoxic–ischemic brain damage by targeting the receptors of 5HT1A and CB2[5]; and to protect PC12 and SH-SYS5 cells from tert-butyl-hydroperoxide-induced oxidative stress, independently of the CB1 and CB2 receptors [6]. CBD also displays better protective activity against glutamate neurotoxicity than either ascorbate or alpha-tocopherol, which suggests that it is a potentially effective antioxidant [7]. CBD also acts as a reactive oxygen species (ROS) scavenger, reducing lipid peroxidation, and restores caspase 12, caspase 3, Bcl-2, and Bax mRNA levels [8], [9], [10]. CBD has recently been approved in the United Kingdom and several other European countries as an important component of an oromucosal spray that is used as a complementary treatment for multiple sclerosis [11]. It has also received orphan drug approval by the U.S. Food and Drug Administration (FDA) to treat refractory childhood epilepsy [12]. To explore the positive effects of CBD on cerebral IRI, we used the immortalized mouse hippocampal neuronal cell line, HT22, and a model of oxygen–glucose deprivation/reperfusion (OGD/R) to mimic the conditions for IRI in vitro. Our results provide novel insight into the neuroprotective properties of CBD, which involves the regulation of the mitochondrial bioenergetics and the glucose metabolism of hippocampal neurons during OGD/R injury.
2. Materials and methods
2.1. Drugs and reagents
Cannabidiol (HPLC purified, 99.8%; dissolved in dimethyl sulfoxide) was purchased from Biotrend (Cologne, Germany). The reagents and materials supplied with each kit are mentioned with each method.
2.2. Cell culture and OGD/R model
HT22 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin (Sigma-Aldrich, St. Louis, MO, USA), in a humidified atmosphere at 37 °C under 5% CO2. Briefly, the HT22 cells were cultured under normal conditions for 24 h, then moved to glucose-free DMEM (Gibco) and placed under ischemic conditions (3% O2, 92% N2, 5% CO2) at 37 °C for 8 h. Finally, the medium was discarded and the cells were cultured in normal medium under normoxic conditions (95% air, 5% CO2) for another 24 h for reperfusion, to induce OGD/R injury. Cells incubated in complete medium under a normoxic atmosphere were used as the control.
2.3. Cell viability
Approximately 3×103 HT22 cells were cultured in 96-well plates under normal conditions for 24 h. The cells were treated with oxygen–glucose deprivation (OGD) for 8 h and then returned to normal culture conditions with or without CBD (1, 2.5, 5, or 10 μM) for another 24 h. Cell viability was evaluated with the Cell Counting Kit-8 (CCK-8) (#CK04; Dojindo, Japan), and all the experiments were performed according to the manufacturer’s instructions. Absorbance was measured at 450 nm with a microplate reader (Synergy H1 Hybrid Reader, BioTek, USA). The optical density values are shown as percentages of the control values.
2.4. Lactate dehydrogenase (LDH) release assay
The release of cytoplasmic LDH indicates the loss of cell membrane integrity, which represents the death of the cell [13]. We used a commercial LDH Cytotoxicity Assay Kit purchased from Jiancheng Bioengineering Institute (#A020-2; Nanjing, China), according to the manufacturer’s instructions. In brief, 20 μl of supernatant from each cell culture well was collected for a coupled enzymatic reaction in which LDH catalyzed the conversion of lactate to pyruvate via the reduction of NAD+ to NADH for 15 min at 37 °C. It was then reacted with 2,4-dinitrophenylhydrazine for another 15 min to form a brownish red product, which was measured spectrophotometrically at 450 nm with a microplate reader (Synergy H1 Hybrid Reader). To measure the total LDH released, the cells were incubated with 100 μl of lysis solution/well at 37 °C for 30 min and then centrifuged to remove the cellular debris. The values are shown as percentages of the total LDH (intracellular plus supernatant LDH).
2.5. Caspase 3 and poly (ADP-ribose) polymerase (PARP) activity assays
Caspase 3 activity was measured with a colorimetric method based on the hydrolysis of acetyl–Asp–Glu–Val–Asp p-nitroanilide (Ac-DEVD-pNA) and the release of the p-nitroaniline (pNA) moiety, with a Caspase 3 Colorimetric Assay Kit (#CASP3C; Sigma-Aldrich). In brief, the cells were harvested with lysis buffer at a concentration of 107 cells per 100 μl, incubated on ice for 20 min, and centrifuged at 16,000× g for 15 min at 4 °C. The caspase 3 activity was detected in 5 μl of supernatant with Ac-DEVD-pNA (2 mM) in reaction buffer at 37 °C for 2 h and the absorbance was read at 405 nm. PARP activity was measured with a commercial ELISA kit purchased from Trevigen (#4684-096-K; Gaithersburg, MD, USA) as described previously [14]. Briefly, the cells were collected and incubated in lysis buffer for 30 min on ice. After they were transferred to the wells of a histone-coated plate, the cell lysates were incubated with a PARP substrate cocktail (containing assay buffer, activated DNA, and NAD+) for 30 min at room temperature. An anti-PAR antibody, a goat anti-mouse IgG antibody–horseradish peroxidase (HRP) conjugate, and a colorimetric substrate were used to generate a signal, which was detected at 450 nm with a microplate reader. The results are expressed as the fold activity relative to the control.
2.6. TUNEL staining
The apoptosis of HT22 cells was evaluated with the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) In Situ Cell Death Detection Kit (#12156792910; Roche, Mannheim, Germany). Cells were fixed with freshly prepared 4% paraformaldehyde for 20 min at room temperature and washed twice with PBS. They were then incubated with 50 μl of TUNEL reaction mixture in a humidified atmosphere for 60 min at 37 °C in the dark. The cells were rinsed three times with PBS and incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min. The images were captured with a fluorescence microscope (Leica DMI 3000 B, Germany). The index of apoptosis is shown as the ratio of TUNEL-positive cells to the total number of cells counted within five randomly chosen fields per condition.
2.7. Mitochondrial ROS detection
The intramitochondrial production of ROS in live HT22 cells was assessed with the MitoSOX Red mitochondrial superoxide indicator (#M36008; Carlsbad, CA, USA). The cells were reacted with a 5 μM working solution of MitoSOX Red for 10 min at 37 °C, and then carefully washed twice with PBS and images were obtained with a fluorescence microscope, and the fluorescence intensity was evaluated with the Image J software (National Institutes of Health, MD, USA). The data are shown as the mean intensities in the entire fields of view in six random graphs per condition and are expressed as the fold intensity relative to the control.
2.8. Malondialdehyde (MDA), Reduced Glutathione (GSH), Superoxide dismutase-1 (SOD1) and Glutathione Peroxidase (GPx) determination assay
HT22 cells were incubated in six-well plates at a density of 4×104 cells/well. After treatment with OGD/R with or without the administration of CBD, the cells were collected, homogenized in PBS, and centrifuged at 1000× g for 10 min at 4 °C. The supernatants were collected to measure the MDA and GSH contents and the activities of SOD1 and GPx with a microplate reader, according to the manufacturer’s instructions (#A001-3, A003-4, A006-2; Jiancheng, Bioengineering Institute, Nanjing, China; #CGP1; Sigma-Aldrich). Briefly, lipid peroxidation was determined by the reaction of MDA with thiobarbituric acid, acetic acid, and sodium dodecyl sulfate at 95 °C for 80 min to form thiobarbituric acid reactive substances (TBARS), which were detected at 530 nm. An aliquot (100 μl) of the supernatant was reacted with 100 μl of a working mixture containing 5,5′-dithiobis(2-nitrobenzoic acid) and 25 μl of NADPH solution (0.12 mg/ml) for 6 min at room temperature, and reduced GSH was detected spectrophotometrically at 405 nm as the yellow product, 5-thio-2-nitrobenzoic acid. Another aliquot (20 μl) of the supernatant was reacted with 200 μl of WST-1 (a highly water-soluble tetrazolium salt) and 20 μl of the enzyme working solution for 20 min at 37 °C to measure the SOD1 activity as the production of water-soluble formazan, detected at 450 nm. The GPx activity in the supernatant was measured by a reaction with the GPx detection working solution from the kit (0.25 mM NADPH, 2.1 mM reduced GSH, 0.5 unit/ml glutathione reductase, and 300 μM tert-butyl hydroperoxide), in which the reduction in NADPH absorbance was indicative of the GPx activity, detected at 340 nm after an initial delay of 15 s and monitored every 10 s for 1 min at 25 °C. A BCA assay (Beyotime Biotechnology, Nantong, China) was performed to normalize the results to the protein content. The data are shown as fold activities of the corresponding controls.
2.9. Measurement of mitochondrial bioenergetics and glucose metabolism
The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured by sequentially adding specific compounds from the Seahorse XF Cell Mito Stress Test Kit and XF Glycolysis Stress Test Kit (#103015-100, 103020-100; Seahorse Bioscience, USA) using the Seahorse XFe24 Extracellular Flux Analyzer, according to the manufacturer’s protocol. Briefly, cells were incubated at a density of 2×104 cells/well in XFe24 microplates. Before the assay, the cells were washed thoroughly with assay medium (#102353-100; Seahorse Bioscience) and incubated in a CO2-free incubator at 37 °C for about 1 h. The microplates were then loaded into the XFe24 Analyzer. Three compounds, oligomycin (1.0 μM), carbonylcyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP; 1.0 μM), and a mixture of rotenone and antimycin A (0.5 μM), were sequentially injected in the OCR measurement to obtain the values for the basal mitochondrial respiration, ATP-linked oxygen consumption rate, maximal respiration, and spare respiratory capacity. ECAR was measured after the sequential injection of glucose (10 mM), oligomycin (1.0 μM), and 2-deoxy-glucose (2-DG, 50 mM) at the specified time points, as the basal ECAR, maximal ECAR, and reserve capacity. After the assays, the microplates were stored and stained with DAPI to calculate the number of cells in each well using the Operetta High-Content Imaging System (PerkinElmer, USA) to normalize the results.
2.10. Quantification of NADPH/NADP+
The NADPH/NADP+ ratio was measured by a commercial NADPH/NADP+ quantification kit (#MAK038; Sigma-Aldrich). Briefly, the cells were extracted with extraction buffer, homogenized, and centrifuged at 10,000× g for 10 min to isolate the NADPH/NADP+-containing supernatant. An aliquot of the supernatant was heated at 60 °C for 30 min to decompose the NADP+, cooled on ice, and spun quickly to remove the precipitate. Another aliquot of the supernatant was not heated. Both aliquots were reacted with NADP+ cycling buffer and enzyme mix (containing glucose-6-phosphate dehydrogenase [G6PDH]) for 5 min at room temperature to convert NADP+ to NADPH. The solutions were then incubated with NADPH developer for 2 h and the absorbance measured at 450 nm. The amount of NADPH (heated sample) and the total NADP+ and NADPH (unheated sample) were quantified from an NADPH standard curve.
2.11. Glucose-6-phosphate dehydrogenase (G6PDH) activity determination
G6PDH is a cytosolic enzyme that catalyzes the conversion of glucose-6-phosphate (G6P) to 6-phosphogluconolactone, consequently supplying reducing energy to cells via the pentose-phosphate pathway. G6PDH activity was detected using a commercial kit by the colorimetric assay (#MAK015; Sigma-Aldrich). In brief, cells were collected and rapidly homogenized in the ice cold PBS and centrifuged at 15,000× g for 10 min. The supernatant was reacted with the G6PDH substrate mix (containing glucose-6-phosphate and NAD+) and the developer mix from the kit for 3 min to convert NAD+ to NADH, generating an intensely colored product with an absorbance at 450 nm. The absorbance was measured again after the reaction was incubated at 37 °C for 30 min. The amount of NADH generated between two time points was determined from an NADH standard curve, and the G6PDH activity was calculated as the nmol of NADH generated per minute per mg protein. The data are expressed as the fold activity relative to the control.
2.12. Statistical analysis
The data are presented as means±SEM, and were analyzed statistically with one-way ANOVA and Tukey’s multiple comparisons test using GraphPad Prism 6.0 (San Diego, CA, USA). A value of p<0.05 was defined as statistically significant.
3. Results
3.1. CBD protects hippocampal cells against OGD/R- induced cytotoxity
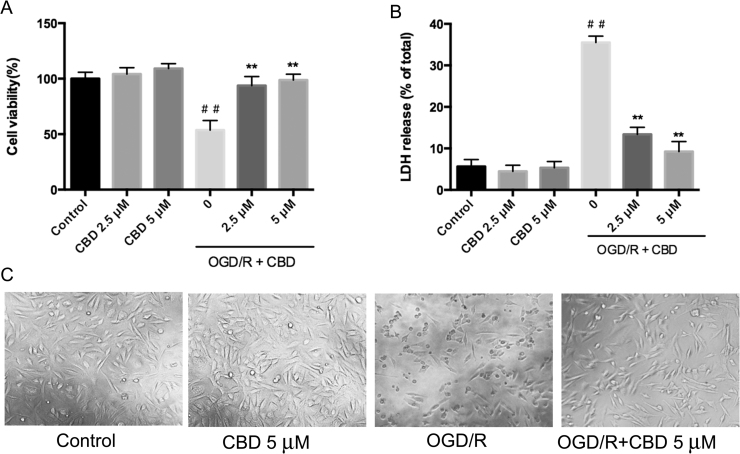
To investigate the neuroprotective effects of CBD on hippocampal cells with OGD/R injury, we first performed cell viability and LDH release assays. As shown in Figs. 1A and B, the average viability of the HT22 cells decreased significantly to 39.5% and the average LDH release increased from 5.6–38.7% after OGD/R insult. Remarkably, CBD reversed the OGD/R-induced cytotoxicity in the HT22 cells, improving cell viability and reducing the release of LDH (Fig. 1A, B; p<0.01, n =5). OGD/R injury also induced morphological changes in the neurons, which became shrunken and rounded. The administration of CBD during reperfusion markedly attenuated the morphological damage and the cells maintained a normal appearance (Fig. 1C). We used 5 μM CBD to perform the subsequent experiments because, according to the results described above, it afforded the most effective protection against OGD/R.
Fig. 1.
CBD reduces OGD/R-induced cytotoxicity in HT22 cells. Cells were treated under OGD-inducing conditions for 8 h and then reoxygenated in the presence of 2.5 or 5 μM CBD for 24 h. (A) Cell viability was detected with a CCK-8 assay. (B) Cell cytotoxicity was determined with an LDH release assay. (C) Cell morphology was evaluated with a biological microscope. Three independent experiments were performed. Data shown are means±SEM, n =5. ##p<0.01 vs control, **p<0.01 vs OGD/R group.
3.2. CBD attenuates OGD/R-induced cell death in hippocampal neurons
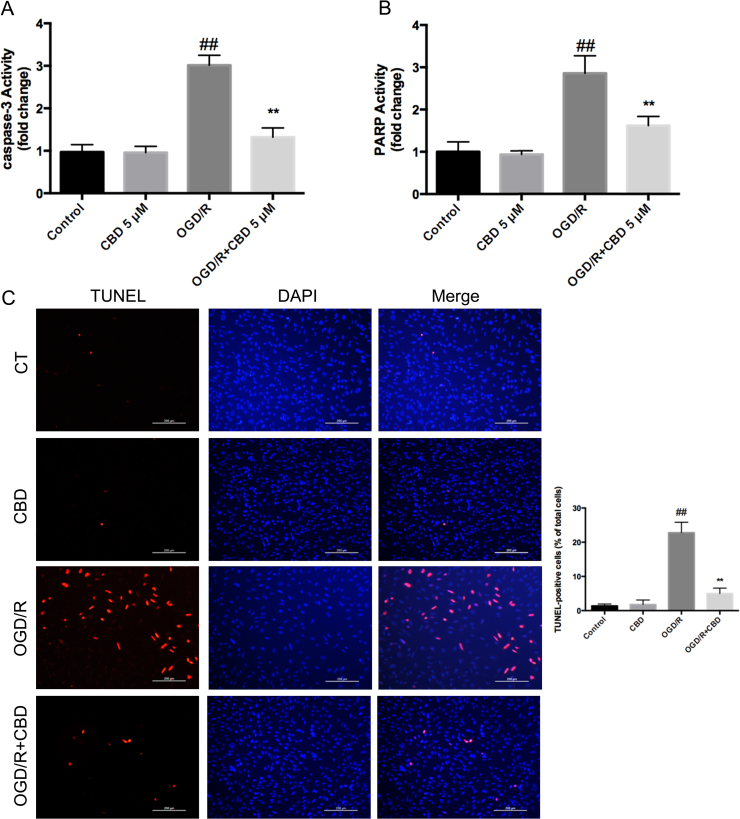
Activated caspase 3 and PARP were significantly upregulated during OGD/R injury, and this was attenuated by the administration of CBD during the course of reperfusion (caspase 3 activity decreased by ~56% and PARP activity by ~43%) (Fig. 2A, B). TUNEL staining of HT22 cells under OGD/R showed a marked increase in apoptotic bodies, which were reduced by ~17.8% in the presence of CBD during reperfusion (p<0.01, n =5) (Fig. 2C). These results demonstrate the protective effects of CBD during neuronal OGD/R, insofar as it attenuated apoptosis and PARP-dependent cell death.
Fig. 2.
CBD (5 μM) administered during reperfusion attenuated OGD/R-induced apoptosis and PARP-dependent cell death in HT22 cells. (A) Caspase 3 activity. (B) PARP activity. (C) TUNEL apoptosis assay was examined with a fluorescence microscope (magnification 10× objective field, scale bar =200 µm). Three independent experiments were performed. Data shown are means±SEM, n=5. ##p<0.01 vs control, **p<0.01 vs OGD/R group.
3.3. CBD reduces OGD/R-induced oxidative stress in hippocampal neurons
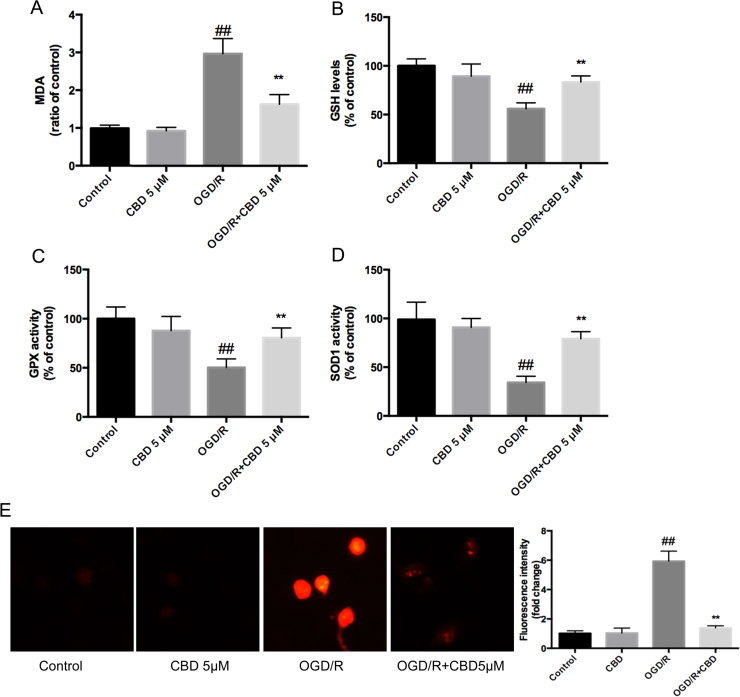
Several researchers have demonstrated that the potent neuroprotective property of CBD is associated with its antioxidant capacity [6], [7], so we explored the effects of CBD on OGD/R-induced oxidative stress by detecting the relevant biomarkers. Significant increases in MDA and mitochondrial ROS levels (Fig. 3A, E), a reduction in the reduced glutathione content (Fig. 3B), and attenuated glutathione peroxidase and SOD1 activities (Fig. 3C, D) relative to the control levels were observed, and the changes were reversed by the administration of CBD. These results demonstrate the outstanding antioxidative ability of CBD against the oxidative stress induced during OGD/R injury (see Fig. 3A–E).
Fig. 3.
CBD administered during the reperfusion period shows potent antioxidant effects in neurons exposed to OGD/R injury. CBD enhanced the intracellular SOD1 and GPx activities, elevated the GSH level, and reduced the MDA level and mitochondrial ROS generation. (A) MDA level. (B) Reduced glutathione (GSH) content. (C) GPx activity. (D) SOD1 activity. (E) MitoSOX fluorescence intensity. Three independent experiments were performed. Data shown are means±SEM, n=6. ##p<0.01 vs control, **p<0.01 vs OGD/R group.
3.4. CBD enhances mitochondrial bioenergetics and regulates glucose metabolism in OGD/R-injured neurons
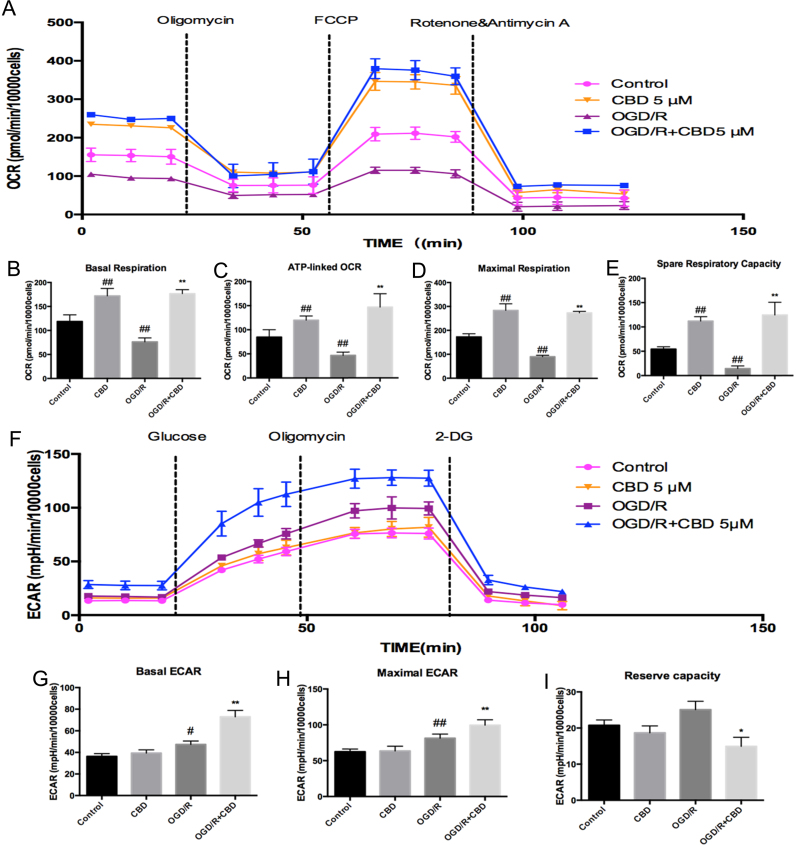
Previous research has demonstrated that global cerebral ischemia/reperfusion causes mitochondrial damage [15]. To identify the effects of CBD on mitochondrial bioenergetics during OGD/R, we measured mitochondrial respiration using the Seahorse XFe24 Extracellular Flux Analyzer. As shown in Fig. 4B, the OGD/R injury in HT22 cells caused a significant decline in their basal respiration compared with the control (p<0.01, n =9), which was largely reversed by treatment with CBD (p<0.01, n =9). The first compound injected in the assay was oligomycin, a complex V inhibitor, used to determine the proportion of ATP-linked OCR in the basal respiration. The next compound injected was FCCP, an uncoupling agent, used to determine the maximal respiration and calculate the spare respiratory capacity. OGD/R insult reduced the ATP production-linked oxygen consumption rate, the maximal respiration, and the spare respiratory capacity to ~55%, ~52%, and ~26%, respectively, compared with the control, and these reductions were strongly reversed by the simultaneous exposure of the cells to CBD in the course of reperfusion (Fig. 4C–E; p<0.01). Marked increases in the four phases of respiration measured were also observed in the normal hippocampal neurons after CBD treatment (Fig. 4B–E; p<0.01), confirming the specificity of CBD in enhancing mitochondrial bioenergetics.
Fig. 4.
CBD enhances mitochondrial respiration and glucose metabolism in OGD/R-injured hippocampal neurons. The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were determined with the Seahorse XFe24 Extracellular Flux Analyzer. (A) OCR was recorded at baseline and after the sequential injection of oligomycin (1 μM), FCCP (1 μM), and a mixture of rotenone and antimycin (1 μM). (B) Basal respiration, (C) ATP production, (D) maximal respiration, and (E) spare respiratory capacity were calculated. (F) ECAR was recorded after the sequential injection of glucose (10 mM), oligomycin (1 μM), and 2-deoxy-glucose (50 mM). (G) Basal ECAR, (H) maximal ECAR, and (I) the reserve capacity were calculated. Three independent experiments were performed. Data shown are means±SEM, n =9. #p<0.05, ##p<0.01 vs control; *p<0.05, **p<0.01 vs OGD/R group.
ECAR was measured to investigate the involvement of CBD in glucose metabolism, during which ATP is also produced via through the Krebs cycle. Moderate increases in the basal and maximal ECAR were observed in HT22 cells under OGD/R relative to the control (p<0.05 and p<0.01, respectively). Intriguingly, greater glucose consumption and an attenuated reserve capacity were observed in the neurons supplemented with CBD during the course of reperfusion compared with those in the OGD/R group (Fig. 4G–I; p<0.01). Considering these data together, we inferred that the cell metabolism was comprehensively enhanced by CBD treatment under OGD/R-inducing conditions.
3.5. CBD enhances the pentose-phosphate pathway of glucose metabolism in OGD/R-injured hippocampal neurons
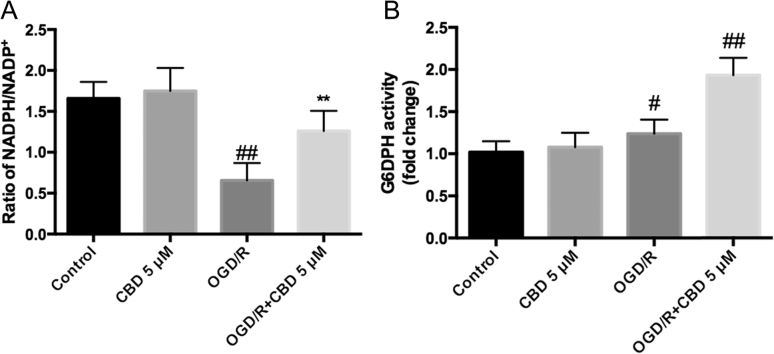
The NADPH/NADP+ ratio and G6PDH activity were measured to further test whether the augmentation of glucose metabolism by CBD occurs via the pentose-phosphate pathway, a major branch of glycolysis that enhances the antioxidant defenses under stress conditions. OGD/R injury reduced the NADPH/NADP+ ratio by ~60% and slightly increased the activity of G6PDH compared with the control (Fig. 5A, B; p<0.01 and p<0.05, respectively, n =6), indicating the depletion of reducing energy in HT22 cells under OGD/R conditions. When neurons were cultured in medium supplemented with CBD during reperfusion, the NADPH/NADP+ ratio was maintained (Fig. 5A) and G6PDH was significantly activated compared with the control (Fig. 5B; p<0.01, n =6). This suggests that CBD helps to maintain the redox balance and enhances the antioxidant defenses by activating the pentose-phosphate pathway in hippocampal neurons during OGD/R injury.
Fig. 5.
CBD maintains the NADPH/NADP+ ratio and enhances the activity of G6DPH in OGD/R-injured HT22 cells. (A) NADPH/NADP+ ratio, and (B) G6DPH activity were quantified with a colorimetric assay (450 nm). Three independent experiments were performed. Data shown are means±SEM, n =6. #p<0.05, ##p<0.01 vs control; **p<0.01 vs OGD/R group.
4. Discussion
Cerebral ischemia is a common cause of death worldwide, after cardiovascular diseases and cancer, and its prevalence increases with increasing age [16]. The consequences of transient cerebral ischemia are often severe and have selectively harmful effects on the vulnerable regions involving the pyramidal neurons of the CA1 hippocampus [17]. Therefore, it is necessary to determine the underlying mechanism of cerebral ischemia/reperfusion injury and to develop effective strategies to circumvent this pathological condition. Oxygen–glucose deprivation/reperfusion is an in vitro model that mimics in vivo ischemia/reperfusion injury and initiates a series of devastating cascades that lead to the overproduction of ROS, the excessive release of excitatory amino acids, disturbance of the ionic balance, the overexpression of proapoptotic factors, the stimulation of a serious inflammatory response, and damage to mitochondrial functions [18], [19]. CBD is a nonpsychoactive component of marijuana, which exerts potent antioxidant and anti-inflammatory effects in vivo and in vitro. It is considered to have a neuroprotective property that is independent of the cannabinoid 1 and 2 receptors [4], [20]. Here, we demonstrate for the first time the potent neuroprotective effects of CBD against OGD/R-injury-induced cytotoxicity, energy crisis, and disturbance of the cellular metabolism by its: (i) attenuation of oxidative stress; (ii) enhancement of mitochondrial bioenergetics; and (iii) activation of the pentose- phosphate pathway to compensate for the diminished antioxidant defenses under stress conditions.
Consistent with many previous studies, cerebral ischemia/reperfusion significantly elevated the ROS content and reduced the antioxidant enzyme activities of neurons, causing oxidative damage and cell death [21], [22]. MDA, a product of lipid peroxidation, is a common and important marker of oxidative stress, which is markedly influenced by the excessive generation of ROS. Cells also have antioxidant defense mechanisms that involve enzymatic components such as GPx and SOD1, and nonenzymatic mechanisms, such as GSH. GSH, SOD1, and GPx act as endogenous free-radical scavengers, whereas GPx also functions biochemically to reduce lipid hydroperoxides to the corresponding alcohols [23]. In this study, OGD/R injury in HT22 cells caused excessive mitochondrial ROS generation, elevated MDA levels, and reductions in the GPx and SOD1 activities and the GSH content. After CBD treatment during the reperfusion period, the levels of mitochondrial ROS and MDA were dramatically reduced, simultaneously with the significant upregulation of GSH and increases in SOD1 and GPx activities. This suggests that neuroprotection is afforded by CBD by reducing the oxidative stress produced under OGD/R conditions. These results are consistent with the attenuation of oxidative damage by CBD in a diabetic retinopathy model [24], in PC12 cells stimulated with β-amyloid [9], and in oligodendrocyte progenitor cells treated with H2O2[8], and with the antioxidant property of CBD in rats with renal ischemia/reperfusion injury [25]. Furthermore, consistent with previous studies [26], [27], [28], we found that OGD/R insult markedly reduced cell viability and induced apoptosis and PARP-dependent cell death, which were significantly attenuated by the administration of CBD.
Mitochondria are the important energy production centers in neurons under physiological conditions, so mitochondrial dysfunction strongly affects neuronal function and survival [29]. A devastating well-known consequence of reperfusion following brain ischemia is grossly enhanced ROS production, followed by severe oxidative stress, which leads to mitochondrial dysfunction. Emerging evidence also suggests that substantially decreasing activities of mitochondrial complexes I and IV followed by irreversible impaired mitochondrial function and a consequently deficient energy supply often occur in the later stages of reperfusion [3], [30]. Moreover, the cerebral ischemia/reperfusion injury also results in delayed neuronal death after reperfusion by releasing apoptogenic factors, including cytochrome C (Cytc) and apoptosis inducing factor (AIF) from mitochondria [31], [32]. According to our OCR data, CBD attenuates the reduction of ATP production-linked OCR and the diminished mitochondrial capacity induced by OGD/R. This is consistent with previous studies that demonstrated that CBD modulates mitochondrial function and biogenesis to protect against doxorubicin-induced cardiomyopathy12; increases the activity of mitochondrial complexes in the rat brain [33]; exerts neuroprotective effects against mitochondrial toxins, and restores intracellular Ca2+ homeostasis in human neuroblastoma cell lines (SH-SY5Y)[34]. Taken together, these data suggest that CBD is a mitochondria-targeting drug that markedly enhances mitochondrial function and bioenergetics, thus exerting potent protective effects against pathological conditions.
Glucose, which is widely thought to be the vital oxidative substrate providing energy to the brain, can be further metabolized by glycolysis or the pentose-phosphate pathway (PPP) after phosphorylating glucose to glucose-6-phate (G6P) by hexokinase [35]. The two pathways share common pools of fructose-6-phosphate and glyceraldehyde-3-phosphate, and both produce pyruvate, which is converted to acetyl-coenzyme A and is fully oxidized through the tricarboxylic acid (TCA) cycle, contributing to energy conservation [36], [37]. Supporting the involvement of CBD in the regulation of glucose metabolism, we found that OGD/R injury moderately stimulated the glucose metabolism in hippocampal neurons, and that CBD supplementation during reperfusion induced greater glucose consumption and lactate release, implying that the stimulation of glucose metabolism by CBD protects hippocampal neurons from energy stress. As we know, neurons are particularly vulnerable to oxidative stress because their antioxidant defenses are weak and their ability to maintain energy homeostasis is poor [38]. Emerging evidence also indicates that neurons have a low capacity to metabolize glucose via glycolysis because they have low levels of PFKFB-3, an enzyme that supports the equilibrium between glycolysis and the pentose-phosphate pathway. Neurons are also more likely to utilize G6P through the pentose-phosphate pathway under oxidative stress, allowing the efficient regeneration of NADPH and the reduction of glutathione disulfide (GSSG) to GSH, which enhances the antioxidant defense system [36], [39], [40]. To further test whether the stimulation of the pentose-phosphate pathway is involved in neuronal glucose metabolism under OGD/R stress, the NADPH/NADP+ ratio and the activity of G6PDH, a rate-limiting enzyme of the pentose-phosphate pathway, were measured. In OGD/R-injured neurons, CBD supplementation increased the G6PDH activity and maintained the NADPH/NADP+ ratio, implicating the activation of the pentose-phosphate pathway in the protection of hippocampal neurons by CBD under oxidative stress. Therefore, we propose, for the first time, that CBD stimulates glucose metabolism through the pentose-phosphate pathway to maintain the redox balance and energy conservation during neuronal ischemia/reperfusion injury.
5. Conclusion
In summary, our results suggest that CBD exerts a potent neuroprotective effect against ischemia/reperfusion injury by attenuating intracellular oxidative stress, enhancing mitochondrial bioenergetics, and optimizing glucose metabolism via the pentose-phosphate pathway, thus strengthening the antioxidant defenses and preserving the energy homeostasis of neurons. More in-depth studies are required to investigate the precise mechanism underlying the success of CBD treatment and to determine the actual role of CBD in cerebral ischemia.
Conflict of interest/disclosure
The authors declare that they have no conflicts of interest regarding this study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81470625, 81470624) and the Natural Science Foundation of Shanghai, China (14ZR1405500).
Contributor Information
Jihong Wu, Email: jihongwu@fudan.edu.cn.
Shenghai Zhang, Email: zsheent_fdu@163.com.
References
- 1.Puyal J., Ginet V., Clarke P.G. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog. Neurobiol. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Chen H., Yoshioka H., Kim G.S., Jung J.E., Okami N., Sakata H., Maier C.M., Narasimhan P., Goeders C.E., Chan P.H. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sims N.R., Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim. Biophys. Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Campos A.C., Fogaca M.V., Sonego A.B., Guimaraes F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharm. Res. 2016 doi: 10.1016/j.phrs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Pazos M.R., Mohammed N., Lafuente H., Santos M., Martinez-Pinilla E., Moreno E., Valdizan E., Romero J., Pazos A., Franco R., Hillard C.J., Alvarez F.J., Martinez-Orgado J. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology. 2013;71:282–291. doi: 10.1016/j.neuropharm.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Harvey B.S., Ohlsson K.S., Maag J.L., Musgrave I.F., Smid S.D. Contrasting protective effects of cannabinoids against oxidative stress and amyloid-beta evoked neurotoxicity in vitro. Neurotoxicology. 2012;33:138–146. doi: 10.1016/j.neuro.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Hampson A.J., Grimaldi M., Axelrod J., Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. (https://www.ncbi.nlm.nih.gov/pubmed/9653176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mecha M., Torrao A.S., Mestre L., Carrillo-Salinas F.J., Mechoulam R., Guaza C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012;3:e331. doi: 10.1038/cddis.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iuvone T., Esposito G., Esposito R., Santamaria R., Di Rosa M., Izzo A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004;89:134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- 10.Esposito G., De Filippis D., Maiuri M.C., De Stefano D., Carnuccio R., Iuvone T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci. Lett. 2006;399:91–95. doi: 10.1016/j.neulet.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Pacher P., Kunos G. Modulating the endocannabinoid system in human health and disease--successes and failures. FEBS J. 2013;280:1918–1943. doi: 10.1111/febs.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao E., Mukhopadhyay P., Cao Z., Erdelyi K., Holovac E., Liaudet L., Lee W.S., Hasko G., Mechoulam R., Pacher P. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 2015;21:38–45. doi: 10.2119/molmed.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobner D. Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J. Neurosci. Methods. 2000;96:147–152. doi: 10.1016/s0165-0270(99)00193-4. 〈https://www.ncbi.nlm.nih.gov/pubmed/10720679〉 [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay P., Rajesh M., Batkai S., Kashiwaya Y., Hasko G., Liaudet L., Szabo C., Pacher P. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am. J. Physiol. Hear. Circ. Physiol. 2009;296:H1466–H1483. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ham P.B., 3rd, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Writing Group M., Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., Das S.R., de Ferranti S., Despres J.P., Fullerton H.J., Howard V.J., Huffman M.D., Isasi C.R., Jimenez M.C., Judd S.E., Kissela B.M., Lichtman J.H., Lisabeth L.D., Liu S., Mackey R.H., Magid D.J., McGuire D.K., Mohler E.R., 3rd, Moy C.S., Muntner P., Mussolino M.E., Nasir K., Neumar R.W., Nichol G., Palaniappan L., Pandey D.K., Reeves M.J., Rodriguez C.J., Rosamond W., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Woo D., Yeh R.W., Turner, C M.B. American heart association statistics, s. stroke statistics, heart disease and stroke statistics-2016 update: aa report from the american heart association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 17.Kumar R., Bukowski M.J., Wider J.M., Reynolds C.A., Calo L., Lepore B., Tousignant R., Jones M., Przyklenk K., Sanderson T.H. Mitochondrial dynamics following global cerebral ischemia. Mol. Cell Neurosci. 2016;76:68–75. doi: 10.1016/j.mcn.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazan N.G., Marcheselli V.L., Cole-Edwards K. Brain response to injury and neurodegeneration: endogenous neuroprotective signaling. Ann. N. Y Acad. Sci. 2005;1053:137–147. doi: 10.1196/annals.1344.011. [DOI] [PubMed] [Google Scholar]
- 19.Doyle K.P., Simon R.P., Stenzel-Poore M.P. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharm. Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y., Zhang L., Sun S., Yi Z., Jiang X., Jia D. Neuroprotective effects of syringic acid against OGD/R-induced injury in cultured hippocampal neuronal cells. Int J. Mol. Med. 2016;38:567–573. doi: 10.3892/ijmm.2016.2623. [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Zhu X., Chen M., Ge Q., Shen Y., Pan S. Resveratrol protects PC12 cells against OGD/ R-induced apoptosis via the mitochondrial-mediated signaling pathway. Acta Biochim Biophys. Sin. 2016;48:342–353. doi: 10.1093/abbs/gmw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller F.L., Lustgarten M.S., Jang Y., Richardson A., Remmen H. Van. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 24.El-Remessy A.B., Al-Shabrawey M., Khalifa Y., Tsai N.T., Caldwell R.B., Liou G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006;168:235–244. doi: 10.2353/ajpath.2006.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fouad A.A., Al-Mulhim A.S., Jresat I. Cannabidiol treatment ameliorates ischemia/reperfusion renal injury in rats. Life Sci. 2012;91:284–292. doi: 10.1016/j.lfs.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Chen X., Deng A., Zhou T., Ding F. Pretreatment with 2-(4-methoxyphenyl)ethyl-2-acetamido-2-deoxy-beta-D-pyranoside attenuates cerebral ischemia/reperfusion-induced injury in vitro and in vivo. PLoS One. 2014;9:e100126. doi: 10.1371/journal.pone.0100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J.M., Gu S.S., Mei W.H., Zhou J., Wang Z.Z., Xiao W. Ginkgolides and bilobalide protect BV2 microglia cells against OGD/reoxygenation injury by inhibiting TLR2/4 signaling pathways. Cell Stress Chaperon-. 2016 doi: 10.1007/s12192-016-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Z., Wu D., Yao J.P., Yao X., Huang Z., Li P., Wan J.B., He C., Su H. Protection against oxygen-glucose deprivation/reperfusion injury in cortical neurons by combining Omega-3 Polyunsaturated Acid with Lyciumbarbarum Polysaccharide. Nutrients. 2016;8 doi: 10.3390/nu8010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rugarli E.I., Langer T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J. 2012;31:1336–1349. doi: 10.1038/emboj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalogeris T., Bao Y., Korthuis R.J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchino H., Ogihara Y., Fukui H., Chijiiwa M., Sekine S., Hara N., Elmer E. Brain injury following cardiac arrest: pathophysiology for neurocritical care. J. Intensive Care. 2016;4:31. doi: 10.1186/s40560-016-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Sanderson T.H., Reynolds C.A., Kumar R., Przyklenk K., Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013;47:9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valvassori S.S., Bavaresco D.V., Scaini G., Varela R.B., Streck E.L., Chagas M.H., Hallak J.E., Zuardi A.W., Crippa J.A., Quevedo J. Acute and chronic administration of cannabidiol increases mitochondrial complex and creatine kinase activity in the rat brain. Rev. Bras. Psiquiatr. 2013;35:380–386. doi: 10.1590/1516-4446-2012-0886. [DOI] [PubMed] [Google Scholar]
- 34.Ryan D., Drysdale A.J., Lafourcade C., Pertwee R.G., Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J. Neurosci. 2009;29:2053–2063. doi: 10.1523/JNEUROSCI.4212-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wamelink M.M., Struys E.A., Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J. Inherit. Metab. Dis. 2008;31:703–717. doi: 10.1007/s10545-008-1015-6. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Rodriguez P., Almeida A., Bolanos J.P. Brain energy metabolism in glutamate-receptor activation and excitotoxicity: role for APC/C-Cdh1 in the balance glycolysis/pentose phosphate pathway. Neurochem Int. 2013;62:750–756. doi: 10.1016/j.neuint.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Bouzier-Sore A.K., Bolanos J.P. Uncertainties in pentose-phosphate pathway flux assessment underestimate its contribution to neuronal glucose consumption: relevance for neurodegeneration and aging. Front Aging Neurosci. 2015;7:89. doi: 10.3389/fnagi.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolanos J.P., Almeida A. The pentose-phosphate pathway in neuronal survival against nitrosative stress. IUBMB Life. 2010;62:14–18. doi: 10.1002/iub.280. [DOI] [PubMed] [Google Scholar]
- 39.Bolanos J.P., Almeida A., Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci. 2010;35:145–149. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Bolanos J.P. Bioenergetics and redox adaptations of astrocytes to neuronal activity. J. Neurochem. 2016 doi: 10.1111/jnc.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]