Abstract
The tuberculin skin test (TST) is the “gold standard” for detecting infection with Mycobacterium tuberculosis. We compared the TST using purified protein derivative to the QuantiFERON-TB test (QFT). Two groups were examined. Group 1 individuals (n = 66) (low risk) were at low risk for exposure to M. tuberculosis and were not Mycobacterium bovis BCG vaccinated. Group 2 (n = 29) include individuals who were likely to have been exposed to a high prevalence of M. tuberculosis infections and were BCG vaccinated. Group 1 individuals were given a TST. Group 2 individuals were not given a TST because of possible adverse reactions. A 10- to 15-mm indurated area 48 h after TST was considered positive. A positive QFT result was defined as a significant gamma interferon response to M. tuberculosis antigen, Mycobacterium avium antigen, and a nonspecific mitogen stimulus and no response in the negative control. In group 1, 60 of 66 individuals (90.9%) were negative by both methods, and 1 person was positive by both methods. There was one QFT-negative, TST-positive case, one QFT-positive, TST-negative case, and three conditional QFT-positive, TST-negative cases. In group 2, 12 of 29 (41.4%) were positive by QFT and considered likely to be TST positive because of prior BCG vaccination. QFT testing in our low-risk group resulted in an agreement of 96.8%, a sensitivity of 50%, and a specificity of 98.4% compared with TST results. QFT testing with TST in low-risk groups can aid in the detection of latent M. tuberculosis infections.
Mycobacterium tuberculosis is a major global public health problem. The detection and monitoring of M. tuberculosis infections are essential to controlling its spread. Current studies reason that without increasing the efforts to control tuberculosis (TB), by the year 2020, approximately 1 billion people will be newly infected, and an estimated 35 million will die from TB worldwide (13). Contemporary estimates argue that one-third of the earth's population is already infected, with the majority suffering from a latent form of infection (16).
The tuberculin skin test (TST) predominates as the method of choice for detection of latent M. tuberculosis infections. Although widely used, it cannot be trusted as a “gold standard” because of the variability of interpretation and false positives and negatives (1, 2). Placement of the purified protein derivative (PPD), subjective reading of the results, and the unwillingness of individuals to return for test interpretation are responsible for many problems associated with its use. In addition, environmental mycobacteria and the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine commonly cause false-positive results (5, 6).
In this study we set out to compare performance of a new kit, QuantiFERON-TB (QFT) (Cellestis Inc. Valencia, Calif.), used for detection of latent M. tuberculosis infection, to the that of the TST with a normal population of patients who were unlikely to be exposed to M. tuberculosis and in individuals who had received BCG vaccination. Immunity to M. tuberculosis infection is primarily a cell-mediated immune response to M. tuberculosis antigens. When the T cells are stimulated by purified protein derivative from M. tuberculosis, they produce the cytokine gamma interferon (IFN-γ), which allows macrophages to kill the intracellular pathogen (14). The QuantiFERON-TB assay detects the in vitro cell-mediated immune response to M. tuberculosis infection by measuring IFN-γ in whole blood that was incubated with M. tuberculosis PPD. An enzyme-linked immunosorbent assay (ELISA) detects the amount of IFN-γ produced by the T cells (16).
MATERIALS AND METHODS
Study population.
The University of Utah Internal Review Board approved this study (IRB no. 12166). We recruited and enrolled 100 individuals for this study in November 2003 at the ARUP Laboratories Central Facility in Salt Lake City, Utah. Neither immunocompromised nor pregnant individuals participated in this study. All study enrollees provided written consent forms and answered questions that included details about age, prior vaccination with BCG, prior knowledge of sensitization to TST, and whether they had had a TST within the previous 12 months. Individuals were 18 or older and were either newly hired individuals required to take a TB test for employment or existing employees requiring a TB test. The study population consisted of 50 men and 50 women with an average age of 31.5 years old. Ages of the patients ranged from 19 to 59. Based on responses to the form questions and a review of medical records, we divided participants into two groups (9). Group 1, individuals who were considered at “low risk,” included participants who satisfied preemployment screening for TB by TST with no known risk factors for exposure to M. tuberculosis infection. Group 2 exclusively included individuals with a history of BCG vaccination history along with possible risk factors for M. tuberculosis infection or exposure.
Blood collection and tuberculin skin test.
Prior to administration of the TST, blood was drawn from each participant into two 5-ml sodium heparin blood tubes. Blood samples were processed for the QFT assay within 4 h, and results were analyzed within 24 h. Participants in the low-risk group received 0.1 ml of PPD (Aplisol; Parkedale Pharmaceuticals, Rochester, Mich.) intradermally, which is the bioequivalent to 5 US units of PPD-S per test dose. It is administered on the top of the forearm approximately 4 in. below the elbow. The skin area must be free of lesions and away from any veins. Trained health care workers interpreted the results 48 h after application according to the American Thoracic Society (ATS) and Centers for Disease Control and Prevention guidelines (3). The positive interpretation of a TST is an area of induration of ≥10 mm in individuals with increased risk factors and ≥15 mm in individuals with no known risk factors for M. tuberculosis infection (5).
QFT assay.
The QFT assay was performed in accordance with the manufacturer's instructions included in the package insert. The testing was conducted in two parts, an overnight culture of blood with stimulation antigens and the subsequent quantification of IFN-γ production by an ELISA. In part one, 1-ml aliquots of heparinized whole blood were incubated in tissue culture wells (Falcon Multiwell, 24-well; catalog number 353047) with different tuberculin PPDs (human and avian), sterile phosphate-buffered saline (no-antigen control), and phytohemagglutinin (positive mitogen control). Following an overnight incubation at 37°C in a humidified atmosphere, the supernatant plasma was harvested. The IFN-γ in the plasma supernatant was subsequently quantified by ELISA. Results were calculated and interpreted according to the manufacturer's instructions. M. tuberculosis infection was indicated by a human response (Table 1, equation 1) of ≥15% and an avian difference (equation 3) of >10%. A M. avium-M. intracellulare complex infection was indicated by a percent avian response (Table 1, equation 2) of >20% and an avian difference of <10%. Results were considered “indeterminate” if the mitogen response (immune response control) was inadequate: Mitogen − Nil value of <0.5 index value. A percent human response of ≥15 and <30% indicated a conditionally positive result. The probability of an infection, in this conditionally positive group, is based on the identified risk factors to individuals for exposure to M. tuberculosis. If an individual had a human response of ≥15%, they would be considered positive if they had an increased risk for M. tuberculosis exposure. Those with a human response of ≥30% would be considered positive if they were at low risk for M. tuberculosis exposure. Calculations were performed using software provided by the kit manufacturer (Analysis Software v1.51 Cellestis Inc.).
TABLE 1.
Equations for determination of QFT resultsa
| Equation no. | Equation |
|---|---|
| 1 | 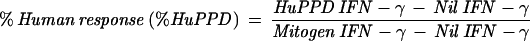 |
| 2 | 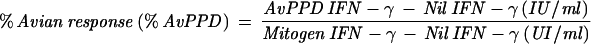 |
| 3 | 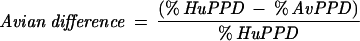 |
AvPPD, avian PPD; HuPPD, human PPD; Nil IFN-γ, negative control.
QuantiFERON-TB time study.
The manufacturer's instructions for the QuantiFERON-TB kit indicate that heparinized whole blood must be processed with 12 h of blood draw. To determine if this time interval could be extended, a time study was performed. Six tubes of whole blood were drawn for six individuals that were members of the previously tested groups. Four of these participants previously had positive results, one had a conditionally positive result, and one had a negative result by QFT. Blood was processed after storage for 12, 24, 48, 72, 96, and 120 h at room temperature. After each incubation period, the samples were processed and plasma was harvested and then stored at −20°C until all time trial incubations were complete. All samples were then assayed for IFN-γ in a single ELISA run.
RESULTS
Although 100 subjects were enrolled in this study, only 95 were included in the final analysis. Five remaining individuals were omitted from this study because they did not return for PPD skin test interpretation.
Standard curve and linearity.
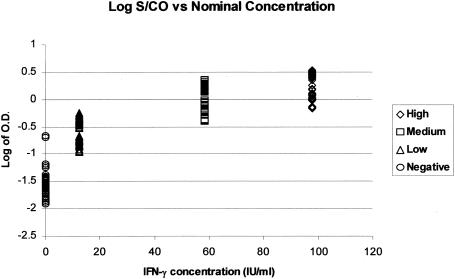
The accuracy of the QuantiFERON-TB test was assessed by measuring the ability of the ELISA to generate a linear curve based on the required standards run with each test. Standards were run in duplicate for smaller runs (<6 patients) and in multiples of four for larger runs. The overall correlation coefficient was based on the individual plate data. This resulted in an r2 value of 0.9992 with a standard deviation of 0.0009 (data not shown), which is well within the requirements of the assay guidelines of an r2 value of ≥0.98. The maximum acceptable difference in zero standards must not vary more than 0.040 (zero standard ± 0.040 change in optical density [OD]). “High,” “medium,” and “low” standards must be within 15% of their individual measured absorbance values (percent coefficient of variation, <15%). The mean absorbance for the high standard must be ≥0.700, as instructed by the manufacturer's guidelines. Figure 1 is a graph plotting the log of the OD values for the three standards (high, medium, and low) plus the negative control used for generating the standard curves during validation (n = 70).
FIG. 1.
Log of OD values of standard curve data points.
Group 1.
Both the QuantiFERON-TB test and the PPD skin test were administered to 66 individuals considered to be at low risk. Sixty of the sixty-six patients (90.9%) tested negative by both assays, and one person (1.5%) tested positive by both methods (Table 2). One patient (1.5%) was positive by QFT and negative by TST, and one individual was negative by QFT and positive by TST. In addition, three enrollees had conditionally positive results by QFT and negative results by TST. Among group 1 members, agreement, sensitivity, and specificity of the QuantiFERON-TB test compared to the TST were 98.6, 50, and 98.4%, respectively. Routine follow-up of TST-positive patients included chest X rays that revealed no active M. tuberculosis.
TABLE 2.
Comparison of the TST to QFT for the total number of low-risk individuals (group 1)a
| QFT result | TST result
|
Total | |
|---|---|---|---|
| POS | NEG | ||
| POS | 1 | 1 | 2 |
| NEG | 1 | 60 | 61 |
| Conditional POS | 0 | 3 | 3 |
| Total | 2 | 64 | 66 |
POS, positive; NEG, negative. Agreement, 96.8%; QFT sensitivity, 50.0%; QFT specificity, 98.4%.
Group 2.
Twenty-nine study patients were not given the TST, as generally recommended, because of possible adverse reactions due to their BCG vaccine history. Twenty-six of twenty-nine individuals in group 2 were immigrants to the United States from other countries and had each received BCG vaccine. The remaining three participants in group 2 were born in the United States and had received the BCG vaccine for reasons related to employment risks. The QuantiFERON assay detected 12 individuals (41.4%) who were positive and who might be considered to have latent M. tuberculosis infection, and 17 (58.6%) were negative (Table 3). Sensitivity of the QuantiFERON assay in patients with prior BCG vaccine history was only 41.8%.
TABLE 3.
QuantiFERON results for BCG-vaccinated people (group 2)a
| QFT result | No. of TST-POS subjects with QFT result |
|---|---|
| POS | 12 |
| NEG | 17 |
| Total | 29 |
POS, positive; NEG, negative. QFT sensitivity, 41.4%.
Time study.
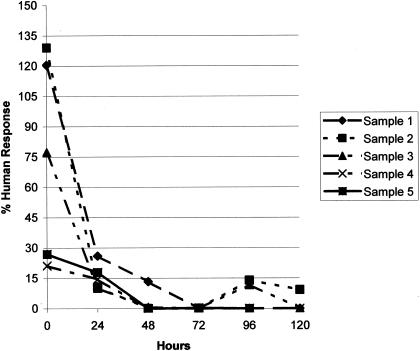
Figure 2 shows results for six individuals with the respective determinations made at six time intervals (12, 24, 48, 72, 96, and 120 h) after blood collection. Sample 1, a previous negative, was negative on all days. Sample 2, a previous conditional positive, was again conditionally positive at 12 h, negative at day 1, and indeterminate on days 4 to 5 due to gross hemolysis. Samples 3 and 4 were previous positives that were both positive at 12 h and then negative from day 1 through day 5. Samples 5 and 6 were previous positives and were conditional positives at 12 h and then negative from day 1 through day 5. Sample 6 was determined to be of a quantity not sufficient for testing at day 5 and could not be analyzed. Hemolysis occurred in all samples in various amounts by days 4 and 5.
FIG. 2.
Time study of IFN-γ detection at timed intervals after blood collection. Values of ≥15% human PPD response are considered positive for individuals with a known risk of M. tuberculosis exposure, and values of ≥30% are considered positive for those with no risk for exposure.
DISCUSSION
The tuberculin skin test is one of the most widely used diagnostic tests ever developed and remains the method of choice for detecting infection due to M. tuberculosis. Detection of cell-mediated immunity to mycobacteria by TST has been used for more than a century as a surrogate marker for infection with M. tuberculosis, both as a diagnostic aid and in epidemiological studies. One problem with the TST is that it is a crude mixture of poorly defined mycobacterial antigens, some of which are shared with M. tuberculosis complex, environmental nontuberculous strains, and the vaccine substrain M. bovis BCG. Despite widespread use of the TST, its disadvantages include the need for patients to return for test interpretation, variability in test administering and reading, booster effect, false-negative results due to intercurrent immunosuppression, and low specificity in those who have been BCG vaccinated previously (4). In addition, false-positive and false-negative reactions to tuberculin PPD can make decisions about preventative therapy problematic. The utility of the tuberculin skin test depends on the prevalence of infection with M. tuberculosis and prevalence of other mycobacteria in the population tested. In low-risk populations, the estimated specificity approached 99%. Sensitivity for the skin test is so variable that estimates remain unclear (6).
The QuantiFERON-TB test was recently developed to overcome some of the limitations of the TST (5, 8, 16). The TST and the QuantiFERON-TB assay are both based on M. tuberculosis purified protein derivative preparations but measure two different reactions. Where the TST is a measurement of delayed-type hypersensitivity on the forearm, the QuantiFERON-TB assay actually measures IFN-γ, an important product of the cell-mediated immune response. We compared these two assays with low-risk individuals with no BCG vaccine history and a probable low level of exposure to M. tuberculosis and in healthy recipients who had received the BCG vaccine as children (7). In agreement with earlier large-scale evaluations of the QuantiFERON-TB test, our study demonstrate a high degree of specificity (98.4%) with nonvaccinated, low-risk donors. This study also demonstrated that 41.4% of healthy BCG-vaccinated individuals have positive results by the QuantiFERON-TB assay (4, 10, 15). Some advantages of the QFT assay include the convenience of having only one visit to a testing site (12). In addition, the inclusion of a positive mitogen control allows the identification of individuals who have negative test results for M. tuberculosis due to their inability to mount a normal in vitro response, which indicates immunosuppression (11). The QFT is more expensive and complex to perform and requires special equipment. In addition, the whole blood must be processed within 12 h of collection, since we showed that positive reactions were negative by 24 h. The QFT assay measures the immune response to tuberculosis, which is primarily cell mediated. The QFT can give false-positive results for patients that have been exposed to nontuberculosis mycobacterial species and to BCG vaccine recipients due to the similarity of the antigens used. The TST false positives have been attributed to the same cross-reactivity and to errors in administering and interpreting the TST. Recent studies to determine which assay is more precise have not been conclusive (3, 4, 7, 8, 10, 16). Proving that a new test is more accurate than the TST for detection of latent M. tuberculosis infection is difficult, since there is no gold standard test against which to measure the accuracy of either the new diagnostics assay or the TST (9). The use of recombinant mycobacterial antigens in place of human PPD may obviate the need for or decrease the number of false-positive results in the QFT assay. This modification will be included in the second generation of the QuantiFERON-TB kit (4, 7).
The requirement for processing the whole blood within 12 h is a major limitation of the QuantiFERON-TB kit in the setting of a reference laboratory, where the time from specimen collection to actual testing often exceeds the 12-h limit.
We found the QuantiFERON-TB assay to have high specificity for the early detection of infection by M. tuberculosis complex organisms with two patient groups. This assay may allow for more-specific risk assessment in population screening and overcome many of the current practical limitations of the TST. In its present form, the QuantiFERON-TB assay results in an unacceptable amount of positivity in a vaccinated population for usefulness in determining latent M. tuberculosis infection under such circumstances. The 12-h time limit on whole-blood processing is also a major weakness in terms of its application in a reference laboratory setting. These two issues lead us to suggest that replacing TST with the QuantiFERON-TB assay is not warranted at this time.
Acknowledgments
This study was in compliance with regulations for research with human subjects and was approved by the University of Utah Institutional Review Board.
This work was supported by the ARUP Institute for Clinical and Experimental Pathology.
REFERENCES
- 1.American Thoracic Society. 1990. Diagnostic standards and classification of tuberculosis. Am. Rev. Respir. Dis. 142:725-735. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376-1395. [DOI] [PubMed] [Google Scholar]
- 3.Bellete, B., J. Coberly, G. L. Barnes, C. Ko, R. E. Chaisson, G. W. Comstock, and W. R. Bishai. 2002. Evaluation of a whole-blood interferon-gamma release assay for the detection of Mycobacterium tuberculosis infection in 2 study populations. Clin. Infect. Dis. 34:1449-1456. [DOI] [PubMed] [Google Scholar]
- 4.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 5.Desem, N., and S. L. Jones. 1998. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin. Diagn. Lab. Immunol. 5:531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huebner, R. E., M. F. Schein, and J. B. Bass, Jr. 1993. The tuberculin skin test. Clin. Infect. Dis. 17:968-975. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, P. D., R. L. Stuart, M. L. Grayson, D. Olden, A. Clancy, P. Ravn, P. Andersen, W. J. Britton, and J. S. Rothel. 1999. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin. Diagn. Lab. Immunol. 6:934-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katial, R. K., J. Hershey, T. Purohit-Seth, J. T. Belisle, P. J. Brennan, J. S. Spencer, and R. J. Engler. 2001. Cell-mediated immune response to tuberculosis antigens: comparison of skin testing and measurement of in vitro gamma interferon production in whole-blood culture. Clin. Diagn. Lab. Immunol. 8:339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMicking, J. D., G. A. Taylor, and J. D. McKinney. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302:654-659. [DOI] [PubMed] [Google Scholar]
- 10.Mazurek, G. H., P. A. LoBue, C. L. Daley, J. Bernardo, A. A. Lardizabal, W. R. Bishai, M. F. Iademarco, and J. S. Rothel. 2001. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 286:1740-1747. [DOI] [PubMed] [Google Scholar]
- 11.Mazurek, G. H., and M. E. Villarino. 2003. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. Morb. Mortal. Wkly. Rep. Recomm. Rep. 52:15-18. [PubMed] [Google Scholar]
- 12.Menzies, R., B. Vissandjee, I. Rocher, and Y. St Germain. 1994. The booster effect in two-step tuberculin testing among young adults in Montreal. Ann. Intern. Med. 120:190-198. [DOI] [PubMed] [Google Scholar]
- 13.Pottumarthy, S., A. J. Morris, A. C. Harrison, and V. C. Wells. 1999. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J. Clin. Microbiol. 37:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raviglione, M. C., D. E. Snider, Jr., and A. Kochi. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 15.Streeton, J. A., N. Desem, and S. L. Jones. 1998. Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int. J. Tuberc. Lung Dis. 2:443-450. [PubMed] [Google Scholar]
- 16.World Health Organization. 2001. Tuberculosis. World Health Organization, Geneva, Switzerland.