Abstract
Photosynthesis produces chemical energy from photon energy in the photosynthetic electron transport and assimilates CO2 using the chemical energy. Thus, CO2 limitation causes an accumulation of excess energy, resulting in reactive oxygen species (ROS) which can cause oxidative damage to cells. O2 can be used as an alternative energy sink when oxygenic phototrophs are exposed to high light. Here, we examined the responses to CO2 limitation and O2 dependency of two secondary algae, Euglena gracilis and Phaeodactylum tricornutum. In E. gracilis, approximately half of the relative electron transport rate (ETR) of CO2-saturated photosynthesis was maintained and was uncoupled from photosynthesis under CO2 limitation. The ETR showed biphasic dependencies on O2 at high and low O2 concentrations. Conversely, in P. tricornutum, most relative ETR decreased in parallel with the photosynthetic O2 evolution rate in response to CO2 limitation. Instead, non-photochemical quenching was strongly activated under CO2 limitation in P. tricornutum. The results indicate that these secondary algae adopt different strategies to acclimatize to CO2 limitation, and that both strategies differ from those utilized by cyanobacteria and green algae. We summarize the diversity of strategies for prevention of photo-oxidative damage under CO2 limitation in cyanobacterial and algal photosynthesis.
Air consists of 21% O2, the concentration of which increased during the evolution of oxygenic phototrophs, in particular the oceanic cyanobacteria, around 2.3 billion years ago1. Due to its electron configuration, O2 has a very high oxidizing potential and is the final electron acceptor in aerobic respiratory electron transport.
Oxygenic photosynthesis uses photon energy to produce sugar from CO2 and H2O, and releases O2 as a waste product. Two photosystems, PSI and PSII, play central roles in this process, which involves an electron transport system located on thylakoid membranes. The reaction centers, P700 and P680, are photo-oxidized via light-harvesting pigments such as chlorophyll (Chl). The oxidized P700 in PSI accepts electrons from PSII via plastoquinone, the cytochrome b6/f complex, and plastocyanin (or cytochrome c6). This electron transport is accompanied by the generation of a proton gradient across the membranes (ΔpH), allowing the production of ATP by ATP synthase2. At the acceptor side of PSI, NADP+ is reduced to NADPH by accepting electrons from P700 through ferredoxin and ferredoxin-NADP+ reductase. O2 is produced via the oxidation of H2O by oxidized P680 in the luminal side of PSII. Together, these reactions are termed ‘photosynthetic electron transport’, and are the source of chemical energy in the forms NADPH and ATP, which are used for CO2 assimilation in the Calvin-Benson cycle3.
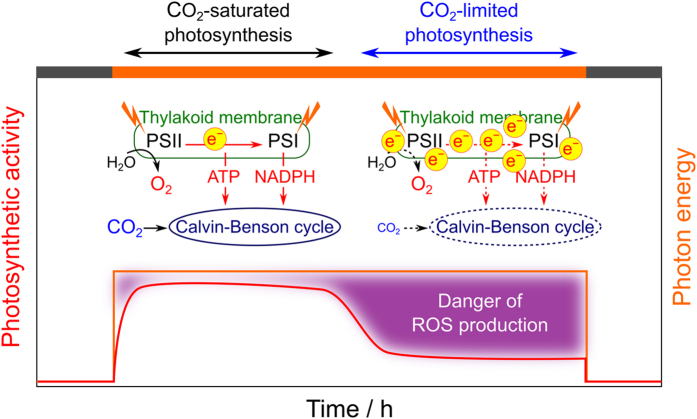
The production and consumption of energy by photosynthetic electron transport and the Calvin-Benson cycle becomes unbalanced without sufficient CO2 (CO2-limited photosynthesis; Fig. 1). Excess photon energy causes the production of reactive oxygen species (ROS), which trigger oxidative damage to PSII and PSI, so-called photoinhibition4,5,6,7.
Figure 1. A simple model of photosynthesis.
Orange line shows photon energy and red line shows photosynthetic activity. Photosynthesis actively occurs when sufficient CO2 is available (CO2-saturated photosynthesis, double-headed black arrow). Under CO2-limited photosynthesis (double-headed blue arrow), excess photon energy accumulates in a photosynthetic electron transport system located on the thylakoid membranes, which causes the production of reactive oxygen species.
It is broadly accepted that O2 is essential, not only as the respiratory electron acceptor, but also as an electron sink for various reactions of photosynthesis: O2-dependent alternative electron flow (AEF)8, including the Mehler-ascorbate peroxidase (MAP) pathway9,10, singlet O2 production in PSII5, flavodiiron protein (FLV) reactions11,12,13, mitochondrial respiration14, and plastidial (or cyanobacterial) respiration15,16. Also, photorespiration can be explained as an O2-dependent AEF in the broad sense17,18,19. A large O2-dependent AEF that replaces photosynthesis can alleviate photoinhibition by dissipating excess energy to O211,12,13,17,18,19,20,21,22. Recently, we showed that an O2-dependent AEF is essential for the oxidation of P700 under CO2 limitation to protect PSI against photo-oxidative damage in the cyanobacterium Synechococcus sp. PCC 7002 (S. 7002)22,23. That is, oxygenic phototrophs accessed O2 to prevent photo-oxidative damage derived from O2. However, both the magnitude and the molecular mechanisms of O2-dependent AEF vary across species in oxygenic phototrophs12,13,18,22,23.
There are alternative mechanisms, which do not depend on O2, that dissipate excess photon energy in oxygenic phototrophs. First, during Chl fluorescence measurements, non-photochemical quenching (NPQ) is observed as a decrease in maximum Chl fluorescence yields (Fm or Fm′). Simply put, NPQ is a process of heat dissipation of photon energy around PSII. The molecular mechanisms of NPQ in various oxygenic phototrophs are diverse and include the xanthophyll cycle, light-harvesting complex II aggregation, and state transition, some of which are activated by ΔpH24,25. The degree of induced NPQ varies widely depending on growth and measurement conditions24,25. Second, the electron transport in the cytochrome b6/f complex has suppressed sensitivity to ΔpH26 or reduced plastoquinone pool27, which is expected to oxidize PSI to alleviate the production of ROS by PSI owing to P700 quenching. Finally, cyclic electron transport (CET) around PSI supports the formation of ΔpH to induce NPQ and down-regulate the electron transport in the cytochrome b6/f complex28. We note that CET is defined as an AEF but does not require O2. In prokaryotic and eukaryotic algae, CET around PSI is suggested to be driven in several pathways, including chloroplast NADPH dehydrogenase (NDH) 1 and 2, and an elusive ferredoxin-plastoquinone reductase29. Further, CET around PSII has been found in the green alga Chlorella pyrenoidosa30,31.
In this study, we measured responses to CO2 limitation of the cyanobacterium Synechococcus elongatus PCC 7942 (S. 7942) and two secondary algae, the Euglenoid Euglena gracilis and the pennate marine diatom Phaeodactylum tricornutum. We aimed to elucidate the diversity of mechanisms to utilize O2 as an alternative electron acceptor in photosynthetic electron transport to CO2 in cyanobacteria and algae. Cyanobacteria are known as the ancestors of chloroplasts, and have evolved into the chloroplasts of various photosynthetic eukaryotes via endosymbiosis. In contrast, the secondary algae are known to be the products of two endosymbiotic events and to have evolved from cyanobacteria along different lineages from that of land plants32. Chloroplasts of E. gracilis are possibly derived from a green plastid-containing organism and are surrounded by a triple, rather than a double, membrane as found in vascular plants and green algae32, which possess Chl a and b as light-harvesting pigments. However, the relative content of Chl b to Chl a in E. gracilis is less than that in vascular plants33. Conversely, P. tricornutum harbors chloroplasts that possibly originated from red algae, and are surrounded by a quadruple membrane32. The light-harvesting complex of P. tricornutum has fucoxanthin-Chl a/c binding proteins containing the carotenoids diadinoxanthin and diatoxanthin, which are involved in NPQ34.
Results
Responses of photosynthetic electron transport to CO2 limitation in S. 7942, E. gracilis, and P. tricornutum
We measured O2 and relative Chl fluorescence in S. 7942, E. gracilis, and P. tricornutum using an O2 electrode and a PAM fluorometer to evaluate the responses of photosynthetic electron transport. We estimated AEF activities in S. 7942, E. gracilis, and P. tricornutum using the relationship between photosynthetic O2 evolution rates and the relative electron transport rate (ETR) at PSII. Photosynthetic O2 evolution rate reflects the activity of photosynthesis (the Calvin-Benson cycle), whereas relative ETR is related to total electron transport activity, including AEF. Actually, we have found that the deletion of FLV2 and 4 (FLV2/4), which is the molecular mechanism of AEF under CO2 limitation, gives the proportional linear relationship between photosynthetic O2 evolution rates and relative ETR in the cyanobacterium Synechocystis sp. PCC 6803 (S. 6803) (Supplemental Fig. S1)13. These rates showed proportional linearity in CO2-saturated conditions in the two secondary algae, but not in S. 7942 (Supplemental Figs S2–S4), which suggests that electron transports at PSII were strongly coupled to photosynthesis in E. gracilis and P. tricornutum when sufficient CO2 was available. In S. 7942, relative ETR was already partially uncoupled from photosynthetic O2 evolution rate during CO2-saturated photosynthesis at supersaturated photon flux densities (Supplemental Fig. S2), indicating that cyanobacterial AEF functions in such situations35. We note that AEF can be reflected in the relative ETR only when the AEF has a high activity level comparable to photosynthesis.
Responses of algal photosynthesis to CO2 limitation were measured by following the method previously described12,13. The responses of photosynthetic parameters to CO2 limitation are shown in Fig. 2, and the original traces used to estimate these parameters are presented in Supplemental Figs S5–S7. The cyanobacterial and algal cells in fresh media were applied to an O2 electrode chamber without adding inorganic carbon sources, and then illuminated with white actinic light (AL). Illumination with AL stimulated photosynthesis, which was accompanied by an increase in O2 in the reaction medium (Supplemental Figs S5–S7). However, CO2 in the medium was gradually removed by algal photosynthesis, as the diffusion of CO2 from the atmosphere into the reaction medium was very slow, compared with the consumption by photosynthetic CO2 assimilation in the experimental system. O2 in the reaction medium began to decrease when CO2 was depleted (Supplemental Figs S5–S7), indicating that photosynthesis was suppressed during the transition to CO2 limitation. The addition of CO2 (as NaHCO3) to the reaction medium restored photosynthetic activity (Fig. 2, Supplemental Figs S5–S7).
Figure 2.
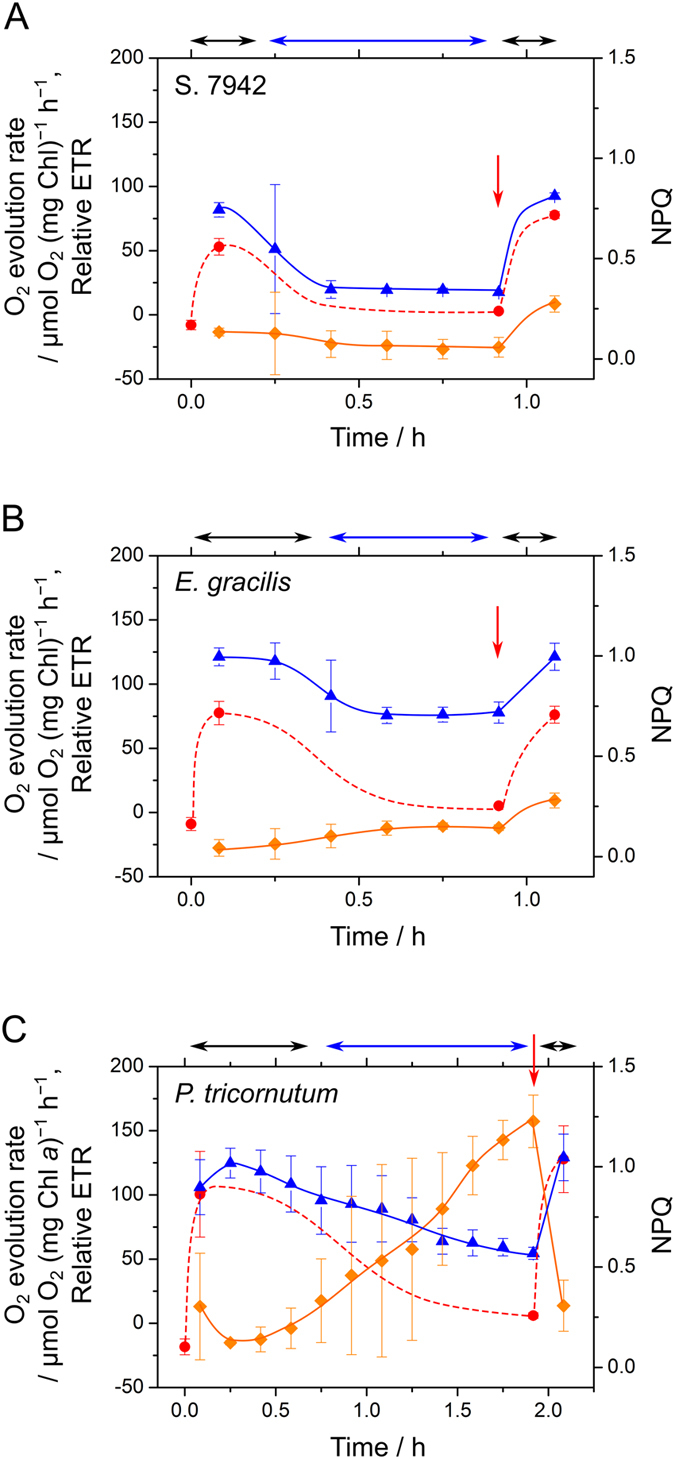
Responses of photosynthesis to CO2 limitation in S. 7942 (A), Euglena gracilis (B), and Phaeodactylum tricornutum (C). The graphs show the time course of O2 evolution rate (red circles), relative electron transport rate (ETR) (blue triangles), and non-photochemical quenching (NPQ) (orange diamonds) in the fresh media containing the cells (10 μg Chl a mL−1). Illumination with white actinic light (AL) (300 μmol photons m−2 s−1 for S. 7942 and E. gracilis; 400 μmol photons m−2 s−1 for P. tricornutum) began at 0. NaHCO3 (final concentration 10 mM) was added at the times indicated by red arrows. The double-headed black and blue arrows show CO2-saturated and CO2-limited photosynthesis, respectively.
During the measurements, the top of the chamber remained open, which enabled O2 and CO2 to diffuse into or out of the reaction medium. This open system relieved excessive increases in O2 in the reaction mixture, which enabled O2 to be measured for longer without passing over an undetectable point of the O2 electrode. We temporarily closed the chamber to exclude the effects of diffusion of O2 for determination of photosynthetic O2 evolution rates (Supplemental Figs S5–S7)13.
In several earlier studies, it was observed that S. 7942 induced little AEF or NPQ in response to CO2 limitation12,23,36. Thus, we used S. 7942 as a control to compare the responses of E. gracilis and P. tricornutum in this study. In S. 7942, the photosynthetic O2 evolution rate decreased in the transition to CO2-limited photosynthesis, which was paralleled by the decrease in the relative ETR without NPQ induction (Fig. 2A, Supplemental Fig. S5). The proportional linear relationship between gross photosynthetic activity and relative ETR indicated that S. 7942 hardly drives AEF in the transition to CO2 limitation (Supplemental Fig. S5). The increase in NPQ after adding NaHCO3 has been observed in a previous study36, although the molecular mechanism was unclear.
In both secondary algae, particularly in E. gracilis, some relative ETR was uncoupled from the O2 evolution rates during CO2-limited photosynthesis (Fig. 2B and C, Supplemental Figs S6 and S7), indicating that an AEF partially replaced photosynthesis during CO2-limited photosynthesis in these algae. Compared with S. 7942, E. gracilis maintained relative ETR uncoupled from the photosynthetic O2 evolution rate during CO2-limited photosynthesis, which reached approximately half that during CO2-saturated photosynthesis (Supplemental Fig. S6). Conversely, NPQ was slightly induced in the transition to CO2 limitation in E. gracilis, which was not alleviated after at least 5 min after NaHCO3 was added (Fig. 2B). These results concurred with those of a previous study34.
In the diatom P. tricornutum, a dramatic induction of NPQ was observed in the transition to CO2 limitation with the suppression of photosynthesis, although the AEF activity was much less, compared with E. gracilis (Fig. 2C). The relaxation of NPQ after adding NaHCO3 was faster than that in E. gracilis (Fig. 2C), which is in agreement with a number of studies of diatomaceous NPQ24,25,34. These data suggest that the NPQ in E. gracilis and P. tricornutum are derived from different molecular mechanisms.
Dependences of relative ETR on O2 under CO2 limitation in S. 7942, E. gracilis, and P. tricornutum
Diverse responses of photosynthetic electron transport to CO2 limitation in S. 7942, E. gracilis, and P. tricornutum (Fig. 2) suggest different strategies of O2 usage when photosynthesis is suppressed. To compare the O2 usage of photosynthetic electron transport in these cyanobacterium and algae, we investigated the dependencies of relative ETR on O2 during CO2-limited photosynthesis. We eliminated O2 in the medium by adding glucose, catalase, and glucose oxidase during CO2-limited photosynthesis using the method described by Shimakawa et al.23. We confirmed in advance that the addition of exogenous glucose during illumination did not affect the O2 evolution rates and relative ETR in these species (Supplemental Table S1)23. The top of the O2 electrode chamber was closed to exclude the effects of diffusion of O2 and CO2 into or out of the reaction medium. It should be noted that there may have been some unintended consequences of using anaerobic conditions. However, removing O2 did not affect relative ETR, at least during CO2-saturated photosynthesis, in E. gracilis or P. tricornutum (Supplemental Fig. S8).
Compared with S. 7942 and P. tricornutum, which required little O2 to drive AEF during CO2-limited photosynthesis (Fig. 3A and C)12,23, E. gracilis showed a biphasic dependence on O2 (Fig. 3B). This indicated that more than two molecular mechanisms functioned as the O2-dependent AEF in E. gracilis. Conversely, P. tricornutum was unlikely to rely on O2-dependent AEF to alleviate photo-oxidative damage under CO2 limitation, compared with E. gracilis. There was residual relative ETR under anaerobic conditions in E. gracilis and P. tricornutum, which might be derived from an O2-insensitive AEF, including CET around PSI and PSII28,29,30,31.
Figure 3.
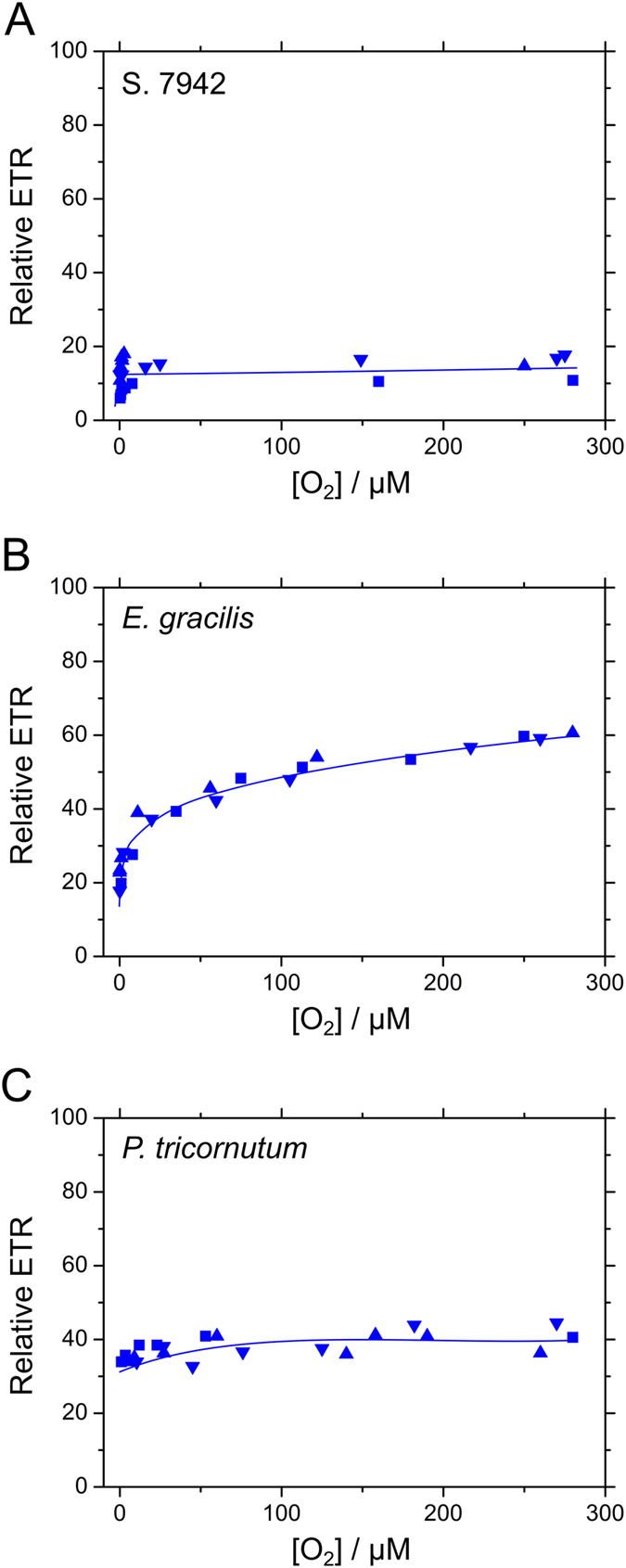
Dependence of relative electron transport rate (ETR) on O2 during CO2-limited photosynthesis in S. 7942 (A), Euglena gracilis (B), and Phaeodactylum tricornutum (C). To remove dissolved O2, d-glucose (5 mM), catalase (250 units mL−1), and glucose oxidase (5 units mL−1) was added to the fresh media containing the cells (10 μg Chl a mL−1). Photon flux densities of white actinic light (AL) were 300 μmol photons m−2 s−1 for S. 7942 and E. gracilis; 400 μmol photons m−2 s−1 for P. tricornutum. Triangles, inverse triangles, and squares represent three independent measurements, respectively.
Discussion
Figure 4 is a summary diagram of our previous and present results12,13,22,23 that presents the diverse O2 usage strategies of photosynthetic electron transport to dissipate excess energy under CO2 limitation in cyanobacteria, green algae, and two classes of algae with secondary plastids. Oxygenic phototrophs possess a number of molecular mechanisms that protect their cells against photo-oxidative damage by ROS. In this study, we focused on the physiological significance of O2 as an alternative ‘safety valve’ in photosynthetic electron transport, and compared responses of photosynthesis to CO2 limitation in genetically, phylogenetically, biologically, and ecologically different cyanobacteria and two classes of algae with secondary plastids. These organisms had different pigment compositions (Supplemental Table S2), which made it difficult to quantitatively compare photosynthetic O2 evolution rates and relative ETR. Therefore, we simply defined the ratio of relative ETR during CO2-limited photosynthesis to that during CO2-saturated photosynthesis as residual relative ETR under CO2 limitation in each species, and summarized the dependencies on O2 as shown in Fig. 4. The cyanobacterium S. 6803, harboring FLV2/4, showed the activation of an O2-dependent AEF during CO2-limited photosynthesis13. This was different from S. 7942, which does not possess FLV2/4 (Fig. 2A)12. Conversely, the marine species S. 7002, which does not harbor FLV2/4, maintained a relatively high electron flux to O2 during CO2-limited photosynthesis owing to the higher contribution of FLV1 and 3 homologs (FLV1/3) to AEF, compared with S. 7942 and S. 680322,23. The green alga Chlamydomonas reinhardtii drives an O2-dependent AEF in the transition from CO2-saturated to CO2-limited photosynthesis, similar to S. 700223. The dependences of relative ETR on O2 under CO2 limitation in S. 6803, S. 7942, S. 7002, and C. reinhardtii have already been reported in Shimakawa et al.23. In addition, in E. gracilis, the electron flux to O2 partially replaced photosynthesis under CO2 limitation, while the dependency on O2 was different from that in S. 6803, S. 7002, and C. reinhardtii (Figs 3B and 4). The biphasic O2 dependency of relative ETR in E. gracilis indicated that this alga might drive the other AEF, which has low affinity for O2 (e.g. photorespiration)37,38, in addition to the AEF that has high O2 affinity. Conversely, the diatom P. tricornutum hardly showed O2 usage (Figs 3C and 4). Compared with cyanobacteria and algae, C3 plants mainly drive photorespiration as an O2-dependent AEF that replaces photosynthesis at the CO2 compensation point18,19, whereas this is not observed in the C4 plant maize18. Overall, there appear to be a number of diverse strategies of O2 utilization that prevent photo-oxidative damage under CO2 limitation, irrespective of the species of oxygenic phototroph, and O2 is essential for some oxygenic phototrophs to protect cells against excess photon energy21,22.
Figure 4. The diversity of O2 usage strategies under CO2 limitation in cyanobacterial and algal photosynthesis.
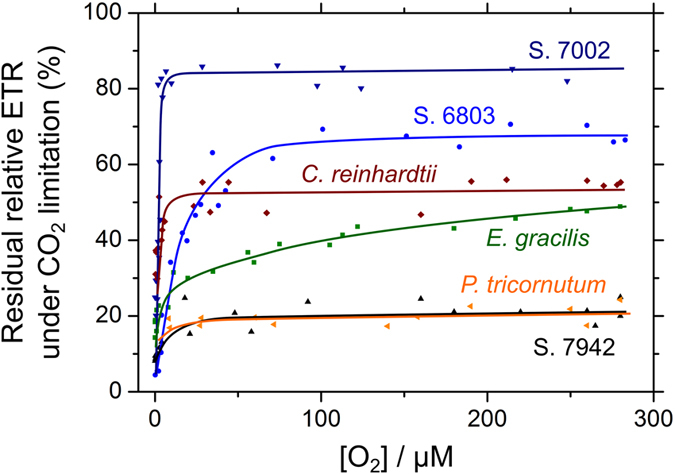
Shown are cyanobacteria: Synechocystis sp. PCC 6803 (S. 6803), S. elongatus PCC 7942 (S. 7942), and Synechococcus sp. PCC 7002 (S. 7002); the green alga C. reinhardtii; the Euglenoid Euglena gracilis; and the diatom Phaeodactylum tricornutum. Cyanobacterial and algal cells were grown under ambient CO2. Residual relative electron transport rate (ETR) under CO2 limitation indicates the ratio of relative ETR during CO2-limited photosynthesis to that during CO2-saturated photosynthesis, which is shown with the dependency on O2 in reference to data in this and previous studies12,13,22,23.
Overall, dissipating photon energy to O2 is not necessarily a universal strategy in oxygenic phototrophs during CO2-limited photosynthesis (Fig. 4). In many oxygenic phototrophs, the MAP pathway and respiratory terminal oxidases reduce O2 at low concentrations, but in most cases, the rates estimated are less than 10% of CO2-saturated gross photosynthesis39,40,41, whereas some species show high activity of MAP pathway in vivo42. In this study, we measured the activities of the MAP pathway under CO2 limitation in S. 7942, E. gracilis, and P. tricornutum by adding exogenous H2O243. The maximum activities reached approximately 70%, 25%, and 10% of the gross photosynthetic O2-evolution rates in S. 7942, E. gracilis, and P. tricornutum, respectively (Supplemental Fig. S9). These estimates can be applied to the dependence of relative ETR on O2 under CO2 limitation in E. gracilis, but not in S. 7942 or P. tricornutum (Fig. 3). That is, both S. 7942 and P. tricornutum probably suppressed the MAP pathway under CO2 limitation, and relied upon alternative strategies to dissipate excess photon energy.
There are mechanisms other than O2-dependent AEF that function in the protection of cells against photo-oxidative damage, which would explain why there are diverse strategies of O2 usage in oxygenic phototrophs. Increased NPQ is broadly used in many oxygenic phototrophs to dissipate excess photon energy, but the molecular mechanisms are unlikely to have the same origin. In the cyanobacterium S. 6803, it is observed that the orange carotenoid protein is expressed and functions in NPQ in response to high light levels44. However, in the transition to CO2 limitation, no induction of NPQ was observed in S. 7942 or S. 6803 (Fig. 2A)12,23,36. The strategy to enhance NPQ under CO2 limitation might not have been widespread in oxygenic phototrophs during their early evolution. The diatom P. tricornutum showed a large increase in NPQ under CO2 limitation (Fig. 2C), which would be strictly related to ΔpH, some carotenoids, and the gene product of lhcXs24,25,45. Conversely, the suppression of electron transport in the cytochrome b6/f complex is stimulated by ΔpH26 and reduced the plastoquinone pool27, both of which can cause the oxidation of P700 to dissipate excess photon energy22. Additionally, O2-insensitive AEF, including CET around PSI28,29 and PSII30,31 may function to alleviate photo-oxidative damage. Furthermore, phototaxis possibly functions as a main strategy to avoid excess photon energy under CO2 limitation in motile algae, such as E. gracilis46. These O2-insensitive strategies to alleviate photo-oxidative damage would enable various oxygenic phototrophs to be independent of O2-dependent AEF. Nevertheless, the questions of the benefit (or cost) of O2 usage to dissipate excess photon energy remains. There are still many questions over the diverse strategies that oxygenic phototrophs use to counter the detrimental effects of sunlight.
Methods
Growth conditions and determination of Chlorophyll
Cyanobacteria and algae were cultured in baffled shake flasks on a rotary shaker (100 rpm) under ambient CO2. For all measurements, cells from the exponential growth phase were used.
S. 7942 was cultured in BG-11 medium47 under light:dark conditions (25 °C, 16 h, 150 μmol photons m−2 s−1, fluorescent lamp: 23 °C, 8 h, dark). To quantify Chl, cells were centrifugally harvested and re-suspended by vortexing in 1 mL 100% (v/v) methanol. After subsequent incubation at room temperature for 5 min, the suspension was centrifuged at 10,000 × g for 2 min. Total Chl was determined from the supernatant48.
E. gracilis Z (NIES-48) was photoautotrophically cultured in Cramer-Myers medium49 under light:dark conditions (25 °C, 16 h, 150 μmol photons m−2 s−1, fluorescent lamp: 23 °C, 8 h, dark). Both Chl a and Chl b were quantified following the above-mentioned method48.
P. tricornutum (UTEX642) was photoautotrophically cultured in the artificial seawater medium described previously, with the addition of 0.31% half-strength Guillard’s ‘F’ solution50,51, under light:dark conditions (22 °C, 14 h, 100 μmol photons m−2 s−1, fluorescent lamp: 20 °C, 10 h, dark). Both Chl a and Chl c were quantified as described above, except that the cells were re-suspended in a 1 mL mixture (10% [v/v] distilled water, 10% [v/v] dimethyl sulfoxide, and 80% [v/v] acetone)52.
Measurement of O2 and Chl fluorescence
Net uptake and evolution of O2 was measured simultaneously with Chl fluorescence. Cell samples in freshly prepared media (2 mL, 10 μg Chl a mL−1) were stirred with a magnetic microstirrer and illuminated with white actinic light (AL) at 25 °C (for S. 7942 and E. gracilis) or 20 °C (for P. tricornutum). A halogen lamp (Xenophot HLX 64625, Osram, München, Germany) from the LS2 light source (Hansatech, King’s Lynn, UK) was used as the white AL source. O2 was monitored continuously using an O2 electrode (Hansatech, King’s Lynn, UK) while the measuring cuvette remained open to allow diffusion of O2 and CO2 between the medium and the air12,13. The top of the cuvette was temporarily closed (1–3 min) while the O2 evolution rate was determined12,13. Representative raw traces of O2 and relative Chl fluorescence in S. 7942, E. gracilis, and P. tricornutum are shown in Supplemental Figs S5A, S6A and S7A, respectively.
The relative Chl fluorescence originating from Chl a was measured using a PAM-Chl fluorometer (PAM-101; Walz, Effeltrich, Germany)53,54. Pulse-modulated excitation was achieved using an LED lamp with a peak emission at 650 nm. Modulated fluorescence was measured at λ > 710 nm (Schott RG9 long-pass filter). The minimum Chl fluorescence (Fo) was determined from illumination using a measuring light (ML). The steady-state fluorescence (Fs) was monitored under AL, and 1,000-ms pulses of saturated light (10,000 μmol photons m−2 s−1) were supplied at arbitrary intervals to determine the maximum variable fluorescence (Fm′). The fluorescence terminology used in this study follows that of van Kooten and Snel (1990)55. The effective quantum yield of PSII, Y(II), was defined as (Fm′ − Fs)/Fm′. Relative ETR at PSII was estimated as the product of Y(II) and photon flux density of white AL. NPQ was calculated as (Fm − Fm′)/Fm′56. For S. 7942, Fm was determined in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea to exclude effects of state transition35.
To measure the dependence of relative ETR on O2 (Fig. 3, Supplemental Fig. S8), we added glucose (5 mM), catalase (250 units mL−1, Wako, from bovine liver), and glucose oxidase (5 units mL−1, Wako, from Aspergillus niger) to the medium with the chamber closed to block the diffusion of air to the medium. After photosynthetic O2 evolution rates decreased to 0, we added these agents and evaluated the relative ETR23.
Activity of the Mehler-ascorbate peroxidase pathway in cyanobacterial and algal cells was estimated from H2O2-dependent O2 evolution rates during CO2-limited photosynthesis43. To exclude the effects of catalase, we added hydroxylamine (HA) to the reaction medium at 0.1 mM (for S. 7942 and P. tricornutum) or 0.5 mM (for E. gracilis).
Measurement of nitrogen
Cyanobacterial and algal cells were centrifugally harvested and dried overnight at 60 °C. Dried pellets were digested using the Kjeldahl method with sulfuric acid and H2O2. Total N content was determined using Nessler’s reagent after adding sodium potassium tartrate and NaOH57.
Additional Information
How to cite this article: Shimakawa, G. et al. Diverse strategies of O2 usage for preventing photo-oxidative damage under CO2 limitation during algal photosynthesis. Sci. Rep. 7, 41022; doi: 10.1038/srep41022 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS, Scientific Research Grant No. 26450079 to C.M.) and the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Scientific Research on Innovative Area No. 22114512 to C.M.). G.S. is supported as a JSPS research fellow (grant no. 16J03443). We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Author Contributions C.M. conceived the original screening and research plans; C.M. and Y.M. supervised the experiments; G.S. performed most of the experiments; Y.M., K.N., M.T., S.S., and C.M. provided technical assistance to G.S.; C.M. and G.S. designed the experiments and analyzed the data; C.M. and G.S. conceived the project and wrote the article with contributions from all the authors; C.M. supervised and complemented the writing.
References
- Kasting J. F. Theoretical constraints on oxygen and carbon dioxide concentrations in the Precambrian atmosphere. Precambrian Res. 34, 205–229 (1987). [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. 41, 445–501 (1966). [DOI] [PubMed] [Google Scholar]
- Trebst A. Energy conservation in photosynthetic electron transport of chloroplasts. Annu. Rev. Plant Physiol. 25, 423–458 (1974). [Google Scholar]
- Murata N., Takahashi S., Nishiyama Y. & Allakhverdiyev S. I. Photoinhibition of photosystem II under environmental stress. Biophys. Biochim. Acta Bioenerg. 1767, 414–421 (2007). [DOI] [PubMed] [Google Scholar]
- Roach T., Na C. S. & Krieger-Liszkay A. High light-induced hydrogen peroxide production in Chlamydomonas reinhardtii is increased by high CO2 availability. Plant J. 81, 759–766 (2015). [DOI] [PubMed] [Google Scholar]
- Sonoike K. Photoinhibition of photosystem I. Physiol. Plant. 142, 56–64 (2011). [DOI] [PubMed] [Google Scholar]
- Sejima T., Takagi D., Fukayama H., Makino A. & Miyake C. Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol. 55, 1184–1193 (2014). [DOI] [PubMed] [Google Scholar]
- Miyake C. Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol. 51, 1951–1963 (2010). [DOI] [PubMed] [Google Scholar]
- Mehler A. H. Studies on reactivities of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch. Biochem. Biophys. 33, 65–77 (1951). [DOI] [PubMed] [Google Scholar]
- Miyake C., Schreiber U., Hormann H., Sano S. & Asada K. The FAD-enzyme monodehydroascorbate radical reductase mediates photoproduction of superoxide radicals in spinach thylakoid membranes. Plant Cell Physiol. 39, 821–829 (1998). [Google Scholar]
- Helman Y. et al. Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr. Biol. 13, 230–235 (2003). [DOI] [PubMed] [Google Scholar]
- Hayashi R. et al. O2-dependent large electron flow functioned as an electron sink, replacing the steady-state electron flux in photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803, but not in the cyanobacterium Synechococcus sp. PCC 7942. Biosci. Biotechnol. Biochem. 78, 384–393 (2014). [DOI] [PubMed] [Google Scholar]
- Shimakawa G. et al. FLAVODIIRON2 and FLAVODIIRON4 proteins mediate and oxygen-dependent alternative electron flow in Synechocystis sp. PCC 6803 under CO2-limited conditions. Plant Physiol. 167, 472–480 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K. & Yoshida K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8, 87–99 (2008). [DOI] [PubMed] [Google Scholar]
- Joët T. et al. Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J. Biol. Chem. 277, 31623–31630 (2002). [DOI] [PubMed] [Google Scholar]
- Lea-Smith D. J. et al. Thylakoid terminal oxidases are essential for the cyanobacterium Synechocystis sp. PCC 6803 to survive rapidly changing light intensities. Plant Physiol. 162, 484–495 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki A. & Takeba G. Photorespiration protects C3 plants from photooxidation. Nature 384, 557–560 (1996). [Google Scholar]
- Sejima T. et al. Post-illumination transient O2-uptake is driven by photorespiration in tobacco leaves. Physiol. Plant. 156, 227–238 (2016). [DOI] [PubMed] [Google Scholar]
- Takagi D., Hashiguchi M., Sejima T., Makino A. & Miyake C. Photorespiration provides the chance of cyclic electron flow to operate for the redox-regulation of P700 in photosynthetic electron transport system of sunflower leaves. Photosynth. Res. doi: 10.1007/s11120-016-0267-5 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang P., Allahverdiyeva Y., Eisenhut M. & Aro E. M. Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS One 4, e5331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y. et al. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. USA 110, 4111–4116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakawa G., Shaku K. & Miyake C. Oxidation of P700 in photosystem I is essential for the growth of cyanobacteria. Plant Physiol. doi: http://dx.doi.org/10.1104/pp.16.382 012277 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakawa G. et al. Diversity in photosynthetic electron transport under [CO2]-limitation: the cyanobacterium Synechococcus sp. PCC 7002 and green alga Chlamydomonas reinhardtii drive an O2-dependent alternative electron flow and non-photochemical quenching of chlorophyll fluorescence during CO2-limited photosynthesis. Photosynth. Res. 130, 293–305 (2016). [DOI] [PubMed] [Google Scholar]
- Goss R. & Lepetit B. Biodiversity of NPQ. J. Plant Physiol. 172, 13–32 (2015). [DOI] [PubMed] [Google Scholar]
- Derks A., Schaven K. & Bruce D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta Bioenerg. 1847, 468–485 (2015). [DOI] [PubMed] [Google Scholar]
- Hope A. B., Valente P. & Matthews D. B. Effects of pH on the kinetics of redox reactions in and around the cytochrome bf complex in an isolated system. Photosynth. Res. 42, 111–120 (1994). [DOI] [PubMed] [Google Scholar]
- Shaku K., Shimakawa G., Hashiguchi M. & Miyake C. Reduction-induced suppression of electron flow (RISE) in the photosynthetic electron transport system of Synechococcus elongatus PCC 7942. Plant Cell Physiol. 57, 1443–1453 (2016). [DOI] [PubMed] [Google Scholar]
- Allen J. F. Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci. 8, 15–19 (2003). [DOI] [PubMed] [Google Scholar]
- Peltier G., Tolleter D., Billon E. & Cournac L. Auxiliary electron transport pathways in chloroplasts of microalgae. Photosynth. Res. 106, 19–31 (2010). [DOI] [PubMed] [Google Scholar]
- Falkowski P. G., Fujita Y., Ley A. & Mauzerall D. Evidence for cyclic electron flow around photosystem II in Chlorella pyrenoidosa. Plant Physiol. 81, 310–312 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C. & Yokota A. Cyclic flow of electrons within PSII in thylakoid membranes. Plant Cell Physiol 42, 508–515 (2001). [DOI] [PubMed] [Google Scholar]
- Falkowski P. G. et al. The evolution of modern eukaryotic phytoplankton. Science 305, 354–360 (2004). [DOI] [PubMed] [Google Scholar]
- Cunningham F. X. Jr. & Schiff J. A. Chlorophyll-protein complexes from Euglena gracilis and mutants deficient in chlorophyll b. Plant Physiol. 80, 231–238 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper-Lindley C. & Björkman O. Fluorescence quenching in four unicellular algae with different light-harvesting and xanthophyll-cycle pigments. Photosynth. Res. 56, 277–289 (1998). [Google Scholar]
- Campbell D., Hurry V., Clarke A. K., Gustafsson P. & Öquist G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 62, 667–683 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S. & Bruce D. Characterization of the non-photochemical quenching of chlorophyll fluorescence that occurs during the active accumulation of inorganic carbon in the cyanobacterium Synechococcus PCC 7942. Photosynth. Res. 49, 251–262 (1996). [DOI] [PubMed] [Google Scholar]
- Jordan D. B. & Ogren W. L. Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature 291, 513–515 (1981). [Google Scholar]
- Yokota A. & Kitaoka S. Rates of glycolate synthesis and metabolism during photosynthesis of Euglena and microalgae grown on low CO2. Planta 170, 181–189 (1987). [DOI] [PubMed] [Google Scholar]
- Trimborn S., Thoms S., Petrou K., Kranz S. A. & Rost B. Photophysiological responses of Southern Ocean phytoplankton to changes in CO2 concentrations: Short-term versus acclimation effects. J. Exp. Mar. Biol. Ecol. 451, 44–54 (2014). [Google Scholar]
- Bailleul B. et al. Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524, 366–369 (2015). [DOI] [PubMed] [Google Scholar]
- Driever S. M. & Baker N. R. The water-water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ. 34, 837–846 (2011). [DOI] [PubMed] [Google Scholar]
- Waring J., Klenell M., Bechtold U., Underwood G. J. C. & Baker N. R. Light-induced responses of oxygen photoreduction, reactive oxygen species production and scavenging in two diatom species. J. Phycol. 46, 1206–1217 (2010). [Google Scholar]
- Miyake C., Michihata F. & Asada K. Scavenging of hydrogen peroxide in prokaryotic and eukaryotic algae: acquisition of ascorbate peroxidase during the evolution of cyanobacteria. Plant Cell Physiol. 32, 33–43 (1991). [Google Scholar]
- Wilson A. et al. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18, 992–1007 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B. et al. An atypical member of the light-harvesting complex stress-related protein family modulates diatom responses to light. Proc. Natl. Acad. Sci. USA 107, 18214–18219 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P. R. et al. High light exposure leads to a sign change of gravitaxis in the flagellate Euglena gracilis. Acta Protozool. 41, 343–351 (2002). [PubMed] [Google Scholar]
- Allen M. M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 4, 1–4 (1968). [DOI] [PubMed] [Google Scholar]
- Grimme L. H. & Boardman N. K. Photochemical activities of a particle fraction P1 obtained from the green alga Chlorella fuska. Biochem. Biophys. Res. Commun. 49, 1617–1623 (1972). [DOI] [PubMed] [Google Scholar]
- Cramer M. & Myers J. Growth and photosynthetic characteristics of Euglena gracilis. Arch. Mikrobiol. 17, 384–402 (1952). [Google Scholar]
- Guillard R. R. L. & Ryther J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8, 229–239 (1962). [DOI] [PubMed] [Google Scholar]
- Guillard R. R. L. Culture of phytoplankton for feeding marine invertebrates. In: Smith W. L. & Chanley M. H. (eds) Culture of Marine Invertebrate Animals, Plenum Press, New York, pp 29–60 (1975). [Google Scholar]
- Jeffrey S. W. & Humphrey G. F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 167, 191–194 (1975). [Google Scholar]
- Schreiber U., Schliwa U. & Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62 (1986). [DOI] [PubMed] [Google Scholar]
- Genty B., Briantais J. M. & Baker N. R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 990, 87–92 (1989). [Google Scholar]
- van Kooten O. & Snel J. F. H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 25, 147–150 (1990). [DOI] [PubMed] [Google Scholar]
- Baker N. R. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113 (2008). [DOI] [PubMed] [Google Scholar]
- Shimakawa G. et al. Respiration accumulates Calvin cycle intermediates for the rapid start of photosynthesis in Synechocystis sp. PCC 6803. Biosci. Biotechnol. Biochem. 78, 1997–2007 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.