Abstract
We compare the circuit and cellular mechanisms for homeostatic plasticity that have been discovered in rodent somatosensory (S1) and visual (V1) cortex. Both areas use similar mechanisms to restore mean firing rate after sensory deprivation. Two time scales of homeostasis are evident, with distinct mechanisms. Slow homeostasis occurs over several days, and is mediated by homeostatic synaptic scaling in excitatory networks and, in some cases, homeostatic adjustment of pyramidal cell intrinsic excitability. Fast homeostasis occurs within less than 1 day, and is mediated by rapid disinhibition, implemented by activity-dependent plasticity in parvalbumin interneuron circuits. These processes interact with Hebbian synaptic plasticity to maintain cortical firing rates during learned adjustments in sensory representations.
This article is part of the themed issue ‘Integrating Hebbian and homeostatic plasticity’.
Keywords: firing rate homeostasis, homeostatic plasticity, somatosensory cortex, whisker, sensory cortex, inhibition
1. Introduction
Experience continually adjusts brain circuits to store information about the sensory world. To function effectively, the brain must balance this ongoing adaptive process with homeostatic mechanisms that maintain firing rates, and perhaps other features of neural activity, within a stable operating range. What are these homeostatic mechanisms, and how do they balance stability with plasticity? Here, we review recent progress on this question, focusing on sensory areas of cortex in rodents. Homeostatic plasticity is clearly evident in visual (V1) and somatosensory (S1) cortex, where it acts to stabilize mean firing rate following experimental manipulation of sensory activity. This process is termed firing rate homeostasis (FRH) [1]. FRH has a dual role: first, it maintains mean firing rate near a constant set point, which may be permissive for appropriate circuit computations. Second, FRH also appears to directly drive some of the classical changes in sensory-evoked spiking and sensory tuning that occur in response to visual or whisker deprivation. Thus, FRH is both a stabilizing process and contributes to adaptive circuit plasticity that stores information about sensory statistics.
Here, we review the evidence for FRH in rodent primary somatosensory (S1) and visual (V1) cortex, and compare the circuit and cellular mechanisms for homeostatic plasticity in each area. Multiple homeostatic mechanisms exist, with strong similarities between S1 and V1. Slow homeostasis is implemented by synaptic scaling in excitatory networks, augmented in some cases by changes in neuronal intrinsic excitability. Rapid homeostasis also exists and is mediated by active plasticity in inhibitory networks. Rapid disinhibition serves a homeostatic role, but also may be a critical gate to enable some features of Hebbian plasticity. Homeostatic mechanisms vary across cortical layers and cell types, perhaps related to variations in baseline firing rate.
What features of circuit and cellular activity are stabilized by FRH are not completely known. The simplest hypothesis is that FRH acts to maintain total mean firing rate (i.e. spontaneous plus sensory-evoked firing) in pyramidal neurons. In this model, FRH would be driven by any sustained change in firing rate, and would adjust spontaneous and sensory-evoked spiking similarly in order to restore the overall firing rate to its set point. This could be implemented by a cell-autonomous mechanism that regulates synaptic strength or intrinsic excitability to maintain a set point of average cytosolic calcium, for example. Alternatively, it is possible that FRH regulates spontaneous and sensory-evoked firing independently, each to their own set point. However, this is more complex and may require that spontaneous and sensory-evoked activity engage different subcircuits. Here, we assume that FRH is driven by, and acts to stabilize, total mean firing rate in each pyramidal cell or the local pyramidal cell network.
2. Firing rate homeostasis mediated by synaptic scaling
FRH in cortical networks was first discovered in cell culture, where pharmacological or genetic manipulation of network activity was found to drive synaptic changes that restore mean firing rate to normal levels [2,3]. The primary mechanism for this effect is homeostatic synaptic scaling of excitatory synapses, in which pyramidal cells globally increase or decrease AMPA receptor-mediated synaptic transmission via GluA2 receptor trafficking, in order to maintain average firing rate or calcium levels near a cell-specific set point [4]. Scaling is a relatively slow process, beginning a few hours after activity manipulation and reaching a plateau over several days. This is in contrast to long-term potentiation (LTP), long-term depression (LTD) or other synapse-specific Hebbian plasticity mechanisms, which occur within minutes of an appropriate induction protocol.
FRH mediated by synaptic scaling is also prominent in vivo, with a similar slow time course. In V1, excitatory synaptic scaling is first detectable in L2/3 pyramidal cells after 2 days of dark rearing or monocular inactivation as a multiplicative increase in miniature excitatory post-synaptic current (mEPSC) amplitude, and is maximal after approximately one week [5–7]. Downscaling of mEPSCs is also detectable during normal development, appearing as a decrease in mEPSC amplitude that occurs as synaptogenesis increases synapse number and mEPSC frequency [5]. The time course of FRH has been quantified in monocular V1 following contralateral eyelid suture (monocular deprivation, MD). Here, the mean firing rate of extracellularly recorded regular spiking (RS) units (putative pyramidal cells) in L2–4 is transiently reduced by MD, with maximal reduction at 2 days, but during continuing MD, mean firing rate returns to baseline levels by 4 days of MD [8] (figure 1a). This restoration coincides with excitatory synaptic scaling in L2/3 pyramidal cells [8]. Remarkably, despite substantial cell-to-cell variability in mean firing rate, FRH causes individual neurons to return to within 15% of their cell-specific baseline firing rate [1]. Thus, V1 neurons show robust cell-specific FRH following visual deprivation. (For additional discussion, see [17].)
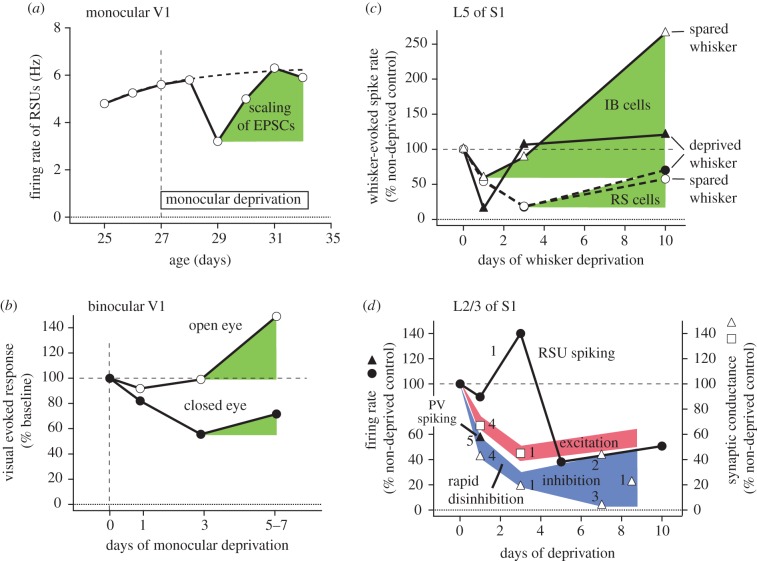
Figure 1.
Forms of firing rate homeostasis in V1 and S1 in vivo. (a) Mean firing rate of regular spiking units (RSU) in L2–L4 of monocular V1, following contralateral monocular deprivation (MD). MD initially reduces firing rates, which then return to normal despite continued deprivation. Dashed curve, schematic of normal firing rate development. Green, FRH attributed to synaptic scaling of mEPSCs. Data from Hengen et al. [8]. (b) Average population response in binocular V1 to open- and closed-eye visual stimuli following contralateral MD. MD rapidly decreases closed-eye visual responses, followed by a slower increase in both open- and closed-eye responses that is attributable to synaptic scaling (green). Points show average data from Frenkel & Bear [9] and Kaneko et al. [10]. (c) Effect of D-row whisker deprivation on whisker-evoked spiking in L5 IB and regular spiking (RS) pyramidal cells. In RS cells, deprivation reduces both deprived and spared whisker responses, followed by partial recovery. IB cells show a similar depression-recovery sequence, with preferential response gain for spared whisker responses. In both cell types, response recovery or potentiation are mediated by synaptic scaling (green). Data from Greenhill et al. [11]. (d) Rapid disinhibition during whisker deprivation in S1. Whisker-evoked spiking in L2/3 pyramidal cells is initially maintained and even transiently increased for 1–3 days following D-row whisker deprivation, prior to subsequent depression (circles). During this 1–3 day period, there is already a substantial weakening of excitatory synaptic drive to L2/3 neurons (red), evident as reduced whisker-evoked EPSCs in L2/3 neurons in vivo and reduced L4-evoked EPSCs in L2/3 pyramidal neurons in S1 slices (squares). Counteracting this loss of excitation is a rapid decrease in inhibition (blue), as reflected by reduced whisker-evoked IPSCs in L2/3 neurons in vivo and reduced L4-evoked and recurrent L2/3-evoked IPSCs in L2/3 pyramidal cells in S1 slices (open triangles). Rapid disinhibition also occurs in V1, as seen by reduced visual-evoked spiking of PV neurons following 1 day of MD (filled triangle). Data from 1: Li et al. [12]; 2: House et al. [13]; 3: Shao et al. [14]; 4: Gainey, SFN abstract [15]; 5: Kuhlman et al. [16].
FRH mediated by synaptic scaling also underlies a prominent slow component of ocular dominance plasticity in V1. In juvenile mice, brief MD (less than 3 days) causes a rapid decrease in closed-eye evoked visual responses in L2/3 and L4 of binocular V1. Longer MD (5–6 days) causes a second, slower effect in which open-eye responses increase [9]. Careful measurement revealed that this late increase occurs in parallel for both open-eye and closed-eye responses, suggesting that it may be a global scaling process in response to visual deprivation and weakening of closed-eye inputs [18]. The early weakening of closed-eye responses represents LTD and other synapse depression mechanisms driven by residual closed-eye thalamic input, as predicted by Hebbian correlation-based rules [19–22]. The delayed increase of both open- and closed-eye inputs is absent in mice lacking tumour necrosis factor (TNF)-α [10], a cytokine that is necessary for homeostatic synaptic scaling [23] (figure 1b). Thus, open-eye response potentiation and the partial recovery of closed-eye responses likely represent FRH mediated by synaptic scaling [24].
In S1, synaptic scaling similarly occurs with a slow time course following whisker deprivation. Trimming or plucking a subset of whiskers (often the D row of whiskers) in juvenile rats or mice causes neurons in deprived S1 columns to rapidly reduce spiking responses to deprived whiskers, and more slowly increase spiking responses to spared surrounding whiskers [25]. The time course for these depression and potentiation components of plasticity is similar to the two components of ocular dominance plasticity in V1. Whisker receptive field plasticity occurs in both L2/3 and L5. In L5, the depression and potentiation components evoked by D-row whisker deprivation are largely segregated between RS and intrinsically bursting (IB) pyramidal cell subtypes, respectively [26]. Brief deprivation (less than 3 days) weakens deprived whisker spiking responses in RS cells, but not in IB cells, which show only a transient weakening that rapidly reverses. With longer deprivation (10 days), both deprived and spared whisker responses increase, with the most prominent potentiation for spared whisker responses in L5 IB cells [11]. This suggests a global homeostatic process such as synaptic scaling, perhaps coupled with additional potentiation of spared responses in IB cells. Consistent with this hypothesis, both the slow potentiation of spared whisker responses and recovery of deprived whisker responses are absent in TNF-α knockout mice, which lack synaptic scaling [11] (figure 1c). Thus, both ocular dominance plasticity in V1, and whisker receptive field plasticity in L5 of S1, involve rapid weakening of deprived inputs mediated, in part, by LTD, and a slower FRH that increases responses to both spared and deprived inputs, and is mediated by synaptic scaling.
In L2/3 of S1, D-row deprivation weakens spiking responses to deprived whiskers, but spared whisker responses do not increase [12,27,28], and scaling of mEPSCs is not observed at the synaptic level even after one week of whisker deprivation [12,29]. Other whisker deprivation paradigms do drive spared whisker response potentiation [25], but whether these are associated with mEPSC scaling is unknown. Why scaling is more prominent in L5 of S1 and L2/3 of V1 than in L2/3 of S1 is unresolved. One possibility is that scaling only occurs in response to a strong reduction in firing rate. Relative to deeper S1 layers and V1, L2/3 of S1 has extremely sparse spiking, with low probability of whisker-evoked firing, low overall firing rate and single-spike whisker responses [30–32]. Trimming the columnar whisker reduces mean firing rate by only 20% [33], which may be insufficient to drive detectable scaling of mEPSCs. (For new results suggesting that scaling may indeed drive slow homeostasis in L2/3 of S1, see [34].)
3. Firing rate homeostasis mediated by rapid disinhibition
As described above, FRH mediated by synaptic scaling is a slow process, unfolding over multiple days of sensory deprivation in vivo [8,10,11]. Recently, a more rapid form of FRH has been discovered in L2/3 of V1 and S1 in vivo that is mediated by disinhibition within cortical networks, and which occurs rapidly following sensory deprivation. Rapid homeostasis is predicted theoretically as an efficient means to prevent positive feedback instability associated with Hebbian plasticity during information storage in cortical networks [35]. In V1, 1 day of MD reduces visually evoked spiking of L2/3 parvalbumin (PV) interneurons, measured in awake behaving mice [16]. This is mediated by a rapid, deprivation-induced reduction in L4 and L5a excitatory synaptic strength onto L2/3 PV neurons [16]. Similarly, visual deprivation rapidly reduces the spontaneous firing rate of L2–4 fast-spiking (presumed PV) interneurons in V1 of awake, freely moving rats [8] and reduces both average spontaneous activity of L2/3 GABAergic interneurons and evoked and spontaneous inhibitory currents in L2/3 pyramidal cells [36]. These changes are associated with an increase in L2/3 pyramidal neuron spiking above control levels [16,36] and an increase in the ratio of L2/3-evoked excitation versus inhibition in pyramidal neurons [36]. These findings strongly suggest that reduced activity in PV circuits, and potentially in other interneuron classes, disinhibits the surrounding excitatory pyramidal cell network, leading to increased pyramidal cell firing rates. Because this firing rate increase in pyramidal cells would compensate for the loss of sensory drive, disinhibition implements FRH at the network level.
Rapid disinhibition is also prominent in L2/3 of S1 in response to deprivation of the D row of whiskers. Brief D-row deprivation in rats does not change (1 day) or slightly increases (3 days) mean single-unit spiking responses to the deprived whiskers, while longer deprivation (5+ days) markedly weakens deprived whisker responses [12] (figure 1d, black line). This weakening process involves LTD [29,37,38]. As mentioned above, D-row deprivation does not potentiate spared whisker responses in L2/3, and there is no evidence for scaling of mEPSCs in L2/3 pyramidal cells even after a week of deprivation [12,29]. Instead, the early 1–3 days period represents rapid FRH mediated by disinhibition. At 3 days of deprivation, whisker-evoked field potentials in L2/3 are already reduced, suggesting that LTD has already occurred and has reduced synaptic drive to L2/3 [12,38]. Consistent with this idea, whisker-evoked EPSCs measured in whole-cell recordings from L2/3 pyramidal cells in vivo are already substantially weakened at 3 days of deprivation [12]. This suggests that a rapid homeostatic mechanism exists in L2/3 to maintain, and transiently elevate, whisker-evoked spiking despite the rapid weakening of excitatory synaptic input by LTD. Rapid disinhibition is this mechanism. At 3 days of deprivation, whisker-evoked inhibitory PSCs (IPSCs) in L2/3 pyramidal cells, measured by whole-cell recording in vivo, are strongly decreased, and whisker-evoked excitation/inhibition ratio is increased [12]. Disinhibition occurs very rapidly, with feed-forward L4–L2/3 inhibition being strongly reduced in just 1 day of deprivation in mouse S1 [15]. Disinhibition persists with longer whisker deprivation [12], mediated by reduced L4 synaptic excitation to PV interneurons [13] and reduced L2/3 recurrent inhibition [14]. Thus, whisker deprivation drives rapid disinhibition in L2/3 of S1, which is mediated by plasticity in PV circuits, and which implements a rapid form of FRH in the surrounding pyramidal cell network (figure 1d). This appears to be the same process that occurs with visual deprivation in V1. A rapid, inhibitory circuit-mediated FRH also occurs in L4 of S1, where overstimulation of a whisker drives increased inhibition and inhibitory synaptogenesis to restore whisker-evoked firing rate [39]. Inhibitory homeostasis may also occur in deeper layers, but this has not been examined.
Disinhibition was originally proposed as a mechanism for the immediate unmasking of novel sensory responses in S1 following digit amputation or inactivation [40]. The current findings differ in that they do not simply reflect an acute loss of sensory drive to inhibitory circuits, but involve experience-dependent, adaptive plasticity of PV circuits that further reduces spiking excitability of these networks within 24 h of deprivation [15,16]. Decreased PV network activity in awake behaving animals presumably reflects a combination of both acute and plastic effects.
Current data implicate PV neurons in rapid disinhibition. Interestingly, PV neurons are uniquely positioned to effectively drive network-level homeostasis, because a single PV neuron receives input from a high proportion of local pyramidal cells, and densely inhibits nearly all of these neurons [41,42]. PV neurons could therefore, in principle, sense average local network activity, and via a cell-autonomous plasticity process, homeostatically regulate a sizeable chunk of a cortical column. PV circuits are well known to modulate sensory gain in cortical networks [43,44]. In this sense, PV-mediated circuit homeostasis would represent an adjustment of sensory gain in response to recent history of activity. Thus, adaptive changes within a small number of PV neurons could rapidly modulate sensory gain and implement FRH in an entire cortical column. This column-level homeostasis would be predicted if PV cells displayed similar sensory tuning as surrounding pyramidal cells. Another interesting functional outcome is predicted if PV cells had broader sensory tuning than their pyramidal cell targets, as may be the case in some systems [45]. In this case, cross-channel compensatory plasticity is predicted in which reduced sensory input on one channel (e.g. one whisker or one visual orientation) drives rapid disinhibition that affects nearby channels. This would elevate sensory gain for surrounding non-deprived columns, which may provide effective behavioural compensation. Whether other interneuron classes besides PV cells are also involved in network homeostasis seems likely, but has not been examined.
In addition to restoring network firing rate, rapid disinhibition may also potentially serve as a gate for subsequent Hebbian plasticity in the excitatory network. Both LTP and LTD are critically dependent on dendritic depolarization in pyramidal cells, and GABA-A inhibition powerfully suppresses both these forms of plasticity [46–48]. An early model of the V1 critical period posited that maturation of inhibition ends the critical period by suppressing LTP and LTD [49–51]. Rapid disinhibition is likely to promote LTP and LTD, thus enabling Hebbian synaptic reorganization in the pyramidal cell network. Consistent with this idea, visual deprivation drives rapid disinhibition in V1 that precedes the loss of visual-evoked spiking in pyramidal cells [8,36,52], and maintaining normal PV spiking levels reduces ocular dominance plasticity [16]. This suggests that disinhibition gates or promotes LTD or other Hebbian depressive mechanisms [53,54]. In S1, the precise timing of excitatory and inhibitory synapse weakening following D-row deprivation has not been determined. However, whisker deprivation is known to promote spike timing-dependent LTP in L2/3 of S1 by weakening inhibition [55], suggesting it may play a similar role.
Rapid disinhibition may be mediated by loss of GABAergic interneuron synapses and axons, which are rapidly plastic in sensory cortex. In V1, 6 h of MD is sufficient to induce a loss of inhibitory spine synapses during the critical period in vivo [52], and several studies report inhibitory synapse loss over 1–2 days after deprivation in adult mice [56–58]. V1 inhibitory synapses show rapid constitutive rates of synapse removal and reformation, which are altered by MD to drive a net loss of inhibition [59]. In S1, whisker deprivation drives retraction of inhibitory axons in deprived whisker columns in as little as 2.5 h [60]. PV (fast-spiking) interneurons also show rapid physiological plasticity of synaptic input and intrinsic excitability [61–63]. PV interneurons remain plastic in adulthood, where this plasticity may regulate some forms of adult learning [64].
4. Intrinsic excitability
Adjustment of intrinsic excitability can also contribute to homeostasis. This was first discovered in response to chronic activity manipulation in cortical cultures [65]. In vivo, visual deprivation can drive a slow, homeostatic increase in intrinsic excitability of pyramidal cells in V1. For example, 6 days of monocular or binocular deprivation increases intrinsic excitability of L2/3 pyramidal neurons in binocular V1, as revealed by increased spiking to somatic current injection (F–I curves). This reflects both higher input resistance and reduced spike threshold [66]. Briefer MD (1–3 days) does not alter intrinsic excitability in binocular V1 [66] but does in monocular V1 [63], perhaps reflecting the fact that binocular V1 is still receiving open-eye input. Thus, homeostatic regulation of pyramidal cell excitability can occur in vivo, although it is a relatively slow process when networks still receive some sensory input. Prolonged deprivation, therefore, drives FRH in L2/3 of binocular V1 through both increased intrinsic excitability and excitatory synaptic scaling [66].
Plasticity of intrinsic excitability also occurs in L5 of S1, where prolonged one to five week whisker deprivation increases burst firing in pyramidal neurons via increased dendritic excitability and downregulation of hyperpolarization-activated cyclic nucleotide-gated channels [67]. In L2/3 of S1, the situation seems different. Two weeks of D-row whisker deprivation does not alter L2/3 pyramidal cell excitability in deprived columns [37]. However, when all but one whisker is plucked, synaptic potentiation is rapidly elicited in the spared column, and this is associated with a modest reduction in L2/3 pyramidal cell intrinsic excitability in the spared column [68]. Thus, in both V1 and L5 of S1, deprivation predominantly drives slow homeostatic changes in pyramidal cell intrinsic excitability.
Intrinsic excitability is also plastic in interneurons, which may contribute to deprivation-induced disinhibition and rapid homeostasis. In motor cortex, 2 days of activity suppression by muscimol reduce intrinsic excitability of fast-spiking, presumed PV, interneurons [69]. In S1, whisker deprivation decreases excitability of L4 fast-spiking cells, measured by F–I curves, but the time course of this effect is not known [70]. This is owing, in part, to regulation of A-type potassium channel currents, which elevates spike threshold [70]. In L2/3, 1 day of D-row whisker deprivation reduces intrinsic excitability of PV neurons, by affecting multiple aspects of near-threshold excitability including spike threshold and first-spike latency [15]. Interestingly, two studies have identified a molecular pathway that could mediate activity-dependent regulation of intrinsic excitability in L2/3 PV cells. In these neurons, the transcription factor Er81 regulates intrinsic excitability by promoting the expression of Kv1.1, which increases spike threshold and increases the latency to first spike after current injection [61,71]. Er81 expression varies across PV cells, with high-expressing cells having more Kv1.1 and elevated spike threshold, and low-expressing cells having a reduced spike threshold and shorter time to first spike [61]. Notably, chandelier cells, a subtype of PV cells with a shorter time to first spike, lack Er81. In acute S1 slices, 2 h of increased network activity (induced by KCl or kainate treatment) reduces Er81 expression and reduces first-spike latency in PV cells, which should promote more PV spiking. Correspondingly, reducing network activity (with nifedipine) increases Er81 expression and PV spike latency, which should reduce overall PV spiking. In vivo, reducing mean network activity by expression of Kir2.1 upregulates Er81 in L2/3 PV neurons, and training mice on a learning task downregulates Er81 [61]. These changes are all appropriate in sign to homeostatically control network spiking by regulating intrinsic excitability of PV neurons. The lack of Er81 in chandelier cells suggests that different subtypes of PV neurons may differentially contribute to network homeostasis. Thus, the Er81–Kv1.1 regulatory pathway is a strong candidate for rapid activity-dependent adjustment of PV intrinsic excitability in order to homeostatically regulate firing rate in local pyramidal cells.
5. Conclusion
FRH occurs by multiple common mechanisms in both S1 and V1, operating on two distinct time scales. Rapid FRH is expressed less than 1 day after sensory deprivation in both areas, and is caused by disinhibition implemented by a reduction in activity of PV interneuron circuits. This reduction is not simply the loss of ongoing sensory drive to PV neurons, but reflects changes in synaptic or intrinsic properties of PV neurons rapidly induced in response to deprivation. The same rapid disinhibition may also gate subsequent Hebbian components of plasticity. A slower FRH process also exists that is mediated by synaptic scaling and, in some cases, homeostatic adjustment of intrinsic excitability, in excitatory neurons. Slow FRH develops gradually over several days, and restores firing rates after early-stage Hebbian plasticity. It is also a major process underlying the slow increase in open-eye or spared-whisker responses that occur following deprivation in V1 and S1. While computational studies have explored the functional consequences of synaptic scaling on network stability and information storage, the consequences of rapid disinhibition remain more poorly understood. We suggest that adaptive regulation of PV inhibition could serve to adjust sensory response gain as a function of recent activity levels, so that networks that were recently inactive respond more strongly to remaining sensory stimuli.
A key feature of homeostasis in both areas is that multiple mechanisms are engaged cooperatively to stabilize cortical firing. Some of these mechanisms may not appear homeostatic if examined in isolation—for example, PV neurons do not stably maintain their own firing rate, but further reduce their firing rate following deprivation, in order to restore firing in the pyramidal cell network. Somehow, this coordinated regulation of multiple neural systems achieves an overall stabilization of cortical firing rate, and potentially other aspects of cortical function. This dynamic, coordinated multi-point control to achieve stability in a complex system has been termed allostasis in organismal biology [72]. How such complex coordination appropriately balances stability and plasticity remains to be discovered.
Authors' contributions
M.G. and D.F. co-wrote the paper.
Competing interests
We have no competing interests.
Funding
This work was supported by NIH R01 NS073912 to D.F. and NIH F32 NS087893 to M.G.
References
- 1.Hengen KB, Torrado Pacheco A, McGregor JN, Van Hooser SD, Turrigiano GG. 2016. Neuronal firing rate homeostasis is inhibited by sleep and promoted by wake. Cell 165, 180–191. ( 10.1016/j.cell.2016.01.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. 1998. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896. ( 10.1038/36103) [DOI] [PubMed] [Google Scholar]
- 3.Burrone J, O'Byrne M, Murthy VN. 2002. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 420, 414–418. ( 10.1038/nature01242) [DOI] [PubMed] [Google Scholar]
- 4.Turrigiano G. 2012. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 4, a005736 ( 10.1101/cshperspect.a005736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. 2002. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat. Neurosci. 5, 783–789. ( 10.1038/nn878) [DOI] [PubMed] [Google Scholar]
- 6.Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. 2006. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat. Neurosci. 9, 1001–1003. ( 10.1038/nn1725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goel A, Lee HK. 2007. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J. Neurosci. 27, 6692–6700. ( 10.1523/JNEUROSCI.5038-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. 2013. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron 80, 335–342. ( 10.1016/j.neuron.2013.08.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frenkel MY, Bear MF. 2004. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron 44, 917–923. ( 10.1016/j.neuron.2004.12.003) [DOI] [PubMed] [Google Scholar]
- 10.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. 2008. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680. ( 10.1016/j.neuron.2008.04.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenhill SD, Ranson A, Fox K. 2015. Hebbian and homeostatic plasticity mechanisms in regular spiking and intrinsic bursting cells of cortical layer 5. Neuron 88, 539–552. ( 10.1016/j.neuron.2015.09.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Gainey MA, Goldbeck JE, Feldman DE. 2014. Rapid homeostasis by disinhibition during whisker map plasticity. Proc. Natl Acad. Sci. USA 111, 1616–1621. ( 10.1073/pnas.1312455111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.House DR, Elstrott J, Koh E, Chung J, Feldman DE. 2011. Parallel regulation of feedforward inhibition and excitation during whisker map plasticity. Neuron 72, 819–831. ( 10.1016/j.neuron.2011.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao YR, Isett BR, Miyashita T, Chung J, Pourzia O, Gasperini RJ, Feldman DE. 2013. Plasticity of recurrent l2/3 inhibition and gamma oscillations by whisker experience. Neuron 80, 210–222. ( 10.1016/j.neuron.2013.07.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gainey MA, Feldman DE. 2016. Rapid disinhibition during whisker map plasticity is mediated by reduced intrinsic excitability of PV interneurons in mouse S1. Soc. Neurosci. Abstract 149.19. [Google Scholar]
- 16.Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. 2013. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501, 543–546. ( 10.1038/nature12485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turrigiano GG. 2017. The dialectic of Hebb and homeostasis. Phil. Trans. R. Soc. B 372, 20160258 ( 10.1098/rstb.2016.0258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. 2007. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron 54, 961–972. ( 10.1016/j.neuron.2007.05.028) [DOI] [PubMed] [Google Scholar]
- 19.Crozier RA, Wang Y, Liu CH, Bear MF. 2007. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc. Natl Acad. Sci. USA 104, 1383–1388. ( 10.1073/pnas.0609596104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. 2003. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat. Neurosci. 6, 854–862. ( 10.1038/nn1100) [DOI] [PubMed] [Google Scholar]
- 21.Smith GB, Heynen AJ, Bear MF. 2009. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Phil. Trans. R. Soc. B 364, 357–367. ( 10.1098/rstb.2008.0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon BJ, Smith GB, Heynen AJ, Neve RL, Bear MF. 2009. Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc. Natl Acad. Sci. USA 106, 9860–9865. ( 10.1073/pnas.0901305106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stellwagen D, Malenka RC. 2006. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059. ( 10.1038/nature04671) [DOI] [PubMed] [Google Scholar]
- 24.Espinosa JS, Stryker MP. 2012. Development and plasticity of the primary visual cortex. Neuron 75, 230–249. ( 10.1016/j.neuron.2012.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glazewski S, Fox K. 1996. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J. Neurophysiol. 75, 1714–1729. [DOI] [PubMed] [Google Scholar]
- 26.Jacob V, Petreanu L, Wright N, Svoboda K, Fox K. 2012. Regular spiking and intrinsic bursting pyramidal cells show orthogonal forms of experience-dependent plasticity in layer V of barrel cortex. Neuron 73, 391–404. ( 10.1016/j.neuron.2011.11.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drew PJ, Feldman DE. 2009. Intrinsic signal imaging of deprivation-induced contraction of whisker representations in rat somatosensory cortex. Cereb. Cortex 19, 331–348. ( 10.1093/cercor/bhn085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foeller E, Celikel T, Feldman DE. 2005. Inhibitory sharpening of receptive fields contributes to whisker map plasticity in rat somatosensory cortex. J. Neurophysiol. 94, 4387–4400. ( 10.1152/jn.00553.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bender KJ, Allen CB, Bender VA, Feldman DE. 2006. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J. Neurosci. 26, 4155–4165. ( 10.1523/JNEUROSCI.0175-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barth AL, Poulet JF. 2012. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 35, 345–355. ( 10.1016/j.tins.2012.03.008) [DOI] [PubMed] [Google Scholar]
- 31.de Kock CP, Bruno RM, Spors H, Sakmann B. 2007. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J. Physiol. 581, 139–154. ( 10.1113/jphysiol.2006.124321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connor DH, Peron SP, Huber D, Svoboda K. 2010. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67, 1048–1061. ( 10.1016/j.neuron.2010.08.026) [DOI] [PubMed] [Google Scholar]
- 33.Celikel T, Szostak VA, Feldman DE. 2004. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat. Neurosci. 7, 534–541. ( 10.1038/nn1222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glazewski S, Greenhill S, Fox K. 2017. Time-course and mechanisms of homeostatic plasticity in layers 2/3 and 5 of the barrel cortex. Phil. Trans. R. Soc. B 372, 20160150 ( 10.1098/rstb.2016.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zenke F, Hennequin G, Gerstner W. 2013. Synaptic plasticity in neural networks needs homeostasis with a fast rate detector. PLoS Comput. Biol. 9, e1003330 ( 10.1371/journal.pcbi.1003330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes SJ, Sammons RP, Jacobsen RI, Mackie J, Keller GB, Keck T. 2015. Subnetwork-specific homeostatic plasticity in mouse visual cortex in vivo. Neuron 86, 1290–1303. ( 10.1016/j.neuron.2015.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen CB, Celikel T, Feldman DE. 2003. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 6, 291–299. ( 10.1038/nn1012) [DOI] [PubMed] [Google Scholar]
- 38.Li L, Bender KJ, Drew PJ, Jadhav SP, Sylwestrak E, Feldman DE. 2009. Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex. Neuron 64, 537–549. ( 10.1016/j.neuron.2009.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knott GW, Quairiaux C, Genoud C, Welker E. 2002. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34, 265–273. ( 10.1016/S0896-6273(02)00663-3) [DOI] [PubMed] [Google Scholar]
- 40.Calford MB, Tweedale R. 1988. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature 332, 446–448. ( 10.1038/332446a0) [DOI] [PubMed] [Google Scholar]
- 41.Fino E, Yuste R. 2011. Dense inhibitory connectivity in neocortex. Neuron 69, 1188–1203. ( 10.1016/j.neuron.2011.02.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Packer AM, Yuste R. 2011. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition?. J. Neurosci. 31, 13 260–13 271. ( 10.1523/JNEUROSCI.3131-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atallah BV, Bruns W, Carandini M, Scanziani M. 2012. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73, 159–170. ( 10.1016/j.neuron.2011.12.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson NR, Runyan CA, Wang FL, Sur M. 2012. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348. ( 10.1038/nature11347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swadlow HA, Gusev AG. 2002. Receptive-field construction in cortical inhibitory interneurons. Nat. Neurosci. 5, 403–404. ( 10.1038/nn847) [DOI] [PubMed] [Google Scholar]
- 46.Artola A, Singer W. 1987. Long-term potentiation and NMDA receptors in rat visual cortex. Nature 330, 649–652. ( 10.1038/330649a0) [DOI] [PubMed] [Google Scholar]
- 47.Tomasulo RA, Ramirez JJ, Steward O. 1993. Synaptic inhibition regulates associative interactions between afferents during the induction of long-term potentiation and depression. Proc. Natl Acad. Sci. USA 90, 11 578–11 582. ( 10.1073/pnas.90.24.11578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner JJ, Alger BE. 1995. GABAergic and developmental influences on homosynaptic LTD and depotentiation in rat hippocampus. J. Neurosci. 15, 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirkwood A, Bear MF. 1994. Hebbian synapses in visual cortex. J. Neurosci. 14, 1634–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirkwood A, Lee HK, Bear MF. 1995. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature 375, 328–331. ( 10.1038/375328a0) [DOI] [PubMed] [Google Scholar]
- 51.Jiang B, Huang ZJ, Morales B, Kirkwood A. 2005. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res. Brain Res. Rev. 50, 126–133. ( 10.1016/j.brainresrev.2005.05.007) [DOI] [PubMed] [Google Scholar]
- 52.Keck T, Scheuss V, Jacobsen RI, Wierenga CJ, Eysel UT, Bonhoeffer T, Hübener M. 2011. Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron 71, 869–882. ( 10.1016/j.neuron.2011.06.034) [DOI] [PubMed] [Google Scholar]
- 53.Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Viegi A, Berardi N, Maffei L. 2010. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J. Neurosci. 30, 361–371. ( 10.1523/JNEUROSCI.2233-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Froemke RC. 2015. Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci. 38, 195–219. ( 10.1146/annurev-neuro-071714-034002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gambino F, Holtmaat A. 2012. Spike-timing-dependent potentiation of sensory surround in the somatosensory cortex is facilitated by deprivation-mediated disinhibition. Neuron 75, 490–502. ( 10.1016/j.neuron.2012.05.020) [DOI] [PubMed] [Google Scholar]
- 56.Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. 2012. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74, 361–373. ( 10.1016/j.neuron.2012.02.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen JL, Nedivi E. 2013. Highly specific structural plasticity of inhibitory circuits in the adult neocortex. Neuroscientist 19, 384–393. ( 10.1177/1073858413479824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, Sommeijer JP, De Zeeuw CI, Hofer SB, Heimel JA, Levelt CN. 2012. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74, 374–383. ( 10.1016/j.neuron.2012.03.015) [DOI] [PubMed] [Google Scholar]
- 59.Villa KL, Berry KP, Subramanian J, Cha JW, Oh WC, Kwon HB, Kubota Y, So PTC, Nedivi E. 2016. Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 89, 756–769. ( 10.1016/j.neuron.2016.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marik SA, Yamahachi H, McManus JN, Szabo G, Gilbert CD. 2010. Axonal dynamics of excitatory and inhibitory neurons in somatosensory cortex. PLoS Biol. 8, e1000395 ( 10.1371/journal.pbio.1000395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dehorter N, Ciceri G, Bartolini G, Lim L, del Pino I, Marin O. 2015. Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science 349, 1216–1220. ( 10.1126/science.aab3415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, Lu B, Sun Q-Q. 2011. A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc. Natl Acad. Sci. USA 108, 12 131–12 136. ( 10.1073/pnas.1105296108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maffei A, Turrigiano GG. 2008. Multiple modes of network homeostasis in visual cortical layer 2/3. J. Neurosci. 28, 4377–4384. ( 10.1523/JNEUROSCI.5298-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donato F, Rompani SB, Caroni P. 2013. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276. ( 10.1038/nature12866) [DOI] [PubMed] [Google Scholar]
- 65.Desai NS, Rutherford LC, Turrigiano GG. 1999. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci. 2, 515–520. ( 10.1038/9165) [DOI] [PubMed] [Google Scholar]
- 66.Lambo ME, Turrigiano GG. 2013. Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J. Neurosci. 33, 8810–8819. ( 10.1523/JNEUROSCI.4502-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Breton JD, Stuart GJ. 2009. Loss of sensory input increases the intrinsic excitability of layer 5 pyramidal neurons in rat barrel cortex. J. Physiol. 587, 5107–5119. ( 10.1113/jphysiol.2009.180943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barth AL, Gerkin RC, Dean KL. 2004. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J. Neurosci. 24, 6466–6475. ( 10.1523/JNEUROSCI.4737-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller MN, Okaty BW, Kato S, Nelson SB. 2011. Activity-dependent changes in the firing properties of neocortical fast-spiking interneurons in the absence of large changes in gene expression. Dev. Neurobiol. 71, 62–70. ( 10.1002/dneu.20811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun QQ. 2009. Experience-dependent intrinsic plasticity in interneurons of barrel cortex layer IV. J. Neurophysiol. 102, 2955–2973. ( 10.1152/jn.00562.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. 2008. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron 58, 387–400. ( 10.1016/j.neuron.2008.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sterling P. 2004. Principles of allostasis: optimal design, predictive regulation, pathophysiology, and rational therapeutics. In Allostasis, homeostasis, and the costs of adaptation (ed. Shulkin J.). Cambridge, UK: Cambridge University Press. [Google Scholar]