Abstract
The involvement of clathrin and associated adaptor proteins in receptor recycling from endosomes back to the plasma membrane is controversial. We have used an in vitro assay to identify the molecular requirements for the formation of recycling vesicles. Cells expressing the asialoglycoprotein receptor H1, a typical recycling receptor, were surface biotinylated and then allowed to endocytose for 10 min. After stripping away surface-biotin, the cells were permeabilized and the cytosol washed away. In a temperature-, cytosol-, and nucleotide-dependent manner, the formation of sealed vesicles containing biotinylated H1 could be reconstituted. Vesicle formation was strongly inhibited upon immunodepletion of adaptor protein (AP)-1, but not of AP-2 or AP-3, from the cytosol, and was restored by readdition of purified AP-1. Vesicle formation was stimulated by supplemented clathrin, but inhibited by brefeldin A, consistent with the involvement of ARF1 and a brefeldin-sensitive guanine nucleotide exchange factor. The GTPase rab4, but not rab5, was required to generate endosome-derived vesicles. Depletion of rabaptin-5/rabex-5, a known interactor of both rab4 and γ-adaptin, stimulated and addition of the purified protein strongly inhibited vesicle production. The results indicate that recycling is mediated by AP-1/clathrin-coated vesicles and regulated by rab4 and rabaptin-5/rabex-5.
INTRODUCTION
Early endosomes are a major sorting station for proteins and membranes in eukaryotic cells (Gruenberg, 2001; Maxfield and McGraw, 2004). They receive material from the cell surface by endocytosis and from the exocytic pathway via the trans-Golgi network (TGN), and they distribute it further to late endosomes and back to the TGN and the plasma membrane. Transport receptors like the transferrin receptor, the low-density lipoprotein (LDL) receptor, and the asialoglycoprotein (ASGP) receptor cycle continuously between the plasma membrane and early endosomes (Spiess, 1990; Trowbridge et al., 1993). Receptor-positive early endosomes can be subdivided into sorting endosomes and recycling endosomes (or endocytic recycling compartment, ERC). Primary endocytic vesicles fuse to sorting endosomes where ligands dissociate from their receptors because of a reduced internal pH. The receptors exit into tubular membranes that form recycling endosomes, whereas the ligands with the main fluid volume mature to late endosomes (“geometry-based sorting”; Maxfield and McGraw, 2004). There seem to be two main recycling pathways from early endosomes to the plasma membrane: a fast one directly from sorting endosomes and a slower one via recycling endosomes (Sheff et al., 1999; Hao and Maxfield, 2000; van Dam et al., 2002).
The mechanisms to generate endosome-derived recycling vesicles are not clear, because there are seemingly contradictory findings. In general, formation of transport vesicles between organelles of the endocytic and secretory pathways requires cytosolic coat proteins to be recruited at the membrane of the donor compartment. Among the established coat complexes, clathrin is classically responsible for endocytosis from the plasma membrane in combination with the adaptor protein (AP) complex AP-2 and for TGN-to-endosome transport with AP-1 adaptors (Hirst and Robinson, 1998). However, clathrin coats were also detected on endosomal membranes positive for the transferrin receptor (Stoorvogel et al., 1996). Consistent with a role of clathrin-coated vesicles in receptor recycling, expression of a temperature-sensitive mutant of dynamin, a GTPase involved in clathrin-coated vesicle release (van der Bliek, 1999), inhibited recycling of transferrin receptor (van Dam and Stoorvogel, 2002). In addition, brefeldin A (BFA), an inhibitor of guanine nucleotide exchange factors of the GTPase ADP-ribosylation factor 1 (ARF1) involved in recruiting AP-1– and AP-3– containing coats, partially inhibited transferrin receptor recycling in nonpolarized cells (van Dam and Stoorvogel, 2002; van Dam et al., 2002). Overexpression of rabaptin-5, an interactor of the γ-subunit of AP-1 (Hirst et al., 2000; Shiba et al., 2002) as well as of rab4 and rab5 (Vitale et al., 1998), was shown to inhibit transferrin receptor recycling, unless γ-adaptin was overexpressed simultaneously (Deneka et al., 2003).
AP-1/clathrin coats on endosomes may, however, be involved in other functions than recycling to the plasma membrane (Hinners and Tooze, 2003): Shiga toxin B-chain, which is transported via endosomes to the Golgi, was found to be colocalized in recycling endosomes with AP-1 and clathrin and accumulated in transferrin receptor-positive endosomes upon BFA treatment (Mallard et al., 1998). In cells lacking the μ1A subunit of AP-1, the mannose-6-phosphate receptor was found to accumulate in endosomes, rather than in the TGN, supporting an AP-1– dependent pathway from endosomes to the TGN (Meyer et al., 2000). Furthermore, retrograde transport of furin and other proteins containing acidic-cluster motifs from endosome-to-TGN is mediated by the protein PACS-1 and its interaction with AP-1 (Crump et al., 2001).
Some experiments directly argued against an involvement of clathrin-coated vesicles in receptor recycling. Perturbation of clathrin coat formation by regulated expression of a dominant-negative clathrin hub domain in HeLa cells reduced endocytosis by ∼50% and caused perinuclear aggregation of early endosomes, but it reduced recycling by only ∼20% (Bennett et al., 2001). Similarly, drug-induced cross-linking of an FK506-binding protein-clathrin light-chain fusion, which dominantly interferes with clathrin function, reduced LDL or transferrin uptake by half, but minimally affected receptor recycling in Chinese hamster ovary (CHO) cells (Moskowitz et al., 2003). Elimination of clathrin heavy-chain in DT40 lymphocytes substantially inhibited receptor-mediated and fluid phase internalization, but it reduced transferrin receptor recycling by only ∼50% (Wettey et al., 2002).
Another consideration is that generally clathrin/adaptor coats mediate cargo concentration by interaction with specific sorting signals, whereas receptor recycling in nonpolarized cells seems to be signal independent. Mutation or deletion of the cytoplasmic domain of the transferrin receptor, for example, resulted in a reduction of endocytosis, but it did not significantly affect its recycling rate (Jing et al., 1990; Johnson et al., 1993). An isoform of the μ1 subunit of AP-1 complexes, μ1B, has recently been discovered in epithelial cells and shown to mediate basolateral sorting of the receptors for transferrin, LDL, and ASGPs in a signal-dependent manner (Ohno et al., 1999; Sugimoto et al., 2002; Fölsch et al., 2003). Indeed, AP-1B was found to mediate specific basolateral recycling from endosomes (Gan et al., 2002), supporting earlier proposals that implicated clathrin/AP-1 coats in polarized cells in recycling transferrin receptor back to the basolateral surface (Futter et al., 1998). Consistent with this, BFA treatment caused loss of polarity in recycling to the cell surface in Madin-Darby canine kidney (MDCK) cells (Futter et al., 1998; Mohrmann et al., 2002).
The apparent discrepancy between experimental results and their interpretations may be due to the complexity and plasticity of the endosomal system. Long-term experiments such as overexpression or elimination of proteins, which takes hours to days to take effect, may cause indirect effects or adaptation. In this study, we have reconstituted the formation of recycling vesicles in vitro by using permeabilized cells. Because the formation of a single cohort of vesicles is analyzed, indirect effects on the organization of endosomes are unlikely to affect the results. We found that formation of endosome-derived vesicles containing ASGP receptor depends on AP-1 and clathrin, requires rab4, and is inhibited by rabaptin-5, an interactor of both AP-1 and rab4.
MATERIALS AND METHODS
Reagents and Antibodies
Cell culture media and reagents were obtained from Invitrogen (Carlsbad, CA). Adenylyl imidodiphosphate (AMP-PNP), guanylyl imidodiphosphate (GMP-PNP), ATP, GTP, creatine kinase, and creatine phosphate were from Roche Diagnostics (Indianapolis, IN); sulfosuccinimidyl-2-(biotinamido)-ethyl-1,3-dithiopropionate (sulfo-NHS-SS-biotin) and avidin-Sepharose were from Pierce Chemical (Rockford, IL); protein A-Sepharose was from Zymed Laboratories (South San Francisco, CA), protein G-Sepharose was from Gerbu (Gaiberg, Germany); and LY294002 was from BIOMOL Research Laboratories (Plymouth Meeting, PA). Mouse monoclonal anti-γ-adaptin (100/3) and anti-α-adaptin (100/2) antibodies, horseradish peroxidase-coupled anti-mouse IgG and anti-rabbit IgG antibodies, reduced glutathione, and BFA were purchased from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal anti-myc (9E10) and anti-clathrin (X22) antibodies were purified using protein A-Sepharose from culture media of hybridomas obatained from American Type Culture Collection (Manassas, VA); anti-α-adaptin (AP6) was from Affinity Bioreagents (Golden, CO); and anti-rabaptin-5 antibodies were from BD Transduction Laboratories (Lexington, KY). Mouse monoclonal anti-rab5 antibody (CL621.3) was a kind gift from Reinhard Jahn (Max Planck Institute, Göttingen, Germany) and Jean Gruenberg (University of Geneva, Geneva, Switzerland), and anti-rab4 antibody was from Bruno Goud (Institut Curie, Paris, France). A rabbit antiserum was raised against a glutathione S-transferase fusion construct with residues 22–756 of δ-adaptin (a gift of Margaret Robinson, University of Cambridge, Cambridge, United Kingdom) (Simpson et al., 1997).
In Vitro Assay for the Formation of Recycling Vesicles
The MDCK (strain II) cell line stably expressing the human ASGP receptor subunit H1 with a C-terminal myc-tag has been described previously (Leitinger et al., 1995). Semiconfluent cells of four 15-cm plates were washed three times with ice-cold phosphate-buffered saline (PBS) supplemented with 0.7 mM CaCl2, 0.25 mM MgCl2 (PBS++), and surface-biotinylated with 1 mg/ml sulfo-NHS-SS-biotin in the same buffer at 4°C for 30 min. The reaction was quenched by washing the cells three times with PBS++, by a 5-min incubation with 50 mM glycine in PBS, and by another three washes with PBS++. The cells were then incubated in prewarmed serum-free medium (minimal essential medium; Invitrogen) containing 20 mM HEPES (pH 7.4) for 10 min at 37°C to allow internalization of biotinylated surface proteins. Cells were rinsed with ice-cold PBS++. Biotin at the cell surface was stripped by two 20-min incubations with 50 mM reduced glutathione, 75 mM NaCl, 75 mM NaOH, 1 mM EDTA, with 1% bovine serum albumin. The cells were rinsed twice with PBS++ and incubated for 5 min with 5 mg/ml iodoacetamide in PBS++ to quench any residual glutathione. After two additional rinses with PBS++, the cells were permeabilized by incubation in swelling buffer (15 mM HEPES/KOH, pH 7.2, 15 mM KCl) for 15 min at 4°C, scraped in transport buffer [20 mM HEPES/KOH, pH 7.2, 90 mM KOAc, 2 mM Mg(OAc)2], and sedimented at 800 × g for 5 min. The broken cells were resuspended twice in stripping buffer [20 mM HEPES/KOH, pH 7.2, 500 mM KOAc, 2 mM Mg(OAc)2] for 10 min on ice, pelleted again, and resuspended in transport buffer.
In a standard assay, permeabilized cells at 0.5 mg/ml protein were incubated with 1.2 mg/ml cytosol and an ATP-regenerating system (2 mM ATP, 4 mM GTP, 12 mM creatine phosphate, 320 μg/ml creatine kinase) in a total volume of 200 μl. The reaction mixture was left on ice for 5 min and then incubated at 37°C for 30 min. Reactions were stopped on ice and centrifuged at 800 × g for 5 min. The supernatants were carefully aspirated and solubilized for 1 h at 4°C with lysis buffer (1% Triton X-100, 0.5% deoxycholate in PBS, 2 mM phenylmethylsulfonyl fluoride) containing protease inhibitor cocktail (500-fold diluted from 5 mg/ml benzamidine, 1 mg/ml pepstatin A, 1 mg/ml leupeptin, 1 mg/ml antipain, 1 mg/ml chymostatin in 40% dimethyl sulfoxide and 60% ethanol) for 1 h. Insoluble material was removed by centrifugation in a microcentrifuge at 14,000 rpm for 10 min. Supernatants were recovered and rotated end over end for 1 h at 4°C with 40 μl of avidin-Sepharose. The beads were washed three times with lysis buffer and boiled in SDS-sample buffer. Proteins were separated by SDS-gel electrophoresis and transferred to polyvinylidene difluoride membrane that was then incubated in blocking buffer (PBS with 0.1% Tween 20 and 5% nonfat dry milk) for 30 min and with anti-myc antibody in blocking buffer for 1 h at room temperature or overnight at 4°C. Antibody was detected using horseradish peroxidase-conjugated anti-mouse secondary antibody and the enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ).
Cytosol and Immunodepletion
Cytosol was obtained from calf brain as the high-speed supernatant after homogenization (Campbell et al., 1984), supplemented with protease inhibitors. For immunodepletion, a 1:1 mixture of protein A- and protein G-Sepharose beads, was first saturated with 5 mg/ml bovine serum albumin overnight at 4°C, washed with transport buffer, incubated overnight with anti-α-adaptin (AP6), anti-δ-adaptin, anti-rabaptin-5, or anti-rab5 antibody in transport buffer, and washed extensively with transport buffer. Fifty microliters of packed beads was then incubated with 200 μl (0.75 mg) of brain cytosol for 3 h at 4°C with gentle rocking. For depletion of AP-1 and rab4, the cytosol was first incubated overnight at 4°C with anti-γ-adaptin or anti-rab4 antibody, respectively, before albumin-saturated protein A/G-Sepharose beads were added for 3 h at 4°C. After centrifugation, the supernatant was collected and the beads were washed. Bound and unbound proteins were analyzed by immunoblotting by using the corresponding specific antibodies (to detect α-adaptin on blots, antibody 100/2 was used). As a control, cytosol was mock treated with beads without antibody.
Protein Purification
Clathrin-coated vesicles were isolated from calf brains as described previously (Campbell et al., 1984). The coats were released and fractionated on a Superose 6 column as described previously (Crottet et al., 2002). Clathrin-containing fractions were collected and dialyzed against 20 mM ethanolamine, pH 8.9, 2 mM EDTA, 1 mM dithiothreitol, and loaded onto a Mono-Q HR 5/5 column (Amersham Biosciences). Elution with a 5-ml linear gradient of 0–150 mM NaCl, followed by a 50-ml gradient of 150–450 mM NaCl in the same buffer yielded pure clathrin (adapted from Ahle et al., 1988). AP-1 was purified from bovine adrenal medulla as described previously (Crottet et al., 2002). Native rabaptin-5/rabex-5 complex was purified from calf brain cytosol according to Horiuchi et al. (1997), followed by immunodepletion of AP-1, which was a major contaminant. His6-tagged rabaptin-5 in complex with rabex-5 purified from Sf9 insect cells infected with recombinant baculovirus as described previously (Lippe et al., 2001), was a generous gift by Marino Zerial (Max Planck Institute, Dresden, Germany).
Electron Microscopy
F(ab′) fragments of anti-ASGP receptor antibodies were covalently attached to Nanogold (Nanoprobes, Yaphank, NY) to minimize steric effects essentially as described by Hainfeld (1987). The IgG fraction of a polyclonal antiserum was isolated with protein A-Sepharose, digested with 4% pepsin in Na-citrate, pH 4.5, for 1 h at 37°C, dialysed against 0.1 M Na-phosphate, pH 6, 0.5 mM EDTA, and reduced for 1 h at room temperature with mercaptoethylamine hydrochloride (13.3 mg/mg antibody). The mixture was desalted on a Sephadex PD-10 gel filtration column equilibrated with 20 mM Na-phosphate, 150 mM NaCl, 1 mM EDTA, pH 6.5, and incubated with threefold molar excess of monomaleimido-Au1.4 nm solution for 18 h at 4°C. Unbound gold particles were separated from antibody conjugates by gel filtration on a Superose-12 column.
Cells were incubated with nanogold-coupled F(ab′) fragments in PBS at 4°C for 1 h, allowed to internalize at 37°C for 10 min, and processed for in vitro vesicle formation. The resulting vesicle supernatant was fixed with 3% formaldehyde and 0.2% glutaraldehyde for 2 h at room temperature and then pelleted by centrifugation at 100,000 × g for 1 h. The pellet was washed with 0.1 M phosphate buffer, pH 7.4, and free aldehyde groups were quenched by incubation in 50 mM NH4Cl for 30 min at room temperature. After three rinses with phosphate buffer, the sample was processed for cryosectioning according to Liou et al. (1996). Briefly, the pellet was mixed with 10% gelatin, cooled on ice, cut into small pieces and infiltrated with 2.3 M sucrose overnight at 4°C, frozen in liquid nitrogen on cutting pins, and cryosectioned at -120°C by using a Leica Ultracut UCT ultramicrotome. Sections were thawed and transferred to Formvar-coated nickel grids. The nanogold marker was enhanced by silver (HQ Silver enhancement kit; Nanoprobes). To localize AP-1 complexes, they were labeled with monoclonal mouse anti-γ-adaptin antibodies followed by goat anti-mouse IgG conjugated to 10-nm colloidal gold (British Biocell International, Cardiff, United Kingdom). Grids were stained and dried as described (Liou et al., 1996) and viewed with a Philips CM10 electron microscope.
RESULTS
In Vitro Formation of Recycling Vesicles Is Dependent on Temperature, Energy, and Cytosol
The ASGP receptor is a hepatic transport receptor responsible for the rapid clearance of galactosyl-terminal glycoproteins from the circulation (Ashwell and Harford, 1982; Spiess, 1990). The cytoplasmic domain of its major subunit H1 contains a tyrosine-based sorting signal for efficient endocytosis and basolateral sorting (Geffen et al., 1993; Fuhrer et al., 1994). To study receptor recycling from endosomes, we used an MDCK cell line stably expressing myc-tagged H1 and monitored the release of vesicles containing H1 from endosomes in broken cells under various conditions. To obtain specificity for early endosomes as the starting compartment for vesicle formation, surface proteins of intact cells were first biotinylated at 4°C with sulfo-NHS-SS-biotin, an impermeant amine-specific reagent coupled to biotin by a disulfide bond. The cells were then incubated for 10 min at 37°C to allow biotinylated H1 to be endocytosed to early/recycling endosomes. Back at 4°C, biotin was stripped from the cell surface by incubation with reduced glutathione, whereas internalized biotinylated H1 was protected. Cells were then permeabilized by swelling and scraping and washed with high-salt buffer to remove cytosol, proteins peripherally associated with the cytosolic face of membranes, and free transport vesicles. These permeabilized, washed cells were incubated with or without added bovine brain cytosol, ATP, and GTP at 37°C for 30 min. Any vesicles released during this incubation were recovered in the supernatant after low-speed centrifugation. On lysis, biotinylated molecules were isolated with avidin-Sepharose and separated by SDS-gel electrophoresis, and H1 was visualized by immunoblot analysis.
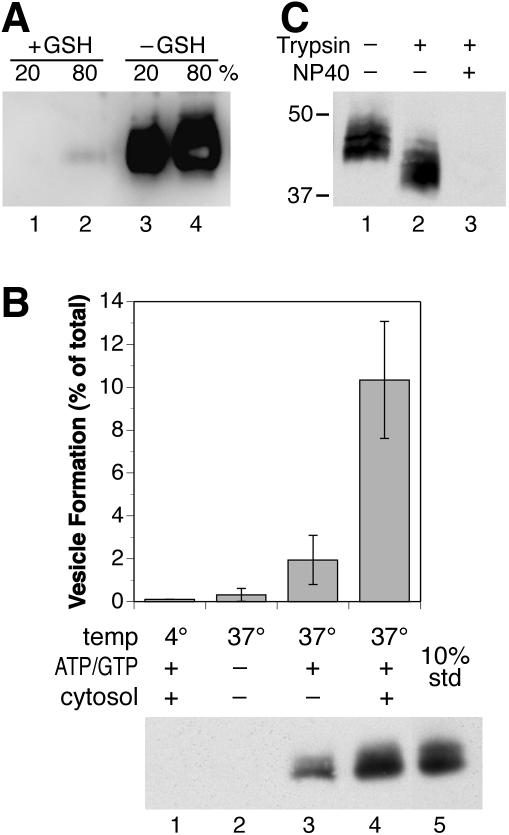
As is shown in Figure 1A in a control experiment without internalization, removal of biotin from the labeled cell surface by reduced glutathione is essentially complete, excluding the possibility that vesicles containing biotinylated H1 generated in the assay may have been derived from the plasma membrane directly. When labeled cells were allowed to internalize before surface-stripping and permeabilization, no vesicles were released upon incubation at 37°C without additions (Figure 1B, lane 2) or when incubated with nucleotides and cytosol at 4°C (lane 1). However, in the presence of ATP, GTP, and 1.2 mg/ml cytosol at 37°C, typically ∼10% of biotinylated H1 in the starting material was recovered in the supernatant (lane 4). In the absence of added cytosol, nucleotides supported a basal release of H1 into the supernatant of typically ∼20% of that in the presence of cytosol (lane 3), most likely due to residual cytosolic proteins and/or coat proteins already recruited to the membranes that had not been removed by the high-salt washes. The amount of H1 released independently of added cytosol was somewhat variable between experiments, most likely because of differences in the removal of cytosolic proteins from the broken cells.
Figure 1.
Biotinylated, internalized ASGP receptor H1 is released from broken cells in a temperature-, energy-, and cytosol-dependent manner in sealed membranes. (A) Cells expressing H1 were surface labeled with sulfo-NHS-SS-biotin on ice, washed, and then incubated with reduced glutathione (+GSH) to release the coupled biotin (lanes 1 and 2) or without glutathione (–GSH; lanes 3 and 4). On cell lysis, biotinylated proteins were isolated with avidin-agarose. Twenty and 80% of each sample were separated by SDS-gel electrophoresis and analyzed by immunoblotting by using anti-myc antibody. Stripping of surface-bound biotin by glutathione is essentially complete. (B) Surface-biotinylated cells were incubated at 37°C for 10 min to allow internalization, chilled in ice, and surface biotin was stripped with reduced glutathione. The cells were then broken and incubated for 30 min at 4 or 37°C, with or without cytosol (1.2 mg/ml) or nucleotides and energy-regenerating system as indicated. Cells were pelleted and the supernatant was lysed and analyzed by avidin precipitation of biotinylated proteins, SDS-gel electrophoresis, and immunoblotting by using anti-myc antibody. Values were normalized to the amount of total labeled H1 by analyzing 10% of a sample before centrifugation as a standard (10% std; lane 5). Average and SD of at least four independent determinations are shown. (C) The supernatant of cells treated as in B, lane 4, was incubated at 4°C for 45 min with or without trypsin in the presence or absence of detergent (NP-40). Trypsin was stopped with soybean trypsin inhibitor. Biotinylated proteins were then isolated, separated by SDS-gel electrophoresis, and analyzed by immunoblotting for myc-tagged H1. The positions of molecular weight markers are indicated (in kilodaltons).
To test whether H1 was released in sealed vesicles, the supernatant of permeabilized cells incubated with exogenous cytosol and nucleotides was subjected to a protease protection assay (Figure 1C). Released H1 was resistant to added trypsin except for the 40-amino acid cytoplasmic portion of H1, resulting in an electrophoretic shift of ∼4 kDa. Only upon solubilization of membranes by detergent was H1 completely digested by trypsin.
The amount of H1-containing vesicles generated in the in vitro reaction depended on the concentration of cytosol used (Figure 2A). It increased with higher cytosol concentrations up to ∼10 mg/ml protein. In most of the following experiments, we used a limiting amount of cytosol of 1.2 mg/ml to more sensitively detect effects of depletion or addition of individual components. In a time-course experiment, formation of H1-containing vesicles was detectable already within 5 min of incubation and reached a maximum after ∼20 min (Figure 2B). Unless specified, vesicles were harvested after 30-min incubation at 37°C in all subsequent experiments. Efficient formation of H1-containing vesicles required the presence of both ATP and GTP (Figure 2C, lanes 1–5), indicating the involvement of ATPase(s) and GTPase(s). The nonhydrolyzable analogues AMP-PNP and GMP-PNP did not substitute for ATP and GTP, respectively (Figure 2C, lanes 8–12), suggesting that nucleotide hydrolysis is essential.
Figure 2.
Vesicle formation depends on cytosol concentration, time, and hydrolyzable ATP and GTP. (A) The assay for the formation of endosomal vesicles was performed using increasing concentrations of cytosol at 37°C in the presence of ATP, GTP, and an ATP-regenerating system. Recovery of biotinylated H1 in the supernatant as determined by immunoblot analysis was quantified and plotted relative to the maximal value. The data represent the average and SD of three independent determinations. (B) Biotinylated broken cells were incubated in the presence of ATP, GTP, and an ATP-regenerating system at 37°C with or without cytosol (12.5 mg/ml) for increasing times. Recovery of biotinylated H1 in the supernatant was plotted relative to the maximal value (•, with cytosol; ×, without cytosol). (C) Vesicle formation was assayed in the presence of cytosol (12.5 mg/ml) with GTP or GMP-PNP (GN), and ATP or AMP-PNP (AN) or without nucleotides. Lanes 1–7 and 8–12 are from separate experiments.
Clathrin and AP-1 Adaptors, but Not AP-2 or AP-3, Are Involved in Generating Recycling Vesicles
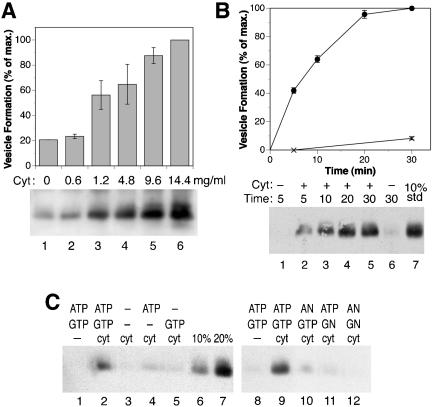
To investigate whether the formation of H1-containing recycling vesicles is mediated by clathrin adaptors, we analyzed the effect of cytosol immunodepleted for AP-1, AP-2, or AP-3 adaptors. On depletion of >80% of AP-1 by using an anti-γ-adaptin antibody (Figure 3A, lane 2), vesicle formation was strongly inhibited by ∼75% compared with the level obtained with control cytosol or mock-depleted cytosol (Figure 3B). Readdition of purified AP-1 isolated from clathrin-coated vesicles of bovine adrenal glands to the depleted cytosol fully restored vesicle formation even beyond the initial extent (Figure 3B, lane 4). This confirms that the inhibition by immunodepletion was due to the removal of AP-1 itself, rather than a component that might have been associated with cytosolic AP-1.
Figure 3.
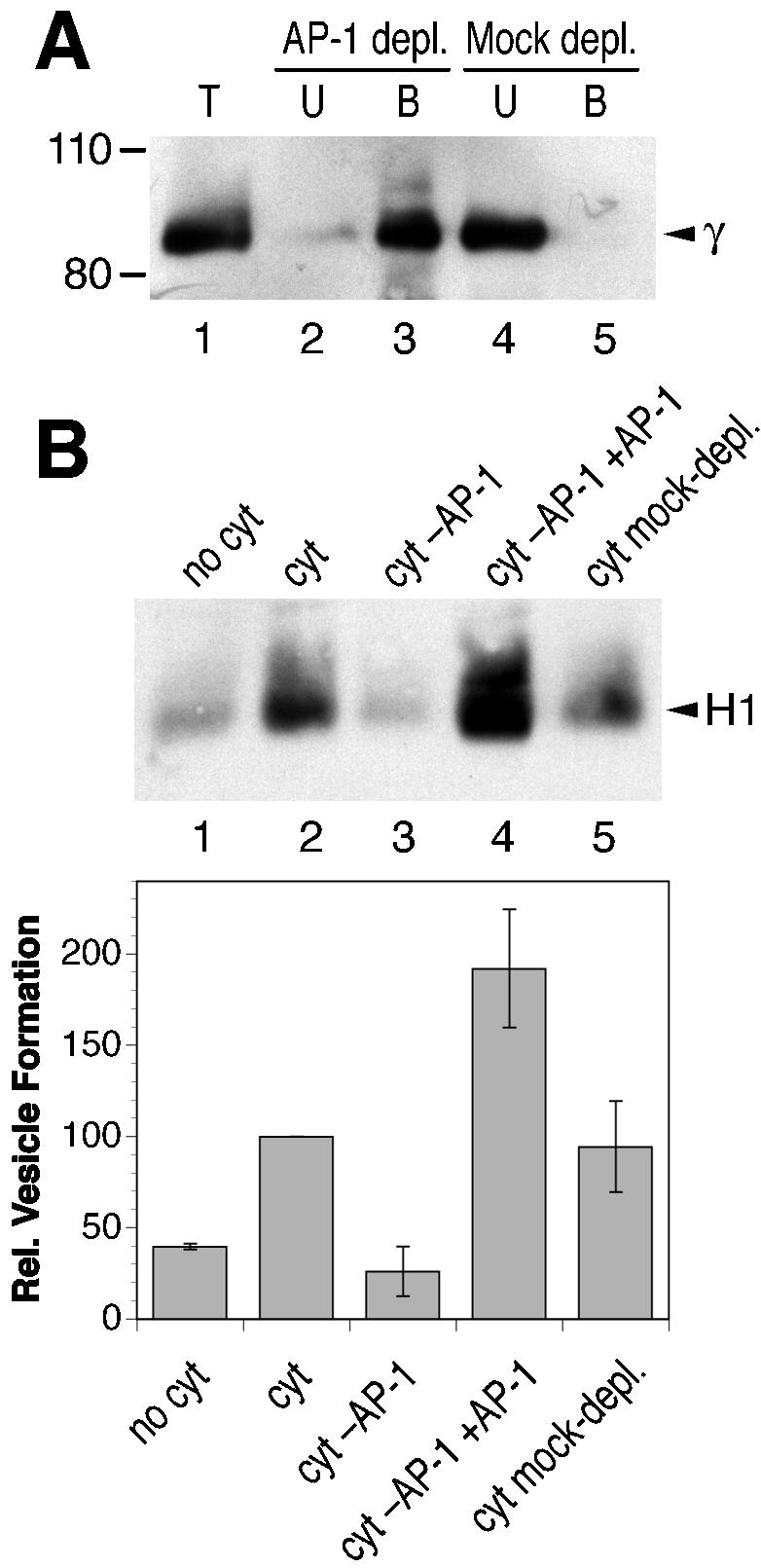
AP-1 adaptors are necessary for endosomal vesicle formation in vitro. (A) Bovine brain cytosol was immunodepleted of AP-1 by using anti-γ-adaptin antibody and protein A/G-Sepharose beads. Total cytosol (T) and corresponding aliquots of the depleted cytosol (unbound fraction, U) and the bound material (B) were analyzed by immunoblotting with anti-γ-adaptin antibody. Depletion efficiency was >80%. The positions of molecular weight markers are indicated (in kilodaltons). (B) Biotinylated permeabilized cells were incubated in the presence of ATP, GTP, and ATP-regenerating system without cytosol (no cyt), with untreated cytosol (cyt; 1.2 mg/ml), with AP-1– depleted cytosol (cyt–AP-1), with AP-1– depleted cytosol that had been supplemented with 12 μg/ml purified AP-1 (cyt–AP-1+AP-1), or with mock-depleted cytosol. Immunoblot analysis of biotinylated H1 in the supernatant after cell pelleting is shown for a representative experiment. Quantitation of three independent experiments (average with SD) is presented below.
In contrast to AP-1, depletion of AP-2 or AP-3 did not affect formation of H1-containing vesicles (Figure 4). AP-2 was depleted to <10% by using an anti-α-adaptin antibody (Figure 4A). Generation of recycling vesicles was unchanged by AP-2 depletion (Figure 4B). Because AP-2 complexes function in endocytosis, this confirms that the H1-containing vesicles generated in our assay were not derived from the plasma membrane. AP-3 adaptors have been localized to endosomal membranes and are involved in protein sorting toward lysosomes (Dell'Angelica et al., 1998). AP-3 was depleted to ∼10% by using an anti-δ-adaptin antibody (Figure 4C). Compared with control cytosol, no significant variation of vesicle formation was observed with AP-3– depleted cytosol (Figure 4D).
Figure 4.
Depletion of AP-2 or AP-3 adaptors does not affect in vitro vesicle formation from labeled endosomes. Bovine brain cytosol was immunodepleted for AP-2 (A) or AP-3 adaptors (C) by using anti-α- and anti-δ-adaptin antibodies, respectively, as in Figure 3A for AP-1. Depletion efficiency was >90% in both cases. Biotinylated permeabilized cells were incubated in the presence of ATP, GTP, and ATP-regenerating system without cytosol (no cyt), with untreated cytosol (cyt; 1.2 mg/ml), with adaptor-depleted cytosol (cyt–AP-2 or -3), or with mock-depleted cytosol (B for AP-2 and D for AP-3). Immunoblot analysis of biotinylated H1 in the supernatant after cell pelleting is shown for a representative experiment. Quantitation of three independent experiments (average with SD) is presented below.
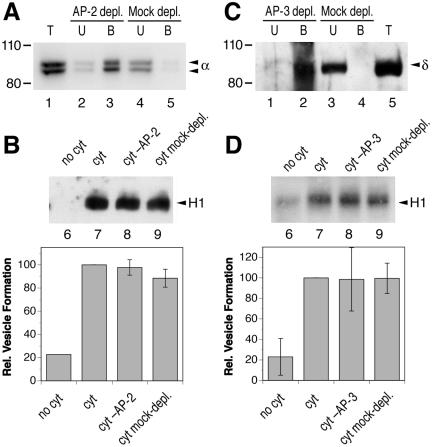
If AP-1 adaptors are part of the coat to form recycling vesicles, clathrin is likely to be involved as well. Supplementing purified clathrin to the cytosol enhanced the formation of H1-containing endosomal vesicles up to more than twofold in a dose-dependent manner (Figure 5A, lanes 1–7). Preincubation of the cytosol with the anti-clathrin antibody X22 (Brodsky et al., 1987) strongly inhibited vesicle formation (Figure 5A, lane 8). X22 has been shown to bind to an epitope in the clathrin heavy chain and to inhibit in vivo both fluid-phase and receptor-mediated endocytosis (Doxsey et al., 1987; Draper et al., 1990). Furthermore, AP-1 recruitment generally involves the small GTPase ARF1 and guanine nucleotide exchange factors sensitive to BFA. Indeed, increasing concentrations of BFA added to the cytosol resulted in increasing inhibition of vesicle formation (Figure 5B), although only at high concentrations. These two findings support the notion that H1-containing recycling vesicles were generated by AP-1/clathrin coats.
Figure 5.
Clathrin stimulates and BFA inhibits in vitro formation of recycling vesicles. (A) Biotinylated permeabilized cells were incubated in the presence of ATP, GTP, and ATP-regenerating system without cytosol, with cytosol (1.2 mg/ml), or with cytosol supplemented with up to 120 μg/ml purified clathrin (0 indicates the addition of solvent only). In a further reaction, cytosol preincubated for 15 min at 4°C with 40 μg/ml anti-clathrin antibody X22 was used. Immunoblot analysis and quantitation of biotinylated H1 in the supernatant after cell pelleting is shown for a representative experiment. (B) Biotinylated and permeabilized cells were incubated with nucleotides, ATP-regenerating system with and without cytosol, and the indicated amount of BFA first at 4°C for 15 min and then for 10 min at 37°C. Immunoblot analysis of biotinylated H1 in the supernatant after cell pelleting is shown together with the quantitation of three independent experiments (average with SD). (C) Biotinylated and permeabilized cells were incubated with nucleotides, ATP-regenerating system with and without cytosol, and up to 100 μM phosphatidyl inositol 3-kinase inhibitor LY294002 (LY; 0 indicates the addition of solvent only). Immunoblot analysis of biotinylated H1 in the supernatant after cell pelleting is shown together with the quantitation of two independent experiments (average with range).
In vivo, transferrin receptor recycling was shown to be partially sensitive to wortmannin and LY294002, inhibitors of phosphatidyl inositol 3-kinase (van Dam et al., 2002). Addition of 50 or 100 μM LY294002, however, had no inhibitory effect on the in vitro formation of endosome-derived vesicles containing biotinylated H1 (Figure 5C).
Visualization of In Vitro-generated Vesicles Containing H1
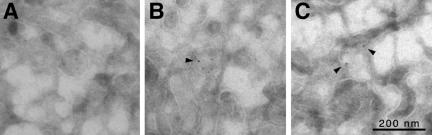
To morphologically characterize the recycling vesicles generated in our assay, H1 at the cell surface was first decorated at 4°C with nanogold-labeled F(ab′) fragments generated from rabbit anti-ASGP receptor immunoglobulins and then allowed to internalize for 10 min. F(ab′) at the cell surface was removed by acid washes, and the cells were then permeabilized and incubated with nucleotides and cytosol as described above. Membranes recovered in the low-speed supernatant were collected, concentrated by high-speed centrifugation, and processed for cryoelectron microscopy. Nanogold particles were enhanced with silver. The presence of nanogold indicated the presence of H1 that had been internalized from the plasma membrane to endosomes before permeabilization of the cells. Labeled membranes generally had a vesicular appearance with a diameter of ∼100 nm (Figure 6).
Figure 6.
Electron microscopic visualization of H1-containing endosomal vesicles. (A) H1 at the cell surface was decorated with nanogold-coupled anti-H1 F(ab′) fragments and allowed to internalize for 10 min. Cells were stripped of surface antibody; permeabilized; incubated with cytosol, nucleotides, and ATP-regenerating system; and sedimented. Membranes in the supernatant were collected by high-speed centrifugation and subjected to cryoelectron microscopy and silver enhancement of nanogold. (B and C) As in A, but in addition decorated with anti-γ-adaptin and a secondary anti-mouse IgG antibody coupled to 10-nm colloidal gold. Arrowheads indicate colloidal gold particles, which have a more dense appearance than silver-enhanced nanogold particles. Bar, 200 nm.
Coat structures were not obvious on any of the membranes in the sample. To test for the presence of AP-1, the cryosections were in addition decorated with anti-γ-adaptin antibody and protein A coupled to 10-nm colloidal gold. Vesicles could be found that were positive for both colloidal gold and nanogold (Figure 6, B and C), consistent with the biochemical evidence that H1 is released from endosomes in vesicles involving AP-1– containing coats.
Rab4 and Rabaptin-5/Rabex-5 Regulate the Formation of Recycling Vesicles
Rab proteins are well-known regulators of endosomal functions (Zerial and McBride, 2001). We tested the requirement of rab4 and rab5, which both are localized to early endosomes, for in vitro vesicle formation by depleting the cytosol by using specific antibodies (Figure 7, A and B). Whereas the formation of H1-containing vesicles was not affected by depletion of rab5, it was strongly inhibited by the removal of rab4 (Figure 7C). An involvement of rab4 in the generation of recycling vesicles is in agreement with previous observations in living cells (van der Sluijs et al., 1992).
Figure 7.
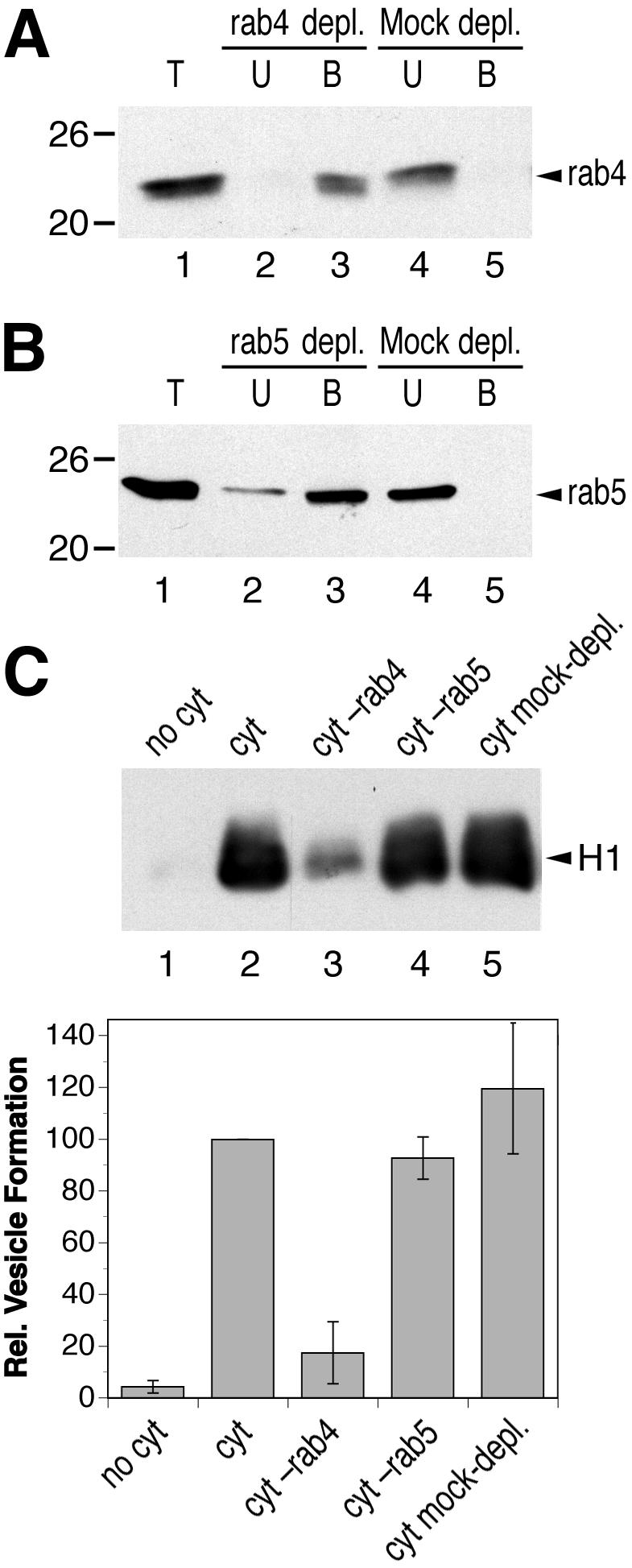
Rab4, but not Rab5, is required for in vitro formation of recycling vesicles. Bovine brain cytosol was immunodepleted for rab4 (A) or rab5 (B) by using specific antibodies as in Figure 3A for AP-1. Depletion efficiency was >90% in both cases. The positions of molecular weight markers are indicated (in kilodaltons). Biotinylated permeabilized cells were incubated in the presence of ATP, GTP, and ATP-regenerating system without cytosol (no cyt), with untreated cytosol (cyt; 1.2 mg/ml), with rab-depleted cytosol (cyt–rab4; cyt–rab5), or with mock-depleted cytosol (C). Immunoblot analysis of biotinylated H1 in the supernatant after cell pelleting is shown for a representative experiment. Quantitation of three independent experiments (average with SD) is presented below.
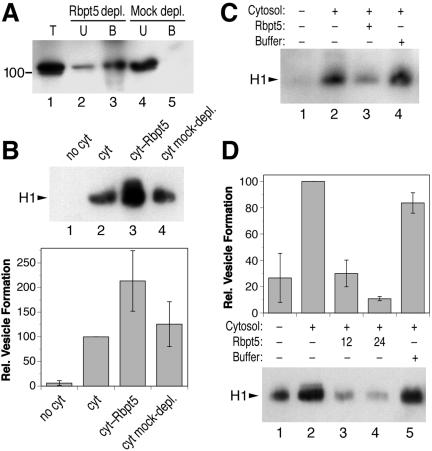
A candidate to connect rab4 function to AP-1/clathrin coat formation on endosomes is the rabaptin-5/rabex-5 complex. Initially, rabaptin-5 was identified as an interactor of rab5 (Stenmark et al., 1995) and rabex-5 as a rab5 guanine nucleotide exchange factor (Horiuchi et al., 1997). Rabaptin-5 in addition was shown to bind to rab4 and AP-1 (Vitale et al., 1998; de Renzis et al., 2002; Deneka et al., 2003). On immunodepletion of rabaptin-5/rabex-5 from the cytosol with an anti-rabaptin-5 antibody (Figure 8A), formation of endosomal vesicles was reproducibly stimulated (Figure 8B), suggesting an inhibitory role of the complex. To test the effect of increased rabaptin-5/rabex-5 concentrations on vesicle formation, the native complex was partially purified from calf brain cytosol according to the procedure by Horiuchi et al. (1997). Because we observed that AP-1, which stimulates vesicle formation, was also enriched together with rabaptin-5/rabex-5, AP-1 was depleted from the preparation by anti-γ-adaptin antibody coupled to protein A/G-Sepharose before use. Addition of enriched rabaptin-5/rabex-5 consistently inhibited in vitro vesicle formation (Figure 8C). To exclude that this effect was due to contaminants, the complex of rabex-5 and His6-tagged rabaptin-5 was expressed using the baculovirus/Sf9 cell system and purified as described previously (Lippe et al., 2001). Addition of 12 or 24 μg/ml rabaptin-5/rabex-5 (corresponding approximately to once or twice the amount already present in the added cytosol, respectively) clearly inhibited the formation of H1-containing vesicles (Figure 8D). The rabaptin-5/rabex-5 complex thus negatively regulates vesicle generation.
Figure 8.
Rabaptin-5/rabex-5 inhibits in vitro formation of recycling vesicles. Bovine brain cytosol was immunodepleted for rabaptin-5/rabex-5 (Rbpt5) by using an antibody directed against rabaptin-5 as in Figure 3A for AP-1 (A). Biotinylated permeabilized cells were incubated in the presence of ATP, GTP, and ATP-regenerating system without cytosol, with untreated cytosol (cyt; 1.2 mg/ml), and with modified cytosol. In B, the effect of the rabaptin-5/rabex-5-depleted cytosol was tested, in C the effect of cytosol supplemented with rabaptin-5/rabex-5 partially purified from calf brain and immunodepleted for AP-1 (approximately doubling the amount of rabaptin-5 already in the cytosol), and in D the effect of adding 12 or 24 μg/ml purified rabaptin-5/rabex-5 produced by the baculovirus system. As controls, cytosol containing the buffer of the corresponding rabaptin-5/rabex-5 preparation was analyzed in parallel. Immunoblot analysis of biotinylated H1 in the supernatant after cell pelleting is shown for a representative experiment, as well as the quantitation of three independent experiments (average with SD) in B and D.
DISCUSSION
AP-1A/Clathrin Coats Mediate Receptor Recycling
One important finding of our study is that in vitro reconstitution of recycling vesicles is clearly dependent on AP-1 adaptors: the formation of H1-containing endosome-derived vesicles is blocked upon AP-1 depletion of the cytosol and restored upon readdition of purified protein. The involvement of AP-1/clathrin coats is further substantiated by the stimulating effect of increased clathrin concentration and by the sensitivity to BFA. These results thus support the earlier observations that transferrin receptor recycling in living cells at least in part was affected by BFA and involved dynamin (van Dam and Stoorvogel, 2002; van Dam et al., 2002). Perturbation of clathrin function in living cells was always found to affect endocytosis more effectively than receptor recycling (Bennett et al., 2001; Wettey et al., 2002; Moskowitz et al., 2003). The reduced recycling rates thus could be interpreted as an indirect result of disturbing clathrin-dependent pathways from the plasma membrane, and from and to the TGN, rather than the result of a direct involvement of clathrin in recycling. However, the existence of at least two major pathways from early endosomes to the plasma membrane (Sheff et al., 1999; Hao and Maxfield, 2000; van Dam et al., 2002) provides an explanation why interference with just one transport mechanism only partially blocks recycling. In addition, compensatory mechanisms are likely to make up for the inhibited route in long-term experiments. In the reconstitution assay used here, normal permeabilized cells, loaded with internalized biotinylated proteins were used for a single round of vesicle formation. This process is unlikely to be significantly affected by other transport events. Consistent with this notion, depletion of AP-2 (which is involved in endocytosis) or AP-3 (which mediates lysosomal sorting from endosomal tubules positive also for AP-1; Peden et al., 2004), or of rab5 (involved in the formation of sorting endosomes, endosome fusion and organization), did not affect the formation of H1-containing endosome-derived vesicles.
Kinetic, pharmacological, and temperature-shift experiments provided evidence for two distinct recycling pathways (Sheff et al., 1999; Hao and Maxfield, 2000; Hunyady et al., 2002; van Dam et al., 2002): a rapid recycling pathway from sorting endosomes that is sensitive to phosphatidyl inositol 3-kinase inhibitors (wortmannin and LY294002), and a slow one from recycling endosomes that is sensitive to BFA and involves dynamin. Because the formation of H1-containing vesicles is insensitive to LY294002 but sensitive to higher concentrations of BFA, it seems plausible that the vesicles generated in our assay correspond to the slow pathway from recycling endosomes. However, there is no evidence on the precise distribution of internalized H1 between sorting and recycling endosomes during the in vitro incubation. In a similar in vitro assay by using CHO cells, endosome-derived vesicles containing transferrin receptor and GLUT4 were generated by a BFA-insensitive but neomycin-sensitive mechanism (Lim et al., 2001). Because endocytic proteins had been internalized at 15°C, a temperature at which transport from sorting to recycling endosomes is blocked (Ren et al., 1998; van Dam et al., 2002), the starting compartment was predominantly sorting endosomes and the vesicles generated thus might have represented the fast recycling pathway.
Basolateral sorting of a subset of transport receptors, among them the receptors of transferrin, LDL, and ASGPs, was recently shown to depend on AP-1B, i.e., AP-1 adaptor complexes containing the epithelial-specific subunit isoform μ1B (Fölsch et al., 1999, 2003; Sugimoto et al., 2002), in particular also in basolateral recycling (Gan et al., 2002). Polarized sorting of these receptors is defective in the kidney epithelial cell line LLC-PK1, which lacks μ1B, but is restored upon μ1B expression. This mechanism was shown to be operative also in MDCK cells (Ang et al., 2003). We have used MDCK cells in our experiments in combination with cytosol from calf brain lacking μ1B. As a result, we reconstituted vesicle formation in the absence of the specifically basolateral coat proteins, a situation like that in LLC-PK1 cells. This mechanism involving the ubiquitous isoform AP-1A is likely to correspond to such a nonpolarized recycling and recycling in nonpolarized cells.
How can the apparent signal-independence of receptor recycling (Jing et al., 1990; Johnson et al., 1993) be reconciled with the involvement of AP-1A/clathrin coats? It has been observed that bafilomycin A1, a blocker of the endosomal proton pump, inhibited recycling of the wild-type, but not of a mutant transferrin receptor lacking its cytoplasmic sorting signals (Johnson et al., 1993). This might suggest that proteins with and without sorting signals depart from different endosomal subcompartments and use different recycling pathways.
Rab4 and Rabaptin-5/Rabex-5 Regulate Recycling Vesicle Formation
Rab GTPases are established regulators of various aspects of endosomal functions and trafficking pathways (Zerial and McBride, 2001). Three rab proteins have been implicated in regulating receptor recycling. Rab4 was implicated in regulating recycling from endosomes to the cell surface in nonpolarized and to the apical surface in polarized cells (van der Sluijs et al., 1992; Mohrmann et al., 2002; Deneka et al., 2003).
In vitro budding of transferrin receptor-containing vesicles from PC12 cells expressing GTPase-deficient or GDP-locked rab4 mutants was stimulated or inhibited, respectively, in comparison with untransfected cells or cells expressing wild-type rab4 (de Wit et al., 2001). For rab11, there is evidence for an involvement in several pathways, from the TGN to the plasma membrane (Chen et al., 1998), and from endosomes to the plasma membrane (Ren et al., 1998) and to the TGN (Wilcke et al., 2000). A constitutively active rab8 mutant was found to interfere specifically with AP-1B localization and function, disturbing basolateral sorting (Ang et al., 2003). With the antibodies available to us, we were able to efficiently remove rab4 and rab5 from cytosol. Only the depletion of rab4 blocked the production of H1-containing vesicles in the in vitro assay, suggesting a rather direct role of this GTPase in the formation of recycling vesicles.
Rab proteins are believed to exert their activity by recruiting specific effector proteins to the membrane domains in which they are localized (Zerial and McBride, 2001; de Renzis et al., 2002). Rabenosyn-5 and rabaptin-5/rabex-5 have been shown to be interaction partners of rab4 as well as rab5 (Vitale et al., 1998; de Renzis et al., 2002). The fact that these proteins can simultaneously bind to both active rab GTPases suggested that they regulate endosomal protein sorting and recycling by defining and connecting endosomal subdomains (de Renzis et al., 2002). In our assay, addition of rabaptin-5/rabex-5 inhibited and depletion stimulated the formation of recycling vesicles. This is not likely to be the result of an indirect effect on rab5 function (e.g., altered activation of rab5 by increased or decreased amounts of its exchange factor rabex-5), because depletion of rab5 itself had no effect on vesicle formation.
Interestingly, rabaptin-5 had been shown to interact also with the γ ear domains of AP-1 and GGAs, a family of ARF1-dependent clathrin adaptors (Hirst et al., 2000; Shiba et al., 2002; Deneka et al., 2003; Mattera et al., 2003). A possible role of rab4 might thus be to recruit rabaptin-5/rabex-5 to recycling endosomes as a docking site for AP-1 adaptors. Our finding that rabaptin-5 depletion stimulates vesicle formation, argues against this model. The rabaptin-5 interaction with GGA was shown to inhibit clathrin binding to GGA in vitro, suggesting a possible role of rabaptin-5 in releasing clathrin from GGA-coated membranes (Mattera et al., 2003). Similarly, rabaptin-5/rabex-5 binding to AP-1 may block the interaction of clathrin with AP-1, which has clathrin binding sites in the hinge regions of β1- and γ-adaptins. Because rab4 is necessary for vesicle formation, i.e., plays a positive role, it might do so by counteracting the inhibitory effect of its interaction partner rabaptin-5/rabex-5. It is therefore conceivable that rab4 triggers the release of rabaptin-5/rabex-5 from AP-1 to free the clathrin binding site of γ-adaptin and to allow the completion of the coat and the formation of a vesicle. In an alternative model, rabaptin-5/rabex-5 might inhibit rab4 function while bound to AP-1. On its displacement by clathrin binding to AP-1, rab4 might be derepressed to perform its function, e.g., the recruitment of the machinery to pinch off the coated bud. These hypotheses remain to be tested experimentally both in vivo and in vitro.
Acknowledgments
We thank Dr. Vivienne Laird for initial experiments; Drs. Bruno Goud, Jean Gruenberg, Reinhard Jahn, Daniel Meyer, Margaret Robinson, Peter van der Sluijs, and Marino Zerial for reagents; and Hans-Peter Hauri for critically reading the manuscript. This work was supported by grant 31-061579.00 from the Swiss National Science Foundation.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–04–0355. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–04–0355.
Abbreviations used: AP, adaptor protein; ARF1, ADP-ribosylation factor 1; ASGP, asialoglycoprotein; BFA, brefeldin A; MDCK, Madin-Darby canine kidney; PBS, phosphate-buffered saline; TGN, trans-Golgi network.
References
- Ahle, S., Mann, A., Eichelsbacher, U., and Ungewickell, E. (1988). Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 7, 919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang, A.L., Fölsch, H., Koivisto, U.M., Pypaert, M., and Mellman, I. (2003). The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J. Cell Biol. 163, 339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell, G., and Harford, J. (1982). Carbohydrate-specific receptors of the liver. Annu. Rev. Biochem. 51, 531-554. [DOI] [PubMed] [Google Scholar]
- Bennett, E.M., Lin, S.X., Towler, M.C., Maxfield, F.R., and Brodsky, F.M. (2001). Clathrin hub expression affects early endosome distribution with minimal impact on receptor sorting and recycling. Mol. Biol. Cell 12, 2790-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky, F.M., Galloway, C.J., Blank, G.S., Jackson, A.P., Seow, H.F., Drickamer, K., and Parham, P. (1987). Localization of clathrin light-chain sequences mediating heavy-chain binding and coated vesicle diversity. Nature 326, 203-205. [DOI] [PubMed] [Google Scholar]
- Campbell, C., Squicciarini, J., Shia, M., Pilch, P.F., and Fine, R.E. (1984). Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry 23, 4420-4426. [DOI] [PubMed] [Google Scholar]
- Chen, W., Feng, Y., Chen, D., and Wandinger-Ness, A. (1998). Rab11 is required for trans-Golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol. Biol. Cell 9, 3241-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crottet, P., Meyer, D.M., Rohrer, J., and Spiess, M. (2002). ARF1-GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol. Biol. Cell 13, 3672-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump, C.M., Xiang, Y., Thomas, L., Gu, F., Austin, C., Tooze, S.A., and Thomas, G. (2001). PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20, 2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Renzis, S., Sönnichsen, B., and Zerial, M. (2002). Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 4, 124-133. [DOI] [PubMed] [Google Scholar]
- de Wit, H., Lichtenstein, Y., Kelly, R.B., Geuze, H.J., Klumperman, J., and van der Sluijs, P. (2001). Rab4 regulates formation of synaptic-like microvesicles from early endosomes in PC12 cells. Mol. Biol. Cell 12, 3703-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica, E.C., Klumperman, J., Stoorvogel, W., and Bonifacino, J.S. (1998). Association of the AP-3 adaptor complex with clathrin. Science 280, 431-434. [DOI] [PubMed] [Google Scholar]
- Deneka, M., Neeft, M., Popa, I., van Oort, M., Sprong, H., Oorschot, V., Klumperman, J., Schu, P., and van der Sluijs, P. (2003). Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. EMBO J. 22, 2645-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey, S.J., Brodsky, F.M., Blank, G.S., and Helenius, A. (1987). Inhibition of endocytosis by anti-clathrin antibodies. Cell 50, 453-463. [DOI] [PubMed] [Google Scholar]
- Draper, R.K., Goda, Y., Brodsky, F.M., and Pfeffer, S.R. (1990). Antibodies to clathrin inhibit endocytosis but not recycling to the trans Golgi network in vitro. Science 248, 1539-1541. [DOI] [PubMed] [Google Scholar]
- Fölsch, H., Ohno, H., Bonifacino, J.S., and Mellman, I. (1999). A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99, 189-198. [DOI] [PubMed] [Google Scholar]
- Fölsch, H., Pypaert, M., Maday, S., Pelletier, L., and Mellman, I. (2003). The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 163, 351-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer, C., Geffen, I., Huggel, K., and Spiess, M. (1994). The two subunits of the asialoglycoprotein receptor contain different sorting information. J. Biol. Chem. 269, 3277-3282. [PubMed] [Google Scholar]
- Futter, C.E., Gibson, A., Allchin, E.H., Maxwell, S., Ruddock, L.J., Odorizzi, G., Domingo, D., Trowbridge, I.S., and Hopkins, C.R. (1998). In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 141, 611-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, Y., McGraw, T.E., and Rodriguez-Boulan, E. (2002). The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat. Cell Biol. 4, 605-609. [DOI] [PubMed] [Google Scholar]
- Geffen, I., Fuhrer, C., Leitinger, B., Weiss, M., Huggel, K., Griffiths, G., and Spiess, M. (1993). Related signals for endocytosis and basolateral sorting of the asialoglycoprotein receptor. J. Biol. Chem. 268, 20772-20777. [PubMed] [Google Scholar]
- Gruenberg, J. (2001). The endocytic pathway: a mosaic of domains. Nature Rev. Mol. Cell. Biol. 2, 721-730. [DOI] [PubMed] [Google Scholar]
- Hainfeld, J.F. (1987). A small gold-conjugated antibody label: improved resolution for electron microscopy. Science 236, 450-453. [DOI] [PubMed] [Google Scholar]
- Hao, M., and Maxfield, F.R. (2000). Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 275, 15279-15286. [DOI] [PubMed] [Google Scholar]
- Hinners, I., and Tooze, S.A. (2003). Changing directions: clathrin-mediated transport between the Golgi and endosomes. J. Cell Sci. 116, 763-771. [DOI] [PubMed] [Google Scholar]
- Hirst, J., Lui, W.W., Bright, N.A., Totty, N., Seaman, M.N., and Robinson, M.S. (2000). A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 149, 67-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst, J., and Robinson, M.S. (1998). Clathrin and adaptors. Biochim. Biophys. Acta 1404, 173-193. [DOI] [PubMed] [Google Scholar]
- Horiuchi, H., et al. (1997). A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149-1159. [DOI] [PubMed] [Google Scholar]
- Hunyady, L., Baukal, A.J., Gaborik, Z., Olivares-Reyes, J.A., Bor, M., Szaszak, M., Lodge, R., Catt, K.J., and Balla, T. (2002). Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J. Cell Biol. 157, 1211-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, S.Q., Spencer, T., Miller, K., Hopkins, C., and Trowbridge, I.S. (1990). Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J. Cell Biol. 110, 283-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L.S., Dunn, K.W., Pytowski, B., and McGraw, T.E. (1993). Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor's internalization motif. Mol. Biol. Cell 4, 1251-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger, B., Hille-Rehfeld, A., and Spiess, M. (1995). Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc. Natl. Acad. Sci. USA 92, 10109-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S.N., Bonzelius, F., Low, S.H., Wille, H., Weimbs, T., and Herman, G.A. (2001). Identification of discrete classes of endosome-derived small vesicles as a major cellular pool for recycling membrane proteins. Mol. Biol. Cell 12, 981-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou, W., Geuze, H.J., and Slot, J.W. (1996). Improving structural integrity of cryosections for immunogold labeling. Histochem. Cell Biol. 106, 41-58. [DOI] [PubMed] [Google Scholar]
- Lippe, R., Miaczynska, M., Rybin, V., Runge, A., and Zerial, M. (2001). Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol. Biol. Cell 12, 2219-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F., Antony, C., Tenza, D., Salamero, J., Goud, B., and Johannes, L. (1998). Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 143, 973-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera, R., Arighi, C.N., Lodge, R., Zerial, M., and Bonifacino, J.S. (2003). Divalent interaction of the GGAs with the Rabaptin-5-Rabex-5 complex. EMBO J. 22, 78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield, F.R., and McGraw, T.E. (2004). Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 5, 121-132. [DOI] [PubMed] [Google Scholar]
- Meyer, C., Zizioli, D., Lausmann, S., Eskelinen, E.L., Hamann, J., Saftig, P., von Figura, K., and Schu, P. (2000). mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 19, 2193-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann, K., Leijendekker, R., Gerez, L., and van der Sluijs, P. (2002). Rab4 regulates transport to the apical plasma membrane in Madin-Darby canine kidney cells. J. Biol. Chem. 277, 10474-10481. [DOI] [PubMed] [Google Scholar]
- Moskowitz, H.S., Heuser, J., McGraw, T.E., and Ryan, T.A. (2003). Targeted chemical disruption of clathrin function in living cells. Mol. Biol. Cell 14, 4437-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, H., Tomemori, T., Nakatsu, F., Okazaki, Y., Aguilar, R.C., Fölsch, H., Mellman, I., Saito, T., Shirasawa, T., and Bonifacino, J.S. (1999). Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 449, 215-220. [DOI] [PubMed] [Google Scholar]
- Peden, A.A., Oorschot, V., Hesser, B.A., Austin, C.D., Scheller, R.H., and Klumperman, J. (2004). Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 164, 1065-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, M., Xu, G., Zeng, J., De Lemos-Chiarandini, C., Adesnik, M., and Sabatini, D.D. (1998). Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA 95, 6187-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff, D.R., Daro, E.A., Hull, M., and Mellman, I. (1999). The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145, 123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba, Y., Takatsu, H., Shin, H.W., and Nakayama, K. (2002). gamma-Adaptin interacts directly with Rabaptin-5 through its ear domain. J. Biochem. 131, 327-336. [DOI] [PubMed] [Google Scholar]
- Simpson, F., Peden, A.A., Christopoulou, L., and Robinson, M.S. (1997). Characterization of the adaptor-related protein complex, AP-3. J. Cell Biol. 137, 835-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess, M. (1990). The asialoglycoprotein receptor - a model for endocytic transport receptors. Biochemistry 29, 10009-10018. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., Vitale, G., Ullrich, O., and Zerial, M. (1995). Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell 83, 423-432. [DOI] [PubMed] [Google Scholar]
- Stoorvogel, W., Oorschot, V., and Geuze, H.J. (1996). A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 132, 21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, H., et al. (2002). Differential recognition of tyrosine-based basolateral signals by AP-1B subunit mu1B in polarized epithelial cells. Mol. Biol. Cell 13, 2374-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge, I.S., Collawn, J.F., and Hopkins, C.R. (1993). Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 9, 129-161. [DOI] [PubMed] [Google Scholar]
- van Dam, E.M., and Stoorvogel, W. (2002). Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 13, 169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam, E.M., Ten Broeke, T., Jansen, K., Spijkers, P., and Stoorvogel, W. (2002). Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J. Biol. Chem. 277, 48876-48883. [DOI] [PubMed] [Google Scholar]
- van der Bliek, A.M. (1999). Functional diversity in the dynamin family. Trends Cell Biol. 9, 96-102. [DOI] [PubMed] [Google Scholar]
- van der Sluijs, P., Hull, M., Webster, P., Male, P., Goud, B., and Mellman, I. (1992). The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70, 729-740. [DOI] [PubMed] [Google Scholar]
- Vitale, G., Rybin, V., Christoforidis, S., Thornqvist, P., McCaffrey, M., Stenmark, H., and Zerial, M. (1998). Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 17, 1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettey, F.R., Hawkins, S.F., Stewart, A., Luzio, J.P., Howard, J.C., and Jackson, A.P. (2002). Controlled elimination of clathrin heavy-chain expression in DT40 lymphocytes. Science 297, 1521-1525. [DOI] [PubMed] [Google Scholar]
- Wilcke, M., Johannes, L., Galli, T., Mayau, V., Goud, B., and Salamero, J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 151, 1207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2, 107-117. [DOI] [PubMed] [Google Scholar]