Abstract
We analyzed biomarkers of lipid peroxidation of the nervous system -F2-dihomo-isoprostanes, F3-neuroprostanes, and F4-neuroprostanes- in urine samples from 158 healthy volunteers ranging from 4 to 88 years old with the aim of analyzing possible associations between their excretion values and age (years). Ten biomarkers were screened in the urine samples by UHPLC-QqQ-MS/MS. Four F2-dihomo-isoprostanes (ent−7-(R)−7-F2t-dihomo-isoprostane, ent−7-epi−7-F2t-dihomo-isoprostane, 17-F2t-dihomo-isoprostane, 17-epi−17-F2t-dihomo-isoprostane), and one DPA-neuroprostane (4-F3t-neuroprostane) were detected in the samples. On the one hand, we found a significant, positive correlation (Rho: 0.197, P=0.015) between the age increase and the amount of total F2-dihomo-IsoPs. On the other hand, the values were significantly higher in the childhood group (4–12 years old), when compared to the adolescence group (13–17 years old) and the young adult group (18–35 years old). Surprisingly, no significant differences were found between the middle-aged adults (36–64 years old) and the elderly adults (65–88 years old). We display a snapshot situation of excretory values of oxidative stress biomarkers of the nervous system, using healthy volunteers representative of the different stages of human growth and development. The values reported in this study could be used as a basal or starting point in clinical interventions related to aging processes and/or pathologies associated with the nervous system.
Abbreviations: OS, (Oxidative stress); ROS, (Reactive oxygen species); PUFAs, (Polyunsaturated fatty acids); F2-dihomo-IsoPs, (F2-dihomo-isoprostanes); F3-NeuroPs, (F3-neuroprostanes); F4-NeuroPs, (F4-neuroprostanes); AdA, (Adrenic acid); DPA, (Docosapentaenoic acid); DHA, (Docosahexaenoic acid)
Keywords: Oxidative stress, Lipid peroxidation, Neuroprostanes, Dihomo-isoprostanes, Age, Urine biomarkers
Graphical abstract
Highlights
-
•
Positive correlation between the age and values of F2-dihomo-isoprostanes was found.
-
•
F2-dihomo-isoprostanes were significantly higher in childhood group when compared to adolescents and young adults.
-
•
F2-dihomo-isoprostanes were not statistically different in middle-aged group and elderly group.
-
•
Neuroprostanes were no detected in healthy volunteers.
1. Introduction
Biomarkers have been increasingly employed in empirical studies of human populations to understand physiological processes that change with age, diseases whose onset appears linked to age, and the aging process itself [1]. The free radical/oxidative stress theory of aging is the most popular explanation of how aging occurs at a molecular level in aerobic biological organisms [2]. This theory of aging consisted of age-related biochemical and physiological decline associated with cumulative oxidative damage to cellular components and tissues, promoting oxidative stress (OS) and leading to lesser longevity [3]. Nowadays, this theory is controversial since there may be interventions independent of reactive oxygen species (ROS) that promote longevity without affecting ROS or OS [2], [4]. Oxidative stress is widely accepted to be a perturbation in the balance of free radicals in a cell and the cell´s ability to cope with the change by means of its antioxidant defense mechanisms [5]. The balance between ROS production and antioxidant defenses determines the degree of OS according to Finkel and Holbrook [6]. Recently, it has been reported that mild stress stimulates endogenous defense systems, which will promote health, but if the stress becomes chronic or is too extensive, it induces cellular damage and/or aging and a shortening of lifespan [7].
The brain and nervous system are prone to OS and are inadequately equipped with antioxidant defense system, which can lead to persistently increased levels of ROS and reactive nitrogen species reacting with the various target molecules (proteins, lipids, and DNA) [8]. The lipids, especially polyunsaturated fatty acids (PUFAs), are vulnerable to oxidation by both enzymatic and non-enzymatic process. In humans, the products of lipid peroxidation have been accepted as toxic mediators, but they are also known to exert diverse biological effects [9]. Solberg et al. [10] mentioned that the determination of OS is complex and requires a quantification of the levels of free radicals or damaged biomolecules. The measurement of F2-isoprostanes (F2-IsoPs) by mass spectrometry has been extensively employed as a marker of oxidant stress and is widely considered to be the gold-standard index of lipid peroxidation in vivo. The measurement of free F2-IsoPs in plasma or urine can be utilized to assess the endogenous formation of IsoPs but not to reveal the organ in which they are formed. Unless determining the levels of IsoPs in the cerebrospinal fluid, which reflects the ongoing metabolic activity of the brain, provides a great opportunity to reveal the occurrence of OS and lipid peroxidation in the brain. However, there are now some IsoPs-like compounds that might be regarded as markers of lipid peroxidation of the nervous system [11]. The F2-dihomo-isoprostanes (F2-dihomo-IsoPs), F3-neuroprostanes (F3-NeuroPs) and F4-neuroprostanes (F4-NeuroPs) are used to analyze the OS status of the nervous system in humans [11], [12], [13]. These biomarkers are formed by a free radical non-enzymatic mechanism from adrenic acid (C22:4 n-6, AdA) [14], docosapentaenoic acid (C22:5 n-6, DPA) [15] and docosahexaenoic acid (C22:6 n-3, DHA) [16] respectively. While DHA is an essential constituent of nervous tissue, highly enriched in neurons, and highly prone to oxidation [17], F4-NeuroPs provide a specific quantification of the OS suffered by neural membranes in vivo [18] and F2-dihomo-IsoPs are potential markers of free radical damage to myelin in the human brain [14]. Recent efforts have focused on the assessment of F2-dihomo-IsoPs, F3-NeuroPs, and F4-NeuroPs as biomarkers in conditions associated with increased OS (particularly in disease conditions) and/or after dietary supplementation with antioxidants [11], [12], [13], [19], [20], [21]. Despite its increasing clinical us to the best of our knowledge the biological variation of these biomarkers in healthy people of different age has not been reported yet. The ability to quantify these compounds in non-invasive samples like urine could shed light on the changes in excretion values of products of lipid peroxidation across a wide age range and may be useful for comparing these values detected in healthy individuals with those obtained diseased individuals. Therefore, the aim of this cross-sectional study was to quantify biomarkers of lipid peroxidation in the nervous system (F2-dihomo-IsoPs, F3-NeuroPs, and F4-NeuroPs) in urine samples from healthy volunteers of different life stage (4–88 years), analyzing possible associations between their values and age intervals.
2. Materials and methods
2.1. Study population
This study was conducted in accordance with the Helsinki declaration. Approval was obtained from the Bioethics Committee of the University Hospital of Murcia. The participants were insured from the Hospital Virgen de la Arrixaca (Murcia, Spain) aged between 4 and 88 years of both genders (n=158). Age was reported at the time of the household interview as the age (years) at the last birthday. The assignment of the age ranges was based on social aging processes (childhood, adolescence, young adulthood, middle-aged adults, and elderly adults) according to Settersten and Mayer [22]. The age categories used in our statistical analyses were 4–12 years (childhood, n=20; mean: 8.20±2.50 years), 13–17 years (adolescence, n=14; mean: 15.73±1.43 years), 18–35 years (young adulthood, n=45; mean: 27.62±4.97 years), 36–64 years (middle-aged adults, n=58; mean: 49.12±9.03 years) and 65–88 years (old age, n=21; mean: 75.61±6.62 years). All volunteers signed the informed consent document (18–88 years old). Volunteers under the age of 18 years had all referred to a doctor clinic for a routine check-up and a parent signed the informed consent document.
Regarding the exclusion criteria, individuals with chronic diseases, under drug treatments, and volunteers with overweight or obesity were excluded from the study. None of the subjects was cigarette smoker an alcoholic and pregnant. All the participants were submitted to clinical examination to confirm their health status. The health status of the participants was considered in the data analysis. The clinical parameters for determining the health status of the individuals are summarized in Supporting information 1.
2.2. Sample collection and preparation
A complete clinical analysis - consisting of hematology, chemistry, and urine chemical analysis - was performed in the volunteers. All samples (blood and urine) were collected, by a nurse at the University Hospital Virgen de la Arrixaca from the subjects early in the morning and under fasting conditions. Blood samples at rest were obtained by venipuncture and were placed in different tubes according to the analytical procedures. The samples were processed within 1 h of collection and stored at −80 °C for the analytical determinations. The hematological parameters were recorded using an automated hematological analyzer (Cell Dyn 3700 and 4000, Abbott, IL, USA) at the clinical analysis service of the University Hospital Virgen de la Arrixaca (Murcia, Spain). One-milliliter from the 24-h urine was used for analysis of the lipid peroxidation biomarkers. The metabolites were normalized as ng mg−1 creatinine and were assayed using the method described by Medina et al. [21]. Clinical parameters results of our volunteers (mean±standard deviations (SD)) are summarized in Table 1.
Table 1.
Biochemical parameters of the volunteers categorized by their life-stage group (n=158).
| Childhood |
Adolescence |
Young adults |
Middle-aged |
Elderly |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (4–12 year) |
(13–17 years) |
(18–35 years) |
(36–64 years) |
(≥65 years) |
|||||||
| Clinical parameters | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Plasma Analysis | Glucose (mg dL−1) | 84.6 | 5.1 | 78.5 | 24.9 | 84.1 | 6.1 | 93.7 | 12.1 | 96.7 | 8.4 |
| Urea (mg dL−1) | 29.2 | 4.8 | 23.7 | 8.6 | 30.5 | 10.0 | 33.7 | 6.4 | 36.2 | 6.5 | |
| Creatinine (serum) (mg dL−1) | 0.5 | 0.1 | 0.7 | 0.3 | 0.8 | 0.2 | 0.8 | 0.1 | 0.8 | 0.2 | |
| Uric acid (mg dL−1) | 3.9 | 0.9 | 4.5 | 1.5 | 4.6 | 1.1 | 4.7 | 1.2 | 4.8 | 1.0 | |
| Cholesterol (mg dL−1) | 170.4 | 24.3 | 126.0 | 45.0 | 172.7 | 74.4 | 173.2 | 22.3 | 173.3 | 23.8 | |
| Total bilirubin (mg dL−1) | 0.5 | 0.3 | 0.5 | 0.2 | 0.5 | 0.2 | 0.5 | 0.2 | 0.5 | 0.2 | |
| Triglyceride (mg dL−1) | 51.4 | 13.4 | 73.7 | 37.8 | 85.1 | 40.2 | 96.5 | 33.3 | 98.5 | 43.5 | |
| Transaminase (ALT) (U/L) | 17.8 | 3.9 | 11.7 | 4.4 | 20.7 | 9.0 | 20.9 | 5.6 | 19.1 | 6.4 | |
| Transaminase (AST) (U/L) | 25.7 | 4.2 | 18.0 | 6.2 | 20.0 | 5.5 | 20.9 | 5.6 | 20.6 | 4.2 | |
| Blood count | Red blood cells (RBCs) (x 106 µL) | 4.8 | 0.2 | 4.6 | 1.5 | 4.9 | 0.4 | 4.8 | 0.5 | 4.7 | 0.4 |
| Leukocytes (x103 µL) | 6.4 | 1.2 | 6.7 | 2.3 | 7.5 | 1.6 | 6.9 | 1.2 | 6.3 | 1.2 | |
| Haemoglobin (g dL−1) | 14.1 | 0.6 | 13.4 | 4.3 | 14.8 | 1.3 | 14.5 | 1.4 | 14.5 | 1.0 | |
| Haematocrit (%) | 41.1 | 1.8 | 40.3 | 13.1 | 44.2 | 4.2 | 43.6 | 1.4 | 43.7 | 3.0 | |
| Platelet (x103 µL) | 273.8 | 42.4 | 234.9 | 81.0 | 245.8 | 49.4 | 263.9 | 50.8 | 240.5 | 51.2 | |
| Urine Analysis | Abnormal results and sediment | Negative | Negative | Negative | Negative | Negative | |||||
Data are represented as means±SD. Childhood, n: 20; Adolescence, n: 14; Young adults, n: 45; middle-aged adults, n: 58; and elderly adults, n: 21.
2.3. Chemicals and Standards
Six NeuroPs (4(RS)−4-F4t-NeuroP, 4-F4t-NeuroP, 10-epi−10-F4t-NeuroP, 10-F4t-NeuroP, 4-epi−4F3t-NeuroP and 4-F3t-NeuroP); as well as, four F2-dihomo-IsoPs (ent−7-(R)−7-F2t-dihomo-IsoP, ent−7-epi-7-F2t-dihomo-IsoP, 17-F2t-dihomo-IsoP, and 17-epi−17-F2t-dihomo-IsoP) were analyzed in this experiment and three deuterated internal standards (d4−4(RS)-F4t-NeuroP, d4−10-epi-10-F4t-NeuroP, and d4−10-F4t-NeuroP) were used for the quality control of the analyses. All standards were synthesized using our published strategies [23], [24], [25]. β-glucuronidase, type H2 from Helix pomatia and BIS-TRIS (Bis-(2-hydroxyethyl)-amino-tris (hydroxymethyl)-methane) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All LC-MS grade solvents were from J.T. Baker (Phillipsburg, NJ, USA). Strata X-AW (100 mg 3 mL−1) solid phase extraction cartridges were purchased from Phenomenex (Torrance, CA, USA).
2.4. UHPLC-QqQ-MS/MS analyses
The separation of NeuroPs and F2-dihomo-IsoPs in the urine samples was performed with an Ultra High-Performance Liquid Chromatography 6460-Triple Quadrupole-tandem Mass Spectrometry (Agilent Technologies, Waldbronn, Germany), using the set up previously described by Medina et al. [21]. Data acquisition and processing were performed using Mass Hunter software version B.04.00 (Agilent Technologies, Waldron, Germany). The identification and quantification of NeuroPs and F2-dihomo-IsoPs were carried out using the authentic markers described by Medina et al. [21].
2.5. Statistical analyses
Quantitative data are presented as mean±SEM (standard error of the mean) or SD (Table 2). Concerning the study population, women and men were analyzed together because no difference between them was detected according to the Student's t-test (data not shown). The Kolmogorov-Smirnov test and Shapiro-Wilk test were applied to assess the distribution of the data. Normality was not established, so non-parametric statistical tests were used for intergroup comparison. Comparison of non-normally distributed groups was carried out using the non-parametric Kruskal-Wallis test. In order to identify differences between the five groups, the Mann-Whitney U test was conducted with a Bonferroni correction, resulting in a significance level set at P<0.005. Correlation between the variables (F2-dihomo-IsoPs) and age (years) was determined using Spearman´s correlation. The statistical analyses were made using the SPSS 21.0 software package (LEAD Technologies, Inc. Chicago, USA). The graphs were plotted using the Sigma Plot 12.0 software package (Systat Software, Inc. Sigma Plot for Windows).
Table 2.
Urinary F4-neuroprostanes, F3-neuroprostanes, and F2-dihomo-isoprostanes (ng mg−1 creatinine) determined in the five study groups of the most representative life-stages in humans.
| Childhood |
Adolescence |
Young adults |
Middle-aged |
Elderly |
Kruskal-Wallis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (4- 12 year) |
(13–17 years) |
(18–35 years) |
(36–64 years) |
(≥65 years) |
|||||||||||
| ng/mg creatinine | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | χ2 | Sig | |||
| From | Number in age groups (W/M) | 20 (9/11) | 14 (7/7) | 45 (23/22) | 58 (31/27) | 21 (10/11) | |||||||||
| A) | 17-epi−17F2t -dihomo-IsoP | 2.14 | 0.18 | 1.63 | 0.22 | 1.68 | 0.12 | 1.74 | 0.11 | 1.93 | 0.18 | 8.37(4) | 0.079 | ||
| AdA | 17-F2t-dihomo-IsoP | 1.74 | 0.14 | 1.34 | 0.17 | 1.41 | 0.09 | 1.53 | 0.08 | 1.71 | 0.15 | 14.67(4) | 0.005 | ||
| n−6 | |||||||||||||||
| ent−7-(R)−7-F2t-dihomo-IsoP | 4.45 | 0.28 | 3.05 | 0.34 | 3.48 | 0.19 | 4.56 | 0.17 | 5.26 | 0.28 | 21.85(4) | ≤0.001 | |||
| ent−7-epi−7-F2t-dihomo-IsoP | 3.49 | 0.44 | 1.98 | 0.53 | 2.51 | 0.30 | 3.08 | 0.27 | 3.29 | 0.46 | 29.86(4) | ≤0.001 | |||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| B) | DHA | 4-(R)-F4t-NeuroP | n/d | – | n/d | – | n/d | – | n/d | – | n/d | – | – | ||
| n−3 | |||||||||||||||
| 10- epi−10-F4t-NeuroP | n/d | – | n/d | – | n/d | – | n/d | – | n/d | – | – | ||||
| 10-F4t-NeuroP | n/d | – | n/d | – | n/d | – | n/d | – | n/d | – | – | ||||
| 4-F4t-NeuroP | n/d | – | n/d | – | n/d | – | n/d | – | n/d | – | – | ||||
| DPA n−6 | 4-epi−4F3t-NeuroP | n/d | – | n/d | – | n/d | – | n/d | – | n/d | – | – | |||
| 4-F3t-NeuroP | n/d | – | n/d | – | 0.87a | 0.57 | 10.48b | 0.85 | 2.15c | 1.17 | – | ||||
A) Dihomo-Isoprostane. Data presented as mean±SEM (Standard error of the mean) ng mg−1 creatinine. Kruskal-Wallis test, χ2: Chi-square (degree of freedom), and P<0.05 was significant. Abbreviations: W: women and M: men.
B) Neuroprostane. Data presented as means±SD (standard deviation) ng mg−1 creatinine. Abbreviations: n/d: not detected. No data on neuroprostanes were found with the only exception of 4-F3t-NeuroP, which was found in a reduced number of volunteers within the experimental groups: young adults (a, detected in 3 women and 7 men), middle age adults (b, detected in 9 women and 6 men), and in elder volunteers (c, detected in 2 women and 6 men).
3. Results
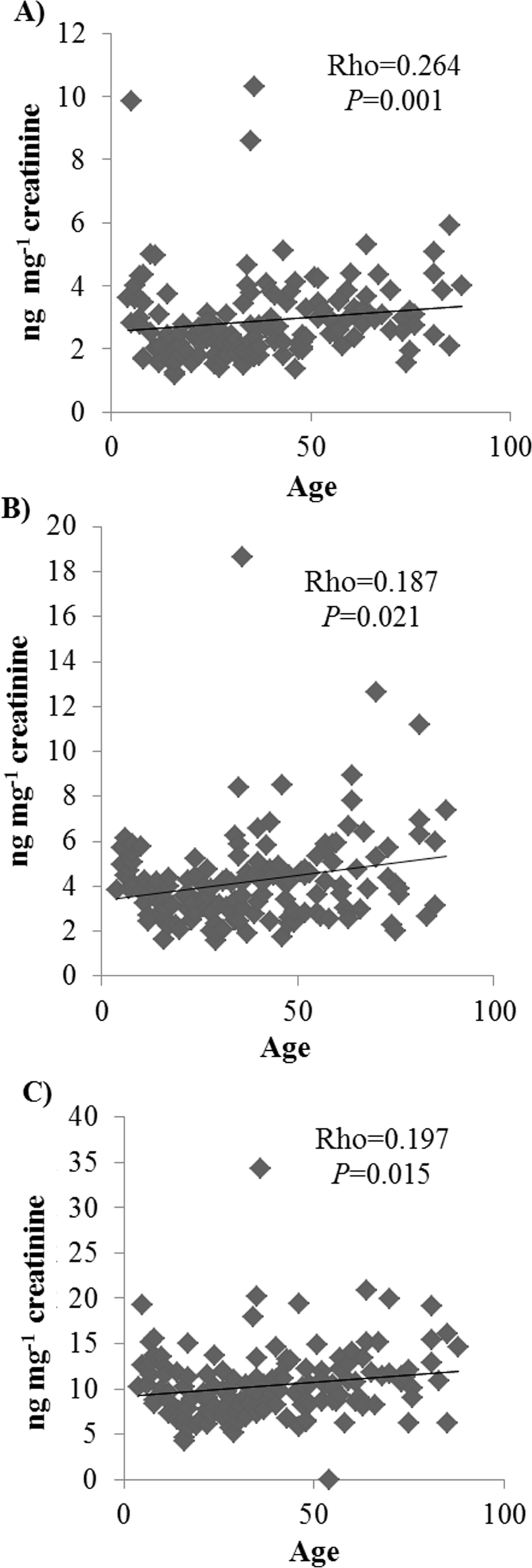
In this study four F2-dihomo-IsoPs stereoisomers (ent−7-(R)−7-F2t-dihomo-IsoP, ent−7-epi−7-F2t-dihomo-IsoP, 17-F2t-dihomo-IsoP, 17-epi−17-F2t-dihomo-IsoP) were detected in the volunteers’ urine in all life-stages, and 4-F3t-NeuroP (derived from n-6 DPA) was detected in the three groups of older volunteers (Table 2). We want to emphasize that the F2-dihomo–IsoPs were detected in the samples from all volunteers of the different life stages (from childhood to old age). The F2-dihomo-IsoPs (data for all volunteers) were correlated with age (4–88 years); the Spearman correlations were: 17-epi−17-F2t-dihomo-IsoP, Rho=0.062, significant level (sig.)=0.448; 17-F2t-dihomo-IsoP, Rho=0.940, sig.=0.253; ent−7-(R)−7-F2t-dihomo-IsoP, Rho=0.264, sig.=0.001; ent−7-epi−7-F2t-dihomo-IsoP, Rho=0.187, sig.=0.021; and total dihomo-IsoPs, Rho=0.197, sig.=0.015 (Fig. 1).
Fig. 1.
Scatterplot showing: A) Ent−7-epi−7-F2t-dihomo-IsoP, B) Ent−7-(R)−7-F2t-dihomo-IsoP, and C) the total sum of F2-dihomo-IsoPs (ng mg−1 creatinine in urine) versus age (years) in our volunteers. The Spearman rank correlation coefficient (Rho) and P-value are shown.
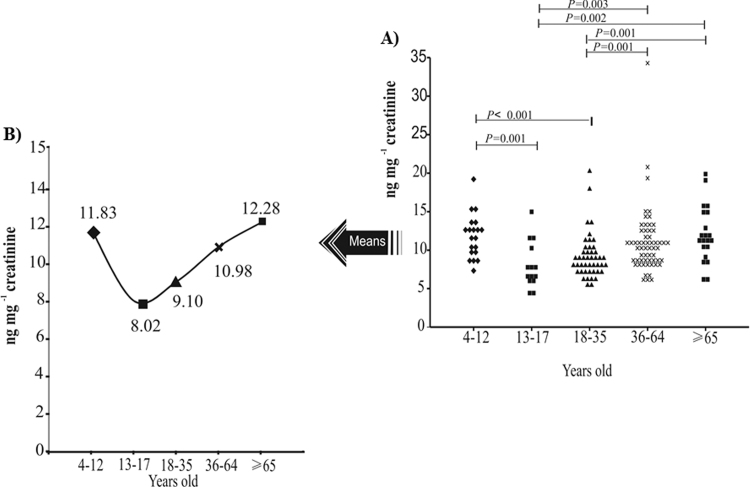
In order to provide a snapshot overview of the values of F2-dihomo-IsoPs in healthy volunteers, we have studied the most representative stages of human growth and development. To determine whether there were statistically significant differences between the groups, a non-parametric analysis of population medians (Kruskal–Wallis test, P<0.05) was performed for each individual analyte (Table 2), as well for the total sum of the four F2-dihomo-IsoPs (Fig. 2). There was a statistically significant difference in 17-F2t-dihomo-IsoP, ent−7-(R)−7-F2t-IsoP, ent−7-epi−7-dihomo-IsoP, and total F2-dihomo-IsoPs when comparing the five groups. Thus, having analyzed the five groups, we used the results for the sum of these four F2-dihomo-IsoPs, since significant P-values were found for most of the biomarkers. The total F2-dihomo-IsoPs ranged from ~ 8.02 ng mg−1 creatinine (age group of 13–17 years old) to ~12.28 ng mg−1 creatinine (age group of 65–88 years old) (Fig. 2). The Mann-Whitney U test showed that among the first stage (childhood) and the later stages of life (middle-aged adults and elderly adults) the F2-dihomo-IsoPs values did not differ statistically; however, it should be noted that the elderly volunteers showed high values comparing to middle-aged. In the middle stage of life (adolescence and young adults) a significant decrease in the levels - compared to the first and last stages of life - was detected (P<0.005) (Fig. 2).
Fig. 2.
The total F2-dihomo-IsoPs content in urine of volunteers (ent−7(R)−7-F2t-dihomo-isoprostane, ent−7-epi-7-F2t-dihomo-isoprostane, 17-F2t-dihomo-isoprostane, and 17-epi−17-F2t-dihomo-isoprostane). The values are expressed as ng mg−1 creatinine and are categorized according to age range. A) Dot plot of the values from all volunteers, by life-stage. Significant P-values (≤0.005) from the Mann-Whitney U test are shown. B) Trend chart plot showing the averages of the values by life-stage.
4. Discussion
The measurement of OS biomarkers using a lipidomics approach has been useful for to compare values detected in a healthy population with those affected with different pathologies [11], [12], [13], [19], [20], [21]. To the best of our knowledge, this is the only study that evaluates F2-dihomo-IsoPs and F4-NeuroPs in a large population of healthy volunteers. The results obtained by correlation tests showed a small but positive association between the concomitant increases of urinary F2-dihomo-IsoPs (total sum) with the age (Fig. 1). Previously, in female volunteers (n=43), no statistically significant correlation (r=0.0841, P=0.40) between age and plasmatic values of F2-dihomo-IsoPs (ent−7(R)-F2t-dihomo-IsoP and 17-F2t-dihomo-IsoP) was observed; the average age was 13.9±6 years (range 1.5–32 years) and the values were 1.0±0.11 pgmL−1 [19]. Our study, with a wider age range and using data from women and men, showed that age may be related to changes in the excretion of F2-dihomo-IsoPs. In addition, individually, ent−7-epi−7-F2t-dihomo-IsoP and ent−7-(R)−7-F2t-dihomo-IsoP also exhibited a slight correlation with age. VanRollins et al. [14] mentioned that AdA is the most important PUFA in white matter and suggested the quantification of F2-dihomo-IsoPs as help to clarify the in vivo contributions of free radical to myelin and axons damage in white matter damage because of aging. Augmented levels of lipid peroxidation and myelin breakdown have been demonstrated in the myelin of older (normal) individuals, compared to younger. Age-related myelin alterations are ubiquitous and the correlations between their frequency and impairments of cognition occur because the conduction velocity along the affected nerve fibers is reduced so that the normal timing sequences within neuronal circuits breakdown [26], [27]. Previous studies also showed an age-related increase in lipid peroxidation in humans and other animals [28], [29], [30], [31], [32]. Besides being a specific component of myelin, AdA also is present in several organs and tissues, including the kidney and the adrenal gland [14], [19]. A study in rats also showed evidence of age-related lipid peroxidation in adrenals [33]. Therefore, our results also may reflect a positive correlation between non-enzymatic oxidation within the kidney and adrenal glands and advanced age.
The precise age of transition among the stages of life is heavily debated and this can vary from person to person [34]. In order to provide a snapshot overview of the values of F2-dihomo-IsoPs in healthy volunteers, we have studied the most representative stages of human growth and development. The growth from childhood into adolescence has been associated with biological changes and could be influenced by the endocrinal and biochemical conditions generated in the pre-pubertal period. Our results are consistent with those obtained by Tamura et al. [35] and Kaneko et al. [36]. These authors studied urinary biomarkers produced under OS from lipids, proteins, DNA, and carbohydrates, and underlined that younger subjects (under the age of 10) were more vulnerable to oxidation than adolescent subjects, since they grow up rapidly, activation of the immune system and are probably exposed to high concentrations of ROS and nitric oxide. When we compared the F2-dihomo-IsoPs values of the young and middle-aged adults, higher OS values in the latter were apparent (Fig. 2). In humans, [37] according to the free radical theory of aging, the inborn aging process produces changes that increase in an exponential manner with age, becoming the major risk factor for disease and death in humans after the age of 28 years in developed countries. In our study, the volunteers aged from 36 to 88 years were divided into two groups - middle-aged (36–64 years) and elderly adults (65–88 years) - that did not differ significantly although, the values provided an ascending trend concomitantly with the age as is observed in Fig. 2.B. In the ZENITH study, European free-living, healthy, older adults (70–88 years) did not appear to be exposed to acute OS [28]. Our results showed a similar slow decline in the antioxidant status of elderly, healthy, free-living adults, and this may be one of the reasons why the values obtained for our group of older adults did not differ significantly from those of the middle-aged group. Currently, it is not clear which levels should be considered “normal” and which represent a serious imbalance between ROS generation and antioxidant defense. Some scientists reported that OS is an adaptation in the aging process and is not so harmful [2], [4]. But, if the damage accumulates throughout the entire lifespan, as a by-product of normal cellular processes or a consequence of inefficient repair systems, this could lead to the diseases associated with the elderly [31], [38]. On the other hand, Soares et al. [39] highlighted the impact of the lifestyle (diet, environment, lifestyle among others factors of life-stage as physiological and/ or metabolic process) has an important role in the accumulation of oxidative damage or in the OS increase apart from growing older. The F2-dihomo-IsoPs have not been used previously as a biomarker of aging; they have been associated mainly with diseases in the elderly, adolescents, and children. Therefore, the normal values in our adults might be useful in subsequent comparisons evaluating OS progression in groups of older individuals.
Finally, regarding NeuroPs derived from DPA or DHA, these lipid metabolites were not detected in samples from healthy and sedentary volunteers. Only 4-F3t-NeuroP, derived from omega 6-DPA, was detected, in a few volunteers in the age range from 18 to 65 years, who represents young adults, middle-aged adults, and elderly volunteers (Table 2). The 4-epimer of 4-F3t-NeuroP was not detected in the urine samples, although its values may have been below the limits of detection (2.95 ng mL−1) and quantification (5.9 ng mL−1) [21] since each is 4-fold higher than the corresponding limit found for 4-F3t-NeuroP. The DPA-NeuroPs were synthesized with the aim of understanding their role in a ω3-depleted organism, as well as to broaden and deepen the study of neuronal OS [15]. In our study the metabolites of DHA n-3 peroxidation (4(RS)−4-F4t-NeuroP, 4-F4t-NeuroP, 10-F4t-NeuroP, and 10-epi−10-F4t-NeuroP) were not detected in the urine samples, suggesting that their values may also have been below the limit of detection. The physiological role of lipid peroxidation is not fully understood; some authors have shown that lipid peroxidation in various organs, including the brain, increases with aging and it is considered a risk factor for neurodegenerative diseases [9], [31]. Thus, further investigation is needed, regarding how or why age or the process of aging can influence the oxidative status of the central nervous system and whether the excretion of oxidative products derived from DPA n-6 and DHA n-3 is scarce in the healthy population, according to age.
5. Conclusions
A significant, positive correlation was found between the increase in age (4–88 years) and the values of ent−7-(R)−7-F2t-dihomo-IsoP, ent-7-epi−7-F2t-dihomo-IsoP, and total F2-dihomo-IsoPs. By dividing the participants into five groups according to social aging processes, we have been able to provide evidence for a decrease in total F2-dihomo-IsoPs during adolescence and the young-adult stage, when compared to childhood. The observation of a spurt in the total F2-dihomo-IsoPs values from the young adults to middle-aged adults is interesting, as is the fact that the elderly group did not show higher values than the middle-aged population. The findings of this work suggest that in healthy and sedentary the values of urinary F4-NeuroPs and F3-NeuroPs were not representative. We are conscious of the limitations inherent in the performance of such analysis in healthy humans due to wide heterogeneity in the expression of OS biomarkers, as a consequence of aging. Nevertheless, this study in a matrix as human urine represents a powerful approach to advance our knowledge of the role of OS in the central nervous system across a wide age range and so it could serve as a baseline for future clinical studies in populations with different disorders.
Declarations of interest
The authors declare that they have no conflict of interest.
Funding
This work was partially funded by the “Fundación Séneca de la Región de Murcia” Grupo de Excelencia 19900/GERM/15.
Acknowledgments
LAGF was awarded a pre-doctoral FPI fellowship (BES2012-060185) by the Spanish government. The authors are grateful to the Clinical Analysis Service of the University Hospital Virgen de la Arrixaca, Murcia (Spain) for its collaboration. We are grateful to Dr. David Walker (native English speaker) for his reviews of the English grammar and style of the current report.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.01.008.
Contributor Information
Sonia Medina, Email: smescudero@cebas.csic.es.
Ángel Gil-Izquierdo, Email: angelgil@cebas.csic.es.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Crimmins E., Vasunilashorn S., Kim J.K., Alley D. Biomarkers related to aging in human populations. Adv. Clin. Chem. 2008;46:161–216. doi: 10.1016/s0065-2423(08)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valavanidis A., Vlachogianni T., Rallis M. The controversy for the validity of the free radical or oxidative stress theory of ageing: recent scientific evidence for oxidative damage at molecular level, animals and ageing population – based studies. Pharmakeftiki. 2015;27(1–2):31–50. [Google Scholar]
- 3.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Vina J., Borras C., Abdelaziz K.M., Garcia-Valles R., Gomez-Cabrera M.C. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid. Redox Signal. 2013;19(8):779–787. doi: 10.1089/ars.2012.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkataraman K., Khurana S., Tai T.C. Oxidative stress in aging-matters of the heart and mind. Int. J. Mol. Sci. 2013;14(9):17897–17925. doi: 10.3390/ijms140917897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 7.Simm A., Klotz L.O. Stress and biological aging: a double-edged sword. Z. Gerontol. Geriatr. 2015;48(6):505–510. doi: 10.1007/s00391-015-0928-6. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 9.Niki E. Biomarkers of lipid peroxidation in clinical material. Biochim Biophys. Acta. 2014;1840(2):809–817. doi: 10.1016/j.bbagen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Solberg R., Longini M., Proietti F., Vezzosi P., Saugstad O.D., Buonocore G. Resuscitation with supplementary oxygen induces oxidative injury in the cerebral cortex. Free Radic. Biol. Med. 2012;53(5):1061–1067. doi: 10.1016/j.freeradbiomed.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Miller E., Morel A., Saso L., Saluk J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxidative Med. Cell Longev. 2014;2014:10. doi: 10.1155/2014/572491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galano J.M., Mas E., Barden A., Mori T.A., Signorini C., De Felice C., Barrett A., Opere C., Pinot E., Schwedhelm E., and others Isoprostanes and neuroprostanes: total synthesis, biological activity and biomarkers of oxidative stress in humans. Prostaglandins Other Lipid Med. 2013;107:95–102. doi: 10.1016/j.prostaglandins.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Vigor C., Bertrand-Michel J., Pinot E., Oger C., Vercauteren J., Le Faouder P., Galano J.M., Lee J.C., Durand T. Non-enzymatic lipid oxidation products in biological systems: assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;964:65–78. doi: 10.1016/j.jchromb.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 14.VanRollins M., Woltjer R.L., Yin H., Morrow J.D., Montine T.J. F2-dihomo-isoprostanes arise from free radical attack on adrenic acid. J. Lipid Res. 2008;49(5):995–1005. doi: 10.1194/jlr.M700503-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auvinet A.-L., Eignerová B., Guy A., Kotora M., Durand T. Total synthesis of 4-F3t-neuroprostane and its 4-epimer. Tetrahedron Lett. 2009;50(13):1498–1500. [Google Scholar]
- 16.Roberts L.J., 2nd, Montine T.J., Markesbery W.R., Tapper A.R., Hardy P., Chemtob S., Dettbarn W.D., Morrow J.D. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 1998;273(22):13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 17.Nourooz-Zadeh J., Liu E.H.C., Yhlen B., Änggåard E.E., Halliwell B. F4 - Isoprostanes as Specific Marker of Docosahexaenoic Acid Peroxidation in Alzheimer's Disease. J. Neurochem. 1999;72(2):734–740. doi: 10.1046/j.1471-4159.1999.0720734.x. [DOI] [PubMed] [Google Scholar]
- 18.Roberts L.J., 2nd, Fessel J.P. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem. Phys. Lipids. 2004;128(1–2):173–186. doi: 10.1016/j.chemphyslip.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 19.De Felice C., Signorini C., Durand T., Oger C., Guy A., Bultel-Poncé V., Galano J.-M., Ciccoli L., Leoncini S., D'Esposito M. F(2)-dihomo-isoprostanes as potential early biomarkers of lipid oxidative damage in Rett syndrome. J. Lipid Res. 2011;52(12):2287–2297. doi: 10.1194/jlr.P017798. (and others) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L., Yin H., Milne G.L., Porter N.A., Morrow J.D. Formation of F-ring isoprostane-like compounds (F3-isoprostanes) in vivo from eicosapentaenoic acid. J. Biol. Chem. 2006;281(20):14092–14099. doi: 10.1074/jbc.M601035200. [DOI] [PubMed] [Google Scholar]
- 21.Medina S., Miguel-Elizaga I.D., Oger C., Galano J.M., Durand T., Martinez-Villanueva M., Castillo M.L., Villegas-Martinez I., Ferreres F., Martinez-Hernandez P., and others Dihomo-isoprostanes-nonenzymatic metabolites of AdA-are higher in epileptic patients compared to healthy individuals by a new ultrahigh pressure liquid chromatography-triple quadrupole-tandem mass spectrometry method. Free Radic. Biol. Med. 2015;79:154–163. doi: 10.1016/j.freeradbiomed.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Settersten R.A., Mayer K.U. The Measurement of Age, Age Structuring, and the Life Course. Ann. Rev. Sociol. 1997;23(1):233–261. [Google Scholar]
- 23.Guy A., Oger C., Heppekausen J., Signorini C., De Felice C., Furstner A., Durand T., Galano J.M. Oxygenated metabolites of n-3 polyunsaturated fatty acids as potential oxidative stress biomarkers: total synthesis of 8-F3t-IsoP, 10-F4t-NeuroP and [D4]-10-F4t-NeuroP. Chemistry. 2014;20(21):6374–6380. doi: 10.1002/chem.201400380. [DOI] [PubMed] [Google Scholar]
- 24.Oger C., Bultel-Ponce V., Guy A., Balas L., Rossi J.C., Durand T., Galano J.M. The handy use of Brown's P2-Ni catalyst for a skipped diyne deuteration: application to the synthesis of a [D4]-labeled F4t-neuroprostane. Chemistry. 2010;16(47):13976–13980. doi: 10.1002/chem.201002304. [DOI] [PubMed] [Google Scholar]
- 25.Oger C., Bultel-Poncé V., Guy A., Durand T., Galano J.-M. Total synthesis of isoprostanes derived from adrenic acid and EPA. Eur. Org. Chem. 2012;2012(13):2621–2634. [Google Scholar]
- 26.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol. Aging. 2004;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Peters A., Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J. Comp. Neurol. 2002;442(3):277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 28.Andriollo-Sanchez M., Hininger-Favier I., Meunier N., Venneria E., O'Connor J.M., Maiani G., Coudray C., Roussel A.M. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: the ZENITH study. Eur. J. Clin. Nutr. 2005;59(Suppl 2):S58–S62. doi: 10.1038/sj.ejcn.1602300. [DOI] [PubMed] [Google Scholar]
- 29.Cui H., Kong Y., Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012;2012:13. doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon H.F., Calabrese V., Scapagnini G., Butterfield D.A. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59(5):478–493. doi: 10.1093/gerona/59.5.m478. [DOI] [PubMed] [Google Scholar]
- 31.Radak Z., Zhao Z., Goto S., Koltai E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol. Asp. Med. 2011;32(4–6):305–315. doi: 10.1016/j.mam.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Rizvi S.I., Maurya P.K. Markers of oxidative stress in erythrocytes during aging in humans. Ann. N.Y. Acad. Sci. 2007;1100:373–382. doi: 10.1196/annals.1395.041. [DOI] [PubMed] [Google Scholar]
- 33.Almeida H., Magalhães M.C., Magalhães M.M. Age-related changes in lipid peroxidation products in rat adrenal gland. AGE. 1998;21(3):119–121. doi: 10.1007/s11357-998-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prenderville J.A., Kennedy P.J., Dinan T.G., Cryan J.F. Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci. 2015;38(1):13–25. doi: 10.1016/j.tins.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Tamura S., Tsukahara H., Ueno M., Maeda M., Kawakami H., Sekine K., Mayumi M. Evaluation of a urinary multi-parameter biomarker set for oxidative stress in children, adolescents and young adults. Free Radic. Res. 2006;40(11):1198–1205. doi: 10.1080/10715760600895191. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko K., Kimata T., Tsuji S., Ohashi A., Imai Y., Sudo H., Kitamura N. Measurement of urinary 8-oxo-7,8-dihydro-2-deoxyguanosine in a novel point-of-care testing device to assess oxidative stress in children. Clin. Chim. Acta. 2012;413(23–24):1822–1826. doi: 10.1016/j.cca.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann. N.Y. Acad. Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 38.Farooqui T., Farooqui A.A. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech. Ageing Dev. 2009;130(4):203–215. doi: 10.1016/j.mad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Soares J.P., Silva A.M., Fonseca S., Oliveira M.M., Peixoto F., Gaivao I., Mota M.P. How can age and lifestyle variables affect DNA damage, repair capacity and endogenous biomarkers of oxidative stress? Exp. Gerontol. 2015;62:45–52. doi: 10.1016/j.exger.2015.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material