Abstract
Memory storage involves activity-dependent strengthening of synaptic transmission, a process termed long-term potentiation (LTP). The late phase of LTP is thought to encode long-term memory and involves structural processes that enlarge the synapse. Hence, understanding how synapse size is graded provides fundamental information about the information storage capability of synapses. Recent work using electron microscopy (EM) to quantify synapse dimensions has suggested that synapses may structurally encode as many as 26 functionally distinct states, which correspond to a series of proportionally spaced synapse sizes. Other recent evidence using super-resolution microscopy has revealed that synapses are composed of stereotyped nanoclusters of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and scaffolding proteins; furthermore, synapse size varies linearly with the number of nanoclusters. Here we have sought to develop a model of synapse structure and growth that is consistent with both the EM and super-resolution data. We argue that synapses are composed of modules consisting of matrix material and potentially one nanocluster. LTP induction can add a trans-synaptic nanocluster to a module, thereby converting a silent module to an AMPA functional module. LTP can also add modules by a linear process, thereby producing an approximately 10-fold gradation in synapse size and strength.
This article is part of the themed issue ‘Integrating Hebbian and homeostatic plasticity’.
Keywords: long-term potentiation, synapse growth, nanoclusters, synaptic module, electron microscopy, super-resolution imaging
1. Introduction
It has long been known that short periods of neural activity can cause persistent changes in the strength of excitatory glutamatergic synapses, a process called long-term potentiation (LTP). Such changes provide a mechanism for at least some forms of long-term memory [1]. Synaptic strength is defined by the size of the postsynaptic response generated by an action potential in the axon that innervates that synapse [2]. The question of how graded such changes in synaptic strength can be bears importantly on how much information the brain can store but has not been experimentally answered. Because it is very difficult to conduct physiological experiments on single synapses in the brain, there have been few relevant studies and these have not yielded a consistent picture; some experiments have pointed to binary storage mechanisms [3–5], while others have suggested a larger number of states [6].
An alternative way to gain information about the gradation of synaptic strength is based on anatomical information. Electron microscopy (EM) has shown that synapse size, as defined by the area of the postsynaptic density (PSD), varies over a very wide range and correlates with the volume of the spine that contains that synapse [7]. Furthermore, synaptic strength, as measured by the response to locally applied glutamate, varies directly with spine volume [2]. There are, therefore, strong reasons to believe that synaptic strength is correlated with synapse size. Thus, studying the size heterogeneity of synapses can provide insight into the gradation of synaptic strength.
Recent work has provided the first evidence that LTP produces an increase in synapse size [8–10]. It has long been known that LTP occurs in two phases, an early phase which lasts on the order of an hour and does not depend on protein synthesis and a late phase that does [11]. Recent EM experiments suggest that early LTP does not involve growth of the PSD, whereas late LTP does [8–10]. Furthermore, analysis of EM images suggests that the growth of a synapse involves an expansion of both the PSD and a presynaptic structure called the presynaptic grid [7]. These two structures are exactly in register and covary in size over a large size range. Thus, growth of a synapse is a trans-synaptic process [7,12]. One limitation of EM is that the same structure cannot be studied both before and after LTP induction, and conclusions must, therefore, be based on statistical analysis. It is thus important that other recent studies using light microscopic analysis of structural proteins of the PSD have been able to follow the incorporation of structural proteins over time after LTP induction [8,9]. These studies show that two structural proteins of the PSD, PSD-95 and Homer, do not increase during early LTP but do increase during late LTP [8,9]. Thus, a strong working hypothesis is that LTP induction produces slowly developing trans-synaptic growth of the synapse and that it is this structural change that encodes long-term memory.
An important recent study [13] provided the first estimate of the number of stable states that could be encoded by synapse size. This study took advantage of the fact that some axons make two synapses onto the same target cell, synapses that are likely to have similar activity history. The main finding is that such synapses have nearly identical size and are on spines of nearly identical size (measured by EM). On the other hand, such pairs differ dramatically in size from each other. A simple model of the data suggested potential rules of memory gradation: (i) that a lower limit on the number of distinguishable size states is 26; and (ii) that these states differ in size by an increment proportional to their size (i.e. distinguishable states do not differ by a quantal value).
The above analysis of synapse structure was based on EM data, but recently super-resolution microscopy [14–17] has revealed other data relevant to understanding the gradation of synaptic size and strength. Various techniques, including stimulated emission depletion (STED) microscopy, single-particle tracking photoactivation localization microscopy (sptPALM), universal point accumulation in nanoscale topography (uPAINT) and direct stochastic optical reconstruction microscopy (dSTORM), have been employed to explore the molecular organization of synapses under the diffraction limit. Notably, it is now clear that synapses contain clusters of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs, approx. 0.1 µm diameter) associated with similar size clusters of the synaptic structural proteins Homer and PSD-95. Various terms have been used to describe these clusters (subcluster, nanodomains, etc.), which we will refer to as nanoclusters. The data show that the number of such nanoclusters varies with synapse size [14,16,17]. These super-resolution studies provide substantial new data about the substructure of the synapse (see also [18]), but there has been no previous attempt to reconcile this data with the EM data [13] described above. Here we have sought to determine whether a model can be developed that is consistent with both lines of experiments. We show that such a model can be developed. This leads us to the conclusion that synapses may grow by quantal addition of trans-synaptic modules and that the number of possible size states is about 10.
2. Results
(a). Analysis of super-resolution data
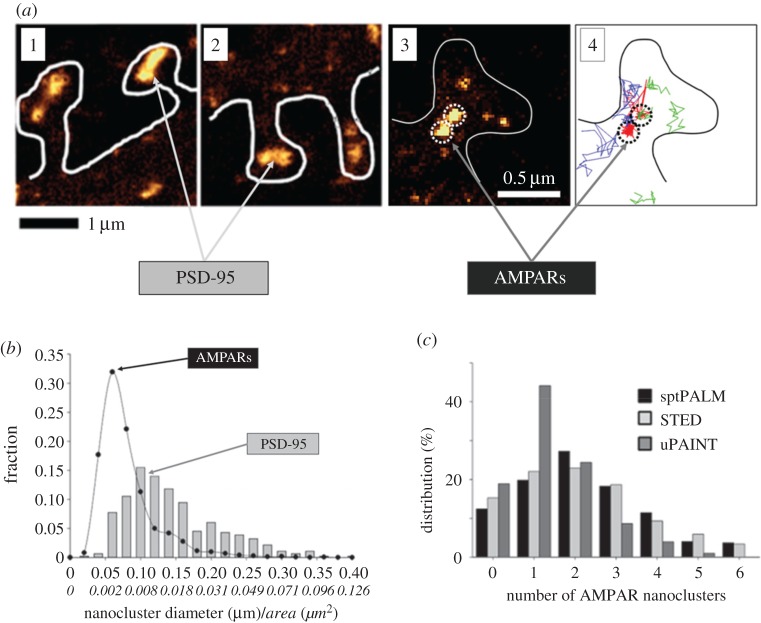
Figure 1a shows images of nanoclusters visualized in [14]. Specifically, (a)1 and (a)2 show PSD-95 nanoclusters in the sptPLAM intensity images of spines having labelled PSD-95. (a)3 shows AMPAR nanoclusters, as visualized using sptPALM, and (a)4 shows the corresponding single-particle trajectories of AMPARs obtained from time-lapse sptPALM recordings. The size of nanoclusters is fairly uniform (figure 1b). AMPAR nanoclusters have a typical length of approximately 70 nm (area approx. 0.004 µm2) [14]. Nanoclusters of PSD-95 are characterized by a diameter of approximately 100 nm (area approx. 0.008 µm2) [14] and are thus roughly similar in size to AMPAR clusters.
Figure 1.
Super-resolution imaging shows that the scaffolding protein, PSD-95 and AMPARs form nanoclusters. (a—1,2) Gallery of super-resolution sptPALM intensity images showing distributed nanoclusters of scaffolding proteins in spines expressing EOS::PSD-95. Synapses contain varying numbers of nanoclusters, with larger synapses containing more nanoclusters. (a—3) sptPALM intensity image of a spine expressing EOS::GluA1. Nanoclusters of AMPARs are outlined by dashed circles. (a—4) Projection of a subset of single-molecule trajectories observed from time-lapse sptPALM recordings in the corresponding spine. Red represents strongly confined trajectories; blue and green represent diffusive and weakly confined trajectories, respectively. (b) Size distribution of AMPAR (closed black circle, GluA1) and PSD-95 (grey bars) nanoclusters obtained by fitting sptPALM images with two-dimensional anisotropic Gaussian functions. Italic tick labels represent estimated area corresponding to the length (principal axis) of the nanoclusters. AMPAR nanoclusters have a typical diameter of approximately 70 nm (approx. 0.004 µm2 in area); PSD-95 nanoclusters are characterized by a length of approximately 100 nm (approx. 0.008 µm2 in area). The relatively small variance of nanocluster sizes is suggestive of a stereotyped modular character of sub-synaptic organization. (c) Probability distribution of number of AMPAR nanoclusters per spine with different imaging techniques (sptPALM, STED and uPAINT). Note the presence of silent synapses that contain no AMPAR nanoclusters. Figures are adapted from Nair et al. [14].
Such visualization of synapses reveals that the number of nanoclusters within a synapse can vary. The probability distribution for the number of AMPAR nanoclusters within a spine is shown in figure 1c; more than 80% of synapses contain one or more AMPAR nanoclusters. Importantly, some synapses have zero AMPAR nanoclusters and thus are likely to correspond to AMPA-silent synapses [19–22].
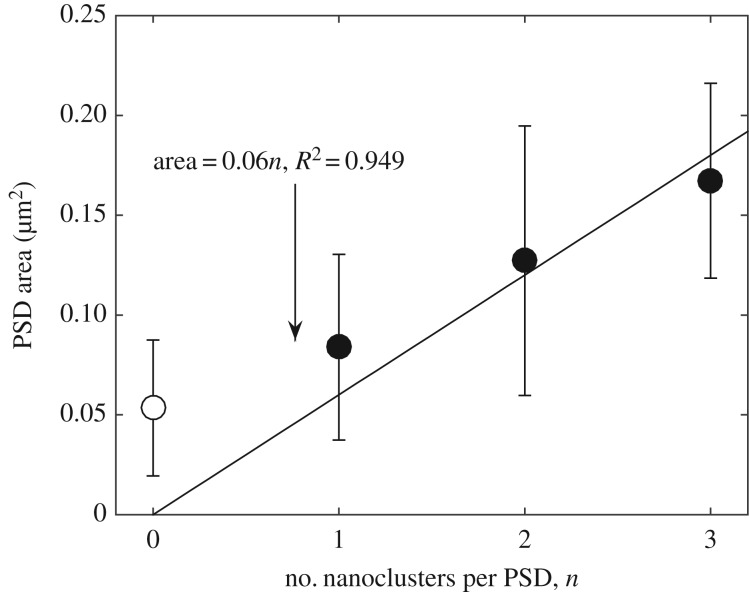
Figure 2 shows how PSD area (as measured in [14] by fitting super-resolution dSTORM images using two-dimensional anisotropic Gaussian functions) varies with the number of AMPAR nanoclusters. The open circle represents silent synapses that contain no AMPAR nanoclusters, whereas solid data points correspond to synapses with one or more AMPAR nanoclusters. In the simplest model, synapses would be composed of an integral number of subunits (we will use the term ‘module’), each of which would contain either no nanocluster or one nanocluster. Fitting the data in figure 2 with a line (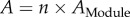 ) through the points for 1, 2, 3 nanoclusters yields a module size of
) through the points for 1, 2, 3 nanoclusters yields a module size of 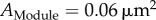 (276 nm in diameter, R2 of the fitting is 0.949). The size of the module is much larger than the typical size of nanoclusters, which is about
(276 nm in diameter, R2 of the fitting is 0.949). The size of the module is much larger than the typical size of nanoclusters, which is about 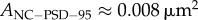 and
and 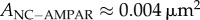 , as shown in figure 1. Thus, by this analysis, about 16% of each module is occupied by the nanocluster (approx. 0.01 µm2); the remaining area (0.05 µm2, 252 nm in diameter) is occupied by material we will term ‘matrix’. Therefore, a silent synapse that only contains matrix should have a typical size of
, as shown in figure 1. Thus, by this analysis, about 16% of each module is occupied by the nanocluster (approx. 0.01 µm2); the remaining area (0.05 µm2, 252 nm in diameter) is occupied by material we will term ‘matrix’. Therefore, a silent synapse that only contains matrix should have a typical size of 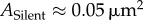 , which is compatible (given the uncertainty) with the open circle data point in figure 2 that shows the size of synapses containing no nanoclusters. Thus, in the simplest theory, the development of a synapse having one nanocluster could arise by adding a nanocluster to a silent synapse having no nanoclusters, i.e. one that contained only matrix. A further important finding is that synapse size is linearly related to the number of nanoclusters (filled circles in figure 2).
, which is compatible (given the uncertainty) with the open circle data point in figure 2 that shows the size of synapses containing no nanoclusters. Thus, in the simplest theory, the development of a synapse having one nanocluster could arise by adding a nanocluster to a silent synapse having no nanoclusters, i.e. one that contained only matrix. A further important finding is that synapse size is linearly related to the number of nanoclusters (filled circles in figure 2).
Figure 2.
Plot of PSD size versus number of AMPAR nanoclusters. PSD sizes were measured from PSD-95 staining using dual-colour dSTORM [14]. The black line is a linear model based on the assumption that synapses are composed of an integer number of modules, each containing matrix material and a single AMPAR nanocluster. Filled circles represent synapses containing AMPAR nanoclusters, and open circle corresponds to silent synapses. Each module is approximately 0.06 µm2 (approx. 276 nm in diameter). The figure is plotted from data in [14]. Error bars indicate cell-to-cell variability. Alternatively, all data points (silent synapses and functional synapses) in figure 2 can also be fitted by a single line as originally done in [14], leading to a module size of approximately 0.04 µm2, which is even smaller than the size of silent synapses. However, as will be described later, other evidence also suggests that silent synapses should be treated as a separate group and should thus be excluded from the linear fit of synapse sizes.
In summary, the data from super-resolution dSTORM imaging [14] are consistent with a model in which silent synapses, which have only matrix, are approximately 0.05 µm2. If a nanocluster (area approximately 0.01 µm2) is added to such a module, the area increases to approximately 0.06 µm2. Larger synapses can be understood as containing an integral number of stereotyped approximately 0.06 µm2 modules, most or all of which contain nanoclusters. This is more consistent with stable size states that differ by a quantal value rather than by a proportional value.
(b). Analysis of electron microscopy data
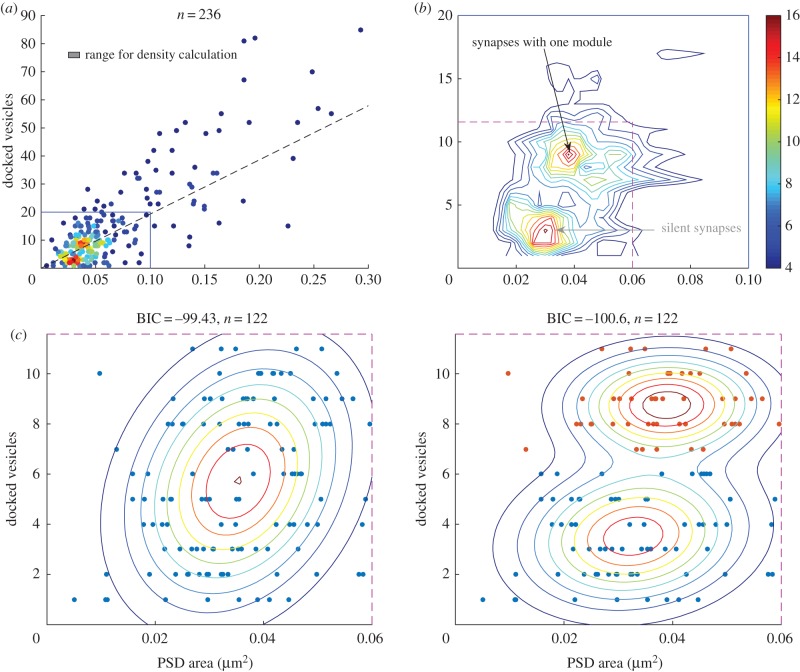
We next considered the analysis of serial section EM data [13] that provides information about synapse area and the number of docked vesicles (defined by the authors as vesicles within 100 nm of the presynaptic grid). The three-dimensional reconstructions were made from these sections [13]. The tissue analysed was from the middle of the stratum radiatum of hippocampal CA1 from three adult male rats (55–65 days old). Plotting number of vesicles versus PSD size, we noted a previously unappreciated aspect of the data: that among the small synapses (blue rectangle region in figure 3a) that constitute most of the data, there appear to be two separable groups. Figure 3b shows the contour plot constructed from figure 3a, which better delineates these two groups. The group of smaller synapses has a synapse area of approximately 0.03 µm2 and only a small number of docked vesicles (approx. three vesicles). The group of slightly larger synapses has an area of approximately 0.04 µm2 but has many more docked vesicles (approx. nine vesicles).
Figure 3.
EM data reveal two distinct groups of small synapses. (a) The number of docked vesicles for 236 synapses is plotted against their PSD areas. The dashed line represents the relation between PSD area and vesicle number as obtained in [13], and the point colour represents the local density of points within the range represented by the box. (b) Contour plot of the scatter plot (region outlined by blue lines in (a) exhibits two clusters of small synapses. The smallest synapses (bottom grey arrow) have an area of approximately 0.03 µm2 and only a small number of docked vesicles (approx. three vesicles). Slightly larger synapses (top black arrow) have an area of approximately 0.04 µm2 but have many more vesicles (approx. nine vesicles). The existence of two distinct types of small synapses, but with only small difference in average size, would suggest that the smaller size synapses might correspond to silent synapses, whereas the slightly larger ones might have a single AMPA-containing module. (c) Scatter points for small synapses are slightly better fit with a two-component (right panel, two groups are marked with different colours) than a one-component Gaussian mixture model (left panel). The smaller group has on average 0.033 µm2 of PSD area and 3.5 vesicles, whereas the larger group has 0.038 µm2 of PSD area and 8.8 vesicles. The fit qualities were evaluated by the BIC, which includes a penalty for more fitting parameters.
Further analysis of these small synapses (area < 0.06 µm2, region outlined by magenta dashed lines in figure 3b) shows that the docked vesicles and PSD area scatter points data are fit somewhat better by a two-component Gaussian mixture model as compared to a one-component model. We obtained similar results if the data were fit in log space. Evaluating the quality of the fits using the Bayes information criterion (BIC), which includes a penalty for more fitting parameters qualities, gave a difference of ΔBIC = 1.2. This value indicates that the observed difference is suggestive, but not demonstrative. While the difference in PSD area of the two groups is small (0.038−0.033 = 0.005 µm2, p = 0.0112), the main separation of the two groups is due to differences in docked vesicle number. On average, the smaller group has 3.5 vesicles, while the larger group has 8.8 vesicles. While the data are strongly suggestive of two groups of synapses, the data are too limited to generate strong statistical support for this assertion; given the limited number of data points, we cannot completely exclude the possibility that the grouping arose by chance. However, it remains noteworthy that the existence of two groups and the average area of each group are consistent with what would be expected from the super-resolution results shown in figure 2. Specifically, it seems likely that the group of synapses with fewer vesicles might correspond to silent synapses lacking any AMPAR (approx. 0.03 µm2, 195 nm in diameter), while the slightly larger synapses (approx. 0.04 µm2, 225 nm in diameter) are ones containing a single AMPAR-containing nanocluster. A larger dataset will be needed to more strongly test this proposal.
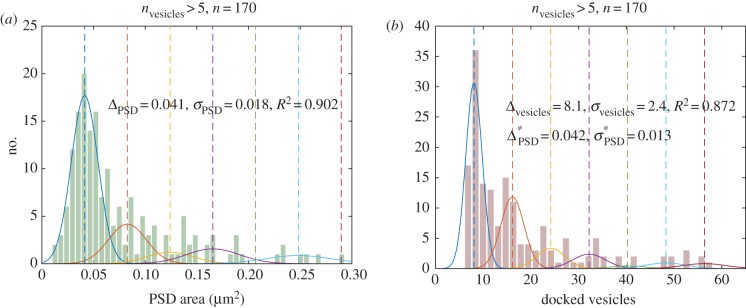
We next sought to determine whether the size distribution of PSDs (or the number of associated vesicles), as measured by EM, might provide direct evidence for a quantal size distribution, as would be predicted on the basis of the model suggested above. The histograms of synapse size and vesicle number are shown in figure 4a,b, respectively (data from CA1 synapses). The quantal nature of synapse growth would predict a series of evenly spaced peaks in these histograms corresponding to an integer number of modules. Because the smallest synapses may have no modules, we eliminated these synapses from the histogram (the synapses shown are associated with more than five vesicles (170 of 236)). Both the histograms for PSD areas and for docked vesicles were fit with a quantal model: 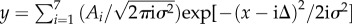 , where i denotes the number of modules. The distribution for synapses with i modules forms a Gaussian peak located at xi = iΔ, where Δ is the quantal increment between adjacent peaks, i.e. the size of a module. Each Gaussian peak is characterized by a width of
, where i denotes the number of modules. The distribution for synapses with i modules forms a Gaussian peak located at xi = iΔ, where Δ is the quantal increment between adjacent peaks, i.e. the size of a module. Each Gaussian peak is characterized by a width of 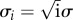 and amplitude of Ai. The normalized amplitude
and amplitude of Ai. The normalized amplitude 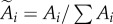 represents the probability of observing a synapse that contains i modules.
represents the probability of observing a synapse that contains i modules.
Figure 4.
Distribution of PSD area and the number of docked vesicles, as obtained by EM. (a) PSD area histogram and (b) docked vesicle histogram of 170 synapses (with more than five docked vesicles). We sought to assess whether these distributions were compatible or incompatible with quantally distributed states, each represented by a Gaussian distribution. Each dashed line marks the peak location, and the solid curve with the same colour represents the individual Gaussian distribution. The quantal increment of PSD area obtained from fitting the PSD area distribution is 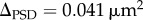 , while the fit to the vesicle number distribution obtained
, while the fit to the vesicle number distribution obtained  , which can be converted to
, which can be converted to 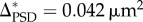 using the relation between PSD area and vesicle number obtained from Bartol et al. [13]. Both estimates for the quantal increment of PSD area are roughly consistent with the super-resolution observations (single module 0.060 µm2). This increment in PSD area is accompanied by the addition of approximately eight vesicles. From the PSD area data, the normalized amplitudes of the first two peaks are
using the relation between PSD area and vesicle number obtained from Bartol et al. [13]. Both estimates for the quantal increment of PSD area are roughly consistent with the super-resolution observations (single module 0.060 µm2). This increment in PSD area is accompanied by the addition of approximately eight vesicles. From the PSD area data, the normalized amplitudes of the first two peaks are 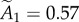 and
and 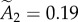 , whereas those from the vesicle data are
, whereas those from the vesicle data are 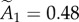 and
and 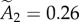 , indicating that about approximately 75% of the synapses contain fewer than two modules.
, indicating that about approximately 75% of the synapses contain fewer than two modules.
Fitting the PSD area and vesicle number distributions with the theoretical distribution described above yields several interesting results. First, the R2 values for PSD area and vesicle number are 0.902 and 0.872, respectively. Second, both histograms, but particularly the vesicle number data, strongly hint at a second peak in the PSD area distribution. Because this peak is twice the size of the first peak, the data are consistent with this part of the distribution being due to synapses having one or two equal size modules. Third, the relative number of synapses in the first (one module) and second (two module) peaks is very similar in figure 4a, b, respectively. These fits suggest that approximately 75% (76% from fitting PSD area, 74% from fitting the vesicles) of the non-silent synapses contain two or fewer modules. Despite this consistency with a quantal distribution, it should be emphasized that the data are too sparse to statistically prove the quantal nature of the distribution. It can be seen in figure 4a that the number of observations contributing to the second peak is only 7 and in figure 4b is only 14. These numbers are too small to prove convincingly that a second quantal peak exists. Thus, the most that can be said at this point is that the data are not inconsistent with quantal peaks and, indeed, provide a suggestion that they exist. To statistically prove the quantal distribution would require more data.
Despite the limitations of these data, they are consistent with the super-resolution data. Specifically, the EM data yield an estimate of Δ, the module size, that is consistent with the super-resolution data. Δ, which was a free parameter in our fit to the EM data, has a value of 0.041 µm2 as calculated from the PSD area distribution and 0.042 µm2, as calculated from the vesicle number distribution. These values are in reasonable agreement with each other and with the value derived from super-resolution work (0.06 µm2 for a non-silent module and 0.05 µm2 for a silent module).
3. Discussion
We have attempted to find a common framework for understanding what EM and super-resolution light microscopy reveal about the rules that govern variation in synapse size. An important observation that strongly constrains possible models is the finding that PSD size is linearly related to the number of nanoclusters of AMPAR (figure 2). This observation is easy to understand in terms of a process that adds stereotyped (quantal) modules to synapses but is difficult to understand if synapses grow by a proportional process. We have been able to obtain estimates of the size of the modular structure from both super-resolution and EM data, and these appear to be reasonably compatible. The super-resolution data [14] show that there are two groups of small synapses, one in which synapses contain no AMPAR nanoclusters (and are therefore silent) and a second group in which synapses are 13% larger and contain a single nanocluster. The EM data [13] similarly reveal two groups: the first (smaller synapses) is notable because its synapses contain very few presynaptic vesicles (two or three vesicles), whereas synapses in the second group are 28% larger and contain nine vesicles on average. To unify the EM and super-resolution data, it seems reasonable to conclude that the smallest synapses observed by both methods correspond to silent synapses. Super-resolution microscopy indicates that these smallest synapses have no nanoclusters of AMPARs and thus are silent. By EM, the smallest synapses have about two or three docked vesicles (note that vesicles must be present at silent synapses to activate N-methyl-d-aspartate (NMDA) channels [23]). The size of these silent synapses, which consist only of what we term ‘matrix’, is approximately 0.03 µm2 (195 nm in diameter) by EM and approximately 0.05 µm2 (252 nm in diameter) by super-resolution. Although these estimations do not match exactly, they are in the same general range and it is possible that such a difference might be methodological. On the one hand, estimation of PSD area using three-dimensional EM data [13] could result in underestimation because of tissue shrinkage during fixation. On the other hand, light microscopy may inherently overestimate size because even a point source has area.
It is important to note that nanoclusters of scaffold proteins and receptors have so far only been identified via super-resolution microscopy, whereas EM analysis has not reported them. The super-resolution microscopy work showed that PSD-95 and AMPAR are present throughout the PSD, but their concentration is elevated within nanoclusters. This elevation was identified based on thresholding of fluorescence intensity and the demonstration that the spatial distribution of proteins is statistically non-random. Such analysis has not yet been applied to EM data but could potentially reveal the nanoclusters.
A definitive demonstration of quantally distributed synapse size could potentially be obtained from the analysis of size distribution of synapses, as measured by EM; these should have quantally spaced peaks. Unfortunately, measurement of synapse size remains tedious, and the number of synapses that have been measured is only a few hundred. Thus, the reported size distribution is noisy (figure 4), precluding any firm conclusions. Nevertheless, when we fit this noisy data with a function that assumes quantally distributed sizes, the fit is reasonably good and the derivation of quantal size (0.041 µm2 by PSD area; 0.042 µm2 by vesicle number) is in reasonable agreement with the values derived from super-resolution light microscopy data (see §2).
These estimates of the size of the elementary module are also compatible with an independent estimate that can be derived from measurement of PSDs in animals in which membrane-associated guanylate kinases (MAGUKs) have been knocked down [24]. Using EM tomography, the authors find that the triple knockdown of MAGUKs (PSD-95, PSD-93 and synapse-associated protein SAP-102) leads to a reduced number of AMPA-type structures and decreased PSD size (from 0.104 ± 0.056 µm2 to 0.032 ± 0.042 µm2). Recordings from such cells reveal that most synapses are silent. It is thus noteworthy that the PSD area in these cells is comparable with the size of the group of smallest synapses of normal cells (figure 3), which we have argued are silent synapses.
Additional information relevant to synapse structure comes from the tomographic study of NMDA receptor (NMDAR) clusters at synapses. Chen et al. [24] identified an NMDAR cluster located at the centre of normal PSDs (these synapses also contained AMPARs) that is 176 nm in diameter. This size matched the observed size of small PSDs lacking AMPARs in the MAGUK knockdown (approx. 180 nm in diameter). This corresponds to an area of 0.03 µm2, again pointing to this as the size of a silent synapse and thus roughly the size of an elementary module.
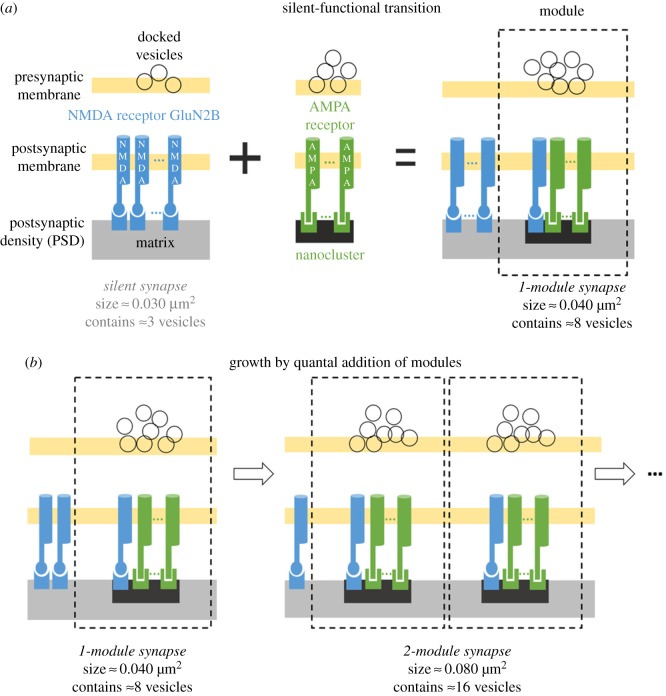
Our analysis of the super-resolution and EM data suggests a linear (quantal) model for synapse growth, as shown in figure 5. The smallest synapses are silent. These have only matrix (no modules) and are associated with two or three vesicles (silent synapse). In an initial LTP-induced growth process, a nanocluster associated with about five vesicles is added. In further growth, integer numbers of modules (matrix + nanocluster) are added. In this linear growth model, memory is structurally encoded by the number of modules. Thus the number of size states is directly associated with the maximum number of modules that can be fitted into a synapse. Given our estimated module size of approximately 0.04 µm2 and the largest measured PSD area [13] (figures 3 and 4), our estimate of the number of distinct synapse size states is approximately 10 (equivalent to 3.3 bits of information). Obviously, a larger size range would be estimated by taking rare outliers in the size distribution that have very small PSD sizes, but attaching physiological importance to rare synapses that are much smaller than the large group of silent synapses seems inappropriate.
Figure 5.
Model for synapse growth by quantal addition of AMPA-containing trans-synaptic modules. (a) Silent synapses are small, with a PSD area of approximately 0.03 µm2 (figure 3b), and are characterized by a matrix with approximately three docked vesicles and no AMPA receptors. A silent synapse (matrix only) can become activated by the addition of an AMPA/PSD-95 nanocluster, which increases the PSD area to approximately 0.04 µm2 (figure 3b) and increases the mean vesicle number to about eight. (b) Additional rounds of synaptic strengthening can occur by adding more functional modules, each contributing about eight vesicles and approximately 0.04 µm2 of PSD area. Note that each nanocluster only takes about 25% of the area of each module, but it is shown as about 50% of the module area solely for the purpose of illustration clarity.
Some comment is warranted about the NMDARs depicted in figure 5. Interestingly, Chen et al. [24] found that, although the number of AMPARs is positively correlated with PSD size, the number of NMDA channels is independent of PSD size [24]. These observations suggest that, during late LTP, increases in PSD size mainly involve the addition of AMPA channels. A recent super-resolution paper has determined that nanoclusters contain GluN2B [15]; we therefore incorporated NMDA receptors into each module in the proposed model. Although LTP induction decreases the current carried by NR2B [25], this appears to involve channel modulation rather than actual loss of NR2B content [26]. Therefore, in the model of figure 5, we suggest that the new modules added after LTP induction have GluN2B-containing nanoclusters. It follows that the GluN2B in the matrix gets depleted, keeping the total number of NMDARs constant. The existence of GluN2B in each nanocluster may be functionally important because GluN2B is a critical binding target of phosphorylated CaMKII [27]. The resulting complex (the CaMKII/NMDAR complex) is critical for the induction [28] and maintenance [29,30] of LTP and may serve as the seed for structural organization of new nanoclusters [27].
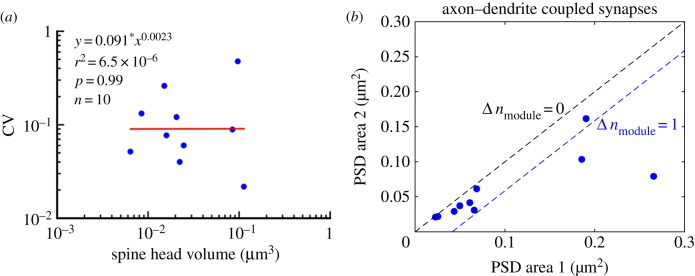
A previous analysis [13] on the EM data analysed here proposed a model in which the functionally distinguishable size states of the PSD vary by a proportional rather than by a quantal increment. The authors investigated the sizes of pairs of synapses making connections between the same presynaptic axon and the same postsynaptic dendrite (axon–dendrite-coupled synapses). They observed closely matched sizes for these pairs, as might be expected based on their common activity history. The authors then used the small size difference between each pair of axon–dendrite-coupled synapses to estimate size variations (CV, coefficient of variation) between functionally similar synapses, noting that the CVs do not show an apparent trend with synapse size (figure 6a), from which they concluded that the absolute variability (and hence limit on distinguishability) increases in proportion to synapse size. Note that volume of the spine head is plotted rather than PSD area, but the two quantities are closely related. Given the evidence for proportional differences in distinguishable size states, the authors used signal detection theory to estimate that the information storage capacity of a synapse is 26 levels (equivalent to 4.7 bits) or greater.
Figure 6.
Variation in synapse size is not inconsistent with the linear growth model. (a) Coefficients of variation (CVs) of spine head volume are plotted for 10 pairs of axon–dendrite-coupled synapses. Based on the lack of a significant size dependence of the CVs, Bartol et al. [13] concluded that a functional change in a synapse involves increasing its size in a proportional manner. (b) PSD areas of these 10 pairs of axon–dendrite-coupled synapses are plotted with the larger synapse on the horizontal axis. The upper blacked dashed line corresponds to the same number of modules and identical PSD areas of the paired synapses. The lower blue dashed line represents the case in which the larger synapse has one more module. Most data points fall between the black and blue lines, indicating that synapses with the same presynaptic and postsynaptic experiences have an almost identical number of modules. This observation is consistent with the linear growth model, where memory is graded by the number of modules contained in a synapse. The plots were generated from data in [13].
The modular growth model that we propose would require that functional states have sizes that differ in a quantal rather than proportional manner, given the linear dependence of synapse size on AMPA nanoclusters [14,16,17] (figure 1). Therefore, we investigated whether the data from Bartol et al. [13] might also be consistent with quantally distributed states. We found that PSD areas from the same dataset can also be accounted for by a quantal (linear) model in which pairs of axon–dendrite-coupled synapses have a number of modules that differ by at most one. As shown in figure 6b, most data points (8 of 10 pairs) fall between the black and blue dashed lines, where the black dashed line denotes identical number of modules, Δnmodule = 0, and the blue dashed line denotes Δnmodule = 1. We conclude that data of Bartol et al. [13] are not incompatible with quantally distributed size states.
4. Other information about gradation of synaptic strength
Recent EM reconstruction work [31] has identified a presynaptic nascent zone, a vesicle-free zone of the presynaptic grid that can be converted to active zone shortly after LTP induction (active zones are regions of the presynaptic grid with docked vesicles and neighbouring non-docked vesicles located less than two vesicle diameters from the presynaptic membrane). Later, after LTP induction, new nascent zones are added as the synapse grows. These observations suggest that small parts of the synapse can be presynaptically silent, a property that is not incorporated into our model (figure 5). One possibility is that, when vesicles are added to nascent zones, this forms a new AMPA-silent module.
The evidence that bears on the question of whether synaptic strength at individual synapses changes by a binary process or by a graded process is very limited because of the difficulty of physiological analysis of single synapses. The work of Petersen [3] demonstrated that potentiation of individual synapses involves a step-like transition from silent to potentiated state, suggesting a binary process. Such step-like changes are much larger than could be accounted for by the addition of a single AMPAR, suggesting that groups of receptors are regulated together. This work was extended by O'Connor et al. [5] to show that plasticity at individual synapses exhibits step-like changes in response amplitudes that are bidirectional. Importantly, a study by Enoki et al. [6] that focused on longer timescales after LTP induction revealed that individual Schaffer collateral synapses are capable of exhibiting more than two levels of synaptic strength. This physiological work is thus consistent with the idea of structural gradation by addition of multiple modules.
5. Conclusion
Rapid progress is now being made in understanding the structure of synapses. The major new insight is that synapses are not uniform structures but, rather, have a trans-synaptic modular composition. Such modular organization was originally predicted on theoretical grounds [22] but now has firm experimental support. Modules may well be units of function in which a transmitter released within a module selectively affects AMPARs within that module [22,32]. These considerations point to the importance of understanding the molecular composition of modules and the way that modules are modified and added after LTP induction. The analysis presented here shows how recent structural findings place strong constraints on models of LTP-induced synaptic modification and open the door for investigation of structure–function relationships.
Acknowledgements
We thank Tom Bartol and Kristen Harris for sharing EM data with us and for useful discussions. We also thank Pengyu Hong for helpful discussion.
Authors' contributions
J.E.L. and M.F.H. conceived the problem and designed the research. K.K.L.L. designed the data analysis code. K.K.L.L., M.F.H. and J.E.L. analysed the data. K.K.L.L., M.F.H. and J.E.L. wrote the paper.
Competing interests
The authors have no competing interests.
Funding
This study was supported by NSF INSPIRE Award number IOS-1526941 (K.K.L.L., M.F.H., J.E.L.); Brandeis Center for Bioinspired Soft Materials, an NSF MRSEC, DMR-1420382 (M.F.H.).
References
- 1.Morris RGM. 2003. Long-term potentiation and memory. Phil. Trans. R. Soc. Lond. B 358, 643–647. ( 10.1098/rstb.2002.1230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. 2010. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 33, 121–129. ( 10.1016/j.tins.2010.01.001) [DOI] [PubMed] [Google Scholar]
- 3.Petersen CC, Malenka RC, Nicoll RA, Hopfield JJ. 1998. All-or-none potentiation at CA3-CA1 synapses. Proc. Natl Acad. Sci. USA 95, 4732–4737. ( 10.1073/pnas.95.8.4732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connor DH, Wittenberg GM, Wang SSH. 2005. Dissection of bidirectional synaptic plasticity into saturable unidirectional processes. J. Neurophysiol. 94, 1565–1573. ( 10.1152/jn.00047.2005) [DOI] [PubMed] [Google Scholar]
- 5.O'Connor DH, Wittenberg GM, Wang SSH. 2005. Graded bidirectional synaptic plasticity is composed of switch-like unitary events. Proc. Natl Acad. Sci. USA 102, 9679–9684. ( 10.1073/pnas.0502332102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enoki R, Hu Y-L, Hamilton D, Fine A. 2009. Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: optical quantal analysis. Neuron 62, 242–253. ( 10.1016/j.neuron.2009.02.026) [DOI] [PubMed] [Google Scholar]
- 7.Lisman JE, Harris KM. 1993. Quantal analysis and synaptic anatomy—integrating two views of hippocampal plasticity. Trends Neurosci. 16, 141–147. ( 10.1016/0166-2236(93)90122-3) [DOI] [PubMed] [Google Scholar]
- 8.Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. 2014. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82, 444–459. ( 10.1016/j.neuron.2014.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer D, Bonhoeffer T, Scheuss V. 2014. Balance and stability of synaptic structures during synaptic plasticity. Neuron 82, 430–443. ( 10.1016/j.neuron.2014.02.031) [DOI] [PubMed] [Google Scholar]
- 10.Ostroff LE, Fiala JC, Allwardt B, Harris KM. 2002. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron 35, 535–545. ( 10.1016/S0896-6273(02)00785-7) [DOI] [PubMed] [Google Scholar]
- 11.Frey U, Morris R. 1997. Synaptic tagging and long-term potentiation. Nature 385, 533–536. ( 10.1038/385533a0) [DOI] [PubMed] [Google Scholar]
- 12.Tang A-H, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. 2016. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 536, 210–214. ( 10.1038/nature19058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartol TM Jr, Bromer C, Kinney J, Chirillo MA, Bourne JN, Harris KM, Sejnowski TJ. 2015. Nanoconnectomic upper bound on the variability of synaptic plasticity. Elife 4, e10778 ( 10.7554/eLife.10778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita J-B. 2013. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J. Neurosci. 33, 13 204–13 224. ( 10.1523/JNEUROSCI.2381-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacGillavry HD, Song Y, Raghavachari S, Blanpied TA. 2013. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron 78, 615–622. ( 10.1016/j.neuron.2013.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukata Y, Dimitrov A, Boncompain G, Vielemeyer O, Perez F, Fukata M. 2013. Local palmitoylation cycles define activity-regulated postsynaptic subdomains. J. Cell Biol. 202, 145–161. ( 10.1083/jcb.201302071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broadhead MJ, et al. 2016. PSD95 nanoclusters are postsynaptic building blocks in hippocampus circuits. Sci. Rep. 6, 24626 ( 10.1038/srep24626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarusawa E, Matsui K, Budisantoso T, Molnár E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. 2009. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J. Neurosci. 29, 12 896–12 908. ( 10.1523/JNEUROSCI.6160-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu GY, Malinow R, Cline HT. 1996. Maturation of a central glutamatergic synapse. Science 274, 972–976. ( 10.1126/science.274.5289.972) [DOI] [PubMed] [Google Scholar]
- 20.Durand GM, Kovalchuk Y, Konnerth A. 1996. Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381, 71–75. ( 10.1038/381071a0) [DOI] [PubMed] [Google Scholar]
- 21.Petralia RS, et al. 1999. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat. Neurosci. 2, 31–36. ( 10.1038/4532) [DOI] [PubMed] [Google Scholar]
- 22.Lisman J, Raghavachari S. 2006. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci. STKE 2006, pre11. ( 10.1126/stke.3562006re11) [DOI] [PubMed] [Google Scholar]
- 23.Liao D, Hessler NA, Malinow R. 1995. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375, 400–404. ( 10.1038/375400a0) [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA, Reese TS. 2015. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc. Natl Acad. Sci. USA 112, E6983–E6992. ( 10.1073/pnas.1517045112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellone C, Nicoll RA. 2007. Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55, 779–785. ( 10.1016/j.neuron.2007.07.035) [DOI] [PubMed] [Google Scholar]
- 26.Swulius MT, Kubota Y, Forest A, Waxham MN. 2010. Structure and composition of the postsynaptic density during development. J. Comp. Neurol. 518, 4243–4260. ( 10.1002/cne.22451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisman J, Raghavachari S. 2015. Biochemical principles underlying the stable maintenance of LTP by the CaMKII/NMDAR complex. Brain Res. 1621, 51–61. ( 10.1016/j.brainres.2014.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barria A, Malinow R. 2005. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48, 289–301. ( 10.1016/j.neuron.2005.08.034) [DOI] [PubMed] [Google Scholar]
- 29.Sanhueza M, McIntyre CC, Lisman JE. 2007. Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor. J. Neurosci. 27, 5190–5199. ( 10.1523/JNEUROSCI.5049-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J. 2011. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J. Neurosci. 31, 9170–9178. ( 10.1523/JNEUROSCI.1250-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell ME, Bourne JN, Chirillo MA, Mendenhall JM, Kuwajima M, Harris KM. 2014. Dynamics of nascent and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus. J. Comp. Neurol. 522, 3861–3884. ( 10.1002/cne.23646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghavachari S, Lisman JE. 2004. Properties of quantal transmission at CA1 synapses. J. Neurophysiol. 92, 2456–2467. ( 10.1152/jn.00258.2004) [DOI] [PubMed] [Google Scholar]