Abstract
Basophils have often been erroneously considered to be minor relatives or blood-circulating precursors of tissue-resident mast cells because of some phenotypic similarity between them, including basophilic secretory granules in the cytoplasm. However, recent studies revealed that the repertoire of serine proteases stored in secretory granules is distinct in them. Particularly, mouse mast cell protease 8 (mMCP-8) is specifically expressed by basophils but not mast cells despite its name. Therefore, mMCP-8 is commonly used as a basophil-specific marker, but its functional property remains uncertain. Here we prepared recombinant mMCP-8 and examined its activity in vitro and in vivo. Purified recombinant mMCP-8 showed heat-sensitive proteolytic activity when α-tubulin was used as a substrate. One intradermal shot of mMCP-8, not heat-inactivated, induced cutaneous swelling with increased microvascular permeability in a cyclooxygenase-dependent manner. Moreover, repeated intradermal injection of mMCP-8 promoted skin infiltration of leukocytes, predominantly neutrophils and, to a lesser extent, monocytes and eosinophils, in conjunction with up-regulation of chemokine expression in the skin lesion. These results suggest that mMCP-8 is an important effector molecule in basophil-elicited inflammation, providing novel insights into how basophils exert a crucial and non-redundant role, distinct from that played by mast cells, in immune responses.
Keywords: cell migration, chemokine, inflammation, leukocyte, serine protease, basophil, microvascular permeability
Introduction
Basophils are the least common granulocytes, representing only ∼0.5% of blood-circulating leukocytes, and therefore have often been neglected in immunological studies (1–3). However, recent studies revealed that basophils play crucial roles, distinct from those by mast cells, in immune responses, including allergic inflammation and protective immunity against parasitic infections (4–7). We previously demonstrated that basophils, but not mast cells, are responsible for the development of IgE-mediated chronic allergic inflammation (IgE-CAI)2 even though basophils account for only ∼2% of cellular infiltrates in the skin lesion, whereas other leukocytes, including eosinophils and neutrophils, are abundant there (8). Basophil depletion before the allergen challenge abolished the development of IgE-CAI, confirming the essential role of basophils (9, 10). Intriguingly, basophil ablation during the progress of IgE-CAI resulted in attenuated skin swelling together with decreased numbers of eosinophils and neutrophils, besides basophils, in the skin lesion, suggesting that basophils may contribute to the recruitment of these proinflammatory cells to the skin lesion (9). Nevertheless, it remains to be determined which molecules derived from basophils are involved in the development of allergic inflammation, including the recruitment of other leukocytes.
Basophils and mast cells are sometimes mixed up, and basophils have been erroneously considered to be minor relatives or blood-circulating precursors of tissue-resident mast cells because of some phenotypic similarity between them, including basophilic secretory granules in their cytoplasm (1–3). Both types of cells store serine proteases in secretory granules and release them in response to various stimuli, such as IgE plus allergens (11–20). Notably, recent studies revealed that the repertoire of serine proteases stored in basophilic granules is distinct in basophils and mast cells. Among the mouse mast cell protease (mMCP) family members, mMCP-8 has been shown to be expressed specifically by basophils but not mast cells, despite its name (14, 20). In contrast, chymases, mMCP-6, and mMCP-7 were expressed only by mast cells but not basophils (20).
mMCP-8 was originally cloned from cDNA of the mouse mastocytoma tumor cell lines and identified as a new subfamily member of murine mast cell serine proteases that does not belong to the authentic chymase and tryptase subfamilies and is rather closely related to cathepsin G and T cell granzymes (13, 21, 22). mMCP-8 showed high sequence similarity with mouse granzyme B in the region critical for substrate specificity, but its physiological substrate(s) is/are still unidentified (13, 23). Because of its unique expression profile confined to basophils, mMCP-8 has been commonly utilized as a specific marker for murine basophils both in vitro and in vivo. For instance, a monoclonal antibody specific for mMCP-8 (TUG8) is a useful tool to detect tissue-infiltrating basophils in tissue sections (20, 24). Gene targeting of the Mcpt8 locus encoding mMCP-8 protein has been used to generate basophil-deficient or basophil reporter mice (24–26). Nevertheless, the functional property of mMCP-8 remains to be clarified, in contrast to extensive studies of the mast cell-specific proteases mMCP-1, mMCP-6, and mMCP-7 (27–33).
In this study, we prepared recombinant mMCP-8 and characterized its activity in vitro and in vivo. Our results illustrated that mMCP-8 is an important effector molecule to induce an inflammatory response with microvascular hyperpermeability and leukocyte infiltration. As far as we are aware, this is the first study to demonstrate the function of the basophil-specific protease mMCP-8 in vivo.
Results
Preparation and Characterization of Recombinant mMCP-8
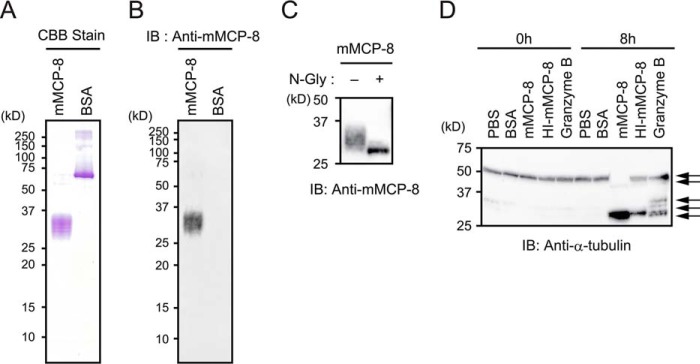
To explore the biological functions of mMCP-8, we first prepared recombinant mMCP-8 proteins by using a baculovirus-mediated expression system. SDS-PAGE analysis of mMCP-8 proteins purified from culture supernatants of mMCP-8-transduced Sf9 cells demonstrated that purified proteins had an apparent molecular mass of 29–36 kDa (Fig. 1A). Purified proteins, but not control BSA, were reacted with the mMCP-8-specific mAb TUG8 in an immunoblot assay (Fig. 1B), indicating that they were indeed mMCP-8. Treatment of purified proteins with N-glycosidase F reduced their apparent molecular mass to 27 kDa (Fig. 1C), in accordance with previous reports that mMCP-8 is an N-glycoprotein (13, 23).
FIGURE 1.
Preparation and characterization of recombinant mMCP-8 protein. A and B, recombinant mMCP-8 protein purified from culture supernatant of mMCP-8-transduced Sf9 cells was resolved by SDS-PAGE, followed by detection with Coomassie Brilliant Blue (CBB) staining (A) or immunoblotting (IB) using an mMCP-8-specific mAb (anti-mMCP-8, B). BSA was used for control experiments. C, purified recombinant mMCP-8 was treated with 100 units/ml N-glycosidase (N-Gly) at room temperature overnight or left untreated, followed by immunoblot analysis using anti-mMCP8 mAb. D, total cell lysates of NIH3T3 cells were incubated at room temperature for 8 h in the presence or absence of the indicated proteins at a concentration of 10 μg/ml. After incubation, the cell lysates were subjected to immunoblot analysis with anti-α-tubulin polyclonal Ab. The bands corresponding to the full-length α-tubulin protein and its degradation products are indicated by arrows. The same set of cell lysates without incubation is shown as 0 h. Data shown are representative of at least three independent experiments.
We next sought to check the protease activity of recombinant mMCP-8 proteins. Although mMCP-8 substrates remain unknown, mMCP-8 shows sequence similarity with mouse granzyme B in the region critical for substrate specificity (13). A previous report that granzyme B could cleave α-tubulin (34) prompted us to assess the protease activity of mMCP-8 by using α-tubulin as a tentative substrate. In accordance with the previous study (34), incubation of NIH3T3 cell lysates with granzyme B, but not control BSA, resulted in the appearance of proteolytic fragments of α-tubulin, as detected by immunoblotting with an α-tubulin-specific antibody (Fig. 1D). Incubation with recombinant mMCP-8 proteins reduced the apparent molecular mass of α-tubulin from 52 to 28 kDa (Fig. 1D). Of note, this activity of mMCP-8 was attenuated by heat treatment of mMCP-8 proteins (Fig. 1D), suggesting that recombinant mMCP-8 proteins had heat-sensitive protease activity.
Intradermal Administration of Recombinant mMCP-8 Induces Cutaneous Swelling with Increased Microvascular Permeability
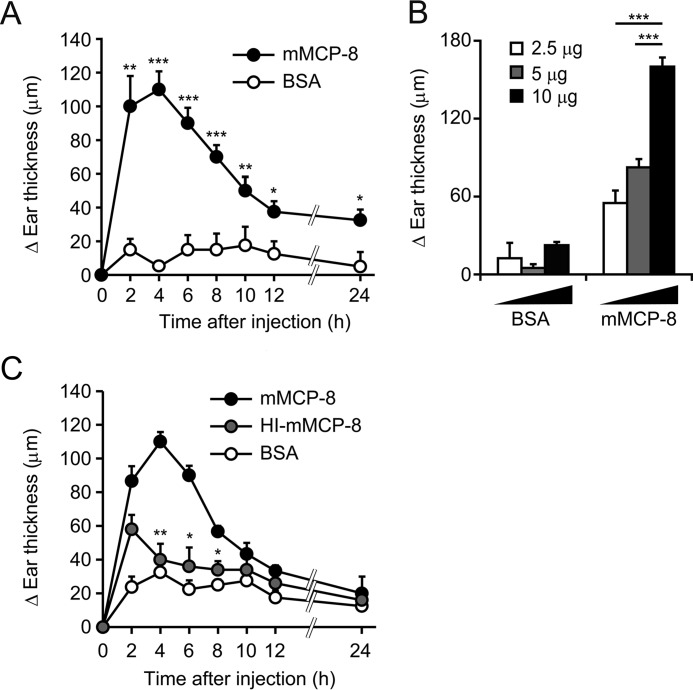
One intradermal shot of 10 μg of recombinant mMCP-8, but not control BSA, into the ear skin of mice induced skin swelling with a peak at 4 h post-injection, followed by gradual attenuation until 24 h post-injection (Fig. 2A). This ear swelling-inducing activity of mMCP-8 was dose-dependent up to 10 μg (Fig. 2B), but no further increase of ear swelling was observed when 20 μg or more mMCP-8 was injected (data not shown). Accordingly, we used 10 μg of recombinant mMCP-8 to induce ear swelling in the following experiments. Heat treatment of recombinant mMCP-8 attenuated the ear swelling-inducing activity of mMCP-8 (Fig. 2C), suggesting that the protease activity of mMCP-8 played an important role in the induction of ear swelling.
FIGURE 2.
Intradermal administration of mMCP-8 provokes a transient cutaneous swelling. C57BL/6 mice were challenged with intradermal administration of mMCP-8, HI-mMCP-8, or control BSA into their ears (right, mMCP-8; left, BSA). A, time course of ear swelling (ΔEar thickness, each time points – 0-h point) after challenge with 10 μg of the indicated proteins (mean ± S.E., n = 4 each). B, ear swelling was measured 4 h after challenge with the indicated doses of proteins (mean ± S.E., n = 4–8 each). C, time course of ear swelling after challenge with 10 μg of the indicated proteins (mean ± S.E., n = 3–8 each). Data shown are representative of at least three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
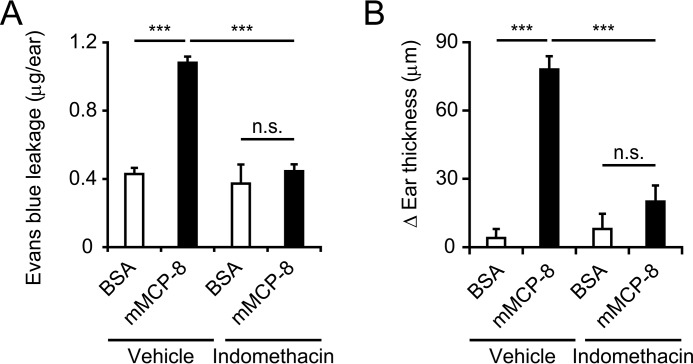
Evans blue dye leakage analysis revealed that intradermal administration of recombinant mMCP-8, but not control BSA, induced an increase in microvascular permeability in the skin lesion (Fig. 3A). This hyperpermeability was abolished when the ear skin was pretreated with indomethacin (Fig. 3A), suggesting that mMCP-8 increased microvascular permeability via COX activation. Indomethacin treatment also abolished the mMCP-8-induced ear swelling (Fig. 3B). Meloxicam, a COX-2 inhibitor, showed a similar inhibitory effect (supplemental Fig. S1). Thus, mMCP-8 appeared to induce cutaneous swelling through COX-mediated microvascular hyperpermeability.
FIGURE 3.
Intradermal administration of mMCP-8 induces vascular hyperpermeability in the skin via COX activation. A and B, C57BL/6 mice were pretreated with topical application of 200 μg of indomethacin or vehicle (ethanol) alone and then challenged with intradermal administration of mMCP-8 or control BSA into their ears. Three hours post-challenge, mice were treated with intravenous injection of Evans blue, and, 2 h later, their ears were subjected to measurement of leaked dye into the skin (mean ± S.E., n = 5 each) (A). Four hours post-challenge, ear swelling (ΔEar thickness, each time point – 0-h point) was measured (mean ± S.E., n = 5 each) (B). Data shown are representative of three independent experiments. ***, p < 0.001; n.s., not significant.
Repeated Intradermal Administration of mMCP-8 Induces Leukocyte Infiltration in the Skin
We then examined whether mMCP-8 triggers inflammation with leukocyte infiltration and accumulation. One intradermal shot of mMCP-8 resulted in no detectable accumulation of CD45+ hematopoietic cells in the swollen skin lesion (Fig. 4A). We assumed that, in basophil-mediated inflammation such as IgE-CAI (8, 9), basophils continuously infiltrate the skin lesion and release mMCP-8 one after another. To mimic this situation in the inflammation site, we repeatedly treated mice with intradermal mMCP-8 at 24-h intervals. After three administrations of mMCP-8, we definitely detected an accumulation of CD45+ hematopoietic cells in the skin lesion by using flow cytometry, predominantly neutrophils and, to a lesser extent, monocytes/macrophages and eosinophils compared with administration of control BSA (Fig. 4, A and B). In accordance with this, immunohistochemical analysis of the skin section revealed an accumulation of Ly-6B+ cells in skin treated with mMCP-8 but not control BSA (Fig. 4C). The number of leukocytes accumulating in the skin lesion increased in a manner dependent on the dose of mMCP-8 injected (supplemental Fig. S2). Importantly, heat-inactivated mMCP-8 did not display such a leukocyte-recruiting ability (Fig. 4D), indicating that the protease activity of mMCP-8 is essential for leukocyte recruitment by mMCP-8.
FIGURE 4.
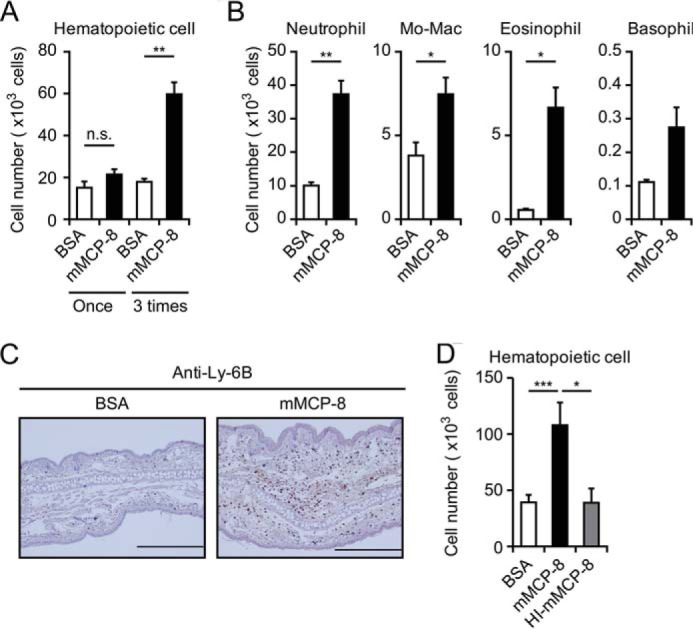
Repeated intradermal administration of mMCP-8 induces leukocyte accumulation in the ear skin. C57BL/6 mice were challenged once or three times at 24 h-intervals with intradermal administration of 10 μg of mMCP-8, HI-mMCP-8, or control BSA in the ear skin. The ears of treated mice were excised 6 h after the last challenge. (A, B, and D) The number of CD45+ hematopoietic cells (A and D) and the indicated cell types (B) isolated from the ear skin are shown (mean ± S.E., n = 3–6 each). C, immunohistochemical analysis using anti-Ly-6B to detect neutrophils and a certain subset of monocytes/macrophages was performed. Scale bars = 200 μm. Data shown are representative of three independent experiments. Mo-Mac, monocytes/macrophages. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
mMCP-8 Up-regulates Chemokine Expression in the Skin
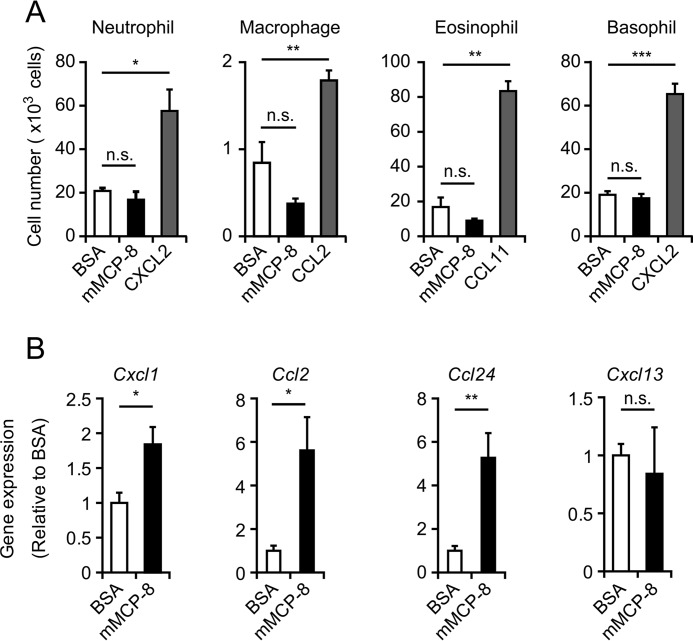
To understand the mechanism underlying the mMCP-8-elicited leukocyte infiltration, we first examined the possibility that mMCP-8 acts directly on leukocytes to promote their migration. To this end, we set up a transwell migration assay in which leukocytes were placed in the upper chamber, whereas mMCP-8, control BSA, or a relevant chemokine was included in the lower chamber. No significant migration of neutrophils, macrophages, eosinophils, or basophils into the mMCP-8-containing chamber was detected, whereas relevant chemokines induced their migration (Fig. 5A). This observation prompted us to explore another possibility: that mMCP-8 acts on skin-resident cells to induce their production of chemokines, which, in turn, attract leukocytes. Indeed, three administrations of mMCP-8 in the skin up-regulated expression of mRNAs encoding the chemokines Cxcl1, Ccl2, and Ccl24 (Fig. 5B), which are known to induce chemotaxis of neutrophils, monocytes/macrophages, and eosinophils, respectively (35), whereas no significant up-regulation of the B cell chemoattractant Cxcl13 was detected. Up-regulated expression of CCL2 in the mMCP-8 injection site was detected at the protein level (supplemental Fig. S3A). Moreover, CCR2 (receptor of CCL2)-deficient mice showed reduced accumulation of monocytes/macrophages in the skin lesion compared with wild-type mice (supplemental Fig. S3B), suggesting a contribution of the CCL2-CCR2 axis to the migration of monocytes/macrophages to the mMCP-8 injection site. Of note, pretreatment of the ear skin with meloxicam prior to each mMCP-8 injection showed no apparent impact on leukocyte accumulation or chemokine expression (supplemental Fig. S4), in contrast to the COX-dependent edematous response (supplemental Fig. S1).
FIGURE 5.
mMCP-8 fails to induce leukocyte migration in vitro, whereas it enhances gene expression of chemokines in the ear skin in vivo. A, the ability of mMCP-8 to induce leukocyte migration was examined by using the transwell system in vitro. The indicated types of cells (5 × 105 cells) were placed in the upper chamber, whereas mMCP-8 (black columns), control BSA (white columns), or the indicated chemokines (gray columns) were included in the culture medium of the lower chamber. The number of cells recovered from the lower chamber after 1.5-h incubation (for neutrophils and macrophages) or 2 h incubation (for eosinophils and basophils) at 37 °C are shown (mean ± S.E., n = 4 each). B, C57BL/6 mice were challenged three times at 24 h-intervals with intradermal administration of 10 μg of mMCP-8 or control BSA in the ear skin. The ears of treated mice were excised 6 h after the last challenge and subjected to Q-PCR analysis to access the gene expression of the indicated chemokines (mean ± S.E., n = 9 each). Data shown are representative of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
Discussion
Mcpt8 is the only known gene that is selectively expressed by mouse basophils (14, 20). Therefore, this gene and its product mMCP-8 are commonly used as basophil-specific markers to identify basophils and generate engineered mice with basophil-specific modification (14, 20, 24–26) even though the biological function of mMCP-8 remains unknown. In this study, we demonstrated that mMCP-8 can provoke an inflammatory response in the skin with increased microvascular permeability and leukocyte infiltration in a protease activity-dependent manner. Considering that basophils play a crucial role in the development of inflammation, including IgE-CAI (8, 9), the basophil-specific protease mMCP-8 could be an important effector molecule involved in the induction of such inflammation.
mMCP-8-elicited cutaneous swelling with microvascular hyperpermeability was almost completely inhibited by indomethacin treatment, indicating that COX-mediated production of prostaglandins likely contributes to the formation of edematous swelling. Although the exact mechanism underlying the mMCP-8-mediated COX activation remains to be determined, the inability of heat-inactivated mMCP-8 in this function suggested that proteolytic cleavage of a protein(s) on target cells may trigger the induction or activation of COX. One intradermal shot of mMCP-8 induced edematous swelling in the skin with no apparent infiltration of leukocytes, whereas three consecutive injections resulted in accumulation of leukocytes. Therefore, mMCP-8-elicited microvascular hyperpermeability in the skin did not seem to directly contribute to leukocyte extravasation and accumulation in the skin. Because the expression of leukocyte-attracting chemokines was up-regulated in the skin lesion after three injections of mMCP-8, it is likely that mMCP-8 at certain amounts persisting for a while activated skin-resident cells to produce chemokines in a protease activity-dependent manner. mMCP-4 and human chymase reportedly show chemotactic activity that directly attracts leukocytes in vitro (36, 37). As far as we examined in the transwell migration assay, mMCP-8 showed no such activity.
A previous study using chromogenic substrates and a phage-displayed random nonapeptide library failed to identify the candidates of mMCP-8 substrates (23), and no further study to identify them has been reported, to our knowledge. In this study, we could show the heat-sensitive protease activity of mMCP-8, as assessed by the proteolysis of α-tubulin. Although α-tubulin may not be a physiological substrate of mMCP-8, further analysis of the amino acid sequence of its proteolytic fragments may give a clue to identify real substrates.
In conclusion, we demonstrated in this study that the basophil protease mMCP-8 can elicit an inflammatory response in the skin with microvascular hyperpermeability and leukocyte infiltration. Considering the basophil-restricted expression and pro-inflammatory activity of mMCP-8 shown here, mMCP-8 may contribute to the non-redundant role of basophils, distinct from that played by mast cells, in immune responses, including allergic inflammation and protective immunity against parasitic infections.
Experimental Procedures
Mice
C57BL/6 and BALB/c mice (7–9 weeks old) were purchased from Japan SLC. Ccr2−/− BALB/c mice were as described previously (38). All animal studies were approved by the Institutional Animal Care and Use Committee of Tokyo Medical and Dental University.
Antibodies
The following Abs were purchased from BioLegend: biotinylated anti-CD49b (DX5), FITC-conjugated anti-CD49b (HMα2), anti-Ly-6G (1A8), allophycocyanin-conjugated anti-CD200R3 (Ba13), anti-F4/80 (BM8), phycoerythrin-Cy7-conjugated anti-CD45 (30-F11), allophycocyanin-Cy7-conjugated anti-Gr-1 (RB6–8C5), Pacific Blue-conjugated anti-CD11b (M1/70), and anti-c-Kit (2B8). Anti-Ly-6B.2 (7/4) and phycoerythrin-conjugated anti-Siglec-F (E50–2440) were from AbD Serotec and BD Biosciences, respectively. Rabbit anti-α-tubulin polyclonal Ab (2144S) was from Cell Signaling Technology. Anti-mMCP-8 (TUG8) was established as reported previously (20). Anti-CD16/32 (2.4G2) was prepared in our laboratory.
Preparation of Recombinant mMCP-8
Recombinant mMCP-8 was prepared by using a baculovirus expression system. In brief, the cDNA fragment encoding mMCP-8 tagged with a FLAG peptide between the natural activation peptide (13), and the first residue of the catalytic domain was inserted into the pFastBac1 baculoviral vector (Invitrogen). The resultant vector was transfected into Sf9 cells to produce a recombinant protein according to the protocol of the manufacturer of the BAC-to-BAC expression system (Invitrogen). The recombinant protein was purified by anti-DYKDDDDK (FLAG) tag antibody beads (Wako) from culture supernatants of transfected Sf9 cells, followed by treatment with enterokinase (Novagen) to cleave off their N-terminal sequences containing the natural activation peptide and FLAG peptide. After removal of enterokinase by EKapture agarose (Novagen) and dialysis with PBS, the purity and identity of the recombinant protein were confirmed by SDS-PAGE, followed by detection with Coomassie Brilliant Blue staining (Nacalai Tesque) and immunoblotting with anti-mMCP-8 mAb, respectively. Before use, both recombinant mMCP-8 and control BSA were treated with polymyxin B using Proteus NoEndo Micro Spin Column Kits (Protein Ark) to remove potential contamination of the endotoxin. In some experiments, a heat-inactivated form of mMCP-8 (HI-mMCP8) was prepared by incubating recombinant mMCP-8 at 95 °C for 1 h.
α-Tubulin Cleavage Assay
The α-tubulin cleavage assay was performed as described previously with some modifications (34). Briefly, 4 × 107 NIH3T3 cells were lysed with 100 μl of PBS containing 0.5% Triton X-100. After removal of insoluble components, the lysates were incubated at room temperature for 8 h in the presence or absence of 10 μg/ml mMCP-8, HI-mMCP-8, granzyme B, or control BSA. The resultant lysates were subjected to SDS-PAGE, followed by immunoblotting with anti-α-tubulin polyclonal Ab. The same set of lysates without incubation were utilized for the 0-h experiment as input.
mMCP-8-induced Cutaneous Inflammation
The indicated amounts of mMCP-8 or control BSA in 10 μl of PBS were intradermally administered, once or three times at 24 h-intervals, into the ear skin of C57BL/6 mice (right, mMCP-8; left, BSA). The ear thickness was measured by a dial thickness gauge (Peacock) at the indicated time points, and the degree of ear swelling was determined by ΔEar thickness (each time point – 0-h point). To evaluate vascular permeability, mice were intravenously injected with 0.5% Evans blue dye in 100 μl of PBS at 3 h after the intradermal administration of mMCP-8 or BSA. Two hours after the dye injection, the ears were excised and incubated in 0.7 ml of formamide at 63 °C overnight to extract the dye leaked into the skin. The amount of the dye in the extracts was determined by a spectrophotometer at 620 nm. For flow cytometric or immunohistochemical analysis to assess cell infiltration or gene expression analysis for chemokines, ears were excised 6 h after the last administration of the reagents. In some experiments, HI-mMCP-8 was used.
Flow Cytometric and Immunohistochemical Analyses
Single-cell suspensions were obtained from ear skins by treatment with 125 units/ml collagenase (Wako) at 37 °C for 2 h. After pretreatment with anti-CD16/32 mAb (2.4G2) and normal rat serum to avoid the nonspecific binding of irrelevant Abs, cells were stained with the indicated combination of Abs on ice for 30 min and analyzed by FACSCanto (BD Biosciences). Each cell lineage was defined as follows: neutrophils (Ly-6G+), eosinophils (Siglec-F+SSChigh), monocyte-macrophages (F4/80+CD11b+ among cells in which both eosinophils and neutrophils were excluded), and basophils (c-kit−CD49b+CD200R3+). For immunohistochemical analysis, ear specimens were fixed and embedded in paraffin, and sections were stained with anti-Ly-6B.2 mAb (7/4) in combination with the appropriate secondary Ab and 3,3′-diaminobenzidine, followed by hematoxylin counterstaining (39, 40).
Quantitative PCR (Q-PCR)
Total RNA was extracted from tissues or isolated cells by RNeasy Mini Kit (Qiagen), followed by cDNA synthesis with reverse transcription using oligo(dT) and random primers. Q-PCR of the cDNA was performed by using the following primer sets: 5′-ACTGCACCCAAACCGAAGTC-3′ and 5′-TGGGGACACCTTTTAGCATCTT-3′ for Cxcl1, 5′-TTAAAAACCTGGATCGGAACCAA-3′ and 5′-GCATTAGCTTCAGATTTACGGGT-3′ for Ccl2, 5′-ATTCTGTGACCATCCCCTCAT-3′ and 5′-TGTATGTGCCTCTGAACCCAC-3′ for Ccl24, 5′-CATAGATCGGATTCAAGTTACGCC-3′ and 5′-TCTTGGTCCAGATCACAACTTCA-3′ for Cxcl13, and 5′-GGCCCTCGACTCTCGCTTTC-3′ and 5′-TGCCAGGACGCGCTTGT-3′ for 36B4. Relative gene expression levels were calculated using standard curves generated by serial dilutions of each cDNA standard and normalized by 36B4 expression levels.
Transwell Migration Assay
Neutrophils were prepared from the bone marrow by using a 62% Percoll gradient as described previously (41). Eosinophils were isolated from the peritoneum of mice that had been treated for 7 days with daily intraperitoneal administration of IL-5 (42). Macrophages were isolated from the peritoneum of mice that had been treated with intraperitoneal administration of 1 ml of 4% thioglycollate broth 3 days before. Mouse bone marrow-derived basophils were generated as described previously (20). In brief, total bone marrow cells were cultured in the presence of 300 pg/ml recombinant IL-3 (BioLegend) for 7 days, followed by purification with biotinylated anti-CD49b (DX5) in combination with the IMag cell separation system (BD Biosciences). The transwell apparatus (Kurabo) consisted of the upper and lower chambers separated by a membrane with 3-μm (for neutrophils) or 5-μm (for other cell types) pore size. Leukocytes (5 × 105 cells) were placed into the upper chamber, whereas 10 μg/ml mMCP-8, chemokines (CXCL2 100 ng/ml, CCL11 200 ng/ml, or CCL2 200 ng/ml), or control BSA was included in the culture medium of the lower chamber. Ninety minutes (for neutrophils and macrophages) or 2 h (for eosinophils and basophils) after incubation at 37 °C, the cells migrating into the lower chamber were counted.
Statistical Analysis
Statistical analysis was performed with unpaired Student's t test. p < 0.05 was considered statistically significant.
Author Contributions
H. T. performed the experiments. H. O. performed the immunohistochemical staining. S. S. generated recombinant mMCP-8. S. Y. provided helpful suggestions. H. T., Y. Y., and H. K. wrote the manuscript. Y. Y. and H. K. designed and supervised the study.
Supplementary Material
Acknowledgments
We thank N. Mukaida (Kanazawa University) and W. A. Kuziel (External Scientific Affairs, Daiichi Sankyo Group, Edison, NJ) for providing Ccr2−/− mice, A. Tomisawa and R. Matsunaga for technical support, all members of the Karasuyama laboratory for helpful discussions; and M. Miki for secretarial assistance.
This work was supported by Research Grant 1A145 from the Japan Science and Technology Agency (to H. K.) and Japan Society for the Promotion of Science Grants 15H05786 (to H. K.) and 15K20969 (to Y. Y.). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. S1–S4.
- IgE-CAI
- IgE-mediated chronic allergic inflammation
- mMCP
- mouse mast cell protease
- COX
- cyclooxygenase
- Ab
- antibody
- Q-PCR
- quantitative PCR
- HI
- heat-inactivated.
References
- 1. Galli S. J. (2000) Mast cells and basophils. Curr. Opin. Hematol. 7, 32–39 [DOI] [PubMed] [Google Scholar]
- 2. Stone K. D., Prussin C., and Metcalfe D. D. (2010) IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 125, S73–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falcone F. H., Haas H., and Gibbs B. F. (2000) The human basophil: a new appreciation of its role in immune responses. Blood 96, 4028–4038 [PubMed] [Google Scholar]
- 4. Sokol C. L., and Medzhitov R. (2010) Emerging functions of basophils in protective and allergic immune responses. Mucosal Immunol. 3, 129–137 [DOI] [PubMed] [Google Scholar]
- 5. Siracusa M. C., Comeau M. R., and Artis D. (2011) New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann. N.Y. Acad. Sci. 1217, 166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karasuyama H., Mukai K., Obata K., Tsujimura Y., and Wada T. (2011) Nonredundant roles of basophils in immunity. Annu. Rev. Immunol. 29, 45–69 [DOI] [PubMed] [Google Scholar]
- 7. Voehringer D. (2013) Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 13, 362–375 [DOI] [PubMed] [Google Scholar]
- 8. Mukai K., Matsuoka K., Taya C., Suzuki H., Yokozeki H., Nishioka K., Hirokawa K., Etori M., Yamashita M., Kubota T., Minegishi Y., Yonekawa H., and Karasuyama H. (2005) Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23, 191–202 [DOI] [PubMed] [Google Scholar]
- 9. Obata K., Mukai K., Tsujimura Y., Ishiwata K., Kawano Y., Minegishi Y., Watanabe N., and Karasuyama H. (2007) Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110, 913–920 [DOI] [PubMed] [Google Scholar]
- 10. Egawa M., Mukai K., Yoshikawa S., Iki M., Mukaida N., Kawano Y., Minegishi Y., and Karasuyama H. (2013) Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity 38, 570–580 [DOI] [PubMed] [Google Scholar]
- 11. Lützelschwab C., Pejler G., Aveskogh M., and Hellman L. (1997) Secretory granule proteases in rat mast cells: cloning of 10 different serine proteases and a carboxypeptidase A from various rat mast cell populations. J. Exp. Med. 185, 13–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hunt J. E., Friend D. S., Gurish M. F., Feyfant E., Sali A., Huang C., Ghildyal N., Stechschulte S., Austen K. F., and Stevens R. L. (1997) Mouse mast cell protease 9, a novel member of the chromosome 14 family of serine proteases that is selectively expressed in uterine mast cells. J. Biol. Chem. 272, 29158–29166 [DOI] [PubMed] [Google Scholar]
- 13. Lützelschwab C., Huang M. R., Kullberg M. C., Aveskogh M., and Hellman L. (1998) Characterization of mouse mast cell protease-8, the first member of a novel subfamily of mouse mast cell serine proteases, distinct from both the classical chymases and tryptases. Eur. J. Immunol. 28, 1022–1033 [DOI] [PubMed] [Google Scholar]
- 14. Poorafshar M., Helmby H., Troye-Blomberg M., and Hellman L. (2000) MMCP-8, the first lineage-specific differentiation marker for mouse basophils: elevated numbers of potent IL-4-producing and MMCP-8-positive cells in spleens of malaria-infected mice. Eur. J. Immunol. 30, 2660–2668 [DOI] [PubMed] [Google Scholar]
- 15. Miller H. R., and Pemberton A. D. (2002) Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology 105, 375–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong G. W., Yasuda S., Morokawa N., Li L., and Stevens R. L. (2004) Mouse chromosome 17A3.3 contains 13 genes that encode functional tryptic-like serine proteases with distinct tissue and cell expression patterns. J. Biol. Chem. 279, 2438–2452 [DOI] [PubMed] [Google Scholar]
- 17. Hallgren J., and Pejler G. (2006) Biology of mast cell tryptase: an inflammatory mediator. FEBS J. 273, 1871–1895 [DOI] [PubMed] [Google Scholar]
- 18. Caughey G. H. (2007) Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 217, 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McNeil H. P., Adachi R., and Stevens R. L. (2007) Mast cell-restricted tryptases: structure and function in inflammation and pathogen defense. J. Biol. Chem. 282, 20785–20789 [DOI] [PubMed] [Google Scholar]
- 20. Ugajin T., Kojima T., Mukai K., Obata K., Kawano Y., Minegishi Y., Eishi Y., Yokozeki H., and Karasuyama H. (2009) Basophils preferentially express mouse mast cell protease 11 among the mast cell tryptase family in contrast to mast cells. J. Leukocyte Biol. 86, 1417–1425 [DOI] [PubMed] [Google Scholar]
- 21. Gallwitz M., and Hellman L. (2006) Rapid lineage-specific diversification of the mast cell chymase locus during mammalian evolution. Immunogenetics 58, 641–654 [DOI] [PubMed] [Google Scholar]
- 22. Gallwitz M., Reimer J. M., and Hellman L. (2006) Expansion of the mast cell chymase locus over the past 200 million years of mammalian evolution. Immunogenetics 58, 655–669 [DOI] [PubMed] [Google Scholar]
- 23. Gallwitz M., Enoksson M., and Hellman L. (2007) Expression profile of novel members of the rat mast cell protease (rMCP)-2 and (rMCP)-8 families, and functional analyses of mouse mast cell protease (mMCP)-8. Immunogenetics 59, 391–405 [DOI] [PubMed] [Google Scholar]
- 24. Wada T., Ishiwata K., Koseki H., Ishikura T., Ugajin T., Ohnuma N., Obata K., Ishikawa R., Yoshikawa S., Mukai K., Kawano Y., Minegishi Y., Yokozeki H., Watanabe N., and Karasuyama H. (2010) Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J. Clin. Invest. 120, 2867–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohnmacht C., Schwartz C., Panzer M., Schiedewitz I., Naumann R., and Voehringer D. (2010) Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33, 364–374 [DOI] [PubMed] [Google Scholar]
- 26. Sullivan B. M., Liang H. E., Bando J. K., Wu D., Cheng L. E., McKerrow J. K., Allen C. D., and Locksley R. M. (2011) Genetic analysis of basophil function in vivo. Nat. Immunol. 12, 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang C., Friend D. S., Qiu W. T., Wong G. W., Morales G., Hunt J., and Stevens R. L. (1998) Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 160, 1910–1919 [PubMed] [Google Scholar]
- 28. Knight P. A., Wright S. H., Lawrence C. E., Paterson Y. Y., and Miller H. R. (2000) Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192, 1849–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., and Adachi R. (2007) The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J. Biol. Chem. 282, 20809–20815 [DOI] [PubMed] [Google Scholar]
- 30. Shin K., Watts G. F., Oettgen H. C., Friend D. S., Pemberton A. D., Gurish M. F., and Lee D. M. (2008) Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 180, 4885–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McNeil H. P., Shin K., Campbell I. K., Wicks I. P., Adachi R., Lee D. M., and Stevens R. L. (2008) The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 58, 2338–2346 [DOI] [PubMed] [Google Scholar]
- 32. Shin K., Nigrovic P. A., Crish J., Boilard E., McNeil H. P., Larabee K. S., Adachi R., Gurish M. F., Gobezie R., Stevens R. L., and Lee D. M. (2009) Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J. Immunol. 182, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamilton M. J., Sinnamon M. J., Lyng G. D., Glickman J. N., Wang X., Xing W., Krilis S. A., Blumberg R. S., Adachi R., Lee D. M., and Stevens R. L. (2011) Essential role for mast cell tryptase in acute experimental colitis. Proc. Natl. Acad. Sci. U.S.A. 108, 290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goping I. S., Sawchuk T., Underhill D. A., and Bleackley R. C. (2006) Identification of α-tubulin as a granzyme B substrate during CTL-mediated apoptosis. J. Cell Sci. 119, 858–865 [DOI] [PubMed] [Google Scholar]
- 35. Griffith J. W., Sokol C. L., and Luster A. D. (2014) Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev. Immunol. 32, 659–702 [DOI] [PubMed] [Google Scholar]
- 36. Tani K., Ogushi F., Kido H., Kawano T., Kunori Y., Kamimura T., Cui P., and Sone S. (2000) Chymase is a potent chemoattractant for human monocytes and neutrophils. J. Leukocyte Biol. 67, 585–589 [DOI] [PubMed] [Google Scholar]
- 37. Terakawa M., Tomimori Y., Goto M., Hayashi Y., Oikawa S., and Fukuda Y. (2005) Eosinophil migration induced by mast cell chymase is mediated by extracellular signal-regulated kinase pathway. Biochem. Biophys. Res. Commun. 332, 969–975 [DOI] [PubMed] [Google Scholar]
- 38. Kuziel W. A., Morgan S. J., Dawson T. C., Griffin S., Smithies O., Ley K., and Maeda N. (1997) Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. U.S.A. 94, 12053–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee P. Y., Wang J. X., Parisini E., Dascher C. C., and Nigrovic P. A. (2013) Ly6 family proteins in neutrophil biology. J. Leukocyte Biol. 94, 585–594 [DOI] [PubMed] [Google Scholar]
- 40. Yamanishi Y., Kitaura J., Izawa K., Kaitani A., Komeno Y., Nakamura M., Yamazaki S., Enomoto Y., Oki T., Akiba H., Abe T., Komori T., Morikawa Y., Kiyonari H., Takai T., et al. (2010) TIM1 is an endogenous ligand for LMIR5/CD300b: LMIR5 deficiency ameliorates mouse kidney ischemia/reperfusion injury. J. Exp. Med. 207, 1501–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Izawa K., Kitaura J., Yamanishi Y., Matsuoka T., Oki T., Shibata F., Kumagai H., Nakajima H., Maeda-Yamamoto M., Hauchins J. P., Tybulewicz V. L., Takai T., and Kitamura T. (2007) Functional analysis of activating receptor LMIR4 as a counterpart of inhibitory receptor LMIR3. J. Biol. Chem. 282, 17997–18008 [DOI] [PubMed] [Google Scholar]
- 42. Karasuyama H., and Melchers F. (1988) Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur. J. Immunol. 18, 97–104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.