Abstract
Plant roots are the first parts of plants to face drought stress (DS), and thus root modification is important for plants to adapt to drought. We hypothesized that the roots of arbuscular mycorrhizal (AM) plants exhibit better adaptation in terms of morphology and phytohormones under DS. Trifoliate orange seedlings inoculated with Diversispora versiformis were subjected to well-watered (WW) and DS conditions for 6 weeks. AM seedlings exhibited better growth performance and significantly greater number of 1st, 2nd, and 3rd order lateral roots, root length, area, average diameter, volume, tips, forks, and crossings than non-AM seedlings under both WW and DS conditions. AM fungal inoculation considerably increased root hair density under both WW and DS and root hair length under DS, while dramatically decreased root hair length under WW but there was no change in root hair diameter. AM plants had greater concentrations of indole-3-acetic acid, methyl jasmonate, nitric oxide, and calmodulin in roots, which were significantly correlated with changes in root morphology. These results support the hypothesis that AM plants show superior adaptation in root morphology under DS that is potentially associated with indole-3-acetic acid, methyl jasmonate, nitric oxide, and calmodulin levels.
Drought stress (DS), which is a key abiotic stress, is considered to be a major threat to crop growth and production worldwide1. Plant roots are usually the first plant parts to encounter DS. They are highly sensitive and responsive to DS conditions, which makes them intimately linked with the drought adaptation of the entire plant2. As a result, root growth and development are important for plants to survive soil DS. A deep and extensive root system enables plants to access moisture at water-absorbed zones in soils, and is thus considered as a vital strategy for drought adaptation3. In response to drought, root hairs often become shorter and more bulbous4. Recent advances in the understanding of DS effects have been achieved based on the evaluation of aboveground (shoot) traits, whereas little information is available about the effects of DS on root morphology and root hair development1.
The plant rhizosphere is inhabited by several soil microorganisms, including arbuscular mycorrhizal (AM) fungi. These fungi, belonging to the Glomeromycota, can colonize the roots of numerous terrestrial plants, forming mutualistic symbioses known as arbuscular mycorrhizas5. A number of studies have confirmed that AM fungi can enhance the drought tolerance of various plants species6,7,8. The potential mechanisms are: (1) direct water absorption by extraradical hyphae9,10, (2) increase in nutrient uptake, especially P11, (3) biochemical changes in osmotic adjustment and antioxidant defence systems12,13,14,15,16, (4) improvement of the soil structure by glomalin-related soil proteins of AM fungi17, and (5) changes in molecule levels, including aquaporins7,18,19, genes involved in oxidative stress20 and proline synthesis21, dehydrin proteins22, and genes involved in stomata development and ABA responses or encoding proteins involved in plant response pathways23,24. The underlying mechanisms of phytohormone responses and root adaptation need to be elucidated.
Plant roots have highly plastic traits that can be modulated by many factors, including AM fungi25. Earlier studies have showed that there are more branches in AM roots than in non-AM controls26,27. Root length, area, and volume were pronouncedly increased by Funneliformis mosseae in Citrus tangerina seedlings28. However, Atkinson et al.29 found no change in the root branching of Trifolium repens by F. mosseae. A decrease in the total root length and root diameter of rice plants was induced by Rhizophagus intraradices30. AM fungi also modulated greater root hair modification to improve aboveground growth31. These root modifications under mycorrhization could be linked to the improved uptake of nutrients32, sugar allocation to roots33, and polyamine metabolism28, independent of common symbiosis signalling34.
Phytohormones are associated with root formation and development, mycorrhizal formation, and response to DS35,36,37. AM symbiosis and methyl jasmonate (MeJA) prevented the inhibition of root hydraulic conductivity in Phaseolus vulgaris exposed to DS, probably through a reduction in root salicylic acid concentrations38. In addition, under unfavourable conditions, plants might stimulate increased strigolactone production to promote symbiosis to deal with the stress8. Mycorrhizal P. vulgaris plants had relatively higher indole-3-acetic acid (IAA) and abscisic acid (ABA) levels than non-mycorrhizal controls under well-watered (WW) and DS conditions38. Little is known about the response of phytohormones to mycorrhization under DS and the subsequent regulation of root morphology by these phytohormones.
Here, we hypothesize that roots of AM plants exhibit better adaptation of morphology in response to DS via modultion of phytohormone levels. The objective of the present study was to confirm the above hypothesis by examining the effects of AM fungi on lateral root formation, root morphology, and root hair development, as well as the relevant phytohormones and their analogues.
Results
Mycorrhizal status
Root colonization by D. versiformis ranged from 37.5% to 55.3% in AM seedlings, and DS treatment significantly (P < 0.05) decreased root colonization by 32.2% compared with WW (Table 1). Similarly, the soil hyphal length was significantly (P < 0.05) 42.3% higher under DS than under WW (Table 1).
Table 1. Mycorrhizal status and plant growth performance of D. versiformis colonized trifoliate orange seedlings in response to different treatments.
| Treatments | Mycorrhizal status |
Plant growth performance |
|||||
|---|---|---|---|---|---|---|---|
| Root colonization (%) | Soil hyphal length (m/g DW soil) | Plant height (cm) | Stem diameter (mm) | Leaf number (#/plant) | Shoot biomass (g FW/plant) | Root biomass (g FW/plant) | |
| WW + AMF | 55.3 ± 8.8a | 0.52 ± 0.06a | 29.1 ± 2.6a | 4.16 ± 0.11a | 22.8 ± 1.2a | 2.47 ± 0.21a | 2.61 ± 0.13a |
| WW–AMF | 0c | 0c | 20.1 ± 2.9c | 3.52 ± 0.07c | 19.4 ± 2.2b | 1.43 ± 0.13c | 1.65 ± 0.16c |
| DS + AMF | 37.5 ± 7.6b | 0.30 ± 0.04b | 25.8 ± 1.2b | 3.80 ± 0.15b | 21.2 ± 1.5ab | 2.13 ± 0.12b | 2.00 ± 0.10b |
| DS–AMF | 0c | 0c | 14.1 ± 1.0d | 3.34 ± 0.11d | 14.3 ± 1.5c | 1.02 ± 0.08d | 1.36 ± 0.08d |
Data (means ± SD, n = 4) followed by different letters indicate significant differences among treatments after using the Duncan’s multiple range test (P < 0.05). Abbreviations: +AMF: inoculation with D. versiformis, –AMF: inoculation without D. versiformis, DS: drought stress, WW: well-watered.
Plant growth performance
Compared with the WW treatment, the DS treatment significantly (P < 0.05) reduced plant height, stem diameter, number of leaves, and shoot and root biomass in both AM and non-AM seedlings. AM seedlings displayed significantly (P < 0.05) better growth traits than non-AM seedlings under WW and DS: 31% and 45% for plant height, 15% and 12% for stem diameter, 15% and 33% for the number of leaves, 42% and 52% for shoot biomass, and 37% and 32% for root biomass, respectively (Table 1).
Leaf water potential (Ψ)
Compared with WW, DS significantly (P < 0.05) reduced leaf Ψ in trifoliate orange, irrespective of the AM fungi status (Fig. 1). Inoculation with AM fungi significantly (P < 0.05) increased leaf Ψ by 25% and 20% under WW and DS conditions, respectively.
Figure 1. Leaf water potential of trifoliate orange (P. trifoliata) seedlings colonized by D. versiformis under well-watered and drought stress conditions.
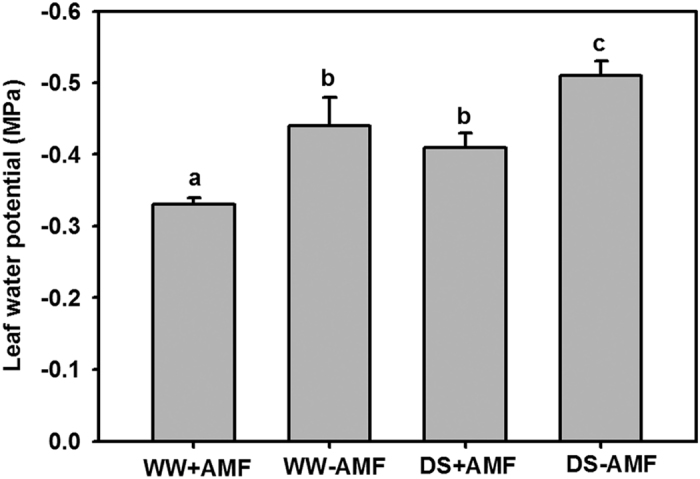
Data (means ± SD, n = 4) followed by different letters above the bars indicate significant differences (P < 0.05) between treatments. Abbreviations: +AMF: inoculation with D. versiformis. −AMF: inoculation without D. versiformis, DS: drought stress, WW: well-watered.
Number of lateral roots
In five-month-old trifoliate orange seedlings, the number of 2nd-order lateral roots was in the dominant position of the number of lateral roots (Fig. 2). DS treatment significantly (P < 0.05) decreased the number of 1st-, 2nd-, and 3rd-order lateral roots in both AM and non-AM seedlings, compared with the WW treatment. Compared with the non-mycorrhizal colonized controls, D. versiformis colonized seedlings showed a significant (P < 0.05) increase by 14%, 39%, and 73% in the number of 1st-, 2nd-, and 3rd-order lateral roots under WW, and by 18%, 28%, and 123% under DS, respectively.
Figure 2. Number of 1st, 2nd and 3rd-order lateral roots of trifoliate orange (P. trifoliata) seedlings colonized by D. versiformis under well-watered and drought stress conditions.

Data (means ± SD, n = 4) followed by different letters above the bars indicate significant differences (P < 0.05) between treatments. Abbreviations: same as for Fig. 1.
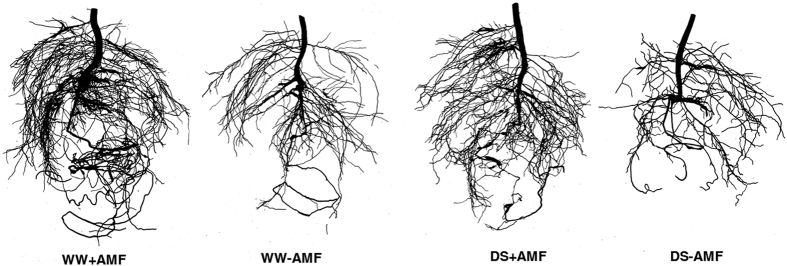
Root morphology
Root morphology of five-month-old trifoliate orange seedlings was significantly reduced by the DS treatment, compared with the WW treatment, while it was increased by mycorrhizal inoculation under the same soil water status as compared with non-mycorrhizal treatment (Table 2; Fig. 3). AM seedlings represented significantly (P < 0.05) higher root length, projected area, surface area, average diameter, volume, tips, forks, and crossings than non-AM seedlings, regardless of whether the seedlings were subjected to DS: 13%, 8%, 9%, 5%, 66%, 323%, 91%, and 107% increase under WW, and 10%, 7%, 8%, 7%, 48%, 201%, 83%, and 79% under DS, respectively (Table 2).
Table 2. Root morphological traits of D. versiformis colonized trifoliate orange seedlings in response to different treatments.
| Treatments | Length (cm) | Projected area (cm2) | Surface area (cm2) | Average diameter (mm) | Volume (cm3) | Tips (#/plant) | Forks (#/plant) | Crossings (#/plant) |
|---|---|---|---|---|---|---|---|---|
| WW + AMF | 240 ± 15a | 13.7 ± 0.3a | 17.6 ± 0.4a | 0.565 ± 0.012a | 1.73 ± 0.06a | 2119 ± 220a | 2562 ± 238a | 765 ± 58a |
| WW − AMF | 213 ± 5b | 12.7 ± 0.1b | 16.2 ± 0.2c | 0.537 ± 0.013b | 1.04 ± 0.11c | 501 ± 84c | 1338 ± 123c | 370 ± 24b |
| DS + AMF | 216 ± 7b | 12.9 ± 0.2b | 16.7 ± 0.2b | 0.548 ± 0.005b | 1.29 ± 0.11b | 889 ± 46b | 2006 ± 90b | 451 ± 63b |
| DS − AMF | 196 ± 8c | 12.1 ± 0.4c | 15.5 ± 0.2d | 0.514 ± 0.004c | 0.87 ± 0.03d | 295 ± 103d | 1097 ± 71d | 252 ± 30c |
Data (means ± SD, n = 4) followed by different letters indicate significant differences among treatments after using the Duncan’s multiple range test (P < 0.05). Abbreviations: same as Table 1.
Figure 3. Root morphology status of trifoliate orange (P. trifoliata) seedlings colonized by D. versiformis under well-watered and drought stress conditions.
Abbreviations: same as for Fig. 1.
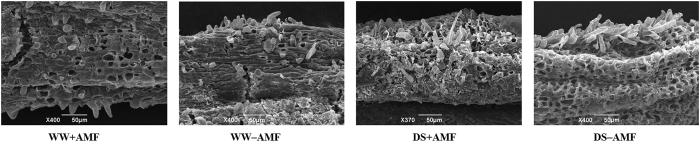
Root hair development
Figure 4 showed the change in root hairs in response to the DS and mycorrhizal inoculation. DS treatment significantly reduced root hair density, while it notably (P < 0.05) increased root hair length and had no effect on root hair diameter, compared with WW (Fig. 5). Compared with the non-AM seedlings, root hair density in mycorrhizal colonized seedlings was significantly (P < 0.05) decreased by 12% under both WW and DS (Fig. 5). Root hair length was significantly (P < 0.05) 22% lower in AM seedlings than in non-AM seedlings under WW, whereas it was significantly 7% higher in AM seedlings than in non-AM seedlings under DS. Root hair length was not affected by AM fungal colonization, irrespective of the soil water status.
Figure 4. Root hair morphology status of trifoliate orange (P. trifoliata) seedlings colonized by D. versiformis under well-watered and drought stress conditions.
Abbreviations: same as for Fig. 1.
Figure 5. Root hair density, root hair length, and root hair diameter of trifoliate orange (P. trifoliata) seedlings colonized by D. versiformis under well-watered and drought stress conditions.
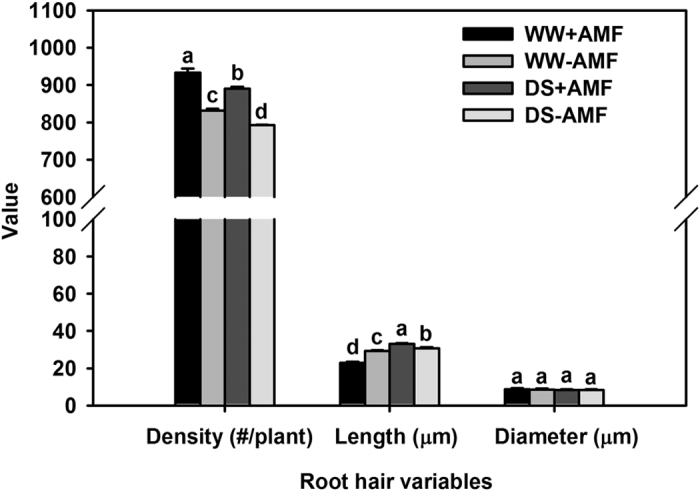
Data (means ± SD, n = 4) followed by different letters above the bars indicate significant differences (P < 0.05) between treatments. Abbreviations: same as for Fig. 1.
Root endogenous phytohormone levels
Mycorrhizal inoculation and DS did not significantly (P < 0.05) alter the root brassinosteroid (BR) and gibberellins (GAs) concentrations (Fig. 6). The DS treatment induced lower accumulation of root IAA and MeJA than the WW treatment, irrespective of the AM status (Fig. 6). However, compared with non-mycorrhizal seedlings, root IAA concentration in mycorrhizal seedlings was increased, by 25% under WW and by 19% under DS. Root MeJA concentration was 24% and 11% higher under WW and DS in AM seedlings than in non-AM seedlings, respectively.
Figure 6. Root BR, GAs, IAA, and MeJA concentration of trifoliate orange (P. trifoliata) seedlings colonized by D. versiformis under well-watered and drought stress conditions.
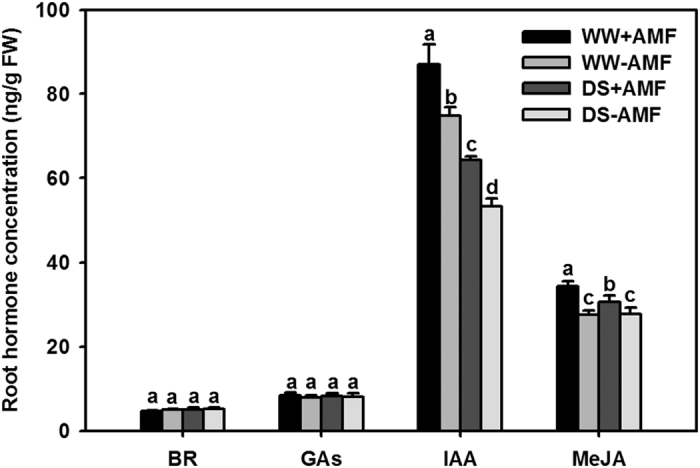
Data (means ± SD, n = 4) followed by different letters above the bars indicate significant differences (P < 0.05) between treatments. Abbreviations: same as for Fig. 1 and BR: brassinosteroid, GAs: gibberellins, IAA: indole-3-acetic acid, MeJA: methyl jasmonate.
Root calmodulin (CaM) and nitric oxide (NO) concentration
The DS treatment significantly (P < 0.05) decreased the root calmodulin (CaM) and nitric oxide (NO) concentration in both AM and non-AM seedlings, as compared with the WW treatment (Fig. 7a,b). Compared with the non-AM fungal colonized controls, D. versiformis-colonized seedlings exhibited significantly (P < 0.05) higher root CaM concentration by 6% and 11% under WW and DS (Fig. 7a), and 219% and 117% higher root NO concentration under WW and DS (Fig. 7b), respectively.
Figure 7. Root CaM and NO concentration of trifoliate orange (P. trifoliata) seedlings colonized by D. versiformis under well-watered and drought stress conditions.
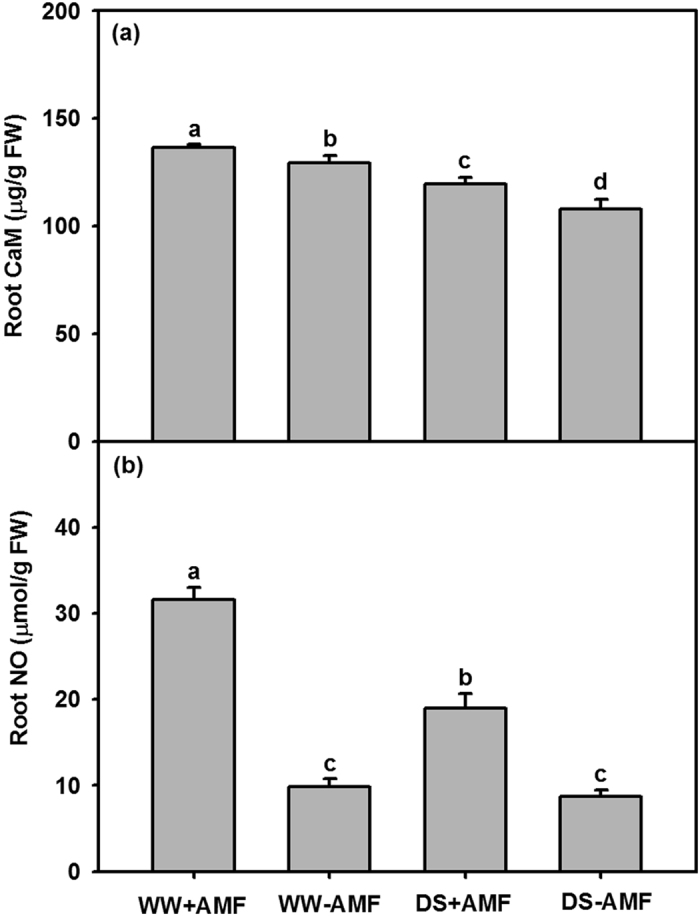
Data (means ± SD, n = 4) followed by different letters above the bars indicate significant differences (P < 0.05) between treatments. Abbreviations: same as for Fig. 1 and CaM: calmodulin; NO: nitric oxide.
Correlation studies
Leaf Ψ and root IAA, MeJA, CaM, and NO, but not BR and GAs, showed a significantly (P < 0.01) positive correlation with the number of 1st-, 2nd-, and 3rd-order lateral roots, root morphological traits, and root hair density. In contrast, they showed a significantly (P < 0.01) negative correlation with root hair length and had no significant correlation with root hair diameter (Table 3).
Table 3. Pearson’s correlations (r) between root morphological traits and leaf Ψ or root chemical variables (n = 16).
| Variables | Root BR | Root GAs | Root IAA | Root MeJA | Root CaM | Root NO | Leaf Ψ |
|---|---|---|---|---|---|---|---|
| Number of lateral roots | |||||||
| 1st | −0.32 | 0.39 | 0.79** | 0.66** | 0.82** | 0.80** | 0.83** |
| 2nd | −0.41 | 0.35 | 0.82** | 0.86** | 0.79** | 0.90** | 0.86** |
| 3rd | −0.40 | 0.23 | 0.93** | 0.61* | 0.93** | 0.71** | 0.79** |
| Root morphology | |||||||
| Length | −0.53* | 0.20 | 0.88** | 0.72** | 0.84** | 0.79** | 0.83** |
| Projected area | −0.37 | 0.23 | 0.91** | 0.73** | 0.87** | 0.76** | 0.85** |
| Surface area | −0.46 | 0.41 | 0.84** | 0.81** | 0.80** | 0.90** | 0.95** |
| Average diameter | −0.29 | 0.35 | 0.81** | 0.74** | 0.72** | 0.82** | 0.88** |
| Volume | −0.28 | 0.30 | 0.80** | 0.90** | 0.72** | 0.94** | 0.89** |
| Tips | −0.38 | 0.21 | 0.92** | 0.78** | 0.85** | 0.84** | 0.84** |
| Forks | −0.36 | 0.28 | 0.71** | 0.90** | 0.66** | 0.95** | 0.85** |
| Crossings | −0.38 | 0.28 | 0.86** | 0.88** | 0.80** | 0.94** | 0.88** |
| Root hair morphology | |||||||
| Density | −0.38 | 0.34 | 0.74** | 0.84** | 0.72** | 0.94** | 0.91** |
| Length | 0.33 | −0.19 | −0.81** | −0.64** | −0.74** | −0.70** | −0.66** |
| Diameter | −0.19 | −0.02 | 0.20 | 0.10 | 0.28 | 0.18 | 0.16 |
**P < 0.01. Abbreviations: BR: brassinosteroid, CaM: calmodulin, GAs: gibberellins, IAA: indole-3-acetic acid, NO: nitric oxide, MeJA: methyl jasmonate, Ψ: water potential.
Discussion
In our study, inoculation with D. versiformis strongly stimulated the formation in 1st-, 2nd-, and 3rd-order lateral roots in trifoliate orange subjected to both WW and DS. This is in agreement with earlier studies reported in Medicago truncatula colonized by Gigaspora margarita27. Such mycorrhizal roots became progressively more branched than non-mycorrhizal controls26. This was confirmed by an increase in the number of tips, forks, and crossings in the present study. This shows that AM plants had a stronger adaptation in terms of lateral root formation than non-AM plants under DS. Mycorrhizal mycelium is mainly localized in the newly formed lateral roots39. Greater number of lateral roots in AM plants would provide better chances of colonization by AM fungi, as well as for absorbing water from the soil to the host plant, as shown in our study through a significant correlation between leaf Ψ and the number of lateral roots. A diffusible factor, ‘Myc factor’, from AM fungi, was found to stimulate lateral root formation27. Our study showed that root IAA, MeJA, CaM, and NO were involved in lateral root formation. As reported by Felten et al.40, mycorrhiza-stimulated lateral root induction is paralleled by an increase in the 1-naphthylphthalamic acid (NPA)-sensitive auxin response at the root apex and in provascular tissues, together with IAA-based auxin signalling.
The present study showed that exposure to DS for six weeks dramatically restricted root morphological development in trifoliate orange seedlings, irrespective of the AM status. This indicates that the volume of soils explored by the whole root systems was dramatically reduced by DS in soils, which is in agreement with earlier studies on rice3. However, the D. versiformis that colonized trifoliate orange seedlings caused an improvement in morphological traits (length, area, volume, diameter, tips, forks, and crossings), compared with the non-AM controls under both WW and DS, indicating a larger extensive distribution of roots in AM seedlings under DS, which can contribute to higher leaf Ψ than non-AM plants41. The improvement of the root system by mycorrhization was also found in split-root trifoliate orange seedlings colonized by Acaulospora scrobiculata and F. mosseae, grown in a two-chambered split-root system42. In general, root morphology, development, and physiology are closely connected with plant growth41. A better root morphology in AM plants would promote plant growth, as shown by the positive effect of AM fungi on plant growth performance in trifoliate orange under both WW and DS. As roots have the ability to grow toward the direction of high water availability in the soil2, a significantly positive correlation of root morphological traits with leaf Ψ suggests that roots of mycorrhizal plants can explore the soil better for water uptake under DS. As a result, AM plants possess a stronger capacity, owing to a better root morphology, to adapt to soil drought than non-AM plants.
Wu et al.31 showed that four AM fungal species (Claroideoglomus etunicatum, D. versiformis, F. mosseae, and R. intraradices) induced a significant increase in root hair density in trifoliate orange seedlings grown in sands. The present study further indicated a strong effect of AM fungal inoculation on the root hair density of trifoliate orange seedlings grown in soils under both WW and DS. Li et al.43 reported that AM fungi mainly enhanced plant drought tolerance by the improvement of P and leaf water status, but root hairs presumably contributed to the shoot P enhancement. The AM-mediated increase in root hair density does not depend on growth substrates and soil water status. On the other hand, AM fungal inoculation affected the root hair length with a significant decrease under WW and with a significant increase under DS, suggesting that the the effect of AM fungus on root hair length under WW can be reversed by DS, possibly due to the increase in nutrient requirements under poor nutrient-movement conditions. This reduction in root hair length under WW is consistent with the results of Sun and Tang44 in Sorghum bicolor plants inoculated with F. mosseae and R. intraradices. The increase in AM-induced root hair length under DS is in agreement with the results of Wu et al.31. We also concluded that higher concentrations of IAA, MeJA, NO, and CaM in roots under mycorrhization stimulate AM fungal colonization under WW and also improve root morphology under DS, indicating different functionings of these phytohormones in mycorrhizal plants exposed to water treatments.
Earlier studies confirmed a negative effect of GAs on root mycorrhizal colonization45,46 and a limited role of BR in determining AM development31. In this study, both AM fungal inoculation and water treatment produced no changes in the root BR and GAs concentration of trifoliate orange seedlings, suggesting that BR and GAs are not key factors involved in mycorrhizal development.
The root IAA level, in this study, was significantly decreased by DS while it was increased by AM fungal colonization, regardless of the soil water status. The mycorrhiza-induced root IAA increase might be due to small amounts of IAA that could be released by spores of AM fungi47. IAA, the major endogenous auxin in plants, is required for root development and root hair formation48. Moreover, auxins participate in the induction of cellular rhizogenic competence, root apical meristem differentiation, and development of the root cap and vasculature49. A mycorrhiza-induced IAA increment in roots would improve root morphology and lead to root hair modification. Therefore, it is reasonable that root IAA was significantly and positively correlated with root morphological traits and root hair density. Root IAA was negatively correlated with root hair length, whereas root hair length was significantly decreased by AM fungi under WW and increased under DS, suggesting that AM-induced IAA changes are dependent on soil water status and the integrated effects of several phytohormones, besides IAA. In addition, auxin accumulation could be involved in plant responses to DS via the activation of signalling pathways and induction of auxin-responsive genes50. The increase in the root IAA by mycorrhization has the potential capacity to enhance drought tolerance of the host plant.
In addition to IAA, NO participates in the induction of root tip elongation and the formation of lateral roots51. It is also a critical molecule for root hair formation through the auxin-signalling cascade52. In this study, in spite of the negative effect of DS on root NO levels, root NO concentrations were significantly increased in response to AM fungal inoculation under both WW and DS. This is consistent with the results of Calcagno et al.53 in Medicago truncatula colonized by Gigaspora margarita and Espinosa et al.54 in olive seedlings inoculated with R. irregularis. It has been established that NO is involved in root mycorrhizal colonization. Furthermore, root NO was significantly and positively correlated with the number of lateral roots, root morphological traits, and root hair density. This implies that mycorrhizal colonization heavily stimulated root NO production, which might play a role in the formation of lateral roots and root hairs. The NO-mediated effect on roots is under the control of auxins52. Further works are needed to clarify the mycorrhiza-induced root improvement by NO-mediated auxin signalling55. In addition, induction of root NO accumulation under mycorrhization might be a part of the mechanism producing a local response to enhance drought tolerance in plants51.
Methyl jasmonate (MeJA) has been identified as a vital cellular regulator to mediate developmental processes (including root hair production), proper arbuscule formation44, and defence responses against stresses (e.g., drought)56. Our study indicated that the root MeJA concentrations in trifoliate orange seedlings were pronouncedly increased by the AM fungal inoculation, irrespective of the soil water status. This is in agreement with previous studies on barley, soybean, and trifoliate orange plants under mycorrhization31,57,58. The root MeJA concentration was significantly and positively correlated with root hair density, but was negatively correlated with root hair length, which is in agreement with our previous work on trifoliate orange31. The increase in root MeJA concentration upon mycorrhizal inoculation might be associated with improving drought tolerance of the host plant, as previously mentioned by Anjum et al.59.
An earlier study had reported an increase in the intracellular CaM in soybean after being colonized by R. intraradices60. The present study also indicated that inoculation with D. versiformis induced the accumulation of root CaM under both WW and DS. Similar results were found in trifoliate orange seedlings colonized by F. mosseae under both WW and DS16. It seems that CaM is involved in the process of root mycorrhizal colonization. Lévy et al.61 further confirmed that Ca2+ spiking and CaM-dependent protein kinases are necessary for mycorrhizal infection. Calmodulin (CaM), a Ca2+ receptor, can bind with Ca2+ as the Ca2+/CaM messenger system to activate downstream target proteins, thereby regulating the generation of reactive oxygen species and modulating transcription factors to maintain homeostasis between different cellular processes62. In the study of Huang et al.16, mycorrhiza-induced CaM mediated the antioxidant enzyme defence system to enhance drought tolerance in plants. Higher root CaM levels in AM plants have the capacity to enhance drought tolerance of the host plant. CaM is a primary decoder of Ca2+ signals63, while the Ca2+ gradient and Ca2+ influxes induce root hair formation and growth64. Root CaM was significantly and positively correlated with root hair density while negatively correlated with root hair length.
In short, our results supported the preceding hypothesis that AM plants had better root adaptation of morphology in response to drought stress, which is potentially associated with AM-induced changes in IAA, MeJA, NO, and CaM. It is therefore suggested that in citriculture, either stimulating the formation and development of AMs or inoculating native AM fungi into citrus orchards, would be vital for the enhancement of drought tolerance and root morphology in citrus trees.
Methods
Plant culture
Seeds of trifoliate orange were sterilized by 70% of ethanol solution for 10 min, rinsed 4 times with distilled water, and pre-germinated in autoclaved (121 °C, 0.11 Mpa, 2 h) river sand under the condition of 27/20 °C day/night temperature, 740 μmol/m2/s photon flux density, and 80% relative humidity. After 4 weeks, three five-leaf-old seedlings were transplanted into a plastic pot (11.5 cm upper diameter × 8.5 cm bottom diameter × 14 cm height) and supplied with 1.8 kg of autoclaved (0.11 Mpa, 121 °C, 2 h) soil + vermiculite mixture (2:1, v/v) on March 28, 2014. The soil, which belongs to the Xanthi-udic Ferralsol soil (FAO system), was collected from a citrus orchard on the Yangtze University campus (30°36′N, 112°14′E, 36 m above sea level). The soil has the characteristics of pH 6.0, 12.1 mg/kg KMnO4-N, 15.7 mg/kg Bray-P, and 22.3 mg/kg neutral NH4OAc-K. Half of the seedlings received the AM fungal inoculated treatment. A 2200-spore dosage of an AM fungus, Diversispora versiformis (P. Karst.) Oehl, G. A. Silva & Sieverd, was applied into the surroundings of the plant roots. The AM fungus was supplied by the Bank of Glomeromycota in China (BGC). The AM fungus was isolated from the rhizosphere of Astragalus adsurgens in Ejin Horo Banner, Inner Mongolia Autonomous Region, China. The spores of the AM fungus were propagated by white clover in a mixture of sand and soil (1:1, v/v) for 12 weeks. For the non-AM fungal treatment, seedlings were supplied the same amount of autoclaved AM fungal inocula plus 2 mL filtrate (25 μm filter) of mycorrhizal inoculum to minimize differences in other microbial communities. AM and non-AM seedlings were placed in a green house on the Yangtze University campus, where the photosynthetic photon flux density was 880 μmol/m2/s, day/night temperature 28/21 °C, and relative humidity 85%. During the experiment, a 30 mL Hoagland solution per pot was used to replace 30 mL distilled water weekly.
Water treatments
After AM fungal inoculation, the seedlings were gravimetrically maintained at 75% of maximum water holding capacity (soil WW status) in the growth substrate for 15 weeks. Subsequently, half of AM and non-AM seedlings were changed to 50% of maximum water holding capacity (soil DS status) in the growth substrate for DS for 6 weeks. The other seedlings were still kept in soil WW status for 6 weeks. After 21 weeks of water treatments, seedlings were harvested.
Experimental design
The experiment was composed of AM fungal inoculations (with or without D. versiformis) and water treatments (WW and DS) with a completely randomized arrangement, for a total of four treatments. Each treatment was replicated four times, for a total of 16 pots.
Determinations of plant growth performance
Before harvest, plant height, stem diameter, and number of leaves per plant were recorded. At harvest, the seedlings were divided into shoots and roots, whose biomass was determined.
Determination of mycorrhizas
One cm long root segments were cleared with 10% (w/v) KOH in 95 °C for 1.5 h and stained with 0.05% (w/v) trypan blue in lactoglycerol for 5 min65. Root AM fungal colonization was calculated as the percentage of AM fungal colonized root lengths against total root lengths.
Determination of root morphology
After washing with tap water, the intact root system of each seedling from each pot was placed in a Regent’s water-proof plexiglass tray without root overlap and then scanned by an EPSON Flatbed Scanner, Epson Perfection V700 (Seiko Epson Corp, Japan). The root images were analyzed by the WinRHIZO 2007d (Regent Instruments Incorporated, Quebec, Canada) for root morphological variables, including total length, projected area, surface area, average diameter, and volume. Lateral roots grew on the taproot were gradually defined as 1st, 2nd, and 3rd order, and the number of different order lateral roots was counted through placing experimental tables.
Determination of root-hair morphology
Root hairs were observed as per the protocol outlined by Wu et al.31. At harvest, the root-hair zone was quickly collected and fixed with 2.5% glutaraldehyde with 0.1 mol/L sodium cacodylate buffer (pH 7.4), dehydrated step by step by alcohol with increasing concentrations, and dried using critical-point drying (CPD). A scanning electron microscope (SEM, model JSM-6390LV, JEOL Co., Japan) was used to acquire the photo of root hairs. The root hair photo was analyzed by the Image J software (National Institutes of Health, Maryland, USA) for density, length, and diameter of root hairs.
Determination of leaf water potential (Ψ)
The leaf water potential (leaf Ψ) was measured by a PSΨPRO Water Potential System with a leaf hygrometer (L-51A-SF, WESCOR).
Determination of root endogenous hormones
Extraction of IAA, GAs, BR, and MeJA was done by the protocol described by Wu et al.31 and determined by the enzyme-linked immunosorbent assay (ELISA). These ELISAs were provided by the Engineering Research Center of Plant Growth Regulator, China Agricultural University.
Root NO and CaM levels were determined by the ELISA assay (CaM: YAD-001, Beijing Dingguochangsheng Biotechnology Co., Ltd, Beijing, China; NO: A012, Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis
Data (means ± SD, n = 4) were statistically analyzed by two-factor variance (ANOVA) in SAS software (SAS Institute Inc., Cary, NC, USA). Data regarding root AM fungal colonization were arcsine transformed prior to ANOVA. The Duncan’s multiple range tests at P < 0.05 were utilized to compare significant differences between treatments.
Additional Information
How to cite this article: Zou, Y.-N. et al. Mycorrhizal trifoliate orange has greater root adaptation of morphology and phytohormones in response to drought stress. Sci. Rep. 7, 41134; doi: 10.1038/srep41134 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This study was supported by the Plan in Scientific and Technological Innovation Team of Outstanding Young, Hubei Provincial Department of Education (T201604) and the National Natural Science Foundation of China (31101513). Thank to Dr. Edward C. Mignot for linguistic advice.
Footnotes
Author Contributions Y.N.Z. and Q.S.W. conceived the experiment, and C.Y.L., Q.D.N., D.J.Z., and P.W. conducted the experiment. All authors analyzed the results. Y.N.Z., P.W., C.Y.L., and Q.S.W. drafted the original manuscript, and Q.S.W. reviewed the manuscript.
References
- Kunert K. et al. Drought stress responses in soybean roots and nodules. Front. Plant Sci. 7, 1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L. M., Wang R. G., Mao G. H. & Koczan J. M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 142, 1065–1074 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Siopongco J., Wade L. J. & Yamauchi A. Fractal analysis on root systems of rice plants in response to drought stress. Environ. Exp. Bot. 65, 338–344 (2009). [Google Scholar]
- Schnall J. A. & Quatrano R. S. Abscisic acid elicits the water-stress response in root hairs of Arabidopsis thaliana. Plant Physiol. 100, 216–218 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. E. & Read D. J. Mycorrhizal symbiosis (Academic Press, San Diego, 2008). [Google Scholar]
- Wu Q. S., Srivastava A. K. & Zou Y. N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 164, 77–87 (2013). [Google Scholar]
- He F., Zhang H. Q. & Tang M. Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 26, 311–323 (2016). [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano J. M. et al. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 39, 441–452 (2016). [DOI] [PubMed] [Google Scholar]
- Allen M. F. Mycorrhizal fungi: Highways for water and nutrients in arid soils. Vadose Zone J. 6, 291–297 (2007). [Google Scholar]
- Zou Y. N., Srivastava A. K., Ni Q. D. & Wu Q. S. Disruption of mycorrhizal extraradical mycelium and changes in leaf water status and soil aggregate stability in rootbox-grown trifoliate orange. Front. Microbiol. 6, 203 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen C. E. & Safir G. R. Increased drought tolerence of mycorrhizal onion plants caused by improved phosphorous nutrition. Planta 154, 407–413 (1982). [DOI] [PubMed] [Google Scholar]
- Wu Q. S. & Xia R. X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 163, 417–425 (2006). [DOI] [PubMed] [Google Scholar]
- Wu Q. S., Xia R. X. & Zou Y. N. Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J. Plant Physiol. 163, 1101–1110 (2006). [DOI] [PubMed] [Google Scholar]
- Tuo X. Q., Li S., Wu Q. S. & Zou Y. N. Alleviation of waterlogged stress in peach seedlings inoculated with Funneliformis mosseae: Changes in chlorophyll and proline metabolism. Sci. Hortic. 197, 130–134 (2015). [Google Scholar]
- Zou Y. N., Huang Y. M., Wu Q. S. & He X. H. Mycorrhiza-induced lower oxidative burst is related with higher antioxidant enzyme activities, net H2O2 effluxes, and Ca2+ influxes in trifoliate orange roots under drought stress. Mycorrhiza 25, 143–152 (2015). [DOI] [PubMed] [Google Scholar]
- Huang Y. M. et al. Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front. Microbiol. 5, 682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y. N., Srivastava A. K., Wu Q. S. & Huang Y. M. Glomalin-related soil protein and water relations in mycorrhizal citrus (Citrus tangerina) during soil water deficit. Arch. Agron. Soil Sci. 60, 1103–1114 (2014). [Google Scholar]
- Porcel R., Aroca R., Azcón R. & Ruiz-Lozano J. M. PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol. Biol. 60, 389–404 (2006). [DOI] [PubMed] [Google Scholar]
- Li T. et al. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 197, 617–630 (2013). [DOI] [PubMed] [Google Scholar]
- Fan Q. J. & Liu J. H. Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in Poncirus trifoliata. Acta Physiol. Plant. 33, 1533–1542 (2011). [Google Scholar]
- Porcel R., Azcón R. & Ruiz-Lozano J. M. Evaluation of the role of genes encoding for Δ1-pyrroline-5-carboxylate synthetase (P5CS) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. Physiol. Mol. Plant Path. 65, 211–221 (2004). [Google Scholar]
- Porcel R., Azcón R. & Ruiz-Lozano J. M. Evaluation of the role of genes encoding for dehydrin proteins (LEA D-11) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. J. Exp. Bot. 56, 1933–1942 (2005). [DOI] [PubMed] [Google Scholar]
- Chitarra W. et al. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. doi: 10.1104/pp.16.00307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. et al. Potential role of D-myo-inositol-3-phosphate synthase and 14-3-3 genes in the crosstalk between Zea mays and Rhizophagus intraradices under drought stress. Mycorrhiza 26, 879–893 (2016). [DOI] [PubMed] [Google Scholar]
- Hodge A., Berta G., Doussan C., Merchan F. & Crespi M. Plant root growth, architecture and function. Plant Soil 321, 153–187 (2009). [Google Scholar]
- Berta G., Fusconi A., Trotta A. & Scannerini S. Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root system of Allium porrum L. New Phytol. 114, 207–215 (1990). [Google Scholar]
- Oláh B., Brière C., Bécard G., Dénarié J. & Gough C. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J. 44, 195–207 (2005). [DOI] [PubMed] [Google Scholar]
- Wu Q. S. et al. Arbuscular mycorrhizas alter root system architecture of Citrus tangerine through regulating metabolism of endogenous polyamines. Plant Growth Regul. 68, 27–35 (2012). [Google Scholar]
- Atkinson D. et al. The influence of arbuscular mycorrhizal colonisation and environment on root development in soil. Eur. J. Soil Sci. 54, 751–757 (2003). [Google Scholar]
- Herdlera S., Kreuzera K., Scheua S. & Bonkowski M. Interactions between arbuscular mycorrhizal fungi (Glomus intraradices, Glomeromycota) and amoebae (Acanthamoeba castellanii, Protozoa) in the rhizosphere of rice (Oryza sativa). Soil Biol. Biochem. 40, 660–668 (2008). [Google Scholar]
- Wu Q. S. et al. Mycorrhiza alters the profile of root hairs in trifoliate orange. Mycorrhiza 26, 237–247 (2016). [DOI] [PubMed] [Google Scholar]
- Schroeder M. S. & Janos D. P. Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 15, 203–216 (2005). [DOI] [PubMed] [Google Scholar]
- Wu Q. S., Li G. H. & Zou Y. N. Improvement of root system architecture in peach (Prunus persica) seedlings by arbuscular mycorrhizal fungi, related to allocation of glucose/sucrose to root. Not. Bot. Horti. Agrobo. 39, 232–236 (2011). [Google Scholar]
- Gutjahr C., Casieri L. & Paszkowski U. Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol. 182, 829–837 (2009). [DOI] [PubMed] [Google Scholar]
- Wittenmayer L. & Merbach W. Plant responses to drought and phosphorus deficiency: contribution of phytohormones in root-related processes. J. Plant Nutri. Soil Sci. 168, 531–540 (2005). [Google Scholar]
- Diego N. D. et al. Metabolites and hormones are involved in the intraspecific variability of drought hardening in radiata pine. J. Plant Physiol. 188, 64–71 (2015). [DOI] [PubMed] [Google Scholar]
- Pozo M. J., López-Ráez J. A., Azcón-Aguilar C. & García-Garrido J. M. Phytohormones as integrators of en vironmental signals in the regulation of mycorrhizal symbioses. New Phytol. 205, 1431–1436 (2015). [DOI] [PubMed] [Google Scholar]
- Sánchez-Romera B., Ruiz-Lozano J. M., Zamarreño A. M., García-Mina J. M. & Aroca R. Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought. Mycorrhiza 26, 111–122 (2016). [DOI] [PubMed] [Google Scholar]
- Tisserant B., Gianinazzi S. & Gianinazzi-Pearson V. Relationships between lateral root order, arbuscular mycorrhiza development, and the physiological state of the symbiotic fungus in Platanus acerifolia. Can. J. Bot. 74, 1947–1955 (1996). [Google Scholar]
- Felten J. et al. The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in Poplar and Arabidopsis through auxin transport and signaling. Plant Physiol. 151, 1991–2005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Chen T. T., Wang Z. Q., Yang J. C. & Zhang J. H. Reprint of “Morphological and physiological traits of roots and their relationships with water productivity in water-saving and drought-resistant rice”. Field Crops Res. 165, 36–48 (2014). [Google Scholar]
- Wu Q. S., Cao M. Q., Zou Y. N., Wu C. & He X. H. Mycorrhizal colonization represents functional equilibrium on root morphology and carbon distribution of trifoliate orange grown in a split-root system. Sci. Hortic. 199, 95–102 (2016). [Google Scholar]
- Li T. et al. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 24, 595–602 (2014). [DOI] [PubMed] [Google Scholar]
- Sun X. G. & Tang M. Effect of arbuscular mycorrhizal fungi inoculation on root traits and root volatile organic compound emissions of Sorghum bicolor. South Afr. J. Bot. 88, 373–379 (2013). [Google Scholar]
- Floss D. S., Levy J. G., Lévesque-Tremblay V., Pumplin N. & Harrison M. J. DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. 110, E5025–E5034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E., Ross J. J., Jones W. T. & Reid J. B. Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Ann. Bot. 111, 769–779 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J. Hormonal responses in host plants triggered by arbuscular mycorrhizal fungi. Arbuscular mycorrhizas: Physiology and Function [ Koltai H. & Kapulnik Y. (eds)] [169–196] (Springer, New York, 2010). [Google Scholar]
- Bhalerao R. P. et al. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 29, 325–332 (2002). [DOI] [PubMed] [Google Scholar]
- Kurepin L. V., Park J. M., Lazarovits G. & Bernards M. A. Burkholderia phytofirmans-induced shoot and root growth promotion is associated with endogenous changes in plant growth hormone levels. Plant Growth Regul. 75, 199–207 (2015). [Google Scholar]
- Jung H., Lee D. K., Choi Y. D. & Kim J. K. OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 236, 304–312 (2015). [DOI] [PubMed] [Google Scholar]
- Corpas F. J. & Barroso J. B. Functions of nitric oxide (NO) in roots during development and under adverse stress conditions. Plants 4, 240–252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M. C., Graziano M., Polacco J. C. & Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal. Behav. 1, 22–33 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno C., Novero M., Genre A., Bonfante P. & Lanfranco L. The exudate from an arbuscular mycorrhizal fungus induces nitric oxide accumulation in Medicago truncatula roots. Mycorrhiza 22, 259–269 (2012). [DOI] [PubMed] [Google Scholar]
- Espinosa F., Garrido I., Ortega A., Casimiro I. & Álvarez-Tinaut M. C. Redox activities and ROS, NO and phenylpropanoids production by axenically cultured intact olive seedling roots after interaction with a mycorrhizal or a pathogenic fungus. PLoS ONE 9, e100132 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. et al. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 168, 343–356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J. J. & Choi Y. D. Methyl jasmonate as a vital substance in plants. Trends Gene. 19, 409–413 (2003). [DOI] [PubMed] [Google Scholar]
- Hause B., Maier W., Miersch O., Kramell R. & Strack D. Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol. 130, 1213–1220 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meixner C. et al. Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 222, 709–715 (2005). [DOI] [PubMed] [Google Scholar]
- Anjum S. A., Wang L., Farooq M., Khan I. & Xue L. Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J. Agron. Crop Sci. 197, 296–301 (2011). [Google Scholar]
- Lorella N. et al. The arbuscular mycorrhizal fungus Glomus intraradices induces intracellular calcium changes in soybean cells. Caryologia 60, 137–140 (2007). [Google Scholar]
- Lévy J. et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303, 1361–1364 (2004). [DOI] [PubMed] [Google Scholar]
- Virdi A. S., Singh S. & Singh P. Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front. Plant Sci. 6, 809 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. J. & Malhó R. Ca2+ signalling in plant cells: the big network. Curr. Opin. Plant Biol. 1, 428–433 (1998). [DOI] [PubMed] [Google Scholar]
- Foreman J. et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446 (2003). [DOI] [PubMed] [Google Scholar]
- Phillips J. M. & Hayman D. S. Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–161 (1970). [Google Scholar]