Abstract
Astrocytes intimately interact with synapses, both morphologically and, as evidenced in the past 20 years, at the functional level. Ultrathin astrocytic processes contact and sometimes enwrap the synaptic elements, sense synaptic transmission and shape or alter the synaptic signal by releasing signalling molecules. Yet, the consequences of such interactions in terms of information processing in the brain remain very elusive. This is largely due to two major constraints: (i) the exquisitely complex, dynamic and ultrathin nature of distal astrocytic processes that renders their investigation highly challenging and (ii) our lack of understanding of how information is encoded by local and global fluctuations of intracellular calcium concentrations in astrocytes. Here, we will review the existing anatomical and functional evidence of local interactions between astrocytes and synapses, and how it underlies a role for astrocytes in the computation of synaptic information.
This article is part of the themed issue ‘Integrating Hebbian and homeostatic plasticity’.
Keywords: gliotransmission, neuron–glia interactions, synaptic homeostasis, synaptic islands, tripartite synapse, sleep
1. Introduction
Astrocytes display a very complex morphology, which is simultaneously their most recognizable hallmark and their most challenging feature. Typically, micron-wide primary and secondary proximal processes (containing the astrocyte marker glial fibrillary acidic protein (GFAP)) emerge from the soma and gradually give rise to fine sub-micron and extremely complex processes. Thus, while they appear intensely branched near the soma, astrocytes actually form a sponge-like network of ultrathin and connected filaments intermingled with neuronal elements, with anatomical organization that lies far beyond the optical diffraction limit. Such an intricate and fine anatomy makes them virtually inseparable from the smallest neuronal compartment and computational unit of the nervous system: the synapse. Indeed, the fine astrocytic protrusions extend to and often enwrap excitatory synapses, as if these peri-synaptic processes were the endpoint specialization of the astrocyte's intense morphological differentiation. Therefore, it is widely accepted that one of the many functions of astrocytes is to interact with synapses. This idea emerged very early in the study of astrocytes, following their first morphological characterization by Rudolph Virchow and Santiago Ramon y Cajal. But surprisingly, little is understood regarding how and why astrocytes are required for proper synaptic function—besides the existence of a thriving and dynamic ‘glial field’ that has accumulated a great wealth of evidence on the functional impact of astrocytes on synaptic properties.
Here, we will provide an overview of the existing anatomical and functional evidence of local interactions between astrocytes and synapses, and how it underlies a role for astrocytes in the computation of synaptic information.
2. The local astrocytic control of dendrites and synapses: structural and functional aspects of synaptic islands
The participation of thin astrocytic protrusions in the architecture and the function of synapses was coined the ‘tripartite synapse’ by our laboratory, more than 15 years ago [1]. This concept positions the peri-synaptic astrocytic processes as a structural, architectural and functional partner of the synapse, and postulates that the synapse can no longer be considered as only engaging two neuronal elements isolated from the rest of the parenchyma.
(a). Morphological interaction of astrocytic processes with synapses
Based on electron microscopy studies employing three-dimensional reconstruction techniques, it appears that only a fraction of synapses are in immediate contact with astrocytic processes at a given time. The numbers vary greatly, but it is estimated that, in rodents, 30–60% of synapses are enwrapped by astrocytes in the neocortex [2,3], while 60–90% of spines are in contact with an astrocytic process in the hippocampus [4,5] and as high as 90% in the somatosensory cortex layer IV [2]. These contacts most commonly occur with the post-synaptic element (i.e. the spine) with the exception of the area CA3 of the hippocampus where synapses are entirely engulfed by extensive astrocytic ensheathment that prevents spillover and spatially isolates individual synapses from each other and from the extrasynaptic space [6]. Importantly, these numbers are unlikely to reflect a steady-state situation and probably only represent a snapshot, at a given time, of a very dynamic system. Indeed, many studies performed in brain slices and in vivo have highlighted the very dynamic nature of astrocytic processes that engage and disengage from synapses spontaneously or in response to physiological (and pathological) stimuli [2,7–9]. Unfortunately, it has proven remarkably challenging to image the ultrathin astrocytic processes because their small size falls beyond the diffraction limit of conventional microscopy. The large GFAP-positive primary branches only account for 10–20% of the total volume of an astrocyte arborization [10]. Therefore, 80–90% of the astrocytic volume and morphology remains beyond the diffraction limit of conventional microscopy and only the inventive use of state-of-the-art techniques by remarkably innovative and skilled experts has recently allowed this major limitation to be partially circumvented [11,12].
(b). Peri-synaptic processes are equipped with the molecular machinery to support synaptic function
Electron microscopy revealed that astrocytic protrusions, including those that contact synapses, express a set of proteins that are involved in synaptic transmission [13]. Among them are glutamate transporters (GLT1) [14], glutamine synthetase [15], aquaporins [16], potassium channels [17], cell adhesion molecules (ephrin) [18] and lactate transporters [19]. These molecules play well-characterized roles in (i) mediating cell-to-cell adhesion and initiating key intracellular pathways following cell-to-cell recognition, (ii) resupplying the pre-synaptic element with the glutamate and GABA precursor glutamine, (iii) providing energy substrates to support synaptic transmission and plasticity, (iv) buffering extracellular potassium resulting from action potential firing and neurotransmitter-gated ion channel opening and (v) ensuring an efficient uptake of glutamate following synaptic transmission, thus limiting heterosynaptic communication as well as excitotoxicity and shaping the synaptic response itself (decay time and desensitization of AMPA receptor-mediated responses). Interestingly, it was shown recently, in cultures, that GLT1 are mobile at the surface of astrocytes and drawn to sources of glutamate [20], suggesting that the molecular machinery of astrocytic processes is not static, but, much like the processes themselves, has the propensity to spatially reorganize and dynamically respond to changes in the immediate environment. Though they intrinsically shape synaptic activity in a dramatic manner [21], the functions listed above are well-established classical roles played by astrocytes at synapses that fit with the dogmatic view of astrocytes as supportive cells.
(c). Peri-synaptic processes and gliotransmission
A revolution in our understanding of astrocyte function has occurred during the past 20 years based on the demonstrations that astrocytic processes also possess signalling machinery, including GABA, glutamate and endocannabinoid receptors, synaptic-like micro-vesicles (SLMVs), SNARE proteins mediating regulated vesicular fusion and transmitters (termed gliotransmitters) such as glutamate, GABA, adenosine/ATP and d-serine. It is thus accepted that by directly sensing neuronal activity (or consequences of neuronal activity), astrocytes engage in synaptic transmission by releasing transmitters that act directly on pre- or post-synaptic neuronal receptors and impact synaptic efficacy, potency or plasticity. Examples include (and are not limited to) the release of ATP, which is rapidly degraded into adenosine by extracellular ectonucleotidases, that acts on pre-synaptic neuronal A1R to inhibit pre-synaptic release [22] or post-synaptic A2R receptors to potentiate synaptic strength [23]; the release of the NMDAR (N-methyl-d-aspartate receptor) co-agonist d-serine that, by modulating the activity of the receptor, determines the rules of synaptic plasticity [6,24]; and the release of glutamate that usually acts on pre-synaptic metabotropic glutamate receptors of various flavours [25] or pre-synaptic NMDARs to modulate synaptic efficacy [26,27].
The modes of release of these transmitters can vary and are often subject to debate. For instance, while SNARE-dependent and VAMP2-mediated vesicular release is the prevailing view of d-serine supply [28], others have suggested that d-serine can be released via transporters (Asc-1) [29] or hemichannels [30]. Similarly, glutamate can be found in SLMVs [31] but can also be released by astrocytes through the Bestrophin1 channel (Best1) [32].
The signalling stimuli reported to elicit the release of each of these gliotransmitters are also inconsistent in the literature and vary among brain regions, age and models, and often among studies. d-serine, for example, was initially reported to be released following stimulation of mGluR by glutamate in cultured astrocytes [33]. However, evidence obtained in brain slices and in vivo suggests that the stimulation of cholinergic muscarinic receptors elicits d-serine release in the somatosensory cortex [34], while noradrenergic receptors could be involved in other regions of the cortex [35,36], and TRPA1 channels could be implicated in the hippocampus [37]. Most of these studies, however, have yet to be replicated, making it difficult to determine whether one of these pathways is the predominant route initiating d-serine release. Very similar situations prevail for the other gliotransmitters and could suggest that there is no such thing as a unique and ubiquitous release signal specific to each gliotransmitter but instead there are diverse spatial and temporal determinants of gliotransmission pathways.
Of interest, transporters expressed by astrocytic processes also play a key role in shaping the action of gliotransmitters. For instance, GLT1 help compartmentalize the synaptic space by delineating the impact of glutamate, and glycine transporters (GlyT1) define the domain of influence of astrocyte-derived d-serine [24,38] by decreasing competition with glycine for binding to the co-agonist site of the NMDAR within the synaptic space. Therefore, an emerging view is that astrocytes contribute to the functional delineation of synaptic and extrasynaptic volumes [38].
(d). Calcium activity in astrocytes
Astrocytes are not electrically excitable, but they display both stimulus-induced and spontaneous (seemingly occurring in the absence of stimulation or neuronal activity) intracellular calcium signals [39]. Calcium is a ubiquitous second messenger for many signalling pathways including those downstream of metabotropic glutamate receptor class I (mGluRI) and purinergic receptors [40]. When activated, these pathways mobilize calcium from internal stores [41]. Transient increases in intracellular calcium can activate many transcription factors and downstream enzymes including PKC, NF-κB, CaMKII and CREB [42]. However, there has been some confusion and controversy in the field as to the functional role of these calcium signals, in particular in gliotransmission. It was generally assumed that the calcium-dependent release of gliotransmitters by astrocytes relies on inositol triphosphate type 2 receptors (IP3R2) and calcium-induced calcium release from internal stores. A well-accepted corollary had thus been that the stimulation of G-protein coupled receptors (GPCRs), recruiting such internal stores, is required for gliotransmission to take place and this was supported by evidence for relatively fast and confined calcium transients in astrocytes that were abolished by IP3R2 deletion [43,44]. However, this view was significantly challenged by observations that (i) deleting IP3R2 in astrocytes or stimulating transgenic GPCRs ectopically expressed on astrocytes was without effect on synaptic transmission and plasticity [45], (ii) calcium stores are absent from the peri-synaptic astrocytic processes and are roughly 500 nm away from the post-synaptic density [46] and (iii) the expression of mGluR5 by astrocytes, thought to be the main route for GPCR-dependent calcium elevations in these cells, is undetectable after the third postnatal week [40]. Along with some uncertainties surrounding the relevance of astrocytic calcium activities observed in in vitro models [40,47], this fuelled a wave of scepticism for the very process of gliotransmission and the validity of the tripartite synapse model was called into question. Since then, the development and use of genetically encoded calcium indicators has enabled faster and more accurate reporting of calcium transients which, coupled with two-photon microscopy imaging of the astrocyte's peripheral processes, has led to the observation that the majority of astrocytic calcium signals, both in vitro and in vivo, occur in previously unmonitored peripheral thin processes, rather than in their soma, and do not require the mobilization of internal calcium stores [35]. Indeed such calcium transients are preserved upon deletion of IP3R2 and partially depend on transmembrane calcium fluxes, probably elicited by surface ionotropic receptors. Similarly, using a two-photon excitation time-resolved microscopy imaging technique (FLIM), Dmitri Rusakov's group has determined that the resting calcium concentration in astrocytic processes is not homogeneous, but highly compartmentalized and spatially regulated [11,12], shedding light on a previously unsuspected and potentially fundamental aspect of calcium encoding in these cells.
Astrocyte calcium activity in slice or in culture models can now be routinely monitored with two-photon fluorescence microscopy. In vivo, however, this investigation is limited to the superficial layers of the rodent cortex (usually layers I and II), because two-photon microscopy only allows imaging 100–200 µm deep below the pial surface of the brain. Our knowledge and exploration of astrocytic calcium activity in vivo thus remains scarce. Yet, such recordings have evidenced calcium signalling in cortical astrocytes of awake and behaving mice that is similar in amplitude, frequency and duration to that observed in vitro [35], suggesting that the basic features of calcium signalling that have been characterized in vitro hold true in vivo. In addition, like in vitro, this activity was completely unaltered in fine astrocytic processes upon IP3R2 deletion [35]. Interestingly, however, some studies (see [48] and [34]) suggest that, unlike in slices, cortical astrocytes mainly respond to sensory-evoked neuromodulator release (such as norepinephrine) rather than to neurotransmitter release (such as glutamate).
Altogether, these observations have revitalized the field by providing evidence that very local and highly dynamic calcium signals are encoded in astrocytic peripheral processes, and are governed by yet unknown mechanisms. Though these advances have sparked a new interest in the understanding of astrocytic calcium signalling, we are still wrestling with understanding how external stimulations are encoded through calcium activity and how such calcium activity later recruits specific gliotransmission pathways, especially in vivo.
(e). Synaptic islands
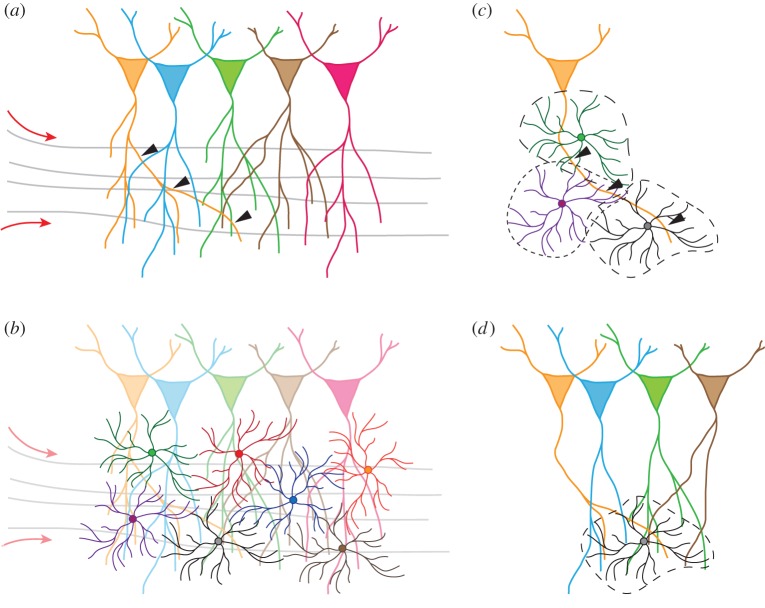
In rodents, astrocytes occupy adjacent but non-overlapping domains of the parenchyma [10,49,50], such that every segment of dendrite within the territory of a given astrocyte, and every individual synapse on this segment, can only be contacted by processes arising from this single astrocyte. The functional consequence of this structural property is that an individual astrocyte handles a defined volume of neuropil and is the sole astrocyte responsible for surveying and influencing neuronal elements within this domain. The volume of this domain varies among brain regions and species, but in rodents it spans from 20 000 to 80 000 µm3 and contains on average 300–600 dendritic segments [10,49–51]. With one to two synapses per µm3 of parenchyma on average [52], the territory of a single astrocyte therefore potentially oversees 20 000–160 000 individual synapses in the rodent brain, over which it exerts an executive and privileged control (figure 1). Regrettably, while the morphological characterization of rodent astrocytes has become extensively detailed, the description of human astrocytes remains very incomplete owing to the very limited amount of data available. Human astrocytes appear much larger and far more complex, ramified and pleomorphic than rodent astrocytes [53,54], which is assumed to be critical to the unique processing capabilities of the human brain. They are also capable of extending very long processes that span nearly 1 mm, a unique and intriguing feature that is not observed in rodent astrocytes and whose function remains mysterious. Yet, besides those major differences, human astrocytes, like rodent astrocytes, are organized in domains with limited overlap, and it is estimated that a single human astrocyte domain contains approximately 270 000 to 2 million synapses [13,53].
Figure 1.
(a) Layout of neuron cell bodies, dendritic network and axonal fibres (grey) transmitting action potentials (red arrows). One dendrite of interest is designated by arrowheads (see below). (b) The mosaic of astrocytic domains is superimposed to the background neuronal network. (c) Different contiguous segments of the same dendrite (arrowheads in A) intersect distinct astrocytic domains. Synapses are controlled independently within each of these domains (synaptic islands). (d) One astrocyte covers portions of different dendrites, belonging to four distinct neurons, surveying the activity of a total 20 000–160 000 synapses.
These observations have led to the proposal that ‘micro-volumes of the nervous system occupied by individual astrocytes have the potential to act as nodes of synaptic modulation and coordination’, a concept we coined ‘synaptic island’ [50, p. 6477]. Indeed, there is abundant evidence that calcium signalling in astrocytes is very compartmentalized and is mostly restricted within individual cells [35,43,44], such that calcium activity does not usually propagate to neighbouring astrocytes through gap junctions. Similarly, second-messenger signalling within an astrocyte (such as IP3-mediated signalling) is a compartmentalized process. Therefore, an individual astrocyte has the potential to affect the function of all (or subsets of) synapses located within its domain, through the mechanisms described above, without disturbing synapses that lie within the domain of the neighbouring astrocytes. As a consequence, a dendrite passing through the domain of two distinct astrocytes will be functionally divided in two contiguous segments governed independently from one another, as far as synapses are concerned. This concept introduces an extra layer of complexity in our understanding of brain computation by superimposing a mosaic of independent (though likely cooperating) astrocyte domains controlling separate volumes of neuropil, on top of the neuronal layout, polarity and connectivity (figure 1). The functional demonstration of such a concept has been obtained by several groups. For instance, Henneberger et al. showed that preventing d-serine synthesis/release in one astrocyte prevented long-term potentiation (LTP) at synapses only located within the domain of this astrocyte while LTP was intact at synapses located in the domain of a neighbouring control-astrocyte [28]. Similarly, taking advantage of the mosaic expression of transgenes by astrocytes in dnSNARE and iBot-Glast-CreERT2 mice, Sultan et al. showed that the spine density of adult-born hippocampal neurons was reduced exclusively on dendritic segments passing through the domain of astrocytes with inhibited release of gliotransmitters [55]. Therefore, both the developmental spine maturation and the function of mature synapses are controlled by astrocytes in a ‘synaptic island’-restricted manner.
As dendrites are lengthy and cross the domain of tens to hundreds of different astrocytes, they gather and integrate various synaptic inputs that are shaped by independent astroglial cells. Consequently, dendritic and somatic integration of synaptic inputs not only represents the computation of multiple pre-synaptic neurons, but also that of the multiple astrocytes that are laid out on the dendritic path. Perturbing the function of astrocytes can thus potentially have an enormous outcome on neuronal computation. Such contribution of astrocytes to information computation in the brain is largely undetermined and would benefit from deeper investigation.
3. Control of synapses by astrocytes in the context of brain states
While neurons are exquisitely equipped to receive and transmit large amounts of information in the millisecond time scale, half of the mammalian brain is made up of astroglia that process information over a much slower time scale [56]. In particular, the speed of astrocyte activity seems unfitted to the millisecond-operated flow of synaptic information, but is highly adequate in the context of brain states. This has led to the idea that astrocytic signalling at synapses could be predominantly involved in processes operating over slower and more long-lasting time scales such as sleep homeostasis.
(a). Sleep homeostasis and astrocyte-derived adenosine
Sleep homeostasis is the mechanism by which a drive for sleep (also termed ‘sleep pressure’) is accumulated during wakefulness as a function of the time elapsed since the last sleep episode. The actual mechanism is incompletely understood, but it is known to originate in the accumulation of sleep-promoting substances in the brain during wakefulness, which increases the pressure to sleep. Sleep pressure is then relieved over periods of sleep during which the levels of sleep-promoting substances rapidly decline. Adenosine is a well-known sleep-promoting signal that drives the homeostatic sleep response, i.e. the increased need for sleep after prolonged periods of wakefulness. It accumulates in the extracellular space during periods of wakefulness/activity or during prolonged forced wakefulness (sleep deprivation) and declines during sleep, thus oscillating throughout the natural sleep/wake cycle. Our laboratory has demonstrated the role that astrocytes play in modulating synaptic activity in the context of sleep homeostasis in mice [22,57–59] by establishing the astrocytic origin of adenosine that accumulates during wakefulness. Indeed, transgenic dnSNARE mice, in which the SNARE-mediated vesicular release of gliotransmitters is inhibited, show deficits in sleep homeostasis. We demonstrated that this effect, recapitulated by the genetic or pharmacological inhibition of the pre-synaptic adenosine A1 receptor (A1R), is due to the reduced availability of adenosine, and thereafter lack of A1R activation, in these mice. While adenosine levels normally rise during wakefulness, they remain unchanged and relatively low in dnSNARE mice throughout the 24 h period, indicating that wakefulness-derived adenosine is of astrocytic origin. Such a lack of adenosine accumulation in dnSNARE mice prevents the wakefulness-induced downscaling of synaptic strength through pre-synaptic A1Rs as well as the modulation of cortical network activity associated with high wakefulness-induced adenosine levels [22,57–59]. Thus, astrocyte-derived adenosine is directly responsible for shaping synaptic activity and neuronal network oscillations across the sleep–wake cycle in rodents. Of importance, we showed that this mechanism is also involved, in mice, in mediating the effects of sleep loss on memory [58,60], a phenomenon that operates over a time scale of hours to days. Similarly, other groups have shown that astrocyte-derived adenosine also mediates the hypnogenic effect of glucose in mice hypothalamus [61].
(b). Synaptic scaling and synaptic plasticity
Another key role of astrocytes, also operating at a slow time scale, is synaptic scaling, which consists of uniform adjustments in the strength of all synapses on a neuron in response to prolonged changes in the cell's electrical activity. This can be achieved in different ways and one of them includes the modulation of synaptic strength via removal or insertion of post-synaptic AMPA receptors. In the hippocampus, this mechanism is independent from the classical LTP/LTD (long-term depression) pathway, and is driven by the release of tumour necrosis factor-alpha (TNF-α) by glia [62–64], presumably in response to decreased levels of ambient glutamate sensed over prolonged periods of time. However, the exact mechanism and molecular actors involved in synaptic scaling are still unknown and debated. In particular, it remains unclear and controversial whether TNF-α is the phasic ‘activity signal’, released upon decreased neuronal activity, that drives synaptic scale-up, or whether it is a ‘permissive signal’ whose presence at tonic levels is simply required to maintain synapses in a plastic state [65]. According to this last view, synaptic scaling, while requiring TNF-α, would be instructed by other signals [65] such as post-synaptic firing [66]. Interestingly, and regardless of the exact signalling role played by TNF-α, a similar and complementary process has been described by the group of Andrea Volterra that involves a pre-synaptic, instead of post-synaptic, regulation of synaptic strength. In this mechanism, TNF-α acts on astrocytes, instead of neurons, to elicit astrocytic vesicular release of glutamate onto pre-synaptic NMDARs, which durably increases pre-synaptic release probability [26,27,67].
An alternative way one could imagine achieving synaptic scaling is by tuning the energy supply to synapses. Astrocytes are responsible for metabolizing glucose in the brain, which they convert into lactate that is then shuttled to neurons as an energy substrate [68]. Interestingly, it has been demonstrated that glucose availability in the brain fluctuates across a 24 h period, with the lowest levels found early during wake phases and increasing amounts found during non-REM (non-rapid eye movement) slow wave sleep [69]. This could shape neuronal and synaptic activity over the sleep/wake cycle by modulating multiple astrocytic functions including the supply of lactate to neurons, as recently suggested by Petit et al. [70]. Alternatively, in the hypothalamus, increases in local glucose availability directly trigger the release of adenosine by astrocytes, which in turn, causes neuronal depolarization and a dilation of local capillaries via A2ARs [61]. Such evidence strongly suggests that glucose (and variations in glucose availability) has signalling properties by itself that can strongly impact astroglial tuning of synaptic and neuronal function.
Similarly, as astrocytes' ability to impact synaptic function relies heavily on their physical interaction with synapses, any change in the degree or incidence of synaptic coverage by astrocytes would certainly modify basal synaptic activity. Interestingly, Bellesi et al. showed that the astrocytic processes retract from synapses during sleep [8]. The consequences of such a reduction in astrocytic coverage of synapses have not been explored, but could include a deceased neurotransmitter re-uptake [21], reduced gliotransmitter supply [9], changes in pre-synaptic control of release probability (through pre-synaptic mGluRs or A1Rs) or increased heterosynaptic communication. The rules that govern synaptic plasticity (BCM model) would also likely be impacted, with a higher spillover of glutamate and a greater recruitment of extrasynaptic NMDARs [8,24,38,71].
(c). Astrocytes sense neuromodulators
Astrocytes are positioned to sense changes in neuromodulator levels, and accumulating evidence has recently demonstrated that they are indeed capable of doing so, including in vivo [48]. It was demonstrated that astrocytes act as a gate for sensory stimulus-specific plasticity in response to changes in acetylcholine or norepinephrine [34,72,73]. Additionally, norepinephrine shifts the gain of astrocytes to the appropriate behavioural state [74], thus shaping local interactions with neurons in a ‘context’-dependent manner. Similarly, the group of Hirase recently showed that astrocytes are involved in mediating transcranial direct current stimulation (tDCS)-induced plasticity by sensing and responding to tDCS-elicited norepinephrine release [36].
Interestingly, neuromodulator signalling often relies on volume transmission that sets a long-range and long-lasting tone of neuromodulator, affecting many targets over prolonged periods of time [75]. The fact that astrocytes respond to neuromodulators therefore further illustrates how their action on synapses is mostly geared towards slower and more durable tuning of synaptic properties.
4. Concluding remarks
It has become increasingly clear that astrocytes are an active partner in the transmission of synaptic information. Their peri-synaptic processes contact and survey thousands of synapses within their spatial domain, which they can influence through the release of gliotransmitters. How the release of such transmitters is governed by calcium fluctuations is still unclear, but the relatively slow kinetics of such calcium activity makes astrocytes more suited to respond to gross changes in neuronal activity and volume transmission of neuromodulators. While further work is certainly needed to better understand the rules that govern the release of precise sets of gliotransmitters, our appreciation of the role of astrocytes in brain computation has grown unceasingly over the past 20 years.
Competing interests
P.H. is the founder of GliaCure. All other authors declare no competing financial interest related to this work.
Funding
Work in our laboratory is supported by a Human Frontier Science Program long-term fellowship (LT000010/2013) and a grant from the Philippe Foundation Inc. awarded to T.P., two NIH/NINDS R01 grants (no. NS037585 and AA020183) awarded to P. H., an NIH/NIMH F31 grant (no. MH106208) pre-doctoral fellowship awarded to J.D. and an NIH/NINDS T32 training grant to M.T. (5T32NS061764-08).
References
- 1.Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. ( 10.1016/S0166-2236(98)01349-6) [DOI] [PubMed] [Google Scholar]
- 2.Bernardinelli Y, et al. 2014. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 24, 1679–1688. ( 10.1016/j.cub.2014.06.025) [DOI] [PubMed] [Google Scholar]
- 3.Reichenbach A, Derouiche A, Kirchhoff F. 2010. Morphology and dynamics of perisynaptic glia. Brain Res. Rev. 63, 11–25. ( 10.1016/j.brainresrev.2010.02.003) [DOI] [PubMed] [Google Scholar]
- 4.Ventura R, Harris KM. 1999. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19, 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witcher MR, Kirov SA, Harris KM. 2007. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55, 13–23. ( 10.1002/glia.20415) [DOI] [PubMed] [Google Scholar]
- 6.Rollenhagen A, Sätzler K, Rodríguez EP, Jonas P, Frotscher M, Lübke JH. 2007. Structural determinants of transmission at large hippocampal mossy fiber synapses. J. Neurosci. 27, 10 434–10 444. ( 10.1523/JNEUROSCI.1946-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. 2014. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J. Neurosci. 34, 12 738–12 744. ( 10.1523/JNEUROSCI.2401-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellesi M, de Vivo L, Tononi G, Cirelli C. 2015. Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol. 13, 66 ( 10.1186/s12915-015-0176-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. 2006. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784. ( 10.1016/j.cell.2006.02.051) [DOI] [PubMed] [Google Scholar]
- 10.Bushong EA, Martone ME, Jones YZ, Ellisman MH. 2002. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng K, Bard L, Reynolds JP, King C, Jensen TP, Gourine AV, Rusakov DA. 2015. Time-resolved imaging reveals heterogeneous landscapes of nanomolar Ca2+ in neurons and astroglia. Neuron 88, 277–288. ( 10.1016/j.neuron.2015.09.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusakov DA. 2015. Disentangling calcium-driven astrocyte physiology. Nat. Rev. Neurosci. 16, 226–233. ( 10.1038/nrn3878) [DOI] [PubMed] [Google Scholar]
- 13.Heller JP, Rusakov DA. 2015. Morphological plasticity of astroglia: understanding synaptic microenvironment. Glia 63, 2133–2151. ( 10.1002/glia.22821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. 1995. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15, 711–720. ( 10.1016/0896-6273(95)90158-2) [DOI] [PubMed] [Google Scholar]
- 15.Derouiche A, Frotscher M. 1991. Astroglial processes around identified glutamatergic synapses contain glutamine synthetase: evidence for transmitter degradation. Brain Res. 552, 346–350. ( 10.1016/0006-8993(91)90103-3) [DOI] [PubMed] [Google Scholar]
- 16.Thrane AS, et al. 2011. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl Acad. Sci. USA 108, 846–851. ( 10.1073/pnas.1015217108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. 2001. An inwardly rectifying K+ channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am. J. Physiol. Cell Physiol. 281, C922–C931. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang Z, Huang J, Cepero ML, Liebl DJ. 2011. Eph signaling regulates gliotransmitter release. Commun. Integr. Biol. 4, 223–226. ( 10.4161/cib.4.2.14507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puchades M, Sogn CJ, Maehlen J, Bergersen LH, Gundersen V. 2013. Unaltered lactate and glucose transporter levels in the MPTP mouse model of Parkinson's disease. J. Parkinsons Dis. 3, 371–385. [DOI] [PubMed] [Google Scholar]
- 20.Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, Groc L, Oliet SH. 2015. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat. Neurosci. 18, 219–226. ( 10.1038/nn.3901) [DOI] [PubMed] [Google Scholar]
- 21.Oliet SH, Piet R, Poulain DA. 2001. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292, 923–926. ( 10.1126/science.1059162) [DOI] [PubMed] [Google Scholar]
- 22.Schmitt LI, Sims RE, Dale N, Haydon PG. 2012. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J. Neurosci. 32, 4417–4425. ( 10.1523/JNEUROSCI.5689-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. 2005. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat. Neurosci. 8, 1078–1086. ( 10.1038/nn1498) [DOI] [PubMed] [Google Scholar]
- 24.Papouin T, et al. 2012. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646. ( 10.1016/j.cell.2012.06.029) [DOI] [PubMed] [Google Scholar]
- 25.Martín R, Bajo-Grañeras R, Moratalla R, Perea G, Araque A. 2015. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734. ( 10.1126/science.aaa7945) [DOI] [PubMed] [Google Scholar]
- 26.Jourdain P, et al. 2007. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339. ( 10.1038/nn1849) [DOI] [PubMed] [Google Scholar]
- 27.Santello M, Bezzi P, Volterra A. 2011. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001. ( 10.1016/j.neuron.2011.02.003) [DOI] [PubMed] [Google Scholar]
- 28.Henneberger C, Papouin T, Oliet SH, Rusakov DA. 2010. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236. ( 10.1038/nature08673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sason H, et al. 2016. Asc-1 transporter regulation of synaptic activity via the tonic release of d-serine in the forebrain. Cereb. Cortex. ( 10.1093/cercor/bhv350) [DOI] [PubMed] [Google Scholar]
- 30.Pan HC, Chou YC, Sun SH. 2015. P2X7 R-mediated Ca2+-independent d-serine release via pannexin-1 of the P2X7 R-pannexin-1 complex in astrocytes. Glia 63, 877–893. ( 10.1002/glia.22790) [DOI] [PubMed] [Google Scholar]
- 31.Bergersen LH, et al. 2012. Immunogold detection of L-glutamate and D-serine in small synaptic-like microvesicles in adult hippocampal astrocytes. Cereb. Cortex 22, 1690–1697. ( 10.1093/cercor/bhr254) [DOI] [PubMed] [Google Scholar]
- 32.Han KS, Woo J, Park H, Yoon BJ, Choi S, Lee CJ. 2013. Channel-mediated astrocytic glutamate release via Bestrophin-1 targets synaptic NMDARs. Mol. Brain 6, 4 ( 10.1186/1756-6606-6-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. 2005. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl Acad. Sci. USA 102, 5606–5611. ( 10.1073/pnas.0408483102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. 2011. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 31, 18 155–18 165. ( 10.1523/JNEUROSCI.5289-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS. 2015. Ca2+ signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717. ( 10.1038/nn.4001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monai H, et al. 2016. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat. Commun. 7, 11100 ( 10.1038/ncomms11100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS. 2013. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 33, 10 143–10 153. ( 10.1523/JNEUROSCI.5779-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papouin T, Oliet SH. 2014. Organization, control and function of extrasynaptic NMDA receptors. Phil. Trans. R. Soc. B 369, 20130601 ( 10.1098/rstb.2013.0601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornell-Bell AH, Thomas PG, Caffrey JM. 1992. Ca2+ and filopodial responses to glutamate in cultured astrocytes and neurons. Can. J. Physiol. Pharmacol. 70(Suppl), S206–S218. ( 10.1139/y92-264) [DOI] [PubMed] [Google Scholar]
- 40.Sun W, et al. 2013. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200. ( 10.1126/science.1226740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashioka S, Wang YF, Little JP, Choi HB, Klegeris A, McGeer PL, McLarnon JG. 2014. Purinergic responses of calcium-dependent signaling pathways in cultured adult human astrocytes. BMC Neurosci. 15, 18 ( 10.1186/1471-2202-15-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellström B, Savignac M, Gomez-Villafuertes R, Naranjo JR. 2008. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol. Rev. 88, 421–449. ( 10.1152/physrev.00041.2005) [DOI] [PubMed] [Google Scholar]
- 43.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. 2011. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284. ( 10.1038/nn.2929) [DOI] [PubMed] [Google Scholar]
- 44.Volterra A, Liaudet N, Savtchouk I. 2014. Astrocyte Ca2+ signalling: an unexpected complexity. Nat. Rev. Neurosci. 15, 327–335. ( 10.1038/nrn3725) [DOI] [PubMed] [Google Scholar]
- 45.Agulhon C, Fiacco TA, McCarthy KD. 2010. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254. ( 10.1126/science.1184821) [DOI] [PubMed] [Google Scholar]
- 46.Patrushev I, Gavrilov N, Turlapov V, Semyanov A. 2013. Subcellular location of astrocytic calcium stores favors extrasynaptic neuron-astrocyte communication. Cell Calcium 54, 343–349. ( 10.1016/j.ceca.2013.08.003) [DOI] [PubMed] [Google Scholar]
- 47.Hamilton NB, Attwell D. 2010. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 11, 227–238. ( 10.1038/nrn2803) [DOI] [PubMed] [Google Scholar]
- 48.Ding F, O'Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. 2013. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394. ( 10.1016/j.ceca.2013.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bushong EA, Martone ME, Ellisman MH. 2004. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int. J. Dev. Neurosci. 22, 73–86. ( 10.1016/j.ijdevneu.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 50.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. 2007. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 27, 6473–6477. ( 10.1523/JNEUROSCI.1419-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberheim NA, Goldman SA, Nedergaard M. 2012. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 814, 23–45. ( 10.1007/978-1-61779-452-0_3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rusakov DA, Harrison E, Stewart MG. 1998. Synapses in hippocampus occupy only 1–2% of cell membranes and are spaced less than half-micron apart: a quantitative ultrastructural analysis with discussion of physiological implications. Neuropharmacology 37, 513–521. ( 10.1016/S0028-3908(98)00023-9) [DOI] [PubMed] [Google Scholar]
- 53.Oberheim NA, et al. 2009. Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287. ( 10.1523/JNEUROSCI.4707-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han X, et al. 2013. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353. ( 10.1016/j.stem.2012.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sultan S, et al. 2015. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88, 957–972. ( 10.1016/j.neuron.2015.10.037) [DOI] [PubMed] [Google Scholar]
- 56.Vardjan N, Parpura V, Zorec R. 2016. Loose excitation-secretion coupling in astrocytes. Glia 64, 655–667. ( 10.1002/glia.22920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. 2009. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc. Natl Acad. Sci. USA 106, 15 037–15 042. ( 10.1073/pnas.0906419106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. 2009. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219. ( 10.1016/j.neuron.2008.11.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pascual O, et al. 2005. Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. ( 10.1126/science.1116916) [DOI] [PubMed] [Google Scholar]
- 60.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. 2011. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J. Neurosci. 31, 6956–6962. ( 10.1523/JNEUROSCI.5761-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scharbarg E, Daenens M, Lemaître F, Geoffroy H, Guille-Collignon M, Gallopin T, Rancillac A. 2016. Astrocyte-derived adenosine is central to the hypnogenic effect of glucose. Sci. Rep. 6, 19107 ( 10.1038/srep19107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. 2002. Control of synaptic strength by glial TNF alpha. Science 295, 2282–2285. ( 10.1126/science.1067859) [DOI] [PubMed] [Google Scholar]
- 63.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. 1998. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391, 281–285. ( 10.1038/34651) [DOI] [PubMed] [Google Scholar]
- 64.Stellwagen D, Malenka RC. 2006. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059. ( 10.1038/nature04671) [DOI] [PubMed] [Google Scholar]
- 65.Steinmetz CC, Turrigiano GG. 2010. Tumor necrosis factor-α signaling maintains the ability of cortical synapses to express synaptic scaling. J. Neurosci. 30, 14 685–14 690. ( 10.1523/JNEUROSCI.2210-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turrigiano GG. 2008. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135, 422–435. ( 10.1016/j.cell.2008.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santello M, Volterra A. 2012. TNFα in synaptic function: switching gears. Trends Neurosci. 35, 638–647. ( 10.1016/j.tins.2012.06.001) [DOI] [PubMed] [Google Scholar]
- 68.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. 2010. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 11, 87–99. ( 10.1038/nrn2757) [DOI] [PubMed] [Google Scholar]
- 69.Dash MB, Bellesi M, Tononi G, Cirelli C. 2013. Sleep/wake dependent changes in cortical glucose concentrations. J. Neurochem. 124, 79–89. ( 10.1111/jnc.12063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petit JM, Magistretti PJ. 2016. Regulation of neuron-astrocyte metabolic coupling across the sleep-wake cycle. Neuroscience 323, 135–156. ( 10.1016/j.neuroscience.2015.12.007) [DOI] [PubMed] [Google Scholar]
- 71.Cooper LN, Bear MF. 2012. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat. Rev. Neurosci. 13, 798–810. ( 10.1038/nrn3353) [DOI] [PubMed] [Google Scholar]
- 72.Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. 2012. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc. Natl Acad. Sci. USA 109, E2832–E2841. ( 10.1073/pnas.1206557109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Navarrete M, Perea G, Fernandez de Sevilla D, Gómez-Gonzalo M, Núñez A, Martín ED, Araque A. 2012. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 10, e1001259 ( 10.1371/journal.pbio.1001259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. 2014. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270. ( 10.1016/j.neuron.2014.04.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirase H, Iwai Y, Takata N, Shinohara Y, Mishima T. 2014. Volume transmission signalling via astrocytes. Phil. Trans. R. Soc. B 369, 20130604 ( 10.1098/rstb.2013.0604) [DOI] [PMC free article] [PubMed] [Google Scholar]