Abstract
Numerous reports have documented that serologic methods are much more sensitive than culture for the diagnosis of pertussis in adolescents and adults. However, a standardized serologic test for pertussis is not routinely available to most clinicians, and the serologic test levels or cutoff points correlated with diseases have not been determined. The goal of the present study was to examine the distribution of immunoglobulin G (IgG) levels against three Bordetella pertussis antigens (pertussis toxin [PT], filamentous hemagglutinin [FHA], and fimbria types 2 and 3 [FIM]) and to determine population-based antibody levels for the purpose of establishing such diagnostic cutoff points. Enzyme-linked immunosorbent assays (ELISAs) were performed with sera from >6,000 U.S. residents aged 6 to 49 years who participated in the Third National Health and Nutrition Examination Survey. Mixture models were developed to identify hypothesized exposure groups and establish diagnostic cutoffs. Quantifiable (>20 ELISA units/ml [EU]) anti-FHA and anti-FIM IgG antibodies were common (65 and 62% of individuals, respectively), but quantifiable anti-PT IgG antibodies were less frequent (16%). Given the distributions of antibody levels, an anti-PT IgG level of ≥94 EU was proposed as the diagnostic cutoff point. Application of this cutoff point to culture-confirmed illness in a prior study investigating cough illness yielded a high diagnostic sensitivity (80%) and specificity (93%). A standardized ELISA for anti-PT IgG with a single serum sample appears to be useful for the identification of recent B. pertussis infection in adolescents and adults with cough illness. The PT cutoff point will be further evaluated in prospective studies of confirmed B. pertussis infection.
Pertussis is underreported in the United States, in part due to difficulties in laboratory confirmation of Bordetella pertussis infection (4, 5, 51). Because infants with pertussis are often hospitalized and B. pertussis is more easily isolated from these patients, they often serve as sentinels for pertussis in a given community (60). However, when serologic studies have been used to investigate the contacts of infants with pertussis, the primary cases have often been identified in adolescents or adults (40).
The diagnosis of pertussis in adolescents and adults is formidable. Isolation of B. pertussis by culture is often unsuccessful after a few weeks of illness, when most patients first present for medical care (8, 11, 14, 18, 26, 32, 48, 54). PCR assays are sometimes used to diagnose pertussis, but these tests are not universally standardized or validated and are also likely to be negative after a few weeks of illness (26). Some investigators have relied on determining antibody levels in a population by using single serum sample to establish diagnostic cutoff points for a positive test result. Because pertussis toxin (PT) is found only in B. pertussis organisms (1), diagnostic enzyme-linked immunosorbent assays (ELISAs) have focused on detecting antibodies to this antigen. One laboratory, the Massachusetts State Laboratory Institute, established a diagnostic cutoff point by using the 99% upper tolerance limit (UTL) of immunoglobulin G (IgG) against PT in 100 subjects ≥11 years of age who had no known recent history of pertussis (29, 64). This cutoff point was applied to a sample of 64 persons aged ≥11 years with bacteriologically confirmed pertussis and resulted in a diagnostic sensitivity of 63% (29). In Massachusetts, serodiagnosis by an anti-PT IgG ELISA with a single serum sample has confirmed a high incidence among adolescents and adults with clinical pertussis (64). Other investigators have used cutoff points for single serum samples derived from the mean + 2 standard deviations (SDs) or the mean + 3 SDs of the anti-PT IgG or the anti-filamentous hemagglutinin (anti-FHA) IgG or IgA ELISA levels (7, 20, 34, 41, 44, 46, 47, 50, 62, 63).
Although commercial serologic tests for the detection of pertussis exist, none have been licensed in the United States for routine diagnostic use. Standardized measurement of the levels of antibodies to B. pertussis antigens has not been performed in a large serologic survey of the U.S. population to establish diagnostic cutoff points for the serodiagnosis of pertussis. After infection, IgG responses to PT and/or FHA can be detected in >90% of infected individuals, and those to fimbria types 2 and 3 (FIM) can be detected in 30 to 60% of infected individuals (37). However, IgA and IgM responses to these antigens occur less frequently, and the diagnostic value of IgM responses has not been established for pertussis (33). To provide the basis for a diagnostic test for acute pertussis in adolescents and adults by use of a single serum sample, we established a validated ELISA (28) to derive diagnostic cutoff points for the levels of IgG against PT, FHA, and FIM in a representative sample of U.S. residents.
(This study was presented in part at the Acellular Pertussis Vaccine Conference, Bethesda, Md., November 2000.)
MATERIALS AND METHODS
Survey design and data collection.
This study was approved by the Institutional Review Board of the Centers for Disease Control and Prevention. We obtained surplus sera collected from persons aged 6 to 49 years who participated in phase 2 (1991 to 1994) of the Third National Health and Nutrition Examination Survey (NHANES III), a nationally representative sample of the U.S. civilian, noninstitutionalized population (9, 39, 55, 56). To produce national estimates, each person sampled was assigned a sampling weight that incorporated the probability of selection and included adjustments for noncoverage and nonresponse.
Of the 9,149 individuals aged 6 to 49 years who were interviewed, 94% (n = 8,604) were examined. Of those examined, 70% (n = 6,040) had a sufficient quantity of serum available for analysis. The rate of serum availability was lower for younger age groups (46% for individuals aged 6 to 9 years, 61% for individuals aged 10 to 14 years, 65% for individuals aged 15 to 19 years) and higher for older age groups (78 to 82%). Participation rates were slightly lower for individuals classified as “other” race or ethnicity (66%) and non-Hispanic blacks (67%) than for non-Hispanic whites (71%) and Mexican-Americans (75%). No response differences by gender were observed.
Laboratory analysis.
The levels of IgG against PT, FHA, and FIM in serum were measured by ELISA methods (32) at Vanderbilt University Medical Center (VUMC) from December 1998 through July 2000. Antibody levels were quantitated with respect to a reference serum sample that was calibrated against the U.S. Reference Pertussis Serum, Human, Lot 3 (HRP3). HRP3 has been assigned unitages of 200 ELISA units/ml (EU) for PT and 200 EU for FHA. Because no official unitage had been assigned to HRP3 for the FIM ELISA, we arbitrarily assigned a unitage of 100 EU, as in prior studies (32). Single lots of the antigens were obtained from GlaxoSmithKline, Rixensart, Belgium (PT and FHA), and from Aventis Pasteur Ltd., Toronto, Ontario, Canada (FIM). These antigens were tested for purity and antigenicity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and ELISA with rabbit polyclonal antibodies provided by the Food and Drug Administration. Their endotoxin concentrations were determined by the Limulus amebocyte lysate assay (Limulus E-Toxate kits; Sigma Chemical Company, St. Louis, Mo.). In each ELISA, the lower limit of detection (LLD) was defined as the lowest amount of antibody detected and was determined to be 1 EU for the PT assay, 1 EU for the FHA assay, and 2 EU for the FIM assay. Each ELISA had an interassay coefficient of variation (CV) of <25%. The lower limit of quantitation (LLQ) was defined as the ELISA value above which the precision of the estimated values stabilized at an acceptable level. The LLQ for all three assays was conservatively estimated to be 20 EU, based on the intra- and interassay variabilities and the parallelism of the sample titration curves to the reference titration curve. Although assays with LLQs below 20 EU have been reported (20, 32), prior studies have shown that most subjects with culture- or PCR-confirmed pertussis have antibody levels that are well above 20 EU (7, 29).
Acceptance criteria for a valid test result were established during assay validation. Reference and control sera were included on every assay plate. Serum samples were retested if assay performance was inconsistent with established acceptance criteria or if the sample titration curves were not parallel to the reference curve (43). Randomly chosen serum samples from participants in NHANES III were assayed in duplicate in every assay run, and intra-assay variability was assessed by evaluating plots of CV versus the mean for duplicate specimens. Assay performance during the study period was monitored by plotting values for the assay parameters versus the date of testing. No significant changes or trends in the values for the control serum samples, assay variability, or parallelism were noted during the course of the study.
Mixture models.
Because pertussis remains an endemic disease (4), we expected some proportion of the population participating in NHANES III to have been recently infected with B. pertussis. From 1991 to 1994, pertussis outbreaks involving several hundred culture- or PCR-confirmed cases were documented throughout the United States (6, 13, 16, 21, 44). ELISA values for children aged 6 to 9 years were excluded from this analysis because elevated antibody levels in this group could have resulted from recent booster doses of diphtheria and tetanus toxoids combined with whole-cell or acellular pertussis vaccine (3). Because pertussis vaccines were not administered to individuals aged >6 years and the levels of antibodies against B. pertussis antigens in serum decline within a few years after vaccination (24, 25) or natural disease (35, 52, 58), we hypothesized that individuals aged 10 to 49 years were more likely than those aged 6 to 9 years to have elevated antibody levels as a result of recent infection rather than vaccination (19, 20, 48, 59). Thus, individuals aged 10 to 49 years who provided serum represented one of several possible exposure groups: (i) no recent exposure to pertussis disease, (ii) past immunization with waning immunity, (iii) prior infection(s) with B. pertussis (or an organism with cross-reacting antigens), or (iv) recent infection with B. pertussis (or an organism with cross-reacting antigens). Assuming that the study subjects fell into one of these or other groups, the distribution of quantitative results of each assay could be modeled as a mixture of the results for several underlying populations.
For each assay, antibody levels were transformed by using the log10 function. A mixture model (31, 42, 53) was then developed that assumed normal distributions for the component populations with antibody levels greater than the LLQ. The model included a component population representing individuals with little or no measurable antibody located entirely at or below the LLQ (36). Models with increasing numbers of groups were fit to determine the best model that could be used to cluster the measured antibody levels. The Bayesian information criterion (BIC) was used as a guideline to decide how many groups should be included in the model (31), with smaller values indicating better fits. The parameters in each model were estimated by maximum likelihood (45). All statistics were weighted to represent the U.S. population (39). Standard errors and variance matrices were estimated by use of the jackknife variance estimator (22).
Diagnostic cutoff points.
For each assay, we assumed that the mixture model selected correctly identified distinct populations and that the component population with the highest antibody levels represented individuals recently exposed to B. pertussis. A cutoff point for the diagnosis of acute pertussis infection was then chosen to attain a high model specificity (99%) with respect to the two component populations with the highest modeled antibody levels. To assess the validity of the cutoff point chosen for each antigen, study subjects were classified into positive and negative groups by using the cutoff point, and the distributions of the levels of antibodies to the other two antigens were evaluated within each group. We also evaluated the alternative cutoff points used in previously published research: assuming a single population, we calculated the mean + 2 SDs or the mean + 3 SDs, the 99th percentile, and the distribution-free 99% UTL (15). For each of these statistics, EU values less than the LLD were assigned a value of one-half the LLD.
Evaluation of PT cutoff point.
We evaluated our PT cutoff point by retrospectively applying it to published data from a prospective study of pertussis among health plan members in Minnesota conducted in 1995 and 1996 (50). We used data obtained from 65 subjects aged 10 to 49 years from whom specimens for culture and acute-phase serum samples were collected within 14 days of cough onset and from whom convalescent-phase serum samples were collected within 26 to 56 days of cough onset. The serologic assays had been performed previously (50) but were performed in the same laboratory used to perform the assays in the present study (VUMC) by using the same methods and primary reference serum sample. The PT cutoff point was evaluated with respect to the B. pertussis culture results.
We also evaluated the PT cutoff point using data from a recent pertussis outbreak among surgical staff at a rural hospital in Ohio (30). In the Ohio outbreak, nasopharyngeal specimens from three suspected pertussis adult patients were tested by a PCR assay at a local laboratory, and the specimens from two adults were found to be positive for B. pertussis DNA. Serum samples were also obtained from 16 adults aged 28 to 54 years who were exposed to B. pertussis or suspected to have pertussis. The serum samples were assayed in the fall of 1999 at VUMC during the period when the serum samples from the NHANES III participants were being tested. We evaluated the PT cutoff point with respect to cough duration.
PT positivity.
For selected characteristics of the NHANES III study subjects, an exponential risk model was fit and a Wald test (22) was performed to assess the association between PT positivity (defined as ≥94 EU) and the characteristic. The month of clinical examination was classified into two groups (July to September versus other months) because pertussis cases were reported more frequently from July to September than during other months (13). To investigate the functional relationship between PT positivity and age at interview, we fit an exponential risk model that included the linear age term (years) and additional spline terms to characterize the trends by age (12).
RESULTS
Study populations.
IgG levels directed against the three B. pertussis antigens were measured in serum samples collected from 6,040 individuals aged 6 to 49 years. Of these, 6,030 had anti-PT IgG values that met the laboratory acceptance criteria for a valid result, 6,018 had valid anti-FHA IgG results, and 6,014 had valid anti-FIM IgG results. For the mixture model analysis, we excluded individuals aged 6 to 9 years to determine a cutoff point: 621 individuals were excluded for the PT assay, 619 were excluded for the FHA assay, and 619 were excluded for the FIM assay. In summary, among individuals aged 10 to 49 years, 5,409 had valid results for PT, 5,399 had valid results for FHA, and 5,395 had valid results for FIM. B. pertussis antigen-specific IgG ELISA values were less than the LLD for 881 (16%) PT tests, 39 (1%) FHA tests, and 863 (16%) FIM tests. ELISA values were less than or equal to the LLQ (20 EU) for 4,577 (85%) PT tests, 3,108 (58%) FHA tests, and 2,781 (52%) FIM tests.
Number of components in mixture models.
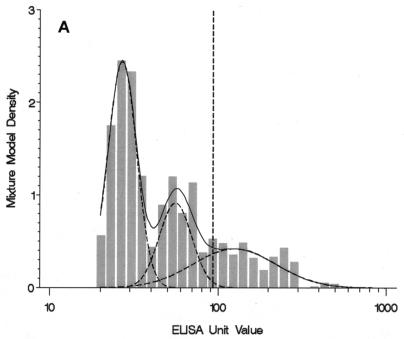
When anti-PT IgG levels were analyzed in the mixture models, the lowest BIC value occurred for the three-component mixture model. However, the four-component model had a BIC value that was relatively close to that for the three-component model (4,731.5 versus 4,729.1), and it identified an additional component with high antibody levels that we speculated represented individuals with recent B. pertussis infection (Table 1). Thus, the four-component model was chosen to represent the distribution of anti-PT IgG levels. The mixture density estimated by the four-component model indicated that the model reflected the frequency distribution of ELISA values greater than the LLQ (Fig. 1A). The first population, with antibody levels less than or equal to the LLQ, included individuals with nondetectable or low levels of anti-PT IgG. The second and third component populations included individuals with moderate antibody levels. The fourth component population included individuals who had relatively high antibody levels. Results from the mixture model suggested that this group comprised 4.23% (95% confidence interval [CI], 2.27 to 6.19%) of the U.S. population aged 10 to 49 years.
TABLE 1.
Summary of component populations in the mixture models for weighted PT, FHA, and FIM assay data, individuals aged 10 to 49 years, NHANES III, 1991 to 1994
| Assay and model | Component population no. | Proportion in component population | Mean EU (mean EU ± 2 SDs) |
|---|---|---|---|
| PT (n = 5,409) | |||
| Three-component mixture | 1 | 0.823 | —a (—) |
| 2 | 0.053 | 27 (19, 37) | |
| 3 | 0.124 | 51 (9, 303) | |
| Four-component mixture | 1 | 0.838 | — (—) |
| 2 | 0.084 | 27 (18, 40) | |
| 3 | 0.036 | 56 (36, 87) | |
| 4 | 0.042 | 126 (40, 392) | |
| FHA (n = 5,399) | 1 | 0.350 | — (—) |
| 2 | 0.650 | 30 (4, 254) | |
| FIM (n = 5,395) | 1 | 0.377 | — (—) |
| 2 | 0.521 | 47 (7, 297) | |
| 3 | 0.102 | 306 (42, 2,209) |
—, values for the first component population were less than or equal to the LLQ (20 EU) and were modeled with a single parameter for proportion in the target population.
FIG. 1.
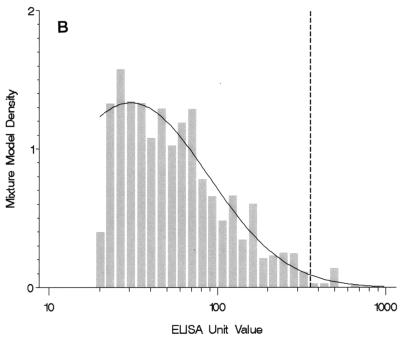
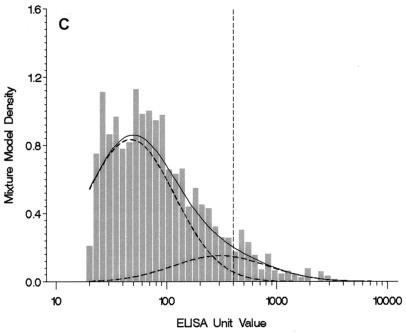
(A) Four-component mixture model fit for weighted anti-PT IgG levels among individuals 10 to 49 years of age (n = 5,409). The histogram graphs the observed data with values greater than the LLQ. Observed values less than or equal to the LLQ (85% of the sample size) and one observed value >1,000 EU are not shown. The solid line plots the mixture model fit for components 2, 3, and 4; component 1 is less than or equal to the LLQ and is not shown. The dashed lines indicate the underlying component populations in the mixture model. A reference dashed line is drawn at the selected cutoff point of 94 EU. (B) Two-component mixture model fit for weighted anti-FHA IgG levels among individuals 10 to 49 years of age (n = 5,399). The histogram graphs the observed data with values greater than the LLQ. Observed values less than or equal to the LLQ (58% of sample size) and two observed values >1,000 EU are not shown. The solid line plots the mixture model fit for component 2; component 1 is less than or equal to the LLQ and is not shown. A reference dashed line is drawn at the selected cutoff point of 358 EU. (C) Three-component mixture model fit for weighted anti-FIM IgG levels among individuals 10 to 49 years of age (n = 5,395). The histogram graphs the observed data with values greater than the LLQ. Observed values less than or equal to the LLQ (52% of sample size) are not shown. The solid line plots the mixture model fit for components 2 and 3; component 1 is less than or equal to the LLQ and is not shown. The dashed lines indicate the underlying component populations in the mixture model. A reference dashed line is drawn at the selected cutoff point of 402 EU.
For anti-FHA IgG levels, the two-component model was chosen to represent the distribution of antibody levels because it had the lowest BIC value (Table 1). The model contained only one population with values greater than the LLQ (Fig. 1B); this group included a large proportion (65%) of the U.S. population aged 10 to 49 years (Table 1).
A three-component model was chosen for the anti-FIM IgG levels because it had the lowest BIC value (Table 1; Fig. 1C). The first component population included individuals with nondetectable or low levels of anti-FIM IgG. The second component population included a large proportion (52%) of the U.S. population aged 10 to 49 years. For the third component population, the mean anti-FIM IgG level was well above that for the second population (306 versus 47 EU). The results from the mixture model suggested that the third component included 10% of the U.S. population aged 10 to 49 years (Table 1).
Cutoff points for diagnosis of pertussis.
We chose a diagnostic cutoff point by assuming that the fourth component population in the four-component mixture model for the anti-PT IgG data represented a group of individuals with true, recent B. pertussis infections, including individuals with atypical or asymptomatic cases. On the basis of a model specificity of 99% with respect to the third and fourth groups in the model, the diagnostic cutoff point for acute infection was chosen to be 94 EU and resulted in a model sensitivity of 70% (Table 2). In contrast, the cutoff point for acute infection defined by the mean for the entire study population + 3 SDs was 436 EU, and the cutoff defined by the 99% UTL was 232 EU, with both values being higher than the cutoff established by using the mixture model. The model-based cutoff point of 94 EU is well above the low end of the distribution for the fourth group in the mixture model (Fig. 1A). For this reason, we calculated a second cutoff point of 49 EU (the fifth percentile of the fourth group) to define a range of ELISA values that might possibly be associated with acute or recent B. pertussis infection. The interval from 49 to 93 EU anti-PT IgG could be used to define a group indeterminate for acute B. pertussis infection.
TABLE 2.
Diagnostic cutoff points for acute pertussis infection based on mixture model or previously published methods applied to weighted PT, FHA, and FIM assay data, individuals aged 10 to 49 years, NHANES III, 1991 to 1994
| Basis of cutoff point and statistic | Cutoff point (EU)a
|
||
|---|---|---|---|
| PT assay (n = 5,409) | FHA assay (n = 5,399) | FIM assay (n = 5,395) | |
| Mixture model with specificity ofb: | |||
| 0.950 | 80 (0.783) | 174 | 214 (0.640) |
| 0.990 | 94 (0.697) | 358 | 402 (0.392) |
| 0.999 | 111 (0.586) | 805 | 812 (0.162) |
| 0.9999 | 128 (0.489) | 1,567 | 1,449 (0.058) |
| Previously published methods | |||
| Mean + 2 SDs | 94 | 229 | 800 |
| Mean + 3 SDs | 436 | 868 | 5,000 |
| 99th percentile | 198 | 287 | 1,163 |
| 99% UTLc | 232 | 311 | 1,386 |
The cutoff points selected are in boldface. Values in parentheses are sensitivities.
For the PT and FIM assays, model specificity and sensitivity were based on the two overlapping normal distributions estimated for the two component populations with the highest antibody levels. For the FHA assay, the mixture model included only one population for which the value was greater than the LLQ.
The 99% UTL (distribution-free) is the upper 95% confidence bound for the 99th percentile of the sampled population.
Because the two-component model for the anti-FHA IgG data included only one component population with values greater than the LLQ, we calculated the cutoff point for FHA as the 99th percentile of the single distribution with values greater than the LLQ (358 EU) (Table 2). The diagnostic cutoff point derived from the three-component model for the anti-FIM IgG data was 402 EU, with a lower model sensitivity (39%) compared with that derived from the mixture model for the anti-PT IgG data (70%).
We evaluated the assumption that the fourth group in the PT mixture model represented individuals recently infected with B. pertussis by examining the distribution of anti-FHA IgG and anti-FIM IgG values for individuals classified as positive for anti-PT IgG. These analyses were restricted to the 5,366 serum samples that had valid results by all three assays. Individuals who had anti-PT IgG levels above the cutoff point of 94 EU had higher levels of both anti-FHA IgG and anti-FIM IgG than those with values below 94 EU (Table 3). The mean level of anti-FHA IgG was 61.2 EU for individuals who were positive for anti-PT IgG, whereas it was only 14.7 EU for individuals who were negative (P < 0.001). In addition, the rate of a positive FHA serologic result was 28.4 times (95% CI, 5.5 to 145.3 times) greater for individuals whose values were above the cutoff point for anti-PT IgG than individuals whose values were below this cutoff point. This pattern of results was also observed for the distribution of anti-FIM IgG levels stratified by positivity for anti-PT IgG (Table 3). Evaluation of the diagnostic cutoff points for the anti-FHA IgG values (≥358 EU) and the anti-FIM IgG values (≥402 EU) produced similar findings (Table 3).
TABLE 3.
Evaluation of a diagnostic cutoff point to define positive IgG results for each assaya by use of the weighted mean IgG level and percent positive for the other two assays within positive and negative groups, individuals aged 10 to 49 years, NHANES III, 1991 to 1994
| Assay and assay resultsd | Sample size (no. of individuals) | Weighted mean IgG level (EU) | P value for weighted meanb | % Positive | RRc (95% CI) |
|---|---|---|---|---|---|
| PT assay | |||||
| (FHA assay) | |||||
| Positive | 144 | 61.2 | <0.001 | 8.5 | 28.4 (5.5-145.3) |
| Indeterminate | 202 | 46.7 | <0.001 | 2.1 | 7.2 (0.6-85.4) |
| Negative | 5,020 | 14.7 | 0.3 | 1.0 (reference) | |
| (FIM assay) | |||||
| Positive | 144 | 65.7 | 0.002 | 15.0 | 4.0 (1.7-9.3) |
| Indeterminate | 202 | 57.5 | 0.001 | 16.9 | 4.5 (2.3-8.9) |
| Negative | 5,020 | 18.9 | 3.7 | 1.0 (reference) | |
| FHA assay | |||||
| (PT assay) | |||||
| Positive | 44 | 28.7 | 0.009 | 40.5 | 15.2 (5.8-40.0) |
| Negative | 5,322 | 4.3 | 2.7 | 1.0 (reference) | |
| (FIM assay) | |||||
| Positive | 44 | 245.6 | <0.001 | 41.0 | 9.5 (4.2-21.2) |
| Negative | 5,322 | 20.1 | 4.3 | 1.0 (reference) | |
| FIM assay | |||||
| (PT assay) | |||||
| Positive | 148 | 10.9 | <0.001 | 9.5 | 3.7 (1.5-9.3) |
| Negative | 5,218 | 4.1 | 2.6 | 1.0 (reference) | |
| (FHA assay) | |||||
| Positive | 148 | 49.2 | <0.001 | 5.4 | 14.6 (3.9-54.6) |
| Negative | 5,218 | 15.1 | 0.4 | 1.0 (reference) |
Data are for 5,366 serum samples valid results by all three assays. A positive IgG result was defined by the cutoff point(s) derived from the mixture model (specificity = 99%): ≥94 EU for PT-positive samples, 49 to 93 EU for PT-indeterminate samples, ≥358 EU for FHA-positive samples, or ≥402 EU for FIM-positive samples.
t test (two-tailed) for the null hypothesis of no difference between the weighted means for the positive (or indeterminate) and negative groups.
RR, relative rate.
Comparison assays are in parentheses.
Clinical evaluation of PT titer cutoff point.
In the Minnesota study, four of the five culture-positive patients had an anti-PT IgG level ≥94 EU, yielding a diagnostic sensitivity of 80% (Table 4). Of the 60 culture-negative patients, 56 had an anti-PT IgG level <94 EU, resulting in a diagnostic specificity of 93%. In the Ohio pertussis outbreak, 10 of 16 health care workers tested had an acute cough illness of >14 days' duration (range, 20 to 42 days); 6 of these 10 adults had anti-PT IgG levels >94 EU. The anti-PT IgG level was <22 EU for the three tested adults who did not have clinical signs of pertussis (cough for 0 days) and for the three adults who had cough for 5 to 7 days.
TABLE 4.
Evaluation of anti-PT IgG cutoff point of 94 EU by using data from a previous study of health plan members aged 10 to 49 years with an acute cough illness, Minnesota, 1995-1996a
| Cultureb result | No. of convalescent-phase serum specimens (EU range):
|
Diagnostic sensitivity (%) | Diagnostic specificity (%) | |
|---|---|---|---|---|
| Positivec | Negative | |||
| Positive | 4 (477-695) | 1 (76) | 80 | 93 |
| Negative | 4 (745-2,241) | 56 (1-85) | ||
Data are from the study in reference 50.
The culture result was used as the reference criterion.
A positive anti-PT IgG result was defined as ≥94 EU.
Positivity for IgG against PT, FHA, and FIM.
Positivity rates were calculated for 5,366 subjects aged 10 to 49 years for whom valid results were obtained by all three assays. The model-based cutoff point for anti-PT IgG (≥94 EU) was exceeded by 2.89% (95% CI, 2.14 to 3.64%) of the U.S. population, the cutoff point for anti-FHA IgG (≥358 EU) was exceeded by 0.60% (95% CI, 0.22 to 0.99%) of the U.S. population, and the cutoff point for anti-FIM IgG (≥402 EU) was exceeded by 4.56% (95% CI, 3.51 to 5.60%) of the U.S. population. Because subjects classified as indeterminate for PT may be used to identify additional pertussis cases, we also determined the percentage of the population classified as indeterminate for PT but positive for FIM (0.63%) or FHA (0.08%).
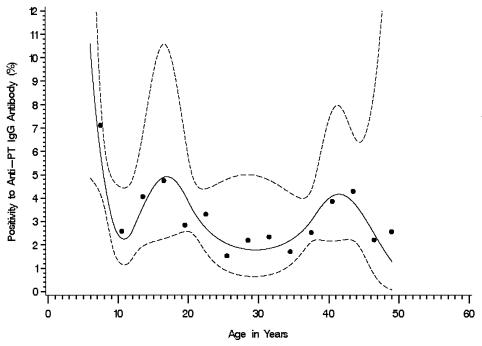
To evaluate associations between PT positivity and selected characteristics of the study subjects, we analyzed the valid anti-PT IgG results for 6,030 subjects, including those aged 6 to 9 years. Subjects aged 6 to 9 years had a statistically significantly (P = 0.016) higher PT positivity rate than those aged 10 to 49 years (6.50 and 2.88%, respectively). The PT positivity rate varied across the entire age range, but the Wald test for an association between positivity and age group was not statistically significant (P = 0.384) (Table 5). The pattern of positivity by age group led us to explore the functional relationship between positivity and age (in years) using an exponential risk model with a quadratic regression spline for age; for the spline function, we chose category boundaries at 9, 12, 20, and 38 years of age. The fitted spline curve suggested a sinusoidal pattern with elevated positivity in the group aged 13 to 20 years and in the group aged 37 to 46 years (Fig. 2). The rate of a positive anti-PT IgG result was not statistically significantly associated (P < 0.05) with other characteristics (Table 5).
TABLE 5.
Weighted rate of a positive anti-PT IgG resulta by selected characteristics among individuals aged 6 to 49 years, NHANES III, 1991 to 1994
| Characteristic | Sample size (no. of individuals) | Estimated population size (in millions) | Weighted rate of a positive anti-PT IgG result (95% CI)b | P valuec |
|---|---|---|---|---|
| Age (yr) at interview | ||||
| 6-9 | 621 | 10.2 | 6.50 (3.71-10.4) | 0.384 |
| 10-14 | 858 | 14.9 | 3.16 (1.52-5.74) | |
| 15-19 | 628 | 10.6 | 4.42 (1.68-9.26) | |
| 20-24 | 755 | 16.0 | 2.76 (1.29-5.11) | |
| 25-29 | 719 | 16.5 | 1.86 (0.15-7.41) | |
| 30-34 | 741 | 18.9 | 2.28 (0.65-5.64) | |
| 35-39 | 680 | 18.4 | 2.68 (0.85-6.27) | |
| 40-44 | 640 | 18.3 | 3.94 (1.73-7.58) | |
| 45-49 | 388 | 11.7 | 2.34 (0.32-7.87) | |
| Race or ethnicity | ||||
| Non-Hispanic white | 1,672 | 97.5 | 3.11 (1.89-4.82) | 0.291 |
| Non-Hispanic black | 2,127 | 16.1 | 3.36 (2.35-4.65) | |
| Mexican-American | 1,926 | 9.2 | 2.28 (1.63-3.10) | |
| Other | 305 | 12.8 | 3.80 (0.41-13.6) | |
| Gender | ||||
| Male | 2,666 | 66.9 | 3.81 (2.57-5.41) | 0.086 |
| Female | 3,364 | 68.7 | 2.52 (1.95-3.19) | |
| Metro residenced | ||||
| Yes | 3,150 | 66.8 | 2.44 (1.72-3.35) | 0.053 |
| No | 2,880 | 68.8 | 3.85 (2.85-5.06) | |
| Poverty indexe | ||||
| < poverty level | 1,754 | 21.0 | 3.54 (1.35-7.42) | 0.855 |
| ≥ poverty level | 3,862 | 108.7 | 3.22 (2.18-4.58) | |
| No. of persons living in household | ||||
| 1-2 | 1,092 | 34.6 | 3.20 (1.71-5.42) | 0.830 |
| 3-4 | 2,527 | 63.0 | 2.89 (1.67-4.64) | |
| ≥5 | 2,411 | 38.0 | 3.55 (1.83-6.15) | |
| Month of clinical exam | ||||
| July to September | 1,491 | 47.0 | 4.31 (2.79-6.32) | 0.101 |
| Other | 4,539 | 88.5 | 2.54 (1.78-3.51) |
Data are for 6,030 serum samples that had valid results by the PT assay.
A positive anti-PT IgG result was defined by the cutoff point derived from the mixture model (specificity = 99%), ≥94 EU. The exact binomial CI modified for the NHANES III sampling design (22).
Wald test for the null hypothesis of no difference in the rate of a positive anti-PT IgG result across subgroups.
Residence in central counties of metropolitan areas of more than 1 million population.
Data on poverty index were missing for 6.9% of the sample.
FIG. 2.
Weighted rate of a positive anti-PT IgG result (≥94 EU) by age at interview (6 to 49 years) (n = 6,030). The solid line indicates the fitted spline curve, and the dashed lines indicate the pointwise 95% confidence bands for the fitted spline curve. Each dot represents the observed data for subjects in a 3-year age group.
DISCUSSION
We examined the distribution of concentrations of antibodies to three B. pertussis antigens in sera from a large representative sample of more than 5,000 individuals aged 10 to 49 years who were participants in NHANES III. In this study, elevated anti-PT IgG levels were relatively infrequent (16% had levels >20 EU), whereas elevated anti-FHA IgG and anti-FIM IgG levels (>20 EU) were more common (65 and 62%, respectively). These results suggest that an elevated level of IgG against PT may be a more specific predictor of B. pertussis infection than an elevated level of IgG against FHA or FIM. Other investigators have reported that IgG against PT is a specific indicator of recent B. pertussis infection (1, 19, 46). Although most individuals infected with B. pertussis produce antibodies to PT, FHA, and FIM, antibodies to FHA and FIM are also observed in response to infection with other Bordetella species and to cross-reacting antigens found on other bacterial species (19, 20, 37, 49, 59). Consequently, assays for IgG against FHA and FIM show less promise as stand-alone diagnostic assays and appear to more useful in helping to confirm B. pertussis infections in individuals with indeterminate anti-PT IgG results.
More traditional approaches to developing a diagnostic cutoff value for anti-PT IgG levels would assume that none of the study population had elevated levels of IgG against PT due to recent B. pertussis infection. These approaches would assume one healthy homogenous population that is lognormally distributed and would use the 99% UTL or the mean + 2 SDs (or the mean + 3 SDs) to define the diagnostic cutoff value (29, 50, 64). However, a more comprehensive approach to discriminating between individuals who have recently been infected with B. pertussis and those who have not been recently infected requires knowledge of the antibody distributions among the individuals in these groups (23, 38). Our mixture model analysis assumed the existence of more than one exposure group in the NHANES III population. This assumption took into consideration the fact that pertussis vaccination is not given to individuals aged >6 years, that documented pertussis outbreaks occurred throughout the United States during the period of collection of the samples from participants in NHANES III, and that waning immunity or recent exposure likely affected the antibody levels. In our four-component mixture model for anti-PT IgG levels, individuals in the fourth group were presumed to have been recently infected with B. pertussis (including individuals who had mild or asymptomatic infection), whereas those in the third group likely had less recent infection. The cutoff point chosen to distinguish between the third and fourth groups with high model specificity (99%) resulted in moderate model sensitivity (70%); a moderate model sensitivity was expected because some individuals with recent or acute B. pertussis infection produce little or no measurable anti-PT IgG (37, 57). Moreover, individuals with levels of anti-PT IgG above the cutoff point were more likely to have high levels of anti-FHA IgG and anti-FIM IgG, which is consistent with recent exposure to B. pertussis.
The mixture model approach yielded a cutoff point for anti-PT IgG of 94 EU. We found that 2.89% of the NHANES III population aged 10 to 49 years had an anti-PT IgG level ≥94 EU, indicating recent or acute infection with B. pertussis. Thus, the prevalence of an elevated anti-PT IgG value in the U.S. population aged 10 to 49 years was 2,890 individuals per 100,000 population. This prevalence rate is higher than previous estimates of annual incidence (41, 50, 61), at least in part because it likely included individuals with an asymptomatic or a mild cough illness and because it likely reflected the gradual decline in the IgG concentrations in the months or years following B. pertussis infection (17, 52). Our results also suggested that adolescents had an elevated rate of PT positivity relative to those of all individuals aged 10 to 49 years, consistent with other studies (2, 50).
We evaluated the PT cutoff point by retrospective application to data from a previously published pertussis study that used very similar assays performed in the same laboratory used for this study. The results support the conclusion that the proposed PT cutoff point is predictive of recent B. pertussis infection with reasonable diagnostic sensitivity and specificity. Although the sample size of 16 people was small, application of the PT cutoff point during the Ohio outbreak suggested that it was useful when it was coupled with clinical criteria. As in the Ohio outbreak, we envision the diagnostic criteria for pertussis by the detection of PT to be used primarily as an epidemiologic tool rather than for individual case management. Comprehensive, prospective evaluations of the PT cutoff point will be carried out in the future to define further the diagnostic sensitivity and specificity of the cutoff point and determine whether a range of ELISA values below the cutoff point can identify additional cases of pertussis.
We believe that a simple, standardized method for anti-PT IgG detection (10) can be developed on the basis of our assay. Transformation of the laboratory technology from our study into a useful diagnostic test will require careful calibration to the working reference sera used in our study. Regional or state health departments could use such a standardized assay to assess pertussis outbreaks and measure the burden of disease in adolescents and adults. Possible future routine vaccination of adolescents and adults with PT-containing vaccines might diminish the ability of serologic tests to diagnose pertussis. At present, however, diagnosis of pertussis with a single serum sample should be used on a broader scale. Timely identification of pertussis cases combined with public health interventions might help prevent the transmission of B. pertussis to infants, who suffer the greatest morbidity and mortality from pertussis (60).
Acknowledgments
We are grateful to Peter Strebel (National Immunization Program [NIP], Centers for Disease Control and Prevention [CDC], Atlanta, Ga.) for guidance on the study concept and design; Melinda Wharton (NIP, CDC) for administrative support; Gerry McQuillan (National Center for Health Statistics, CDC, Hyattsville, Md.) for facilitating access to serum samples from participants in NHANES III and public-use data files; Sandra Yoder, Patricia McGraw, and Roy Miner at the Vanderbilt University Medical Center for establishing the ELISAs and testing the serum samples from participants in NHANES III; Brian Plikaytis (National Center for Infectious Diseases [NCID], CDC, Atlanta, Ga.) for helpful discussions and work on transformations for forming standard reference curves to calculate antibody concentrations during the validation of the laboratory assays; Barry Graubard (National Cancer Institute, Bethesda, Md.) for advice on statistical analysis of the NHANES III data; and Gary Sanden (NCID, CDC), Greg Wallace (NIP, CDC), George Reed (National Eye Institute, National Institutes of Health), Carl Frasch (Center for Biologics Evaluation and Research, Food and Drug Administration), Trudy Murphy (NIP, CDC), John Moran (NIP, CDC), Ben Schwartz (NIP, CDC), and Susan Chu (NIP, CDC) for useful comments on earlier versions of the manuscript.
This study was supported by a contract from CDC to Vanderbilt University Medical Center (200-97-0606).
During the conduct of this work, none of the authors had a commercial or other association that might pose a conflict of interest.
REFERENCES
- 1.Aricò, B., and R. Rappuoli. 1987. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J. Bacteriol. 169:2847-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cattaneo, L. A., G. W. Reed, D. H. Haase, M. J. Wills, and K. M. Edwards. 1996. The seroepidemiology of Bordetella pertussis infections: a study of persons ages 1-65 years. J. Infect. Dis. 173:1256-1259. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1997. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children—recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 46(RR-7):1-25. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. Pertussis—United States, 1997-2000. Morb. Mortal. Wkly. Rep. 51:73-76. [PubMed] [Google Scholar]
- 5.Cherry, J. D. 1999. Epidemiological, clinical, and laboratory aspects of pertussis in adults. Clin. Infect. Dis. 28(Suppl. 2):S112-S117. [DOI] [PubMed] [Google Scholar]
- 6.Christie, C. D. C., M. L. Marx, C. D. Marchant, and S. F. Reising. 1994. The 1993 epidemic of pertussis in Cincinnati—resurgence of disease in a highly immunized population of children. N. Engl. J. Med. 331:16-21. [DOI] [PubMed] [Google Scholar]
- 7.de Melker, H. E., F. G. A. Versteegh, M. A. E. Conyn-van Spaendonck, L. H. Elvers, G. A. M. Berbers, A. van der Zee, and J. F. P. Schellekens. 2000. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 38:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewanowich, C. A., L. W.-L. Chui, M. G. Paranchych, M. S. Peppler, R. G. Marusyk, and W. L. Albritton. 1993. Major outbreak of pertussis in northern Alberta, Canada: analysis of discrepant direct fluorescent-antibody and culture results by using polymerase chain reaction methodology. J. Clin. Microbiol. 31:1715-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezzati, T. M., J. T. Massey, J. Waksberg, A. Chu, and K. R. Maurer. 1992. Sample design: third National Health and Nutrition Examination Survey. National Center for Health Statistics. Vital Health Statistics, series 2, no. 113. Government Printing Office, Washington, D.C. [PubMed]
- 10.Giammanco, A., A. Chiarini, P. A. C. Maple, N. Andrews, R. Pebody, N. Gay, R. M. Ölander, F. Fivet-Groyne, S. Baron, A. Tischer, S. Swidsinski, J. Schellekens, and E. Reizenstein. 2003. European sero-epidemiology network: standardisation of the assay results for pertussis. Vaccine 22:112-120. [DOI] [PubMed] [Google Scholar]
- 11.Greco, D., S. Salmaso, P. Mastrantonio, M. Giuliano, A. E. Tozzi, A. Anemona, M. L. Ciofi degli Atti, L. Marta, A. Giammanco, P. Panei, W. C. Blackwelder, D. L. Klein, and S. G. F. Wassilak. 1996. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N. Engl. J. Med. 334:341-348. [DOI] [PubMed] [Google Scholar]
- 12.Greenland, S. 1995. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology 6:356-365. [DOI] [PubMed] [Google Scholar]
- 13.Güris, D., P. M. Strebel, B. Bardenheier, M. Brennan, R. Tachdjian, E. Finch, M. Wharton, and J. R. Livengood. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin. Infect. Dis. 28:1230-1237. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson, L., H. O. Hallander, P. Olin, E. Reizenstein, and J. Storsaeter. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 334:349-355. [DOI] [PubMed] [Google Scholar]
- 15.Hahn, G. J., and W. Q. Meeker. 1991. Statistical intervals: a guide for practitioners, p. 53-99. John Wiley & Sons, Inc., New York, N.Y.
- 16.Hardy, I. R. B., P. M. Strebel, M. Wharton, and W. A. Orenstein. 1994. The 1993 pertussis epidemic in Cincinnati. N. Engl. J. Med. 331:1455-1456. (Letter.) [DOI] [PubMed] [Google Scholar]
- 17.Heininger, U., J. D. Cherry, and K. Stehr. 2004. Serologic response and antibody-titer decay in adults with pertussis. Clin. Infect. Dis. 38:591-594. [DOI] [PubMed] [Google Scholar]
- 18.Heininger, U., K. Klich, K. Stehr, and J. D. Cherry. 1997. Clinical findings in Bordetella pertussis infections: results of a prospective multicenter surveillance study. Pediatrics 100:e10. (Electronic article.) [DOI] [PubMed] [Google Scholar]
- 19.Hodder, S. L., J. D. Cherry, E. A. Mortimer, A. B. Ford, J. Gornbein, and K. Papp. 2000. Antibody responses to Bordetella pertussis antigens and clinical correlations in elderly community residents. Clin. Infect. Dis. 31:7-14. [DOI] [PubMed] [Google Scholar]
- 20.Jansen, D. L., G. C. Gray, S. D. Putnam, F. Lynn, and B. D. Meade. 1997. Evaluation of pertussis in U.S. Marine Corps trainees. Clin. Infect. Dis. 25:1099-1107. [DOI] [PubMed] [Google Scholar]
- 21.Kenyon, T., H. Izurieta, S. T. Shulman, E. Rosenfeld, M. Miller, R. Daum, and P. M. Strebel. 1996. Large outbreak of pertussis among young children in Chicago, 1993: investigation of potential contributing factors and estimation of vaccine effectiveness. Pediatr. Infect. Dis. J. 15:655-661. [DOI] [PubMed] [Google Scholar]
- 22.Korn, E. L., and B. I. Graubard. 1999. Analysis of health surveys. John Wiley & Sons, Inc., New York, N.Y.
- 23.Kramer, M. S. 1988. Clinical epidemiology and biostatistics, p. 201-219. Springer-Verlag, New York, N.Y.
- 24.Lambert, H. J. 1965. Epidemiology of a small pertussis outbreak in Kent County, Michigan. Public Health Rep. 80:365-369. [PMC free article] [PubMed] [Google Scholar]
- 25.Le, T., J. D. Cherry, S.-J. Chang, M. D. Knoll, M. L. Lee, S. Barenkamp, D. Bernstein, R. Edelman, K. M. Edwards, D. Greenberg, W. Keitel, J. Treanor, and J. I. Ward. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J. Infect. Dis. 190:535-544. [DOI] [PubMed] [Google Scholar]
- 26.Lievano, F. A., M. A. Reynolds, A. L. Waring, J. Ackelsberg, K. M. Bisgard, G. N. Sanden, D. Guris, A. Golaz, D. J. Bopp, R. J. Limberger, and P. F. Smith. 2002. Issues associated with and recommendations for using PCR to detect outbreaks of pertussis. J. Clin. Microbiol. 40:2801-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind-Brandberg, L., C. Welinder-Olsson, T. Lagergård, J. Taranger, B. Trollfors, and G. Zackrisson. 1998. Evaluation of PCR for diagnosis of Bordetella pertussis and Bordetella parapertussis infections. J. Clin. Microbiol. 36:679-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynn, F., G. F. Reed, and B. D. Meade. 1996. Collaborative study for the evaluation of enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clin. Diagn. Lab. Immunol. 3:689-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchant, C. D., A. M. Loughlin, S. M. Lett, C. W. Todd, L. H. Wetterlow, R. Bicchieri, S. Higham, P. Etkind, E. Silva, and G. R. Siber. 1994. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis, and vaccine effectiveness. J. Infect. Dis. 169:1297-1305. [DOI] [PubMed] [Google Scholar]
- 30.McCall, C., K. Bisgard, F. B. Pascual, A. MacMurtray, S. Lane, and F. Smith. 2001. Outbreak of pertussis in a rural Ohio hospital, September 1999, abstr. 62. Abstr. Natl. Immunization Conf. Centers for Disease Control and Prevention, Atlanta, Ga.
- 31.McLachlan, G., and D. Peel. 2000. Finite mixture models. John Wiley & Sons, Inc., New York, N.Y.
- 32.Meade, B. D., A. Deforest, K. M. Edwards, T. A. Romani, F. Lynn, C. H. O'Brien, C. B. Swartz, G. F. Reed, and M. A. Deloria. 1995. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 96:570-575. [PubMed] [Google Scholar]
- 33.Meade, B. D., C. M. Mink, and C. R. Manclark. 1990. Serodiagnosis of pertussis, p. 322-329. In C. R. Manclark (ed.), Proceedings of the 6th International Symposium on Pertussis, DHHS publication (FDA) 90-1164. U.S. Department of Health and Human Services, United States Public Health Service, Food and Drug Administration, Bethesda, Md.
- 34.Miller, E., D. M. Fleming, L. A. E. Ashworth, D. A. Mabbett, J. E. Vurdien, and T. S. J. Elliott. 2000. Serological evidence of pertussis in patients presenting with cough in general practice in Birmingham. Commun. Dis. Public Health 3:132-134. [PubMed] [Google Scholar]
- 35.Mortimer, E. A. 1990. Pertussis and its prevention: a family affair. J. Infect. Dis. 161:473-479. [DOI] [PubMed] [Google Scholar]
- 36.Moulton, L. H., and N. A. Halsey. 1995. A mixture model with detection limits for regression analyses of antibody response to vaccine. Biometrics 51:1570-1578. [PubMed] [Google Scholar]
- 37.Müller, F.-M. C., J. E. Hoppe, and C.-H. Wirsing von König. 1997. Laboratory diagnosis of pertussis: state of the art in 1997. J. Clin. Microbiol. 35:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy, E. A., and H. Abbey. 1967. The normal range—a common misuse. J. Chronic Dis. 20:79-88. [DOI] [PubMed] [Google Scholar]
- 39.National Center for Health Statistics. 1994. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Vital and health statistics, series 1, no. 32, July. DHHS publication (PHS) 94-1308. Government Printing Office, Washington, D.C.
- 40.Nelson, J. D. 1978. The changing epidemiology of pertussis in young infants. The role of adults as reservoirs of infection. Am. J. Dis. Child. 132:371-373. [DOI] [PubMed] [Google Scholar]
- 41.Nennig, M. E., H. R. Shinefield, K. M. Edwards, S. B. Black, and B. H. Fireman. 1996. Prevalence and incidence of adult pertussis in an urban population. JAMA 275:1672-1674. [PubMed] [Google Scholar]
- 42.Parker, R. A., D. D. Erdman, and L. J. Anderson. 1990. Use of mixture models in determining laboratory criterion for identification of seropositive individuals: application of parvovirus B19 serology. J. Virol. Methods 27:135-144. [DOI] [PubMed] [Google Scholar]
- 43.Plikaytis, B. D., P. F. Holder, L. B. Pais, S. E. Maslanka, L. L. Gheesling, and G. M. Carlone. 1994. Determination of parallelism and nonparallelism in bioassay dilution curves. J. Clin. Microbiol. 32:2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenthal, S., P. Strebel, P. Cassiday, G. Sanden, K. Brusuelas, and M. Wharton. 1995. Pertussis infection among adults during the 1993 outbreak in Chicago. J. Infect. Dis. 171:1650-1652. [DOI] [PubMed] [Google Scholar]
- 45.SAS Institute Inc. 1999. The NLP Procedure. In SAS/OR user's guide: mathematical programming, version 8. SAS Institute Inc., Cary, N.C.
- 46.Schmitt-Grohé, S., J. D. Cherry, U. Heininger, M. A. Überall E. Pineda, and K. Stehr. 1995. Pertussis in German adults. Clin. Infect. Dis. 21:860-866. [DOI] [PubMed] [Google Scholar]
- 47.Senzilet, L. D., S. A. Halperin, J. S. Spika, M. Alagaratnam, A. Morris, B. Smith, and the Sentinel Health Unit Surveillance System Pertussis Working Group. 2001. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clin. Infect. Dis. 32:1691-1697. [DOI] [PubMed] [Google Scholar]
- 48.Stehr, K., J. D. Cherry, U. Heininger, S. Schmitt-Grohé, M. Überall S. Laussucq, T. Eckhardt, M. Meyer, R. Engelhardt, P. Christenson, and the Pertussis Vaccine Study Group. 1998. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics 101:1-11. [DOI] [PubMed] [Google Scholar]
- 49.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]
- 50.Strebel, P., J. Nordin, K. Edwards, J. Hunt, J. Besser, S. Burns, G. Amundson, A. Baughman, and W. Wattigney. 2001. Population-based incidence of pertussis among adolescents and adults, Minnesota, 1995-1996. J. Infect. Dis. 183:1353-1359. [DOI] [PubMed] [Google Scholar]
- 51.Sutter, R. W., and S. L. Cochi. 1992. Pertussis hospitalizations and mortality in the United States, 1985-1988. Evaluation of the completeness of national reporting. JAMA 267:386-391. [PubMed] [Google Scholar]
- 52.Teunis, P. F. M., O. G. van der Heijden, H. E. de Melker, J. F. P. Schellekens, F. G. A. Versteegh, and M. E. E. Kretzschmar. 2002. Kinetics of the IgG antibody response to pertussis toxin after infection with B. pertussis. Epidemiol. Infect. 129:479-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson, T. J., P. J. Smith, and J. P. Boyle. 1998. Finite mixture models with concomitant information: assessing diagnostic criteria for diabetes. Appl. Stat. 47:393-404. [Google Scholar]
- 54.Trollfors, B., J. Taranger, T. Lagergård, L. Lind, V. Sundh, G. Zackrisson, C. U. Lowe, W. Blackwelder, and J. B. Robbins. 1995. A placebo-controlled trial of a pertussis-toxoid vaccine. N. Engl. J. Med. 333:1045-1050. [DOI] [PubMed] [Google Scholar]
- 55.U.S. Department of Health and Human Services, National Center for Health Statistics. 1996. Third National Health and Nutrition Examination Survey, 1988-1994, NHANES III household youth data file (CD-ROM). Public use data file documentation no. 77550. Centers for Disease Control and Prevention, Hyattsville, Md.
- 56.U.S. Department of Health and Human Services, National Center for Health Statistics. 1996. Third National Health and Nutrition Examination Survey, 1988-1994, NHANES III household adult data file (CD-ROM). Public use data file documentation no. 77560. Centers for Disease Control and Prevention, Hyattsville, Md.
- 57.van der Zee, A., C. Agterberg, M. Peeters, F. Mooi, and J. Schellekens. 1996. A clinical validation of Bordetella pertussis and Bordetella parapertussis polymerase chain reaction: comparison with culture and serology using samples from patients with suspected whooping cough from a highly immunized population. J. Infect. Dis. 174:89-96. [DOI] [PubMed] [Google Scholar]
- 58.Versteegh, F. G. A., J. F. P. Schellekens, A. F. Nagelkerke, and J. J. Roord. 2002. Laboratory-confirmed reinfections with Bordetella pertussis. Acta Paediatr. 91:95-99. [DOI] [PubMed] [Google Scholar]
- 59.Vincent, J. M., J. D. Cherry, W. F. Nauschuetz, A. Lipton, C. M. Ono, C. N. Costello, L. K. Sakaguchi, G. Hsue, L. A. Jackson, R. Tachdjian, P. A. Cotter, and J. A. Gornbein. 2000. Prolonged afebrile nonproductive cough illnesses in American soldiers in Korea: a serological search for causation. Clin. Infect. Dis. 30:534-539. [DOI] [PubMed] [Google Scholar]
- 60.Vitek, C. R., F. B. Pascual, A. L. Baughman, and T. V. Murphy. 2003. Increase in deaths from pertussis among young infants in the United States in the 1990s. Pediatr. Infect. Dis. J. 22:628-634. [DOI] [PubMed] [Google Scholar]
- 61.Ward, J. 2001. Acellular pertussis vaccine in adolescents and adults, abstr. 1291. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 62.Wirsing von König, C. H., D. Gounis, S. Laukamp, H. Bogaerts, and H. J. Schmitt. 1999. Evaluation of a single-sample serological technique for diagnosing pertussis in unvaccinated children. Eur. J. Clin. Microbiol. Infect. Dis. 18:341-345. [DOI] [PubMed] [Google Scholar]
- 63.Wright, S. W., K. M. Edwards, M. D. Decker, and M. H. Zeldin. 1995. Pertussis infection in adults with persistent cough. JAMA 273:1044-1046. [PubMed] [Google Scholar]
- 64.Yih, W. K., S. M. Lett, F. N. des Vignes, K. M. Garrison, P. L. Sipe, and C. D. Marchant. 2000. The increasing incidence of pertussis in Massachusetts adolescents and adults, 1989-1998. J. Infect. Dis. 182:1409-1416. [DOI] [PubMed] [Google Scholar]