Abstract
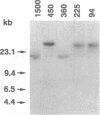
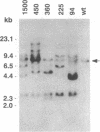
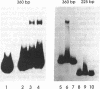
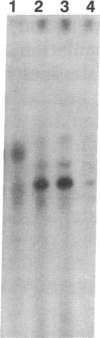
Plant cutin monomers trigger, and glucose suppresses, the expression of the cutinase gene of pathogenic fungi. To identify the cutinase promoter region responsible for induction by the unique plant components, a promoter analysis was done with transformants. Plasmids were constructed that contained (i) the 5' flanking region of the cutinase gene or its deletion mutants from Fusarium solani pisi fused with a chloramphenicol acetyltransferase (CAT) reporter gene and (ii) a constitutive promoter fused with a hygromycin phosphotransferase gene. Hygromycin-resistant transformants of F. solani pisi generated by electroporation were assayed for CAT activity inducible by cutin hydrolysate and for glucose repression of this induction. CAT was induced in a glucose-repressible manner when fused with a 360-base-pair (bp), or longer, segment of the 5' flanking region of the cutinase gene, and deletion of the next 135 bp abolished this induction. Gel retardation assays showed that a protein(s) in nuclear extract from the fungus bound to the 5' flanking region of cutinase gene, and this binding was also abolished when the same 135-bp segment was deleted. These results show that the -225 to -360 segment of the cutinase gene contains a cis-acting regulatory element that binds trans-acting factor(s) in the nuclei. Treatment of the nuclear extract with immobilized phosphatase abolished binding to the promoter, suggesting that binding required a phosphorylated form of the protein. With isolated nuclei, phosphorylation of a protein occurred only in the presence of both cutin monomer and the fungal protein factor. The presence of protein kinase inhibitor H7 during the preincubation of nuclei with the monomer and protein factor inhibited cutinase gene transcription. These results suggest that cutin monomer causes phosphorylation of a transcription factor that binds to the -225 to -360 segment of the cutinase gene and enhances transcription of this gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham J. R. Transformation in fungi. Microbiol Rev. 1989 Mar;53(1):148–170. doi: 10.1128/mr.53.1.148-170.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin L., Kolattukudy P. E. Metabolism of a plant wax paraffin (n-nonacosane) by a soil bacterium (Micrococcus cerificans). J Gen Microbiol. 1968 May;51(3):457–463. doi: 10.1099/00221287-51-3-457. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Lin T. S., Kolattukudy P. E. Induction of a biopolyester hydrolase (cutinase) by low levels of cutin monomers in Fusarium solani f.sp. pisi. J Bacteriol. 1978 Feb;133(2):942–951. doi: 10.1128/jb.133.2.942-951.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Dickman M. B., Kolattukudy P. E. Structure of the cutinase gene and detection of promoter activity in the 5'-flanking region by fungal transformation. J Bacteriol. 1989 Apr;171(4):1942–1951. doi: 10.1128/jb.171.4.1942-1951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge T., Nishimura S., Kobayashi H. Efficient integrative transformation of the phytopathogenic fungus Alternaria alternata mediated by the repetitive rDNA sequences. Gene. 1990 Jun 15;90(2):207–214. doi: 10.1016/0378-1119(90)90181-p. [DOI] [PubMed] [Google Scholar]
- Turgeon B. G., Garber R. C., Yoder O. C. Development of a fungal transformation system based on selection of sequences with promoter activity. Mol Cell Biol. 1987 Sep;7(9):3297–3305. doi: 10.1128/mcb.7.9.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshuk C. P., Kolattukudy P. E. Mechanism by which contact with plant cuticle triggers cutinase gene expression in the spores of Fusarium solani f. sp. pisi. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1704–1708. doi: 10.1073/pnas.83.6.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshuk C. P., Seip E. R., Payne G. A., Adkins C. R. Genetic transformation system for the aflatoxin-producing fungus Aspergillus flavus. Appl Environ Microbiol. 1989 Jan;55(1):86–90. doi: 10.1128/aem.55.1.86-90.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]