Abstract
The proinflammatory cytokine tumour necrosis factor-alpha (TNFα) has long been characterized for its role in the innate immune system, but more recently has been found to have a distinct role in the nervous system that does not overlap with other proinflammatory cytokines. Through regulation of neuronal glutamate and GABA receptor trafficking, TNF mediates a homeostatic form of synaptic plasticity, but plays no direct role in Hebbian forms of plasticity. As yet, there is no evidence to suggest that this adaptive plasticity plays a significant role in normal development, but it does maintain neuronal circuit function in the face of several types of disruption. This includes developmental plasticity in primary sensory cortices, as well as modulating the response to antidepressants, chronic antipsychotics and drugs of abuse. TNF is also a prominent component of the neuroinflammation occurring in most neuropathologies, but the role of TNF-mediated synaptic plasticity in this context remains to be determined. We tested this in a maternal immune activation (MIA) model of neurodevelopmental disorders. Using TNF−/− mice, we observed that TNF is not required for the expression of abnormal social or anxious behaviour in this model. This indicates that TNF does not uniquely contribute to the development of neuronal dysfunction in this model, and suggests that during neuroinflammatory events, compensation between the various proinflammatory cytokines is the norm.
This article is part of the themed issue ‘Integrating Hebbian and homeostatic plasticity’.
Keywords: cytokine, homeostatic plasticity, maternal immune activation, inflammation
1. Introduction
Tumour necrosis factor-alpha (TNFα) is a proinflammatory cytokine and a well-characterized part of the innate immune system. The central nervous system was initially viewed as immune privileged, which would limit the role of cytokine signalling to pathological conditions [1], but it has become clear that cytokines are produced centrally under basal conditions and impact neuronal function [2]. TNFα is produced as a 26 kDa transmembrane proprotein, assembled as a trimer [3], which is then cleaved by TNFα converting enzyme (TACE, otherwise known as ADAM17) to release a 17 kDa soluble fragment [4]. The membrane-bound form can signal directly to TNF receptors [5], particularly the TNFR2 receptor typically found on endothelial and immune cells [6], whereas the ubiquitously expressed TNFR1 can bind both the membrane and soluble forms of TNF [5]. The effects on synaptic function (below) typically occur through neuronal TNFR1 signalling [7,8], though this characterization is incomplete.
(a). Tumour necrosis factor-mediated synaptic plasticity in the brain
TNF was first identified in the regulation of AMPA receptor (AMPAR) trafficking on hippocampal pyramidal cells, where an acute application of exogenous TNF drives the rapid exocytosis of AMPARs [9,10]. In particular, there is an increase in the surface and synaptic content of calcium-permeable GluA2-lacking AMPARs [7,10]. On the other hand, NMDA receptors do not seem to be affected, as judged by synaptic localization [9] or whole cell currents [11]. However, longer-term treatments with TNF have been reported to either increase [12,13] or decrease [14] NMDA currents, suggesting that NMDARs may be indirectly modulated. Subsequent work found that TNF can drive the simultaneous endocytosis of GABA-A receptors (GABARs), resulting in a substantial shift in the balance of excitation-to-inhibition (E/I balance) [7]. These changes are owing to direct activation of TNFR1 on neurons [7,8]. While other proinflammatory cytokines are also able to traffic glutamate receptors [7,15], it is unclear whether other cytokines are constitutively released under basal conditions. However, endogenous TNF regulates receptor trafficking in an activity-dependent fashion [16]. Basal release of TNF outside the context of inflammation has been established in both dissociated neuronal cultures and acute brain slices, including hippocampal, striatal and cortical preparations [9,17,18]. TNF may also regulate pre-synaptic neurotransmitter release, in addition to regulating post-synaptic receptor content. Exogenous TNF can increase the frequency of miniature excitatory post-synaptic currents (mEPSCs) [9,19], which would be consistent with an increase in glutamate release probability. However, TNF also decreases the frequency of miniature inhibitory post-synaptic currents (mIPSCs) [8], so TNF may decrease release probability at GABAergic synapses. The dose of TNF required to see changes in miniature event frequency is typically lower than the amount needed to see changes in miniature event amplitude [8,19], suggesting these effects are separable. However, the effects on pre-synaptic release may be indirect, through the release of gliotransmitters from astrocytes [20].
The abovementioned data were all from pyramidal cells and TNF appears to have a differential effect on GABAergic neurons. TNF treatment of hippocampal interneurons did not increase the surface expression of AMPARs [10]. The principal cell type in the striatum is medium spiny neurons (MSNs), which are also GABAergic. These cells respond to exogenous TNF treatment with the endocytosis of synaptic AMPARs and a preferential removal of calcium-permeable AMPARs [17]. GABARs have not been examined on either cell type to date.
Receptor trafficking is an underlying mechanism of synaptic plasticity, which comes in two basic forms—Hebbian plasticity and homeostatic synaptic plasticity (HSP). Hebbian plasticity is thought to be the synaptic mechanism of learning and memory, and involves activity-dependent changes at individual synapses. HSP is part of a circuit-level negative feedback system, where glutamatergic and GABAergic synaptic strengths are inversely regulated to stabilize circuit function. HSP and Hebbian plasticity are theoretically both required for normal circuit function: HSP is designed to maintain circuit function, whereas Hebbian plasticity is designed to change circuit function, so that the same input will now lead to a different output/behaviour (a minimalistic framing of learning). These processes must be balanced—pure HSP would prevent any learning, while if Hebbian processes dominate, then the network becomes very unstable [21,22]. Given the specificity of TNF to HSP and not Hebbian plasticity [16,23], this is a potential route to probe the role of HSP in neurodevelopment and behaviour.
TNF appears not to be essential for normal development, as mice lacking TNF or TNF receptors are largely normal in terms of cytoarchitecture and baseline behaviours. A number of subtle behavioural changes have been reported in TNF and TNF receptor knockout mice, with inconsistent results. For example, spatial learning is either better [24], worse [25] or unchanged [26] in TNF mutant animals. Similarly, changes in baseline anxiety and depressive-like behaviours (such as the open field, elevated plus maze, light/dark box and forced swim test) are sometimes [26–28] but not always observed [29,30]. Overall, the behavioural phenotype is quite mild, which suggests that HSP (at least the TNF-mediated form of it) is not required for the majority of normal development, and perhaps only becomes engaged when circuit function is substantially perturbed. Such perturbations can reveal roles for TNF in development. For example, TNF is required for some forms of developmental plasticity in sensory cortices [18,23,31,32], where it is necessary for part of the re-normalization of circuit function when sensory input is substantially altered. During monocular deprivation in adolescent (but not adult) mice, there is an initial loss of closed eye responses in the primary visual cortex that presumably occurs through Hebbian mechanisms. There is a subsequent gain of response to the open eye, which is at least partially homeostatic in nature and requires TNF signalling [18,32]. Further, abnormal elevations in TNF can perturb normal development [33,34], with reduced dendritic length and complexity in cultured neurons, and premature synapse maturation and stabilization resulting in less refinement during development in vivo. This would suggest that inflammatory stimuli during development could have consequences for later neural function.
TNF also appears to be required for the development of adaptive behaviours in the adult, particularly in response to chronic psychoactive drugs. We have recently shown that TNF is required for the behavioural response to antidepressants, as measured by the ability of fluoxetine and desipramine to reduce immobility in the forced swim and tail suspension tests [30]. This suggests a role for cytokines in regulating depressive behaviours. Further, TNF mediates synaptic changes within the striatum and acts to reduce the behavioural consequences of chronic administration of antipsychotics [17] and drugs of abuse [35]. As noted above, TNF drives the endocytosis of AMPARs on the GABAergic MSNs of the striatum, reducing excitatory synaptic strength [17]. Chronic administration of classic antipsychotics (such as haloperidol) leads to synaptic potentiation within the striatum, and results in the development of dyskinetic motor problems, characterized by uncontrolled movements particularly of the face and neck. However, the chronic presence of drug seems to induce a homeostatic response, where TNF is elevated in the striatum and reduces both excitatory synaptic strength on the MSNs and the severity of the dyskinetic behaviour [17]. Blocking TNF signalling, even acutely, will cause an increase in dyskinesic movements. Similar changes are seen during the repeated administration of cocaine [35], which likewise causes synaptic potentiation within the striatum that accompanies the sensitization of the locomotor response to the drug [36]. Cocaine also activates striatal microglia, which increase TNF production that, in turn, reduces excitatory synaptic strength on MSNs and decreases the locomotor sensitization to cocaine [35]. Importantly, increasing the microglial response can reduce previously established cocaine-induced sensitization [35]. As the development of addiction is thought to be an aberrant form of learning [36], these data suggest that Hebbian and homeostatic plasticity can act to counterbalance each other, with a TNF-mediated homeostatic response acting to limit the changes induced by overactive Hebbian-type learning.
Thus, TNF can actively modify behaviour in the adult animal, which could suggest that abnormal elevation of TNF in an adult could likely have behavioural consequences. We have explored the role of TNF in a variety of behaviours without specific immune activation; here, we sought to test whether TNF was essential for the behavioural changes induced by an inflammatory state. To do this, we used the maternal immune activation model of neurodevelopmental disorders.
(b). Implications for tumour necrosis factor-alpha in a maternal immune activation model of neurodevelopment disorders
Maternal infection during pregnancy, referred to as maternal immune activation (MIA), is an environmental risk factor for both schizophrenia and autism [37,38]. In animal models, MIA is typically achieved by injecting pregnant mice with the viral mimic and immunostimulant, polyinosinic : polycytidylic acid (poly I : C), although bacterial mimic lipopolysaccharide (LPS) is also used. Offspring from injected pregnant mice develop key behavioural phenotypes as well as molecular and cellular signatures that are indicative of neurodevelopmental disorders, including schizophrenia and autism. The phenotype of the MIA offspring depends on whether the mother's immune system is activated mid or late gestation, which can shift the model from schizophrenia to autistic phenotypes [37]. However, the behavioural and cellular phenotypes of these disorders overlap greatly in animal models.
MIA triggers long-term behavioural alterations in the offspring. Importantly, infectious agents do not act directly on the developing fetal brain, but instead activate several immune signalling pathways and lead to primed immune function in the offspring via increased expression of cytokines and other immune factors, which is maintained in adulthood [39–42]. This altered immune status may contribute to abnormal neurodevelopment and/or continued synaptic dysfunction in MIA offspring. Moreover, changes in immune function are prominent in autism- and schizophrenia-related disorders [38,40,43], including elevated levels of TNF [43–48]. In the MIA animal model, the exact immune signature in MIA offspring depends on the type of stimulation and timing of the immune activation in the mother [38]. Importantly, though, most proinflammatory cytokines, including TNF, are elevated at various stages of post-natal development and in adulthood in most versions of MIA [37]. This is observed in both serum [39,49,50] and the brain [41,50], and in response to immune stimulation [42,50–52].
The elevated levels of TNF in this model suggest that TNF could contribute to the behavioural abnormalities of MIA offspring. This could occur as part of the ongoing dysfunction induced by the immune activation or as an adaptive mechanism to offset disrupted neuronal development. Further, glial activation has been observed in MIA models, with both microglia [50,53] and astrocyte function [53,54] being affected. Increased expression of inflammatory modulators and morphological changes indicative of an activated state are observed in microglia from adult MIA offspring [50,55,56]. Because TNF is released by activated microglia and astrocytes depending on the context [30,35], altered glia function in MIA offspring would also suggest that TNF-mediated plasticity could be involved in an MIA model. Behavioural abnormalities (in MIA or other models) are presumed to result from synaptic changes that change neuronal circuit function. Reports do, indeed, indicate that MIA leads to alterations in synaptic transmission and plasticity in the brain [51,57–62]. TNF could provide a mechanism for these changes, perhaps acting as a dysregulated version of HSP. TNF is critical for maintaining E/I balance following changes in circuit activity [16], resulting from activity-dependent trafficking of AMPAR and GABAR receptors. Altered synaptic transmission in MIA models has been reported as an increase in mEPSC amplitude and decrease in mEPSC frequency [63]. An MIA-induced reduction in neural connectivity has also been reported. In cultured cortical neurons from MIA offspring, spine density and mEPSC frequency are decreased [64]; similarly, a reduction in the number and turnover of dendritic spines in vivo has also been demonstrated [65]. Importantly, the altered structural and functional synaptic connectivity in MIA offspring is prevented by early anti-inflammatory treatment with ibudilast for the first two post-natal weeks [65].
As both a proinflammatory cytokine and mediator of HSP, TNF provides an avenue to study the potential role of HSP in a neurodevelopmental model of psychiatric disorders. Importantly, previous studies have indicated that TNF does not play a role in the early transmission of inflammatory signalling to the fetus [66,67] before post-natal development. Additionally, in the poly I : C model, TNF levels are reported to decrease initially in the fetal brain [34,67,68]. Hence, if TNF-mediated plasticity regulates the neurodevelopment of the MIA offspring, then this regulation would likely occur after the animal was born.
Core symptoms of both autism and schizophrenia include deficits in social interaction [43,52,69–72]. In mouse models, this is investigated by measuring the preference mice typically exhibit for an unfamiliar mouse over an unfamiliar object or empty chamber [73]. This preference is significantly reduced in poly I : C offspring. Investigations into gene × environment interaction have frequently used the social approach test and found interactions between genotype and maternal immune activation [52,74,75], although some studies have focused on other behavioural read-outs [76,77]. Previous evidence has suggested that TNF signalling does contribute to this behaviour as TNF−/− mice showed a baseline preference for a stranger mouse when compared with an empty containment cup in the three-chamber paradigm, as measured by total interaction time [78]. Interestingly, the same authors did not report behavioural differences for TNFR1−/− or TNFR2−/− mice.
It is less clear whether or not TNF signalling basally contributes to anxiolytic behaviour in the elevated plus maze. Some reports demonstrate no difference in TNF−/− mice [29,78] but TNF−/− mice are also reported to be generally less exploratory and more anxious in the elevated plus maze (EPM) test [26]. However, TNFR1 and TNFR2 knockout (KO) mice have lower levels of anxiety as judged by an increased time spent in the centre of the open field test and in the light compartment of the light–dark box test [27]. Interestingly, anxiety behaviours are hypothesized to be related to E/I imbalance, especially in the limbic areas of the brain [79]. Further, maternal immune activation increases limbic expression of GABA receptors, which correlates with observed decreased exploratory behaviour [80]. MIA also results in decreased GABAergic transmission in the medial prefrontal cortex [81].
Here, we tested the contribution of TNF to the MIA-induced behavioural changes in social approach and the EPM. However, TNF−/− mice had similar MIA-induced changes to their wild-type (WT) counterparts, suggesting that TNF signalling is not essential to the development or expression of these immune-induced behavioural changes.
2. Methods
(a). Breeding
All mouse lines were initially acquired from Jackson Labs. Females used for timed mating experiments were eight to 12 weeks old (on the C57BL/6 J background; the TNF−/− mice were backcrossed for a minimum of six generations by Jackson Labs). Breeding pairs for TNF−/− mice (in-house colony) are maintained by backcrossing to WT C57BL/6 J mice. At the time of the experiments performed here, the TNF KO colony had been in-house for three generations, and backcrossed to an in-house C57BL/6 J colony every second generation. Timed pregnancies were set up by placing bedding from a male mouse in cages for four to five females 2–3 days before mating. Groups of four to five females were then placed overnight in a cage with one male. Successful mating was verified the next morning by the presence of a vaginal plug, and the day after was referred to as gestational day 0.5. Because a plug does not always indicate that a female is pregnant, female mice were also weighed at gestation day (GD) 12.5 and only those with significant weight gain were injected with saline or poly I : C. Pregnant dames on GD 12.5 received either a single intraperitoneal injection of 5 mg kg−1 poly I : C (potassium salt at 1 mg ml−1; Sigma-Aldrich) or vehicle (sterile saline). The dose of poly I : C was chosen based on previous studies in C57BL/6 J mice [43,82,83]. All solutions were freshly prepared on the day of administration and injected with a volume of 5 ml kg−1. Animals were returned to their home cages immediately after the injection procedure. Resulting offspring are weaned at post-natal day 24–28. Behavioural data were obtained from male offspring from two separate cohorts. The social approach test was performed at eight weeks of age, and the elevated plus maze was subsequently performed at 12 weeks of age. The first cohort contained n = 4 wild-type (WT) dames (poly I : C, n = 2; saline, n = 2) and n = 4 TNF KO dames (poly I : C, n = 2; saline, n = 2). This cohort comprised 12 WT males and 15 KO males (WT saline, n = 7; WT poly I : C, n = 5; KO saline, n = 8; KO poly I : C, n = 7), and all males from the first cohort were put through the social approach test only. The second cohort contained n = 3 WT dames (poly I : C, n = 1; saline, n = 2) and n = 4 TNF KO dames (poly I : C, n = 2; saline, n = 2). This cohort comprised 12 WT males and 14 KO males (WT saline, n = 8; WT poly I : C, n = 5; KO saline, n = 5; KO poly I : C, n = 7), and all males from the second cohort were put through the social approach test and the elevated plus maze.
(b). Behavioural testing—social interaction
The social interaction test apparatus was made of Plexiglas and consisted of two identical chambers (20 × 20 cm) connected to each other by a third chamber (7 × 20 cm). The Plexiglas walls of the apparatus were covered with different patterns (stripes or polka dots) on each side. We first established that mice had no preference for either side. All animals were habituated to the test apparatus on the day of testing to reduce novelty-related locomotor hyperactivity. Two plastic containment cups (with holes to allow permeation of olfactory cues) were left empty during habituation. Each test mouse was gently placed in the middle chamber and allowed us to explore the apparatus for 5 min. Following habituation, the test mouse was isolated (using Plexiglas sliders) in the middle chamber, whereas an unfamiliar male mouse of the same age (eight weeks) and background (C57BL/6 J) from a non-experimental cage was placed inside one of the plastic containment cups located in one of the chambers. The plastic containment cup in the opposite chamber was left empty. Subjects were allowed to explore the three-chamber apparatus for 10 min. Each mouse received one trial, and all testing chambers were cleaned thoroughly with 30% ethanol between testing sessions. Experiments were conducted in dim lighting provided by two incandescent red lights approximately six feet from the ground on either side of the testing arena.
(c). Behavioural testing—elevated plus maze
One month after the social approach test, the second cohort of mice from saline- or poly I : C-treated mothers were tested in the elevated plus maze. The apparatus used for the elevated plus maze test comprised two open arms (25 × 5 × 0.5 cm) across from each other and perpendicular to two closed arms (25 × 5 × 16 cm) with a centre platform (5 × 5 × 0.5 cm). The open arms had a very small (0.5 cm) wall to decrease the number of falls, whereas the closed arms had a high (16 cm) wall to enclose the arm. The entire apparatus was 50 cm above the floor, and made of light grey Plexiglass. During testing, the mouse was placed in the centre area of the maze with its head directed towards a closed arm and allowed to move freely in the maze for 5 min. Each mouse received one trial. Experiments were conducted under dim lighting provided by two incandescent red lights on either side of the testing arena approximately six feet from the ground. Mice that fell off the elevated plus maze (two KO poly I : C mice) were excluded from the analysis. The trials were recorded using a video camera attached to a computer, and the number of entries into each arm and the time spent in the open arms manually scored. These measurements serve as an index of anxiety-like behaviour.
(d). Behavioural analysis
Behavioural tests were monitored and recorded with a camera placed above the three-chamber apparatus and EPM. Videos were analysed with Ethovision XT video-tracking software from Noldus. Behavioural data were coded by personalizing Arena Tracking settings. In the social approach test, the animal was measured for the time spent in each chamber (e.g. the centre and the chambers with the stranger mouse or empty containment cup), as well as a proximal circular zone around both containment cups in the opposite corners of the chambers. Because the Ethovision software measures only the centre point of the mouse, the proximal zone for each cup was set at 3 cm, which corresponds to the distance the centre point of the mouse would be from the cup if the mouse's head were immediately adjacent to the cup. The total time and entries (frequency) in different zones, as well as the total distance travelled during the test, were collected. For the EPM test, we collected the total time and entries into the open and closed arms as well as the centre zone.
(e). Statistical analysis
Data were analysed in JMP11 using a two-way (factorial) analysis of variance (ANOVA) to compare the main effects of genotype (WT or KO) and treatment (saline or poly I : C), and the interaction between genotype and treatment. Pairwise comparisons were made using a post hoc Fisher's least significant difference test. A value of p < 0.05 was used for the significance level.
3. Results
To investigate the role of TNF in the behavioural changes in adult MIA offspring, we tested social approach in TNF mutant mice, using a poly I : C MIA model. Abnormalities in social approach and social novelty in the three-chamber test are some of the most reported and validated behavioural changes in offspring from poly I : C-injected mothers [37]. Initially, we verified that we were able to reproduce these findings, showing that WT mice from saline-treated mothers (controls) exhibit normal social preference for a novel mouse over an empty chamber (figure 1a–d). We calculated a preference ratio for a proximal zone around the cup with the mouse versus the empty cup (figure 1a,b). The preference ratio takes into account both the time spent proximal to the cup with mouse and proximal to the empty cup. MIA is known to reduce the normal social preference in the offspring [66,69]. This social behaviour deficit is consistent in offspring from immune-stimulated rodents (single LPS or poly I : C injection) across different time-points during gestation [40,43,52,66]. We were able to demonstrate that the WT adult offspring from poly I : C-treated mothers displayed a reduced social preference towards un unknown stranger mouse, consistent with previous results (figure 1c, two-way ANOVA effect of treatment: F1,46 = 4.51, p < 0.05). Social preference was also measured by the time spent in the chamber with the mouse, as a fraction of the total testing time (10 min) spent exploring the entire apparatus. The main effect of poly I : C treatment was again significant (F1,46 = 19.94, p < 0.0001), with the WT poly I : C mice spending significantly less time with the stranger mouse compared with saline controls.
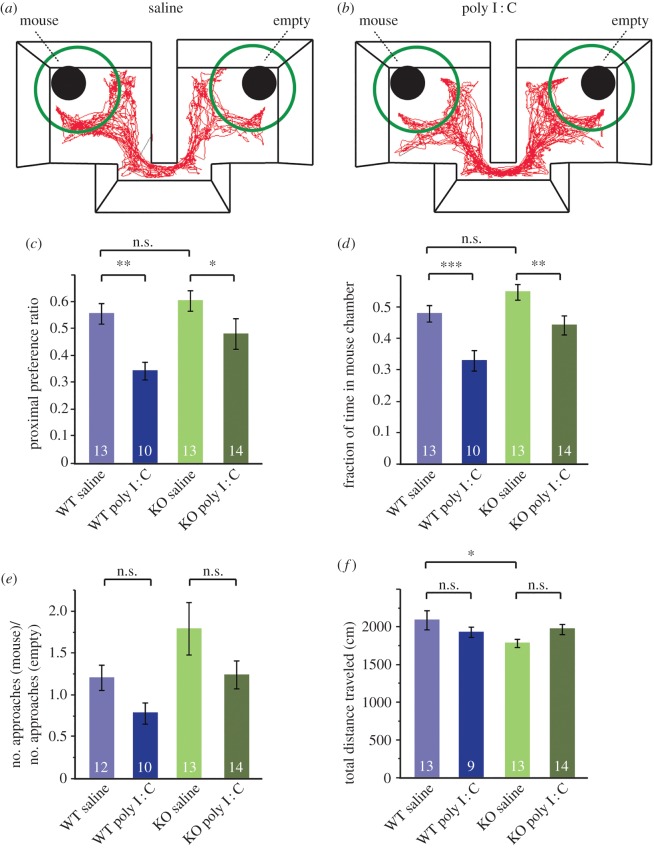
Figure 1.
TNF is not required for loss of social preference in MIA offspring. (a) Mice were tested in a three-chambered social approach task. An unfamiliar stranger mouse was enclosed in an aerated enclosure in one main chamber, with an empty enclosure in the opposite chamber. The separate enclosures are weighed down to prevent escape. The subject mouse was placed in the smaller middle chamber and can freely explore. The red track is a representative Ethovision trace collected over 10 min from a mouse from the WT saline group. (b) Comparison with a track from a mouse from the WT poly I : C group. A shift in preference to the chamber and proximal zone (green circle) with the empty enclosure is visible. (c) A preference ratio was calculated for the time spent in close proximity (green circles in a and b) with the enclosure containing the stranger mouse when compared with an empty enclosure for WT and KO offspring from saline or poly I : C treated mothers. Preference ratio was calculated as: (time proximal to mouse)/(time proximal to mouse and empty). Both WT (p = 0.0022) and KO (p = 0.045) offspring from saline-injected mothers spend significantly more time exploring the enclosure with the stranger mouse than offspring from poly I : C injected mothers (two-way ANOVA: effect of poly I : C yielded F1,46 = 4.51, p < 0.05). The interaction effect of genotype and treatment was insignificant: F1,46 = 0.995, p = 0.32. N for each group is indicated in the corresponding bar of the graph. (d) Data are means of the fraction of 10 min spent in the chamber containing the stranger mouse. The main effect of poly I : C treatment was again significant: F1,46 = 19.94, p < 0.0001. Further comparisons between groups showed that WT poly I : C mice spent proportionally less time in the chamber with the mouse (p = 0.0009) than their saline-treated counterparts, as did the TNF KO poly I : C mice (p = 0.0087). There was again no significant effect of genotype × treatment interaction: F1,46 = 0.58, p = 0.45. (e) Ratio of the frequency of approaches to the containment cup with the mouse to the frequency of approaches to the empty containment cup. A number greater than 1 indicates an overall larger number of ‘social’ approaches. A two-way ANOVA yields an effect of both treatment (F1,45 = 4.97; p = 0.031) as well as genotype (F1,45 = 6.07; p = 0.018), but no interaction effect between treatment and genotype (F1,45 = 0.097, p = 0.76). Post hoc pairwise analysis however shows no significant decrease in ratio of social approaches with poly I : C in either WT or KO mice. (f) Locomotor values during the 10 min duration of the social approach test measured as the total distance travelled (cm). A two-way ANOVA showed neither a significant effect of genotype (F1,45 = 2.41, p = 0.13) nor treatment (F1,45 = 0.033, p = 0.86) on total locomotion, but a trend for genotype × treatment interaction was evident whereby poly I : C increased locomotion in KO offspring but decreased locomotion in WT offspring (F1,45 = 4.04, p = 0.051). There was a significant decrease in baseline locomotion in the KO saline group compared with the WT saline group (p = 0.012).
We then tested the contribution of TNF to the MIA-induced loss of social preference, using TNF−/− mice. We did observe a significant effect of genotype for both measurements of sociability (proximal approach: F1,46 = 4.23; chamber time: F1,46 = 10.11, p < 0.01), but this was owing to differences in the poly I : C groups and not to differences in baseline social preference between WT and TNF−/− mice. But critically, we did not observe a genotype-dependent response to the poly I : C treatment. For both the proximal preference ratio (figure 1c) and fraction of time in the mouse chamber (figure 1d), the interaction effect of genotype and treatment was insignificant (proximal approach: F1,46 = 0.995, p = 0.32; chamber time: F1,46 = 0.58, p = 0.45), demonstrating that KO offspring responded in the same way to MIA as WT offspring in the three-chamber social approach test. As in WT offspring, the KO poly I : C group exhibited a significantly lower preference ratio for the stranger mouse (p = 0.045) and spent less time in the chamber with the stranger mouse (p = 0.0087). These results indicate that TNF is not required for the development and expression of social preference in adult mice, nor is it essential for the loss of normal social preference in MIA offspring.
We expected that the decreased time spent exploring and/or approaching the containment cup with the mouse would be accompanied by an overall decrease in frequency of entering the proximal zone, compared with entries into the proximal zone of the empty containment cup. We computed the ratio of social to non-social approaches (figure 1e), and observed an overall shift to non-social approaches for MIA-treated animals (two-way ANOVA, effect of treatment: F1,45 = 4.97; p = 0.031), as well as slightly higher numbers of approaches in the KO animals (effect of genotype: F1,45 = 6.07; p = 0.018). But while we observed similar shifts in both WT and KO animals from poly I : C-treated backgrounds, none of the individual changes were significant in post hoc analysis. So overall, MIA had similar effects on WT and TNF−/− mice, and we do not observe any significant interaction between TNF and maternal poly I : C treatment (F1,45 = 0.097, p = 0.76).
We also analysed the locomotor behaviour of all the groups tested in the social approach paradigm and did not observe a change in locomotion based on treatment. We did observe a slight difference in baseline locomotion between WT and KO mice (from saline-treated mothers) in the three-chamber apparatus (figure 1f, p = 0.012). We have not previously observed a decreased locomotor phenotype in TNF−/− mice (as measured in the open field test [35]), and also do not observe a baseline change in exploratory behaviour in TNF KO mice in the EPM test (figure 2g), which suggests that the baseline exploratory difference in KO mice is specific to the three-chamber apparatus.
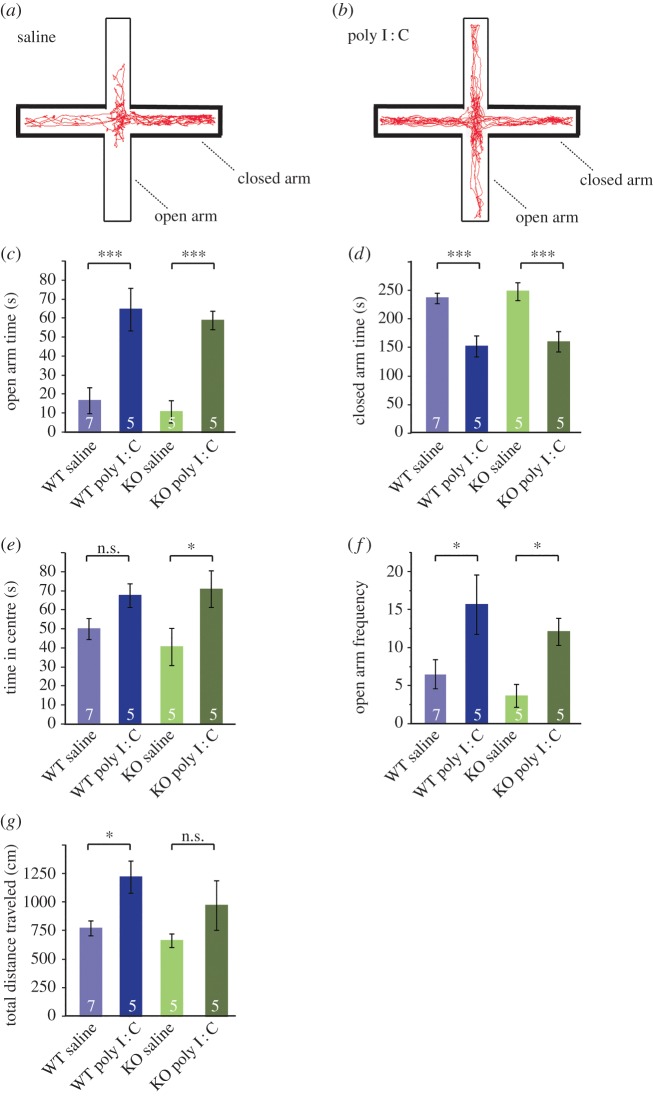
Figure 2.
TNF is not required for the change in anxiety-related behaviour induced by MIA. (a) The diagram shows the experimental methods to examine a mouse staying in the open arms (thick lines) versus closed arms (thin lines) in an elevated plus-maze. Red track is representative Ethovision trace collected over 5 min in a mouse from the WT saline group. (b) Red track representative of a mouse from the WT poly I : C group. An obvious increase in exploratory behaviour in the open arms is visible. (c) Open arm time in seconds measured over 5 min of testing for WT and KO offspring from saline or poly I : C injected mothers. Maternal injection with poly I : C significantly increases openarm time, with no significant differences between genotype and no interaction between poly I : C and genotype (two-way ANOVA, treatment: F1,18 = 39.87, p < 0.0001; genotype: F1,18 = 0.59, p = 0.45; interaction: F1,18 = 0, p = 0.997). Open arm time increases fourfold in the WT poly I : C group (p = 0.0002) and fivefold in the KO poly I : C group (p = 0.0004). (d) Closed arm time in seconds measured over 5 min of testing for WT and KO offspring from saline or poly I : C-injected mothers. Maternal injection with poly I : C significantly correspondingly decreases closedarm time, with no significant differences between genotype and no interaction between poly I : C and genotype (two-way ANOVA, treatment: F1,18 = 33.6, p < 0.0001; genotype: F1,18 = 0.43, p = 0.52; interaction: F1,18 = 0.020, p = 0.89). Closed arm time decreases approximately 50% in both the WT poly I : C group and KO poly I : C group when compared with respective saline groups (WT: p = 0.0006; KO: p = 0.0008). (e) Time in centre of EPM over 5 min. Maternal injection with poly I : C significantly increases exploratory time in the centre of the maze, with no significant differences between genotype and no interaction between poly I : C and genotype (two-way ANOVA, treatment: F1,18 = 9.66, p = 0.0061; genotype: F1,18 = 0.16, p = 0.70; interaction: F1,18 = 0.69, p = 0.42). There was only a significant increase in KO mice with poly I : C (p = 0.015) and not in WT mice (p = 0.11). (f) The total number of entries into the open arms, as measured by the location of the centre of the mouse with Ethovision. The frequency of open arm entries was significantly increased in the poly I : C groups from both WT (p = 0.012) and KO mice (p = 0.030). Two-way ANOVA effect of treatment: F1,18 = 3.63, p = 0.0019; effect of genotype: F1,18 = −1.33, p = 0.20; effect of interaction: F1,18 = −0.16, p = 0.88. (g) Locomotor values during the 5 min duration of the EPM test measured as the total distance travelled (cm). Poly I : C treatment significantly increased locomotion in the WT offspring (p = 0.019), but not the KO offspring (p = 0.12). Two-way ANOVA effect of treatment: F1,18 = 8.74, p = 0.0084; effect of genotype: F1,18 = 1.92, p = 0.18; effect of interaction: F1,18 = 0.31, p = 0.59.
We next compared the effect of MIA on the expression of anxiety-like behaviours in WT and TNF−/− mice, which typically is increased in adult offspring from immune-stimulated mothers [81,84]. We observed no significant differences in baseline anxiolytic behaviour between saline and TNF−/− mice in any of the measurements, looking at time or frequency in the different zones of the EPM (figure 2c–f). In contrast to previous findings on the effect of maternal immune activation on anxiolytic behaviour in the elevated plus maze, we observed a very pronounced and significant effect of poly I : C treatment on exploration time in the EPM open arms (two-way ANOVA effect of treatment: F1,18 = 39.87, p < 0.0001), accompanied by a significant decrease in time spent in the EPM closed arms (two-way ANOVA effect of treatment: F1,18 = 33.6, p < 0.0001). This was significant for both WT and TNF−/− offspring, and there was no evidence for an interaction between maternal immune activation and genotype (two-way ANOVA effect of interaction open arm time: F1,18 = 0, p = 0.997; closed arm time: F1,18 = 0.020, p = 0.89). TNF−/− offspring from poly I : C mothers may become slightly more exploratory as they spend significantly more time in the centre (figure 2e, p = 0.015). However, both genotypes demonstrate the same frequency to the open arms, as well as a similar locomotor response to poly I : C (figure 2f,g), and there is no evidence of an interaction between genotype and maternal immune activation for time in centre, frequency to open arms and total locomotion (respectively, effect of interaction F1,18 = 0.69, p = 0.42; F1,18 = 0.16, p = 0.88; F1,18 = 0.31, p = 0.59). Overall, we conclude that maternal immune activation results in decreased anxiety-like behaviour in the elevated plus maze, and that TNF is dispensable for the development of this immune-driven behavioural change.
4. Discussion
It is known that TNF has important neuroregulatory functions [7,9,11] and that it modulates homeostatic plasticity in vitro [16] and in vivo [18,32]. Further work is needed to establish the role of TNF-mediated plasticity in different models of disease, under both non-inflammatory and inflammatory contexts. Previously, we have shown that TNF-mediated plasticity is required for normal behavioural response to antidepressants [30], and that TNF mediates several adaptive responses in the striatum when circuit activity is acutely perturbed by drugs of abuse [35] or antipsychotics [17]. Our goal here was to evaluate the role of TNF-mediated regulation of circuit function during inflammatory neurodevelopment conditions, using the MIA model of neuropsychiatric disorders.
We saw no differential MIA-induced behavioural deficits in TNF−/− mice compared with WT mice, and conclude that TNF-mediated signalling is not critical for the long-term alterations in neurodevelopment caused by MIA. Specifically, our results indicate that neither the social indifference nor the changes in anxiety seen in MIA models require TNF signalling at the time-point and dose of poly I : C investigated. At least by these measures, TNF is dispensable for the development or maintenance of MIA-induced behavioural change in this model.
We should note that in our hands, offspring of MIA mothers displayed a lower level of anxiety, as measured in the EPM. Although studies to date largely indicate that MIA offspring exhibit higher levels of anxiety [74,81,84], the genetic background of mice plays a large role in the behavioural outcome of MIA [52], particularly for anxiety-related phenotypes [84]. Specifically, MIA was found to increase anxiety-like behaviour of offspring in NMRI mice but not in C57BL/6 J mice (as used here) [84]. Interestingly, NMRI offspring displayed behaviour consistent with elevated anxiety only in the elevated plus-maze but not in other paradigms such as the open field and light–dark box [84]. Strain-treatment interactions were also observed between C57BL/6 J and BTBRT + tf/J mice for other behavioural deficits in the MIA model, including decreased sociability and increased repetitive/stereotyped behaviour [52]. Importantly, offspring from both strains respond to maternal poly I : C injections with increased exploratory behaviour in the EPM. Other studies have reported no change in anxiety behaviour in the elevated plus maze with maternal poly I : C treatment alone [55,74,85], which could be owing to differences in genetic background. However, we again report no differences in the way WT and TNF−/− offspring respond to MIA (figure 2). Our interpretation is that TNF-mediated plasticity does not play a unique adaptive role in the dysregulated neurodevelopment of MIA offspring.
Another finding from our study was, in fact, that TNF−/− showed a slight but significantly decreased baseline locomotor behaviour in the social approach task, which was not significantly altered by MIA. However, TNF−/− have no differential locomotion in the open field test [17,35]; nor did we see this difference in the EPM. This result might indicate that TNF−/− mice have more exploratory behaviour when social cues are present, although the number of approaches to the ‘social’ containment cup and the ‘non-social’ containment cup did not differ from WT mice. Most studies do not report any alteration in spontaneous locomotor activity in the offspring of poly I : C-treated rodents as measured in the open field test [70,71,86], although both increased [87] and decreased [88] locomotor activity in poly I : C offspring have been reported.
Differences in behavioural abnormalities do critically depend on the timing of poly I : C treatment, which may be owing to differences in the fetal brain cytokine response at earlier stages of development (e.g. GD9) versus later stages (e.g. GD17) [82,83]. Cytokine expression and TNF, in particular, seem to be more elevated after an acute poly I : C injection at GD17 compared with GD9; however, GD9 injection favoured an overall more proinflammatory cytokine profile [82]. From a behavioural perspective, we also did not want to bias our behavioural analysis towards either autism (later time-point) or towards schizophrenia (earlier time-point) [37]. For example, some behaviours of interest are not present in animals from immune-activated mothers at later gestation times, including poly I : C-induced decreases in exploratory behaviour, observed at GD9 and GD12 but not GD17 [37]. Deficits in social approach have also been consistently established by mid-gestation MIA [52,66,69], but are also seen at with MIA at GD9 [74] and at GD17 [89–91].
It is important to note that the timing and intensity of the MIA, as well as additional genetic factors, can have an effect on cellular and behavioural phenotypes [38], so investigation of further paradigms (e.g. with LPS at a later stage of gestation) are required to confirm our results. Nonetheless, many MIA-induced behavioural changes, especially deficits in social approach and interaction, have been replicated in several laboratories despite the use of different species (rats or mice), different ages of gestational activation, and different doses and routes of administration (reviewed in [37,38,43]). Investigation of further MIA-induced behavioural abnormalities will further elucidate whether TNF function contributes uniquely to neurodevelopmental abnormalities resulting from prenatal neuroinflammation. For instance, peripheral levels of TNF in adult MIA offspring were positively correlated with repetitive, compulsive-like behaviour in the marble-burying test [40]. Unfortunately, we were not able to experimentally obtain reliable behavioural read-outs in the marble-burying test used in other studies. MIA has also been shown to affect both the acute locomotor response to drugs of abuse [91,92] and drug-induced place preference [91,93].
Acutely, MIA causes a strong inflammatory response in both the mothers and fetuses, although the cytokine profile depends both on whether LPS or poly I : C is used [37] and when the immune stimulant is administered [82,83]. In the poly I : C model, an increase in IL-1β is observed in the fetal brain 24 h after maternal injection [94] but TNF in the fetal brain is actually decreased 24 h after maternal poly I : C injection [68] and also in the neonatal brain [34]. This may be a fetal mechanism to adapt to the maternal immune activation and occurs through α- and β-crystallin downregulation of TNF [67]. Importantly, TNF is significantly elevated in adult MIA offspring in the poly I : C model, both in the brain [41] and periphery [39]. In an LPS rat model however, TNF is generally increased in the fetal brain [95–97]. MIA increases the levels of other proinflammatory cytokines in the periphery [42,50] and in the fetal brain, including interleukin 6 (IL-6) [50,98]. However, the acute changes in TNF do not likely affect the long-term consequences of MIA, because IL-6 alone reproduces the effect of poly I : C [66] and because neonatal challenge with TNF produces no apparent behavioural alterations in adult offspring [99]. These findings again support our rationale to use TNF−/− mice to specifically examine the contribution of TNF-mediated plasticity during development to behavioural abnormalities caused by MIA.
Overall, the long-term neuroinflammatory effects of MIA in adolescent and adult offspring are quite mild [83,100]. Adult MIA offspring do have elevated levels of peripheral TNF [39,49] and an elevated TNF response to immune challenges that depends on genetic background [52]. However, this is not a consistent finding [40], and elevated TNF expression in the adult brain is not always observed in the MIA model [41]. In the mouse poly I : C model, however, IL-1β and IL-6 are increased in the adult offspring ([41,52,82,83,101] but see [40]) and these other cytokines may play a more predominant role in the long-term behavioural consequences of the MIA model.
Outside of an inflammatory context, TNF has established roles in the development of the nervous system, including regulation of progenitor proliferation and neurogenesis [102], sympathetic innervation [103] and normal development of the hippocampus [24]. Increasing TNF levels during development have been shown to dysregulate dendrite and synapse formation [33,34]. Importantly, these effects also occur with increased levels of other proinflammatory cytokines IL-6 and IL-1β. In the context of neurodevelopment IL-6, IL-1β and TNF have all been shown to have the same effect on primary dendrite number, dendritic nodes and total dendrite length during the development of neurons [34]. So during MIA, other cytokines may largely be able to compensate for the deletion of TNF.
Therefore, two possibilities appear to be explanations for why genetic deletion of TNF has no effect on the behavioural changes observed in MIA offspring. The first is that TNF has no role in the neurodevelopment of the circuits underlying the behaviours we examined, either by not being elevated in those tissues or by not impacting the circuit function. In favour of this idea is that development is largely normal in TNF and TNFR knockout mice, though subtle differences are detected, and the examined behaviours were largely normal in offspring from saline-treated TNF−/− mothers compared with those from saline-treated WT mothers. However, other studies have demonstrated that MIA can reveal functions of gene deletions or mutations that do not cause behavioural phenotypes on their own [52,74–77].
The second possibility is that during even mild models of neuroinflammation, proinflammatory cytokines are coregulated and act in conjunction with each other. In this model, no single cytokine has a unique role, and the other cytokines can compensate for the loss of one. For example, artificial elevation of TNF during development dysregulates dendrite and synapse formation [33,34], but these effects also occur with increased levels of other proinflammatory cytokines IL-6 and IL-1β. Thus, interfering with TNF under conditions when several proinflammatory cytokines are elevated will not have distinct effects. Although current data do not allow us to distinguish between these possibilities, we favour the second option and propose that the impact of TNF signalling will vary owing to context. Under basal non-inflammatory conditions, TNF acts as the sole cytokine mediator of HSP, and thus interfering with TNF signalling will prevent the circuit normalization we have observed under other experimental paradigms. However, under inflammatory conditions (such as MIA), TNF is part of a suite of cytokine signalling with overlapping functions. Interfering with TNF signalling under these conditions would not have distinct effects, as we observed here. Clearly, elucidating the role of TNF-mediated plasticity in other behavioural models of neuroinflammation and neurodevelopment is needed to expand on these findings.
Acknowledgements
We thank Alexandre Trottier for expert technical assistance.
Ethics
All animal procedures were approved by the Montreal General Facility Animal Care Committee, in accordance with the guidelines of the Canadian Council for Animal Care.
Authors' contributions
The project was conceived and experiments designed by S.C.K. and D.S. S.C.K. performed the experiments and analysed the data. D.S. and S.C.K. wrote the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by CIHR and NSERC (D.S.), and the Neuroinflammation Training Programme (S.C.K).
References
- 1.Barker CF, Billingham RE. 1977. Immunologically privileged sites. Adv. Immunol. 25, 1–54. ( 10.1016/S0065-2776(08)60930-X) [DOI] [PubMed] [Google Scholar]
- 2.Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. 2000. Cytokine signals propagate through the brain. Mol. Psychiatry 5, 604–615. ( 10.1038/sj.mp.4000813) [DOI] [PubMed] [Google Scholar]
- 3.Smith RA, Baglioni C. 1987. The active form of tumor necrosis factor is a trimer. J. Biol. Chem. 262, 6951–6954. [PubMed] [Google Scholar]
- 4.Black RA, et al. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385, 729–733. ( 10.1038/385729a0) [DOI] [PubMed] [Google Scholar]
- 5.Grell M, et al. 1995. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83, 793–802. ( 10.1016/0092-8674(95)90192-2) [DOI] [PubMed] [Google Scholar]
- 6.Wajant H, Pfizenmaier K, Scheurich P. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65. ( 10.1038/sj.cdd.4401189) [DOI] [PubMed] [Google Scholar]
- 7.Stellwagen D, Beattie EC, Seo JY, Malenka RC. 2005. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J. Neurosci. 25, 3219–3228. ( 10.1523/JNEUROSCI.4486-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pribiag H, Stellwagen D. 2013. TNF-α downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABAA, receptors. J. Neurosci. 33, 15 879–15 893. ( 10.1523/JNEUROSCI.0530-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EBeattie C, et al. 2002. Control of synaptic strength by glial TNFα. Science 295, 2282–2285. ( 10.1126/science.1067859) [DOI] [PubMed] [Google Scholar]
- 10.Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH. 2005. Tumor necrosis-factor-α (TNF-α) induces rapid insertion of Ca2+-permeable α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp. Neurol. 193, 384–393. ( 10.1016/j.expneurol.2004.12.026) [DOI] [PubMed] [Google Scholar]
- 11.He P, Liu Q, Wu J, Shen Y. 2012. Genetic deletion of TNF receptor suppresses excitatory synaptic transmission via reducing AMPA receptor synaptic localization in cortical neurons. FASEB J. 26, 334–345. ( 10.1096/fj.11-192716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki Y, Zhang L, Cheng JK, Ji RR. 2008. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 28, 5189–5194. ( 10.1523/JNEUROSCI.3338-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han P, Whelan PJ. 2010. Tumor necrosis factor alpha enhances glutamatergic transmission onto spinal motoneurons. J. Neurotrauma 27, 287–292. ( 10.1089/neu.2009.1016) [DOI] [PubMed] [Google Scholar]
- 14.Furukawa K, Mattson MP. 1998. The transcription factor NF-κB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-α in hippocampal neurons. J. Neurochem. 70, 1876–1886. ( 10.1046/j.1471-4159.1998.70051876.x) [DOI] [PubMed] [Google Scholar]
- 15.Lai AY, Swayze RD, El-Husseini A, Song C. 2006. Interleukin-1β modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J. Neuroimmunol. 175, 97–106. ( 10.1016/j.jneuroim.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 16.Stellwagen D, Malenka RC. 2006. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059. ( 10.1038/nature04671) [DOI] [PubMed] [Google Scholar]
- 17.Lewitus GM, Pribiag H, Duseja R, St-Hilaire M, Stellwagen D. 2014. An adaptive role of TNFα in the regulation of striatal synapses. J. Neurosci. 34, 6146–6155. ( 10.1523/JNEUROSCI.3481-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. 2008. Tumor necrosis factor-α mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680. ( 10.1016/j.neuron.2008.04.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassi F, Mileo AM, Monaco L, Punturieri A, Santoni A, Eusebi F. 1994. TNF-α increases the frequency of spontaneous miniature synaptic currents in cultured rat hippocampal neurons. Brain Res. 659, 226–230. ( 10.1016/0006-8993(94)90883-4) [DOI] [PubMed] [Google Scholar]
- 20.Santello M, Bezzi P, Volterra A. 2011. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001. ( 10.1016/j.neuron.2011.02.003) [DOI] [PubMed] [Google Scholar]
- 21.Keck T, et al. 2017. Integrating Hebbian and homeostatic plasticity: the current state of the field and future research directions. Phil. Trans. R. Soc. B 372, 20160158 ( 10.1098/rstb.2016.0158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zenke F, Gerstner W. 2017. Hebbian plasticity requires compensatory processes on multiple timescales. Phil. Trans. R. Soc. B 372, 20160259 ( 10.1098/rstb.2016.0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee AX, Hsu Y-T, Chen L. 2017. A metaplastic view of the interaction between homeostatic and Hebbian plasticity. Phil. Trans. R. Soc. B 372, 20160155 ( 10.1098/rstb.2016.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golan H, Levav T, Mendelsohn A, Huleihel M. 2004. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb. Cortex 14, 97–105. ( 10.1093/cercor/bhg108) [DOI] [PubMed] [Google Scholar]
- 25.Baune T, Wiede F, Braun A, Golledge J, Arolt V, Koerner H. 2008. Cognitive dysfunction in mice deficient for TNF- and its receptors. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 1056–1064. ( 10.1002/ajmg.b.30712) [DOI] [PubMed] [Google Scholar]
- 26.Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, Nabeshima T. 2000. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-α gene: implications for emotional behavior. J. Neuroimmunol. 111, 131–138. ( 10.1016/S0165-5728(00)00375-1) [DOI] [PubMed] [Google Scholar]
- 27.Patel A, Siegel A, Zalcman SS. 2010. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain Behav. Immun. 24, 1276–1280. ( 10.1016/j.bbi.2010.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simen BB, Duman CH, Simen AA, Duman RS. 2006. TNFα signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol. Psychiatry 59, 775–785. ( 10.1016/j.biopsych.2005.10.013) [DOI] [PubMed] [Google Scholar]
- 29.Fursenko DV, Khotskin NV, Kulikov VA, Kulikov AV. 2016. Behavioral phenotyping of mice deficient in the tumor necrosis factor. Russ. J. Genet. Appl. Res. 6, 400–404. ( 10.1134/S2079059716040067) [DOI] [Google Scholar]
- 30.Duseja R, Heir R, Lewitus GM, Altimimi HF, Stellwagen D. 2014. Astrocytic TNFα regulates the behavioral response to antidepressants. Brain Behav. Immun. 44, 187–194. ( 10.1016/j.bbi.2014.09.012) [DOI] [PubMed] [Google Scholar]
- 31.Glazewski S, Greenhill S, Fox K. 2017. Time-course and mechanisms of homeostatic plasticity in layers 2/3 and 5 of the barrel cortex. Phil. Trans. R. Soc. B 372, 20160150 ( 10.1098/rstb.2016.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranson A, Cheetham CE, Fox K, Sengpiel F. 2012. Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proc. Natl Acad. Sci. USA 109, 1311–1316. ( 10.1073/pnas.1112204109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee RH, et al. 2010. Neurodevelopmental effects of chronic exposure to elevated levels of pro-inflammatory cytokines in a developing visual system. Neural Dev. 5, 2 ( 10.1186/1749-8104-5-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilmore JH, Jarskog FL, Vadlamudi S, Lauder JM. 2004. Prenatal infection and risk for schizophrenia: IL-1β, IL-6, and TNFα inhibit cortical neuron dendrite development. Neuropsychopharmacology 29, 1221–1229. ( 10.1038/sj.npp.1300446) [DOI] [PubMed] [Google Scholar]
- 35.Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, Stellwagen D. 2016. Microglial TNF-α suppresses cocaine-induced plasticity and behavioral sensitization. Neuron 90, 483–491. ( 10.1016/j.neuron.2016.03.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luscher C, Malenka RC. 2011. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69, 650–663. ( 10.1016/j.neuron.2011.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer U. 2014. Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 75, 307–315. ( 10.1016/j.biopsych.2013.07.011) [DOI] [PubMed] [Google Scholar]
- 38.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. 2014. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 10, 643–660. ( 10.1038/nrneurol.2014.187) [DOI] [PubMed] [Google Scholar]
- 39.Han X, Li N, Meng Q, Shao F, Wang W. 2011. Maternal immune activation impairs reversal learning and increases serum tumor necrosis factor-α in offspring. Neuropsychobiology 64, 9–14. ( 10.1159/000322455) [DOI] [PubMed] [Google Scholar]
- 40.Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P. 2014. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav. Immun. 38, 220–226. ( 10.1016/j.bbi.2014.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garay PA, Hsiao EY, Patterson PH, McAllister AK. 2013. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 31, 54–68. ( 10.1016/j.bbi.2012.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. 2012. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl Acad. Sci. USA 109, 12 776–12 781. ( 10.1073/pnas.1202556109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson PH. 2009. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 204, 313–321. ( 10.1016/j.bbr.2008.12.016) [DOI] [PubMed] [Google Scholar]
- 44.Hope S, Ueland T, Steen NE, Dieset I, Lorentzen S, Berg AO, Agartz I, Aukrust P, Andreassen OA. 2013. Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder. Schizophr. Res. 145, 36–42. ( 10.1016/j.schres.2012.12.023) [DOI] [PubMed] [Google Scholar]
- 45.Goldsmith DR, Rapaport MH, Miller BJ. 2016. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696–1709. ( 10.1038/mp.2016.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. 2007. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 36, 361–365. ( 10.1016/j.pediatrneurol.2007.01.012) [DOI] [PubMed] [Google Scholar]
- 47.Li X, et al. 2009. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 207, 111–116. ( 10.1016/j.jneuroim.2008.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. 2007. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry 7, 551 ( 10.1186/1471-244X-7-46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basta-Kaim A, et al. 2012. Maternal immune activation leads to age-related behavioral and immunological changes in male rat offspring – the effect of antipsychotic drugs. Pharmacol. Rep. 64, 1400–1410. ( 10.1016/S1734-1140(12)70937-4) [DOI] [PubMed] [Google Scholar]
- 50.Krstic D, et al. 2012. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflamm. 9, 151 ( 10.1186/1742-2094-9-151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giovanoli S, Weber-Stadlbauer U, Schedlowski M, Meyer U, Engler H. 2016. Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain Behav. Immun. 55, 25–38. ( 10.1016/j.bbi.2015.09.015) [DOI] [PubMed] [Google Scholar]
- 52.Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. 2013. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry 3, e240 ( 10.1038/tp.2013.16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. 2002. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology 26, 204–215. ( 10.1016/S0893-133X(01)00360-8) [DOI] [PubMed] [Google Scholar]
- 54.Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, Thuras P. 2002. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol. Psychiatry 7, 633–640. ( 10.1038/sj.mp.4001046) [DOI] [PubMed] [Google Scholar]
- 55.Giovanoli S, et al. 2013. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339, 1095–1099. ( 10.1126/science.1228261) [DOI] [PubMed] [Google Scholar]
- 56.Juckel G, Manitz MP, Brüne M, Friebe A, Heneka MT, Wolf RJ. 2011. Microglial activation in a neuroinflammational animal model of schizophrenia—a pilot study. Schizophr. Res. 131, 96–100. ( 10.1016/j.schres.2011.06.018) [DOI] [PubMed] [Google Scholar]
- 57.Ducharme G, Lowe GC, Goutagny R, Williams S. 2012. Early alterations in hippocampal circuitry and theta rhythm generation in a mouse model of prenatal infection: implications for schizophrenia. PLoS ONE 7, e29754 ( 10.1371/journal.pone.0029754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Escobar M, et al. 2011. Early, time-dependent disturbances of hippocampal synaptic transmission and plasticity after in utero immune challenge. Biol. Psychiatry 70, 992–999. ( 10.1016/j.biopsych.2011.01.009) [DOI] [PubMed] [Google Scholar]
- 59.Lowe GC, Luheshi GN, Williams S. 2008. Maternal infection and fever during late gestation are associated with altered synaptic transmission in the hippocampus of juvenile offspring rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1563–R1571. ( 10.1152/ajpregu.90350.2008) [DOI] [PubMed] [Google Scholar]
- 60.Oh-Nishi A, Obayashi S, Sugihara I, Minamimoto T, Suhara T. 2010. Maternal immune activation by polyriboinosinic-polyribocytidilic acid injection produces synaptic dysfunction but not neuronal loss in the hippocampus of juvenile rat offspring. Brain Res. 1363, 170–179. ( 10.1016/j.brainres.2010.09.054) [DOI] [PubMed] [Google Scholar]
- 61.Roumier A, Pascual O, Béchade C, Wakselman S, Poncer J-C, Réal E, Triller A, Bessis A. 2008. Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS ONE 3, e2595 ( 10.1371/journal.pone.0002595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patrich E, Piontkewitz Y, Peretz A, Weiner I, Attali B. 2016. Maternal immune activation produces neonatal excitability defects in offspring hippocampal neurons from pregnant rats treated with poly I:C. Sci. Rep. 6, 19106 ( 10.1038/srep19106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito HT, Smith SE, Hsiao E, Patterson PH. 2010. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav. Immun. 24, 930–941. ( 10.1016/j.bbi.2010.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elmer BM, Estes ML, Barrow SL, McAllister AK. 2013. MHCI requires MEF2 transcription factors to negatively regulate synapse density during development and in disease. J. Neurosci. 33, 13 791–13 804. ( 10.1523/JNEUROSCI.2366-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coiro P, et al. 2015. Impaired synaptic development in a maternal immune activation mouse model of neurodevelopmental disorders. Brain Behav. Immun. 50, 249–258. ( 10.1016/j.bbi.2015.07.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. 2007. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10 695–10 702. ( 10.1523/JNEUROSCI.2178-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K. 2012. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl. Psychiatry 2, e98 ( 10.1038/tp.2012.24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ratnayake U, Quinn T, LaRosa DA, Dickinson H, Walker DW. 2014. Prenatal exposure to the viral mimetic poly I:C alters fetal brain cytokine expression and postnatal behaviour. Dev. Neurosci. 36, 83–94. ( 10.1159/000362205) [DOI] [PubMed] [Google Scholar]
- 69.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. 2012. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26, 607–616. ( 10.1016/j.bbi.2012.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zuckerman L, Weiner I. 2005. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J. Psychiatr. Res. 39, 311–323. ( 10.1016/j.jpsychires.2004.08.008) [DOI] [PubMed] [Google Scholar]
- 71.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. 2006. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol. Psychiatry 59, 546–554. ( 10.1016/j.biopsych.2005.07.031) [DOI] [PubMed] [Google Scholar]
- 72.O'Tuathaigh CM, Kirby BP, Moran PM, Waddington JL. 2010. Mutant mouse models: genotype–phenotype relationships to negative symptoms in schizophrenia. Schizophr. Bull. 36, 271–288. ( 10.1093/schbul/sbp125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silverman JL, Yang M, Lord C, Crawley JN. 2010. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 11, 490–502. ( 10.1038/nrn2851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abazyan B, et al. 2010. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol. Psychiatry 68, 1172–1181. ( 10.1016/j.biopsych.2010.09.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ibi D, et al. 2010. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav. Brain Res. 206, 32–37. ( 10.1016/j.bbr.2009.08.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vuillermot S, Joodmardi E, Perlmann T, Ove Ogren S, Feldon J, Meyer U. 2012. Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments. J. Neurosci. 32, 436–451. ( 10.1523/JNEUROSCI.4831-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu WL, Adams CE, Stevens KE, Chow K-H, Freedman R, Patterson PH. 2015. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav. Immun. 46, 192–202. ( 10.1016/j.bbi.2015.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camara ML, Corrigan F, Jaehne EJ, Jawahar MC, Anscomb H, Baune BT. 2015. Tumor necrosis factor alpha and its receptors in behaviour and neurobiology of adult mice, in the absence of an immune challenge. Behav. Brain Res. 290, 51–60. ( 10.1016/j.bbr.2015.04.040) [DOI] [PubMed] [Google Scholar]
- 79.Nuss P. 2015. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. Treat. 11, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. 2006. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience 143, 51–62. ( 10.1016/j.neuroscience.2006.07.029) [DOI] [PubMed] [Google Scholar]
- 81.Canetta S, et al. 2016. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol. Psychiatry 21, 956–968. ( 10.1038/mp.2015.222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer U, Feldon J, Schedlowski M, Yee BK. 2006. Immunological stress at the maternal–foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav. Immun. 20, 378–388. ( 10.1016/j.bbi.2005.11.003) [DOI] [PubMed] [Google Scholar]
- 83.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. 2008. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 22, 469–486. ( 10.1016/j.bbi.2007.09.012) [DOI] [PubMed] [Google Scholar]
- 84.Babri S, Doosti MH, Salari AA. 2014. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain Behav. Immun. 37, 164–176. ( 10.1016/j.bbi.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 85.Li WY, Chang YC, Lee LJ, Lee LJ. 2014. Prenatal infection affects the neuronal architecture and cognitive function in adult mice. Dev. Neurosci. 36, 359–370. ( 10.1159/000362383) [DOI] [PubMed] [Google Scholar]
- 86.Zuckerman L, Rehavi M, Nachman R, Weiner I. 2003. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacologyy 28, 1778–1789. ( 10.1038/sj.npp.1300248) [DOI] [PubMed] [Google Scholar]
- 87.Howland JG, Cazakoff BN, Zhang Y. 2012. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience 201, 184–198. ( 10.1016/j.neuroscience.2011.11.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van den Eynde K, et al. 2014. Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav. Brain Res. 258, 179–186. ( 10.1016/j.bbr.2013.10.005) [DOI] [PubMed] [Google Scholar]
- 89.Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. 2010. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology 35, 2462–2478. ( 10.1038/npp.2010.129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Labouesse MA, Dong E, Grayson DR, Guidotti A, Meyer U. 2015. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics 10, 1143–1155. ( 10.1080/15592294.2015.1114202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Labouesse MA, Langhans W, Meyer U. 2015. Abnormal context–reward associations in an immune-mediated neurodevelopmental mouse model with relevance to schizophrenia. Transl. Psychiatry 5, e637 ( 10.1038/tp.2015.129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zager A, Mennecier G, Palermo-Neto J. 2012. Maternal immune activation in late gestation enhances locomotor response to acute but not chronic amphetamine treatment in male mice offspring: role of the D1 receptor. Behav. Brain Res 232, 30–36. ( 10.1016/j.bbr.2012.03.036) [DOI] [PubMed] [Google Scholar]
- 93.Richtand NM, Ahlbrand R, Horn PS, Chambers B, Davis J, Benoit S. 2012. Effects of prenatal immune activation and peri-adolescent stress on amphetamine-induced conditioned place preference in the rat. Psychopharmacology 222, 313–324. ( 10.1007/s00213-012-2646-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arrode-Bruses G, Bruses JL. 2012. Maternal immune activation by poly I:C induces expression of cytokines IL-1β and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. J. Neuroinflamm. 9, 83 ( 10.1186/1742-2094-9-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. 2001. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr. Res. 47, 27–36. ( 10.1016/S0920-9964(00)00032-3) [DOI] [PubMed] [Google Scholar]
- 96.Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. 2006. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol. Psychiatry 11, 47–55. ( 10.1038/sj.mp.4001748) [DOI] [PubMed] [Google Scholar]
- 97.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. 2000. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr. Res. 47, 64–72. ( 10.1203/00006450-200001000-00013) [DOI] [PubMed] [Google Scholar]
- 98.Pratt L, Ni L, Ponzio NM, Jonakait GM. 2013. Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: role of interleukin-6. Pediatr. Res. 74, 393–401. ( 10.1038/pr.2013.126) [DOI] [PubMed] [Google Scholar]
- 99.Nawa H, Takei N. 2006. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci. Res. 56, 2–13. ( 10.1016/j.neures.2006.06.002) [DOI] [PubMed] [Google Scholar]
- 100.Smolders S, Smolders SMT, Swinnen N, Gärtner A, Rigo J-M, Legendre P, Brône B. 2015. Maternal immune activation evoked by polyinosinic:polycytidylic acid does not evoke microglial cell activation in the embryo. Front. Cell. Neurosci. 9, 301 ( 10.3389/fncel.2015.00301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Golan H, Stilman M, Lev V, Huleihel M. 2006. Normal aging of offspring mice of mothers with induced inflammation during pregnancy. Neuroscience 141, 1909–1918. ( 10.1016/j.neuroscience.2006.05.045) [DOI] [PubMed] [Google Scholar]
- 102.Iosif RE, et al. 2006. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J. Neurosci. 26, 9703–9712. ( 10.1523/JNEUROSCI.2723-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kisiswa L, Osório C, Erice C, Vizard T, Wyatt S, Davies AM. 2013. TNFα reverse signaling promotes sympathetic axon growth and target innervation. Nat. Neurosci. 16, 865–873. ( 10.1038/nn.3430) [DOI] [PMC free article] [PubMed] [Google Scholar]