ABSTRACT
Myeloid cell leukemia 1 (MCL-1) is a prosurvival BCL-2 protein family member highly expressed in hematopoietic stem cells (HSCs) and regulated by growth factor signals that manifest antiapoptotic activity. Here we report that depletion of MCL-1 but not its isoform MCL-1S increases genomic instability and cell sensitivity to ionizing radiation (IR)-induced death. MCL-1 association with genomic DNA increased postirradiation, and the protein colocalized with 53BP1 foci. Postirradiation, MCL-1-depleted cells exhibited decreased γ-H2AX foci, decreased phosphorylation of ATR, and higher levels of residual 53BP1 and RIF1 foci, suggesting that DNA double-strand break (DSB) repair by homologous recombination (HR) was compromised. Consistent with this model, MCL-1-depleted cells had a reduced frequency of IR-induced BRCA1, RPA, and Rad51 focus formation, decreased DNA end resection, and decreased HR repair in the DR-GFP DSB repair model. Similarly, after HU induction of stalled replication forks in MCL-1-depleted cells, there was a decreased ability to subsequently restart DNA synthesis, which is normally dependent upon HR-mediated resolution of collapsed forks. Therefore, the present data support a model whereby MCL-1 depletion increases 53BP1 and RIF1 colocalization at DSBs, which inhibits BRCA1 recruitment, and sensitizes cells to DSBs from IR or stalled replication forks that require HR for repair.
KEYWORDS: MCL-1, BCL-2, HR, ICL, apoptosis, 53BP1, DSB repair
INTRODUCTION
MCL-1 is a member of the prosurvival BCL-2 family and plays an important role in the regulation of the intrinsic or mitochondrial apoptotic pathway by inhibiting both BH3-only proteins and the proapoptotic proteins (1–3). MCL-1 is mainly located at the outer mitochondrial membrane and inhibits the progression of apoptosis by sequestering executioner proapoptotic proteins BAK and BAX, which are capable of inducing pore formation in the mitochondrial membrane. The subsequent release of cytochrome c into the cytoplasm activates caspases which are responsible for the majority of the macromolecular degradation observed during apoptosis (3). Suppression of BAK and BAX polymerization by MCL-1 is prevented either by MCL-1 degradation or by saturating and inhibiting the MCL-1 binding sites on BAK/BAX with BH3 proteins or mimetics.
Under normal growth conditions, MCL-1 is important for mouse embryonic survival (4) and critical for the survival of neutrophils, lymphocytes, hematopoietic stem cells, and neurons (5). MCL-1 overexpression is the hallmark of several cancers, including hematological malignancies as well as solid tumors. Elevated cellular MCL-1 expression correlates with resistance to drug toxicity and ionizing radiation (IR), whereas its inhibition sensitizes cells to both. The BCL-2 family of proteins is characterized by the presence of BCL-2 homology (BH) domains (1, 2). The MCL-1 protein itself is unique among BCL-2 members in also containing multiple N-terminal PEST motifs in addition to BH1, BH2, BH3, and C-terminal transmembrane (TM) domains. PEST is a signature of short-lived proteins degraded by the ubiquitin pathway, which explains the shorter half-life of MCL-1 than for other BCL-2 proteins (3). MCL-1 also has a smaller isoform (MCL-1S) that has only a BH3 domain and lacks the BH1, BH2, and TM domains (6, 7).
BCL-2 family members have been reported to affect DNA damage repair (8–10), and MCL-1 depletion can decrease Chk1 phosphorylation and increase phosphorylated H2AX (γ-H2AX) in etoposide-treated cells (11). Moreover, MCL-1 has also been shown to interact with several DNA damage response (DDR) proteins, including γ-H2AX, NBS1, and Ku70 (10, 12, 13), but the molecular details as to how MCL-1 may regulate DNA double-strand break (DSB) repair have not been established. We report here that MCL-1 deficiency impairs DNA DSB repair by homologous recombination (HR) and inhibits the resolution of stalled replication forks.
RESULTS
Depletion of MCL-1 increases genomic instability and decreases cell survival postirradiation.
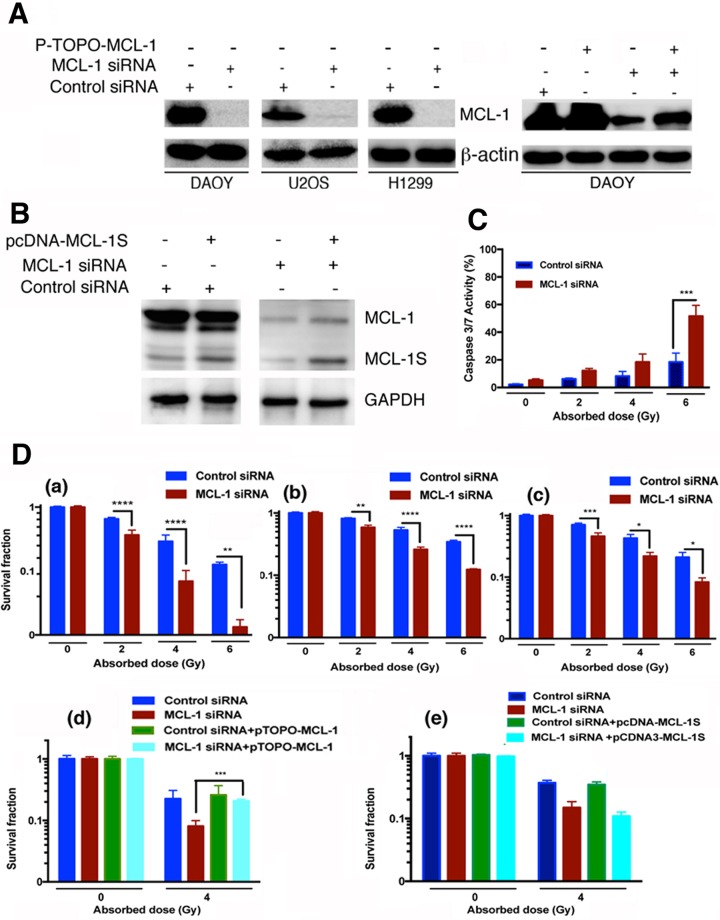
Inhibition of MCL-1 sensitizes cancer cells to chemotherapeutic drugs and IR (12, 14–16). Consistent with the current literature, we found that MCL-1 depletion (Fig. 1) increased caspase 3/7 activity in irradiated DAOY cells (Fig. 1C) (other cell data not shown) and IR-induced killing of DAOY (Fig. 1Da), U2OS (Fig. 1Dc), and H1299 (Fig. 1Db) cells, as assayed by clonogenic survival (17). However, the increase in caspase 3/7 activity was observed only when the cells were exposed to a higher IR (6-Gy) dose, and there was no change in caspase activity at lower doses (2 Gy and 4 Gy). Ectopic expression of MCL-1 (pTOPO-MCL-1) in DAOY cells after depletion of endogenous MCL-1 restored normal radioresistance in the cells (Fig. 1Dd). Expression of pcDNA3-MCL-1S in cells depleted of both MCL-1 and MCL-1S (Fig. 1De) did not restore normal radioresistance, confirming the protective role of MCL-1 but not MCL-1S in postirradiation cell survival.
FIG 1.
Ionizing radiation response in cells with and without MCL-1. (A) The left side shows Western blots representative of MCL-1 knockdown in three cell lines using MCL-1 siRNA, and the right side shows Western blot analysis of MCL-1 levels in control siRNA- and MCL-1 siRNA-treated cells transfected with plasmid pTOPO-MCL-1 to rescue the endogenous MCL-1 knockdown phenotype. (B) The Western blots represent the knockdown of the isoforms of MCL-1 (MCL-1 and MCL-1S) and expression of MCL-1S using pcDNA-MCL-1S. (C) Caspase 3/7 activity in control and MCL-1-depleted cells 48 h postirradiation. (D) Clonogenic survival after exposure of cells to graded IR doses. (a) DAOY cells; (b) H1299 cells; (c) U2OS cells; (d) clonogenic survival after irradiation of DAOY cells with 4 Gy of IR with and without knockdown of endogenous MCL-1 and ectopic expression of MCL-1; (e) clonogenic survival after irradiation of DAOY cells with 4 Gy of IR with and without knockdown of endogenous MCL-1 and MCL-1S and ectopic expression of MCL-1S.
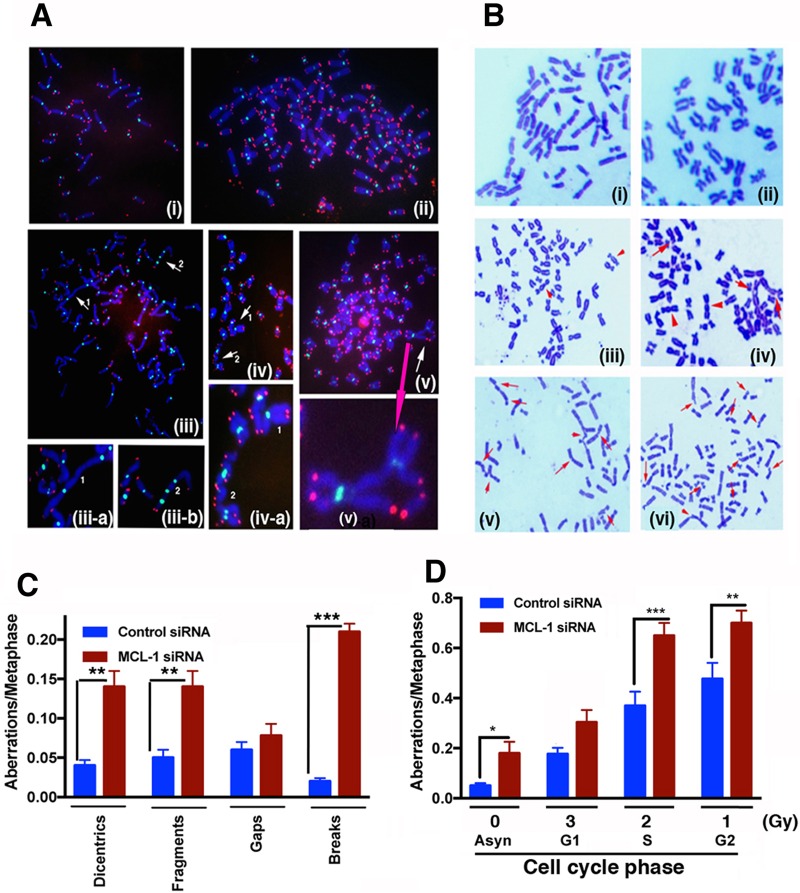
To determine whether the increased radiosensitivity of MCL-1-depleted cells was due to defective DNA damage repair, we measured chromosome aberrations in MCL-1-depleted and control cells before and after irradiation. Genomic integrity is partly dependent upon telomeres whose dysfunction can lead to telomere fusions that produce di- or multicentric chromosomes. We examined telomeres and centromeres by fluorescent in situ hybridization (FISH) analysis and found that MCL-1 depletion results in telomeric signal loss along with dicentric or multicentric chromosome formation and aneuploidy (Fig. 2A, Bi to iii, and C). Similarly, treatment of MCL-1-depleted cells with IR increased the number of cells with aneuploidy and chromosome aberrations (Fig. 2Biv to vi and D). Depletion of MCL-1, therefore, increases the level of spontaneous genomic instability as well as the level of IR-induced genomic instability.
FIG 2.
Chromosome aberration analysis. (A) Telomere and centromere FISH. (i) Control; (ii) MCL-1-depleted cells showing polyploidy; (iii-a) dicentric-type chromosome aberrations; (iii-b) tricentric-type chromosome aberrations; (iv) triradial chromosomes; (v) quadriradial chromosomes. White arrows indicate sites of chromosome damage; the pink arrow indicates the magnified version. (B) (i) Control metaphases; (ii) control siRNA metaphases; (iii to vi) cells depleted of MCL-1. In panel iii, arrowheads show spontaneous dicentric-type aberrations. For panels iv to vi, cells were irradiated, and the following phase-specific aberrations are indicated: G1-type aberrations, e.g., dicentrics and fragments (iv); S-phase-type aberrations, e.g., radials (v); and G2-type aberrations, e.g., gaps and breaks (vi). (C) Categories of spontaneous chromosome aberrations scored included dicentrics, centric rings, interstitial deletions-acentric rings, and terminal deletions. (D) Histogram showing G1-, S-, and G2-phase aberrations. P values were calculated by 2-way analysis of variance (ANOVA) using GraphPad Prism. P values are shown as follows: *, ≤0.05; **, ≤0.01; and ***, ≤0.001.
Cell cycle phase-specific chromosome aberrations were determined based on the frequency of IR-induced chromosomal and chromatid-type aberrations observed at metaphase. G1-specific aberrations detected at metaphase were mostly of the chromosomal type and included a high frequency of dicentrics (Fig. 2Biv). S-phase type aberrations detected at the metaphase were of both the chromosomal and chromatid types (Fig. 2Bv). G2-type aberrations detected at the metaphase were mainly of the chromatid type, with the least number of dicentrics (Fig. 2Bvi). To determine G1-type chromosome damage, cells were treated with 3 Gy and aberrations were scored at the metaphase as described previously (17). After correction for the level of spontaneous aberrations in MCL-1-depleted cells (Fig. 2A, Bii, and D), the IR-induced G1-phase aberrations were not significantly different from control cells (Fig. 2D). To measure defective DNA repair in MCL-1-depleted cells in other phases of the cell cycle, S- and G2-phase chromosome aberrations were evaluated. For S-phase-specific chromosome aberration measurements, cells were treated with 2 Gy of IR and metaphases collected 4 to 6 h after irradiation. Cells depleted of MCL-1 collected postirradiation displayed higher frequencies of chromatid and chromosomal aberrations at metaphase (P < 0.001) than control cells (Fig. 2B and C). Furthermore, when cells were treated with 1 Gy of IR, and metaphases were collected 1 to 2 h after irradiation, depletion of MCL-1 resulted in higher G2 (P < 0.001)-phase-specific chromosome aberrations than in control cells. These results suggest that aberrations are higher in the S and G2 phases of the cell cycle, in which homologous recombination (HR) repair is the predominant mode of DNA repair.
MCL-1 is retained in the nucleus postirradiation through increased chromatin association and contributes to DDR regulation.
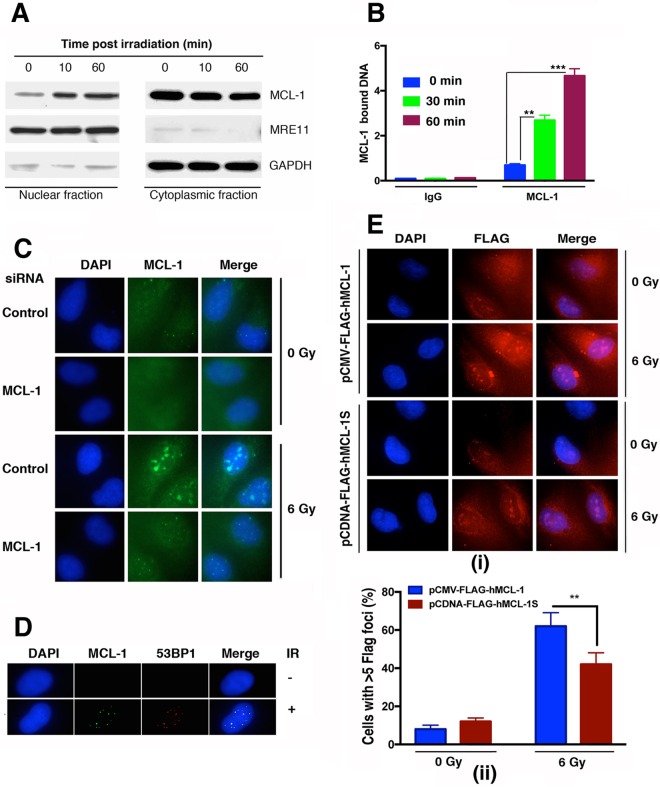
MCL-1 is predominantly a cytosolic protein, and its role in apoptosis in the cytosol is well understood (18), but the DDR occurs largely in the nucleus. To determine whether IR exposure alters the cellular distribution of MCL-1, cells were irradiated with 6 Gy, nuclear and cytoplasmic fractions were prepared, and the relative MCL-1 protein distribution in each was determined by Western blotting. Prior to irradiation, MCL-1 was largely detected in the cytoplasmic fraction, with relatively low levels present in the nuclear fraction (Fig. 3A). However, as soon as 10 min postirradiation, MCL-1 nuclear levels were increasing, and by 60 min, they had increased still further. Measurement by chromatin immunoprecipitation (ChIP) analysis (Fig. 3B) of MCL-1-associated DNA beginning immediately posttreatment indicated that radiation induced a significant increase in chromatin-associated MCL-1, with continued increases through 30 and 60 min postirradiation. Immunostaining for MCL-1 detected IR-induced nuclear foci (Fig. 3C) that colocalized with the DNA damage response protein 53BP1 (Fig. 3D). In order to determine whether MCL-1 or MCL-1S is primarily involved in nuclear formation of foci in response to DNA damage, pCDNA-FLAG-MCL1S and pCMV-FLAG-hMCL-1 were expressed in DAOY cells. Cells ectopically expressing FLAG-tagged MCL-1 or MCL-1S were immunostained with FLAG antibody postirradiation. Cells expressing FLAG-MCL-1 had more formation of foci per cell than did cells expressing FLAG–MCL-1S (Fig. 3E), indicating that MCL-1 is the major protein in repair foci and likely the primary MCL-1 protein associating with DNA postirradiation. These results indicate that IR induces an increase in nuclear MCL-1 levels, probably through increased chromatin binding and nuclear retention. Moreover, at least a portion of this retention is at sites associated with DNA damage repairosome formation (53BP1 foci), suggestive of a role for MCL-1 in DNA repair.
FIG 3.
Increased nuclear MCL-1 levels and colocalization with DNA repair foci in irradiated cells. (A) Western blot analysis for MCL-1 in cytoplasmic and nuclear fractions prepared from control and irradiated DAOY cells. (B) MCL-1 association with chromatin after cells were exposed to IR. ChIP was performed with MCL-1 antibody, and DNA was quantified by absorbance at 260/280 nm by NanoDrop 2000 as described earlier (35) (C) For immunostaining with MCL-1 antibody, DAOY cells were fixed with 100% methanol (5 min) and then blocked in 1% BSA–10% normal goat serum–0.3 M glycine–0.1% phosphate-buffered saline with Tween 20 (PBST) for 1 h. The cells were the incubated with Alexa Fluor 488-tagged MCL-1 antibody at a 1/250 dilution overnight at 4°C. Nuclear DNA was labeled in blue with DAPI. As is evident from the immunostaining, MCL-1 forms distinct foci postirradiation which disappear after MCL-1 knockdown. (D) Immunostaining for colocalization of MCL-1 with 53BP1 was done, which shows that MCL-1 is recruited at the sites of DNA double-strand breaks. (E) (i) pCDNA-FLAG-hMCL-1S and pCMV-FLAG-hMCL-1 were expressed in DAOY cells, and immunostaining was done for FLAG to depict the efficiency of focus formation of FLAG–MCL-1 or FLAG–MCL-1S postirradiation. (ii) Cells with more than 5 FLAG–MCL-1 or FLAG–MCL-1S foci are represented. P values are shown as follows: **, ≤0.01, and ***, ≤0.001.
Depletion of MCL-1 alters the DNA damage response.
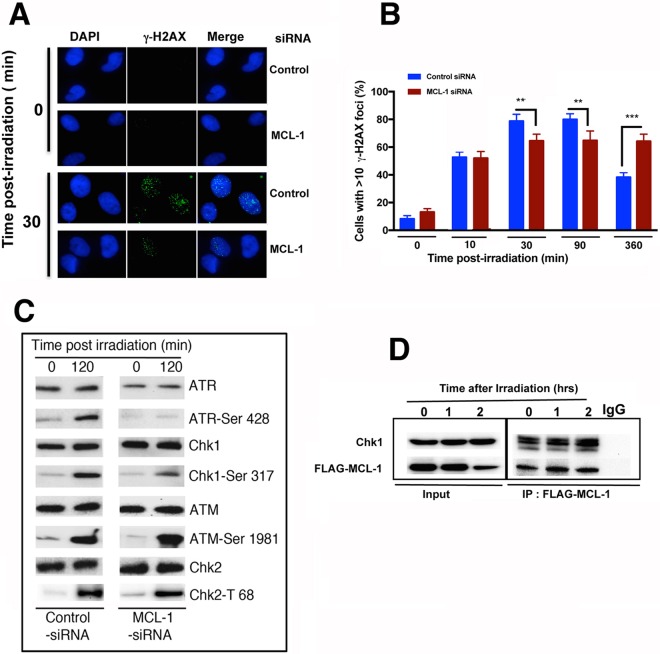
To determine whether the increase in IR-induced chromosomal aberrations in MCL-1-depleted cells was due to a defective DNA damage response or DSB repair process, we examined whether depletion of MCL-1 affected IR-induced phosphorylation of H2AX at serine 139 (γ-H2AX), a surrogate marker for DNA damage detection and/or repair. At 10 min postirradiation, the initial level of γ-H2AX foci in MCL-1-depleted cells was similar to that in control cells; however, by 30 min and 90 min postirradiation, control cells exhibited a further increase in foci, while MCL-1-depleted cells had little or no increase and then contained significantly fewer γ-H2AX foci overall (Fig. 4A and B). More significantly, and in contrast to the case with control cells, the number of γ-H2AX foci did not decline by 360 min postirradiation, the latter observation indicating defective DNA repair. The reduced number of cells with γ-H2AX foci seen in MCL-1-depleted cells could be due to reduced activity of either ATM or ATR, two kinases activated by IR which subsequently phosphorylate H2AX Ser139. ATM can be activated by IR throughout the cell cycle (19); however, depletion of MCL-1 did not affect the IR-induced increase in phosphorylated ATM-Ser1981 levels, an indication of normal ATM activation (Fig. 4C). IR-induced phosphorylation of ATR was largely absent in MCL-1-depleted cells (Fig. 4C). When the levels of the downstream effectors and phosphorylation targets of ATR (Chk1-Ser 317) and ATM (Chk2-Thr68) following irradiation were compared, it was found that MCL-1 depletion reduced Chk1-Ser317 phosphorylation but not Chk2-Thr68 phosphorylation, further supporting the argument that decreased focus appearance of γ-H2AX is due to ATR dysfunction in MCL-1-depleted cells.
FIG 4.
Altered DNA damage response in MCL-1-depleted cells. (A and B) Exponentially growing cells were irradiated with 2 Gy, and the appearance or disappearance of γ-H2AX foci was determined by immunostaining. (C) Phosphorylation status of ATR, Chk1, ATM, and Chk2 in MCL-1-depleted and parental cells postirradiation was determined by Western blotting with corresponding antibodies as described in Materials and Methods. (D) Coimmunoprecipitation experiment showing Chk1 interaction with MCL-1 increases postirradiation. P values were calculated by 2-way ANOVA using GraphPad Prism. P values are shown as follows: **, ≤0.01; ***, ≤0.001; and ****, ≤0.0001.
Previous studies have reported an interaction between MCL-1 and Chk1 in etoposide-treated HeLa cells and that this interaction is important in ATR-mediated regulation of Chk1 phosphorylation (11). In order to determine whether MCL-1 interacts with Chk1 in irradiated cells, FLAG–MCL-1 was transiently overexpressed in cells and immunoprecipitated from cell extracts, and the associated Chk1 was detected by Western blotting with anti-Chk1 antibody. As shown in Fig. 4D, Chk1 and MCL-1 coimmunoprecipitated and IR, like etoposide, induced a small increase in the interaction.
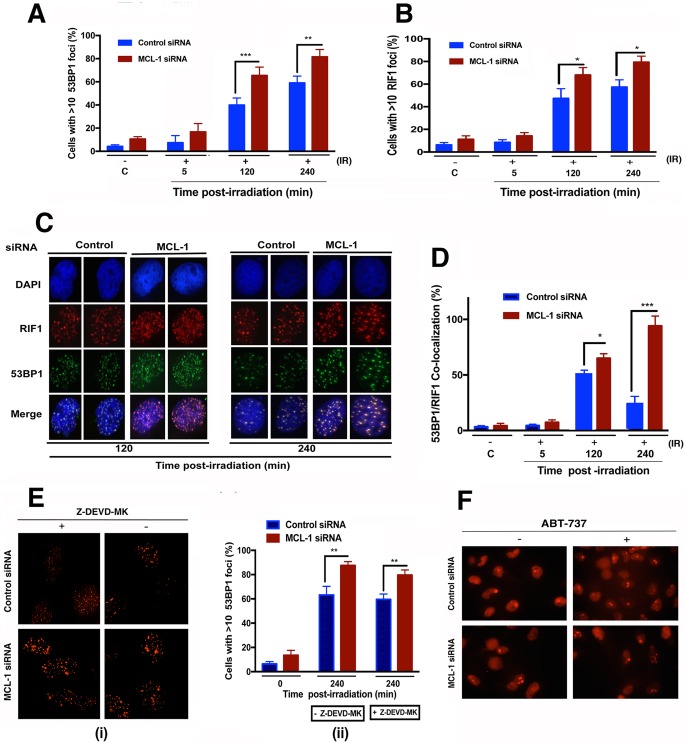
Increased IR-induced 53BP1/RIF1 foci in MCL-1-depleted cells.
Formation of the 53BP1/RIF1 complex enhances DSB repair by nonhomologous end joining (NHEJ) and blocks the recruitment of resection proteins associated with HR pathway repair (20). To test whether depletion of MCL-1 alters the formation of 53BP1/RIF1 foci at DSBs, cells with and without MCL-1 depletion were exposed to 4 Gy of IR and examined for 53BP1 and RIF1 focus formation at 2 and 4 h postirradiation. In MCL-1-depleted cells, there was greater formation of 53BP1 and RIF1 foci postirradiation than in nondepleted cells (Fig. 5A and B). There was also a higher frequency of 53BP1 and RIF1 focus colocalization in MCL1-depleted cells than in controls (Fig. 5C and D), and the number of colocalized foci did not diminish with time as observed in control cells. These results are consistent with the fact that NHEJ in MCL-1-depleted cells is little affected, as no significant increase in G1-specific aberrations was observed (Fig. 2D).
FIG 5.
MCL-1 regulates recruitment of 53BP1 and RIF1 to IR-induced DSBs. (A and B) Control and MCL-1-depleted cells were treated with 4 Gy, fixed postirradiation, and immunostained for 53BP1 and RIF1. (C) Coimmunostaining for 53BP1 and RIF1 was done for fixed cells postirradiation. (D) 53BP1 and RIF1 foci were counted for 3 sets of 25 cells, and the percent colocalized 53BP1/RIF1 foci was calculated relative to total number of foci (53BP1 plus RIF1). (E) Pretreatment of cells with caspase 3 inhibitor Z-DEVD-MK has no effect on 53BP1 focus levels postirradiation in MCL-1-depleted cells. (F) Treatment of cells with BH3 mimetic ABT-737 (10 μM) for 24 h did not induce 53BP1 focus formation in either control or MCL-1-depleted cells. P values were calculated by 2-way ANOVA using GraphPad Prism. P values are shown as follows: *, ≤0.05; **, ≤0.01; and ***, ≤0.001.
Exposure to 6 Gy of IR can induce caspase 3/7 activation in MCL-1-depleted cells (Fig. 1C). To test whether inhibition of the executioner caspase 3 had any effect on 53BP1 focus formation and hence repair of damaged DNA, MCL-1-depleted cells were pretreated for 4 h with 10 μM Z-DEVD-MK and then exposed to 6 Gy of irradiation. Pretreatment with Z-DEVD-MK had no significant effect on 53BP1 focus formation compared to that in untreated cells at 4 h postirradiation (Fig. 5E). Moreover, treatment of MCL-1-depleted or control cells for 24 h with a 10 μM concentration of the BH3 mimetic ABT-737 (which neutralizes BCL-2, BCL-XL, and BCL-W) did not induce any 53BP1 focus formation in these cells. These two results confirm that the contribution of MCL-1 to DNA repair is independent of its classical antiapoptotic functions. Locking or enhancing the apoptotic pathway with nongenotoxic drugs did not alter the level of IR-induced DNA damage in MCL-1-depleted cells. Since the frequency of 53BP1 and RIF1 colocalization is higher in MCL-1-depleted cells and 53BP1 contributes to repair pathway selection, we next examined how MCL-1 depletion affects localization of proteins involved in DSB repair by HR.
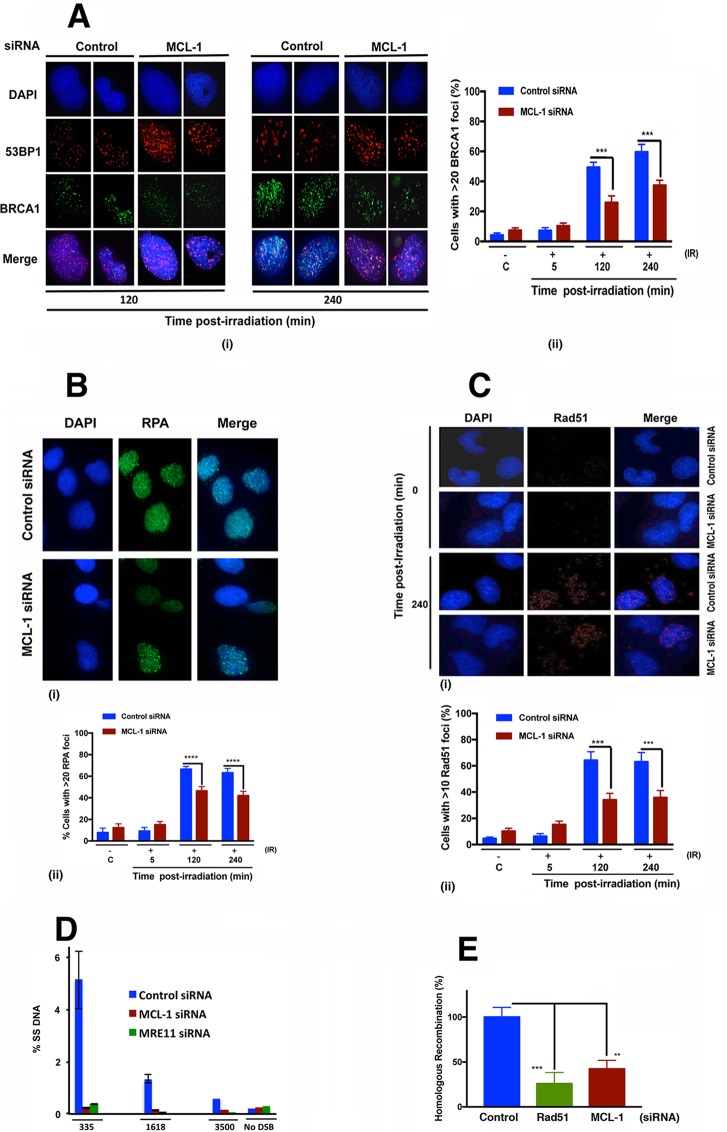
MCL-1 depletion reduces DSB repair by HR.
Repair of DSBs by HR involves BRCA1 recruitment to replace 53BP1, which is known to inhibit the MRN and CtIP complex-mediated end resection step of HR repair (21). Resection is followed by loading of RPA onto the exposed single-stranded DNA (ssDNA), with RPA subsequently being displaced to allow for loading and polymerization of Rad51, to initiate the homologous pairing and strand exchange steps of HR (22). Since we observed an increased number of 53BP1 and RIF1 foci in MCL-1-depleted cells (Fig. 5A and B), we determined whether MCL-1 depletion affected BRCA1 focus formation (Fig. 6A). The frequency of 53BP1/RIF1 colocalized foci in control cells treated with 6 Gy of IR (Fig. 5C) was low, while formation of BRCA1 foci was high (Fig. 6Ai). In contrast, the frequency of 53BP1/RIF1 colocalizing foci postirradiation was high in MCL-1-depleted cells, whereas BRCA1 focus formation was decreased. We further looked at the percentage of cells with >20 BRCA1 foci in control and MCL-1-depleted cells postirradiation and found that more control cells (60%) had greater than 20 foci per cell than did MCL-1-depleted cells (35%) (Fig. 6Aii). BRCA1 facilitates the recruitment of RPA and Rad51 postirradiation. The recruitment of RPA to the ssDNA was detected by RPA focus formation postirradiation (10 Gy), and fewer cells were detected with greater than 20 RPA foci in MCL-1-depleted cells (40%) than was the case with control cells (70%) (Fig. 6B). Similarly, the frequency of cells with >10 Rad51 foci was also lower in MCL-1-depleted cells (30%) than in the control cells (60%) (Fig. 6C). To determine whether the decrease in RPA and Rad51 focus formation in MCL-1-depleted cells postirradiation was due to decreased ssDNA production as a result of defective resection, we did a quantitative resection assay using the ER-AsiSI system (23). In this resection assay system, MRE11-depleted cells were used as a positive control for loss of resection. Compared to the level of ssDNA in control cells, depletion of MCL-1 resulted in an approximately 20-fold decrease in ssDNA product at the site nearest (335 nucleotides [nt]) the AsiSI-induced DSB. Similarly lower levels of ssDNA production were measured at sites more distant from the AsiSI/DSB site (1,618 and 3,500 nt) compared to DNA resection levels in control cells (Fig. 6D). The decrease in DNA resection in MCL-1-depleted cells was similar to the decrease observed in MRE11-depleted cells, and MRE11 is known to be required for DNA end resection during DSB repair by HR. A direct examination of the requirement for MCL-1 in DSB repair by HR was carried out by using a DR-GFP assay, in which reconstitution of a defective green fluorescent protein (GFP) gene is dependent upon HR repair of an introduced DSB (Fig. 6E). As measured by this assay, the efficiency of HR-mediated DSB repair in MCL-1-depleted cells was decreased 60% relative to HR repair efficiency in control cells.
FIG 6.
HR is defective in MCL-1-depleted cells. Cells were irradiated with 6 Gy and coimmunostained with 53BP1 and BRCA1 antibodies. (A) (i) 53BP1 and BRCA1 staining; (ii) histogram of BRCA1 foci. (B) (i) RPA staining; (ii) histogram. (C) (i) Rad51 staining; (ii) histogram. (D) A quantitative resection assay was done using the ER-AsiSI system, as described in Materials and Methods. (E) The HR assay was performed by DR-GFP reporter assay as described in Materials and Methods. P values were calculated by 2-way ANOVA using GraphPad Prism. P values are shown as follows: **, ≤0.01; ***, ≤0.001; and ****, ≤0.0001.
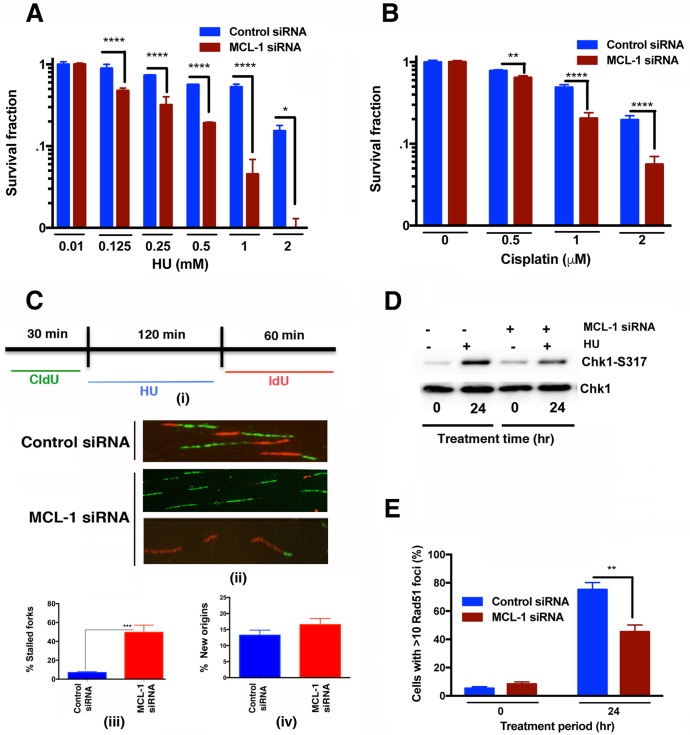
Loss of MCL-1 increases DNA replication fork stalling under stress conditions.
Hydroxyurea (HU) treatment induces replication stress by depleting cellular nucleotide pools, inhibiting DNA replication, and the resulting replication fork stalling, in turn, disrupts the replication machinery, leading to fork collapse and the generation of DSBs that are subsequently repaired by Rad51-mediated HR (24). Since depletion of Chk1 is known to affect resolution of stalled replication forks (25) and MCL-1 interacts with Chk1, we tested whether MCL-1-depleted cells have a greater sensitivity to HU-induced replicative stress. MCL-1-depleted and control cells were treated with increasing doses of HU (0 to 2,000 μM), and clonogenic survival was measured (Fig. 7A). MCL-1-depleted cells displayed significantly increased sensitivity toward HU. In addition, survival of MCL-1-depleted cells after treatment with cisplatin, which induces replication stress by producing intra- or interstrand DNA cross-links (ICLs) that stall DNA replication forks, was also decreased in comparison to that of control cells (Fig. 7B). Replication stress in MCL-1-depleted cells was measured more directly by means of a DNA fiber assay (Fig. 7C). Control and MCL-1-depleted cells were pulse-labeled with 5-iododeoxyuridine (IdU), treated with HU for 2 h to induce replicative stress by nucleotide pool depletion, and then washed and pulse-labeled with 5-chlorodeoxyuridine (CldU). After removal of the HU block, MCL-1-depleted cells were observed to have fewer contiguous ldU/CldU signals (Fig. 7Ci), indicating that the restart of previously initiated replication origins was decreased compared to that in control cells, which readily resumed DNA synthesis (Fig. 7Cii). Moreover, we also observed a higher percentage of stalled DNA replication forks in MCL-1-depleted cells than in control cells (Fig. 7Ciii), while there was no effect of MCL-1 depletion on initiation of new origins of replication (Fig. 7Civ). Failure to resume DNA replication at stalled forks implies defective resolution, which is dependent upon HR repair. We used immunolocalization to measure DSB repairosome sites that had progressed into HR repair after HU-induced replicative stress, as indicated by Rad51 focus formation, and found that MCL-1 depletion decreased formation of foci (Fig. 7D). Moreover, the phosphorylation status of Chk1 post-HU treatment was measured by Western blotting, and the level was decreased in MCL-1-depleted cells (Fig. 7E), further suggesting that HR is defective in MCL-1-depleted cells treated with agents that induce replication stress.
FIG 7.
Decreased reinitiation of stalled DNA replication forks in MCL-1-depleted cells. (A and B) Clonogenic survival assay after treatment of control and MCL-1-depleted cells with hydroxyurea (HU) (125 to 2,000 μM) and cisplatin (0.5 to 2 μM), respectively. (C) (i) DNA labeling and HU treatment protocol for single DNA fiber analysis. (ii) Representative images of replication tracks from MCL-1-depleted and parental cells. (iii) Quantification of stalled forks determined by fiber analysis with only IdU signal after 2 h of HU treatment. MCL-1 cells show the least incorporation of CldU and hence maximum frequency of cells with stalled forks. (iv) Quantification of new origins as determined by CldU signal after 2 h of HU treatment. (D) Western blot showing the Chk1-S317 phosphorylation status in MCL-1-depleted cells treated with hydroxyurea (2 mM). (E) Immunostaining of cells to detect Rad51 foci in control and MCL-1 knockdown cells after treatment of these cells with HU for 24 h. P values were calculated by 2-way ANOVA using GraphPad Prism. P values are shown as follows: *, ≤0.05; **, ≤0.01; ***, ≤0.001; and ****, ≤0.0001.
DISCUSSION
MCL-1 is predominantly present in the cytosol, where it is a well-established regulator of the mitochondrial or intrinsic pathway of apoptosis (2). Nuclear localization of MCL-1 or MCL-1S has also been detected and shown to be involved in cell cycle regulation and the DNA damage response (6, 7, 11). Depletion of MCL-1 enhances cancer cell sensitivity to IR and chemotherapeutic drugs (14), with the IR resistance previously being attributed to resistance to apoptotic death. In this study, we examined an additional direct role for MCL-1 in DNA damage repair involving the HR pathway. Consistent with the current literature (26), we found that MCL-1 depletion enhances cell killing. MCL-1 is mainly concentrated in the cytoplasm, but we found that nuclear levels increase following IR, likely the result of increased chromatin binding. More specifically, we found for the first time that MCL-1 localized to sites of DNA damage, as indicated by colocalization with 53BP1 foci.
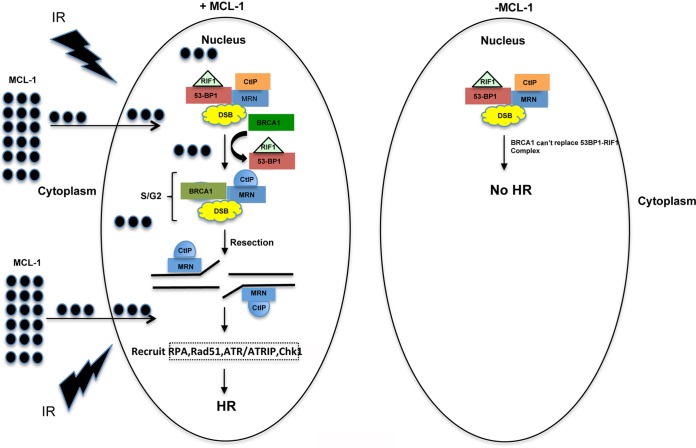
MCL-1-depleted cells have reduced survival even at radiation doses less than 6 Gy; however, an increase in caspase 3/7 activity was observed at 6 Gy and above, suggesting that apoptosis is activated only when MCL-1-depleted cells are irradiated with higher IR doses. Consistent with the reduced survival, MCL-1 depletion also increases spontaneous genomic instability and IR-induced chromosomal aberrations, specifically those aberrations arising from defective repair during the S and G2 phases of the cell cycle. The decrease in γ-H2AX foci in MCL-1-depleted cells at 30 and 60 min postirradiation also indicates that in the absence of MCL-1, DSB signaling is partially impaired. Our data indicate that the decrease in survival and increase in genomic instability in the absence of MCL-1 may be due to impaired DNA repair processes (Fig. 8). The failure of MCL-1S expression to rescue cell survival and its tendency to form fewer foci than with MCL-1 postirradiation suggest that BCL-2 homology domains (BH1 and BH2) which are absent in this isoform are important for cell survival as well as DNA damage repair.
FIG 8.
MCL-1-deficient cells are defective in the DNA repair pathway. The left side shows that translocation of MCL-1 to the nucleus contributes to repair of damaged DNA by the HR pathway, whereas the right side shows that cells deficient in MCL-1 increase the recruitment of 53BP1/RIF1 complex at the DNA damage sites, which prevents the repair of DNA by the HR pathway. Our results show that MCL-1 regulates three important steps in the HR pathway: (i) increase in 53BP1/RIF1 localization at the DNA damage sites, (ii) decrease in DNA end resection or formation of ssDNA, and (iii) decrease in ATR/Chk1 phosphorylation.
The increased colocalization of 53BP1 and RIF1 and decreased BRCA1 focus formation suggest a shift in the DNA repair pathway toward NHEJ in MCL-1-depleted cells. 53BP1 is known to inhibit DNA end resection during the G1 phase of the cell cycle, thus suppressing DSB repair by the HR pathway. Repair during G1 phase is accomplished predominantly by NHEJ, as a homologous DNA template is not available to carry out HR repair (27–29). When a template DNA strand is available during the S/G2 phases of the cell cycle, BRCA1 recruits HR-related proteins to activate the resection process and initiate HR-based DNA repair (30). Our results demonstrate that MCL-1 depletion affects the HR pathway by enhancing the colocalization of 53BP1 and RIF1 to block the recruitment of BRCA1, which is essential for DSB repair by HR.
The impaired recruitment of HR-related proteins in MCL-1-depleted cells is quite obvious, as the frequency of cells with BRCA1, RPA, and Rad51 foci is significantly lower. The lower frequency of RPA and Rad51 foci seen in MCL-1-depleted cells suggests that MCL-1 may be critical for DNA end resection, an early step in HR-mediated repair.
Recent studies suggest that the ssDNA tails produced during DNA end resection activate DNA damage checkpoints that slow down cell cycle progression and allow time for repair. ATR, through its associated protein ATRIP complex, has been shown to bind the ssDNA-RPA complex after DNA end resection, which, in turn, activates ATR (31). ATR then phosphorylates Chk1 to activate the checkpoint pathway and DNA damage repair (32, 33). In MCL-1-depleted cells, ATR autophosphorylation as well as Chk1 phosphorylation is impaired, possibly due to deceased levels of RPA-coated ssDNA for ATR/ATRIP complex binding and activation. Chk1 phosphorylation is required for interaction with Rad51, and Chk1 inhibition reduced Rad51 focus formation (33). Our results demonstrating that MCL-1 depletion decreased Chk1 phosphorylation and also reduced Rad51 focus formation provide further support for MCL-1 regulation of the HR pathway.
Hydroxyurea-induced replication stress, a result of deoxynucleoside triphosphate (dNTP) pool depletion, initially causes replication fork stalling that can progress to fork collapse. In human cells, agents which induce replication fork stalling (such as HU, campothecin, or thymidine) utilize HR to resolve collapsed forks and enable cell survival (24). Chromatin fiber analysis suggests that MCL-1-depleted cells form higher levels of stalled/collapsed forks than control cells. Our results are consistent with previous findings and elucidate the role of MCL-1 in regulation of replication stress. Rad51 has been shown to have two distinct roles during early and late replication blocks. It promotes restart of replication forks when forks are still intact, and it contributes to fork-associated DSB repair after replication fork collapse (24). The low residual levels of Rad51 foci and increased stalled forks in MCL-1-depleted cells after treatment with HU suggest that the stalled forks were not being resolved and thus DSB repair by HR did not take place. These results further support an important role for MCL-1 in IR-induced DSB repair as well as repair in response to replication stress. In summary, the present results reveal a new role for MCL-1, unrelated to its direct antiapoptotic function, in HR-mediated DSB repair and stalled replication fork resolution. Thus, a combinatorial approach of depleting MCL-1 in conjunction with IR may provide an important therapeutic improvement for treatment of cancers that are resistant to IR or drugs inducing replication stress.
MATERIALS AND METHODS
DAOY, H1299, and U2OS cells were maintained and transfected with plasmids as described previously (17, 34). Resection assay ER-AsiSI U2OS cells were a gift from Tanya T. Paull (University of Texas at Austin, TX). MCL-1 overexpression plasmid pTOPO-MCL-1 was purchased from Addgene (Cambridge, MA). pCMV-Flag-hMCL-1 (Addgene plasmid 25392) was a gift from Roger Davis. pCDNA3-MCL-1S was a kind gift from Jeehyeon Bae (School of Pharmacy, Chung-Ang University, Seoul, Republic of Korea). SMARTPOOL:on-target plus MCL-1 small interfering RNA (siRNA) and scrambled control siRNA were obtained from Dharmacon Research (Lafayette, CO). ABT-737 (S1002) was purchased from Selleckchem. Z-DEVD-FMK was purchased from R & D Systems (FMK004). Lipofectamine RNAiMAX transfection reagent was purchased from Life Technologies (Carlsbad, CA). Antibodies used in this study were as follows: from Cell Signaling, Chk1 (2360), pChk1 (Ser317) (12302S), Chk2 (2662S), pChk2 (Thr68) (2661), ATR (2790S), p-ATR (Ser428), and pATM-Ser-1981 (13050S) antibodies; from Santa Cruz, MCL-1 (sc819), 53BP1 (sc-22760), ATM (sc-7230), and BRCA1 (sc-642) antibodies; from Abcam, MCL-1 (ab197529), RPA-70 (ab79398), and Rad51 (ab63801) antibodies; from Genetex, MRE11 (GTX70212) antibody; and from Bethyl Laboratories, RIF1 (A300-5671) antibody. Colony-forming assay and metaphase chromosome analysis were carried out as described previously (35).
Immunofluorescence microscopy.
Cell culture in chamber slides, fixation, and immunostaining were done as previously described (27, 36). To deplete MCL-1 in DAOY, H1299, and U2OS cells, the cells were transfected with MCL-1-specific siRNA, allowed to grow for 48 to 72 h, and then treated with the desired DNA-damaging agent. For immunostaining, cells were fixed in 4% paraformaldehyde (PFA) after 5 min in extraction buffer. Fluorescent images of foci were captured by a standard procedure (27). Sections through nuclei were captured, and the images were obtained by projection of the individual sections as previously described (37). The results shown are from three independent experiments.
DNA replication restart assay.
Exponentially growing cells were pulsed with 50 mM 5-iododeoxyuridine (IdU) for 20 min, washed three times with phosphate-buffered saline (PBS), treated with 2 mM hydroxyurea (HU) for the desired periods, washed three times with PBS, incubated in fresh medium containing with 50 mM 5-chlorodeoxyuridine (CldU) for 20 min, and then washed three times in PBS. DNA fiber spreads were made by a modified procedure described previously (38). Briefly, cells labeled with IdU and CldU were mixed with unlabeled cells in a ratio of 1:10, and 2-μl cell suspensions were dropped onto a glass slide and then mixed with a 20 μl of hypotonic lysis solution (10 mM Tris-HCl [pH 7.4], 2.5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 0.5% Nonidet P-40) for 8 min. Air-dried slides were fixed, washed with 1× PBS, blocked with 5% bovine serum albumin (BSA) for 15 min, and incubated with primary antibodies against IdU and CldU (rat monoclonal antibody [MAb] anti-IdU [1:150 dilution]; Abcam and mouse MAb anti-CldU [1:150 dilution; BD]) and secondary antibodies (anti-rat Alexa Fluor 488-conjugated [1:150 dilution] and anti-mouse Alexa Fluor 568-conjugated [1:200 dilution] antibodies) for 1 h each. Slides were washed with 1× PBS with 0.1% Triton X-100 and mounted with Vectashield mounting medium without 4′,6-diamidino-2-phenylindole (DAPI). ImageJ software was used to analyze the DNA fibers.
Quantitative resection assay using ER-AsiSI system.
A quantitative resection assay to measure ssDNA intermediates was performed as described previously (39). ER-AsiSI U2OS cells were mixed with 0.6% low-melting-temperature agarose (FMC) in PBS at a concentration of 6 × 106 cells per ml. Fifty microliters of cell suspension was solidified as an agar ball by dropping on Parafilm. The agar ball was serially treated with 1 ml of EDTA-sarcosine-proteinase K (ESP) buffer (0.5 M EDTA, 2% N-lauroylsarcosine, 1 mg/ml of proteinase K, and 1 mM CaCl2 [pH 8.0]) and high-salt (HS) buffer (1.85 M NaCl, 0.15 M KCl, 5 mM MgCl2, 2 mM EDTA, 4 mM Tris, and 0.5% Triton X-100 [pH 7.5]) for 20 h each time at 16°C with rotation, followed by washing with 1 ml of phosphate buffer (8 mM Na2HPO4, 1.5 mM KH2PO4, 133 mM KCl, and 0.8 mM MgCl2 [pH 7.4]) for 1 h at 4°C with rotation. After heating of the agar ball at 70°C for 10 min, it was diluted 15-fold with 70°C double-distilled water (ddH2O) and mixed with 2× NEB restriction enzyme buffer 4. Sixty microliters of genomic DNA sample was digested with 60 U of restriction enzyme BsrGI (New England BioLabs) or mock digested at 37°C overnight, and 3-μl quantities of mock- or BsrGI-digested samples were used as templates in quantitative PCRs (qPCRs) with 20-μl mixtures.
ACKNOWLEDGMENTS
We thank the members of Pandita laboratory at the Houston Methodist Research Institute for their support during the execution of this work.
This work was supported by funds from the Houston Methodist Research Institute and MD Anderson Cancer Center, Houston, TX, and National Institutes of Health grants CA129537 and GM109768.
We declare no conflicts of interest.
REFERENCES
- 1.Gross A, McDonnell JM, Korsmeyer SJ. 1999. BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 2.Shamas-Din A, Kale J, Leber B, Andrews DW. 2013. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb Perspect Biol 5:a008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas LW, Lam C, Edwards SW. 2010. Mcl-1; the molecular regulation of protein function. FEBS Lett 584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 4.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. 2000. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev 14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Lømo J, Smeland EB, Krajewski S, Reed JC, Blomhoff HK. 1996. Expression of the Bcl-2 homologue Mcl-1 correlates with survival of peripheral blood B lymphocytes. Cancer Res 56:40–43. [PubMed] [Google Scholar]
- 6.Bae J, Leo CP, Hsu SY, Hsueh AJ. 2000. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem 275:25255–25261. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- 7.Jamil S, Sobouti R, Hojabrpour P, Raj M, Kast J, Duronio V. 2005. A proteolytic fragment of Mcl-1 exhibits nuclear localization and regulates cell growth by interaction with Cdk1. Biochem J 387:659–667. doi: 10.1042/BJ20041596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Vaithiyalingam S, Shi Q, Chazin WJ, Zinkel SS. 2011. BID binds to replication protein A and stimulates ATR function following replicative stress. Mol Cell Biol 31:4298–4309. doi: 10.1128/MCB.05737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Gao F, May WS, Zhang Y, Flagg T, Deng X. 2008. Bcl2 negatively regulates DNA double-strand-break repair through a nonhomologous end-joining pathway. Mol Cell 29:488–498. doi: 10.1016/j.molcel.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie M, Park D, You S, Li R, Owonikoko TK, Wang Y, Doetsch PW, Deng X. 2015. Bcl2 inhibits recruitment of Mre11 complex to DNA double-strand breaks in response to high-linear energy transfer radiation. Nucleic Acids Res 43:960–972. doi: 10.1093/nar/gku1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamil S, Mojtabavi S, Hojabrpour P, Cheah S, Duronio V. 2008. An essential role for MCL-1 in ATR-mediated CHK1 phosphorylation. Mol Biol Cell 19:3212–3220. doi: 10.1091/mbc.E07-11-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamil S, Stoica C, Hackett TL, Duronio V. 2010. MCL-1 localizes to sites of DNA damage and regulates DNA damage response. Cell Cycle 9:2843–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlikowska P, Leray I, de Laval B, Guihard S, Kumar R, Rosselli F, Porteu F. 2010. ATM-dependent expression of IEX-1 controls nuclear accumulation of Mcl-1 and the DNA damage response. Cell Death Differ 17:1739–1750. doi: 10.1038/cdd.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song L, Coppola D, Livingston S, Cress D, Haura EB. 2005. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther 4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- 15.Mattoo AR, FitzGerald DJ. 2013. Combination treatments with ABT-263 and an immunotoxin produce synergistic killing of ABT-263-resistant small cell lung cancer cell lines. Int J Cancer 132:978–987. doi: 10.1002/ijc.27732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattoo AR, Zhang J, Espinoza LA, Jessup JM. 2014. Inhibition of NANOG/NANOGP8 downregulates MCL-1 in colorectal cancer cells and enhances the therapeutic efficacy of BH3 mimetics. Clin Cancer Res 20:5446–5455. doi: 10.1158/1078-0432.CCR-14-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh M, Hunt CR, Pandita RK, Kumar R, Yang CR, Horikoshi N, Bachoo R, Serag S, Story MD, Shay JW, Powell SN, Gupta A, Jeffery J, Pandita S, Chen BP, Deckbar D, Lobrich M, Yang Q, Khanna KK, Worman HJ, Pandita TK. 2013. Lamin A/C depletion enhances DNA damage-induced stalled replication fork arrest. Mol Cell Biol 33:1210–1222. doi: 10.1128/MCB.01676-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perciavalle RM, Opferman JT. 2013. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol 23:22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandita TK, Lieberman HB, Lim DS, Dhar S, Zheng W, Taya Y, Kastan MB. 2000. Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene 19:1386–1391. doi: 10.1038/sj.onc.1203444. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann M, Lottersberger F, Buonomo SB, Sfeir A, de Lange T. 2013. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science 339:700–704. doi: 10.1126/science.1231573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daley JM, Sung P. 2014. 53BP1, BRCA1, and the choice between recombination and end joining at DNA double-strand breaks. Mol Cell Biol 34:1380–1388. doi: 10.1128/MCB.01639-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasin M, Rothstein R. 2013. Repair of strand breaks by homologous recombination. Cold Spring Harb Perspect Biol 5:a012740. doi: 10.1101/cshperspect.a012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikoshi N, Pandita RK, Mujoo K, Hambarde S, Sharma D, Mattoo AR, Chakraborty S, Charaka V, Hunt CR, Pandita TK. 2016. β2-Spectrin depletion impairs DNA damage repair. Oncotarget doi: 10.18632/oncotarget.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. 2010. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol Cell 37:492–502. doi: 10.1016/j.molcel.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilsker D, Petermann E, Helleday T, Bunz F. 2008. Essential function of Chk1 can be uncoupled from DNA damage checkpoint and replication control. Proc Natl Acad Sci U S A 105:20752–20757. doi: 10.1073/pnas.0806917106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfsperger F, Hogh-Binder SA, Schittenhelm J, Psaras T, Ritter V, Bornes L, Huber SM, Jendrossek V, Rudner J. 2016. Deubiquitylating enzyme USP9x regulates radiosensitivity in glioblastoma cells by Mcl-1-dependent and -independent mechanisms. Cell Death Dis 7:e2039. doi: 10.1038/cddis.2015.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A, Hunt CR, Hegde ML, Chakraborty S, Chakraborty S, Udayakumar D, Horikoshi N, Singh M, Ramnarain DB, Hittelman WN, Namjoshi S, Asaithamby A, Hazra TK, Ludwig T, Pandita RK, Tyler JK, Pandita TK. 2014. MOF phosphorylation by ATM regulates 53BP1-mediated double-strand break repair pathway choice. Cell Rep 8:177–189. doi: 10.1016/j.celrep.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann M, de Lange T. 2014. 53BP1: pro choice in DNA repair. Trends Cell Biol 24:108–117. doi: 10.1016/j.tcb.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panier S, Boulton SJ. 2014. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol 15:7–18. [DOI] [PubMed] [Google Scholar]
- 30.Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, Xu X, Deng CX, Finkel T, Nussenzweig M, Stark JM, Nussenzweig A. 2010. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou L, Elledge SJ. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 32.Raynard S, Niu H, Sung P. 2008. DNA double-strand break processing: the beginning of the end. Genes Dev 22:2903–2907. doi: 10.1101/gad.1742408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sørensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T. 2005. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol 7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 34.Yang F, Van Meter TE, Buettner R, Hedvat M, Liang W, Kowolik CM, Mepani N, Mirosevich J, Nam S, Chen MY, Tye G, Kirschbaum M, Jove R. 2008. Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Mol Cancer Ther 7:3519–3526. doi: 10.1158/1535-7163.MCT-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandita RK, Sharma GG, Laszlo A, Hopkins KM, Davey S, Chakhparonian M, Gupta A, Wellinger RJ, Zhang J, Powell SN, Roti Roti JL, Lieberman HB, Pandita TK. 2006. Mammalian Rad9 plays a role in telomere stability, S- and G2-phase-specific cell survival, and homologous recombinational repair. Mol Cell Biol 26:1850–1864. doi: 10.1128/MCB.26.5.1850-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal M, Pandita S, Hunt CR, Gupta A, Yue X, Khan S, Pandita RK, Pratt D, Shay JW, Taylor JS, Pandita TK. 2008. Inhibition of telomerase activity enhances hyperthermia-mediated radiosensitization. Cancer Res 68:3370–3378. doi: 10.1158/0008-5472.CAN-07-5831. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Sharma GG, Young CS, Agarwal M, Smith ER, Paull TT, Lucchesi JC, Khanna KK, Ludwig T, Pandita TK. 2005. Involvement of human MOF in ATM function. Mol Cell Biol 25:5292–5305. doi: 10.1128/MCB.25.12.5292-5305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson DA, Pombo A. 1998. Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J Cell Biol 140:1285–1295. doi: 10.1083/jcb.140.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Caron P, Legube G, Paull TT. 2014. Quantitation of DNA double-strand break resection intermediates in human cells. Nucleic Acids Res 42:e19. doi: 10.1093/nar/gkt1309. [DOI] [PMC free article] [PubMed] [Google Scholar]