Abstract
As early secreted antigenic target of 6 kDa (ESAT-6) of Mycobacterium tuberculosis (Mtb) is an essential virulence factor and macrophages are critical for tuberculosis infection and immunity, we studied ESAT-6 stimulated IL-6 production by macrophages. ESAT-6 stimulated significantly higher IL-6 secretion by murine bone marrow derived macrophages (BMDM) compared to culture filtrate protein 10 kDa (CFP10) and antigen 85A. Polymyxin B, an LPS blocker, did not affect ESAT-6 stimulated macrophage IL-6 production. ESAT-6 but not Pam3CSK4 induced IL-6 by TLR2 knockout BMDM. ESAT-6 induced phosphorylation and DNA binding of STAT3 and this was blocked by STAT3 inhibitors but not by rapamycin. STAT3 inhibitors suppressed ESAT-6-induced IL-6 transcription and secretion without affecting cell viability. This was confirmed by silencing STAT3 in macrophages. Blocking neither IL-6Rα/IL-6 nor IL-10 affected ESAT-6-induced STAT3 activation and IL-6 production. Infection of BMDM and human macrophages with Mtb with esat-6 deletion induced diminished STAT3 activation and reduced IL-6 production compared to wild type and esat-6 complemented Mtb strains. Administration of ESAT-6 but not CFP10 induced STAT3 phosphorylation and IL-6 expression in the mouse lungs, consistent with expression of ESAT-6, IL-6 and phosphorylated-STAT3 in Mtb-infected mouse lungs. We conclude that ESAT-6 stimulates macrophage IL-6 production through STAT3 activation.
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), has become one of the deadliest pathogens in human history. This is mainly due to lack of effective TB vaccine, widespread human immunodeficiency virus (HIV) co-infection in TB endemic countries and development of drug resistance by Mtb. Furthermore, development of strategies by Mtb to evade host immunity to persist and cause disease1 is also a critical contributing factor. Therefore, deciphering the interaction between immune cells and the virulence factors of Mtb is the first step toward development of an effective vaccine and finding novel therapeutics for TB control.
Recent studies have established that secretion of early secreted antigenic target 6-kDa (ESAT-6) and its molecular partner, culture filtrate protein 10 kDa (CFP10), through ESAT-6 (ESX)-1 secretion system, is essential for Mtb pathogenesis2, but how ESAT-6 and CFP10 cause pathology remains obscure. Although tissues destruction due to cell damage is potentially one of the mechanisms by which ESAT-6 causes pathology because ESAT-6 causes lysis of alveolar epithelial cells and macrophages3,4 and apoptosis5 and necrosis of macrophages6, our previous studies suggest that ESAT-6 mediated regulation of host immune responses may play a role in Mtb pathogenesis. ESAT-6 directly inhibits T cell IFN-γ production by activation of p38 MAPK7,8 and indirectly through reprogramming of antigen presenting cells to produce less IL-12p70, an essential IFN-γ stimulating cytokine, and more IL-23, IL-1β and probably IL-6, the Th17 supporting cytokines8,9. We also showed that ESAT-6 induces IL-8 production by lung epithelial cells to promote granuloma formation10. These findings suggest that ESAT-6 has the potential to manipulate host immunity during Mtb infection.
Alveolar macrophages play key roles in TB infection by acting both as an intracellular niche for and as a first line defense against Mtb infection by phagocytosis of Mtb, production of cytokines and presenting Mtb antigens to initiate and regulate Mtb specific adaptive immunity11,12,13. It was suggested that virulent Mtb manipulates host immune responses through macrophages14.
Interleukin (IL)-6 is a multifunctional cytokine produced by various cell types including macrophages, to regulate normal physiological processes15, such as hematopoiesis, acute phase inflammatory response and immune responses. However, dysregulation of IL-6 production is associated with various diseases, such as cancer16 and HIV infection17. Macrophages from TB patients produce higher levels of IL-6 than those from healthy subjects18 and elevated circulating IL-6 levels were found in the patients with far-advanced pulmonary TB lesions19. Indeed, initial identification of IL-6 was accomplished by investigating IL-6 purified from the culture supernatants of purified protein derivative stimulated pleural effusion cells from patients with tuberculous pleurisy20. Furthermore, infection of macrophages by mycobacterial species induces IL-6 which is responsible for suppression of Th1 responses21 and suppression of Mtb infected and non-infected bystander macrophage responses to IFN-γ22. IL-6 also inhibits IFN-γ induced autophagy in Mtb infected macrophages23. Thus, these findings clearly indicate that virulent Mtb may upregulate IL-6 production, especially by macrophages, to regulate host immunity and susceptibility to TB. Therefore, we examined whether ESAT-6 induces IL-6 production by macrophages and the role of signal transducer and activator of transcription (STAT)3 in this process. We demonstrated that ESAT-6 induces IL-6 production by macrophages through activation of STAT3.
Results
ESAT-6 stimulates IL-6 production by macrophages
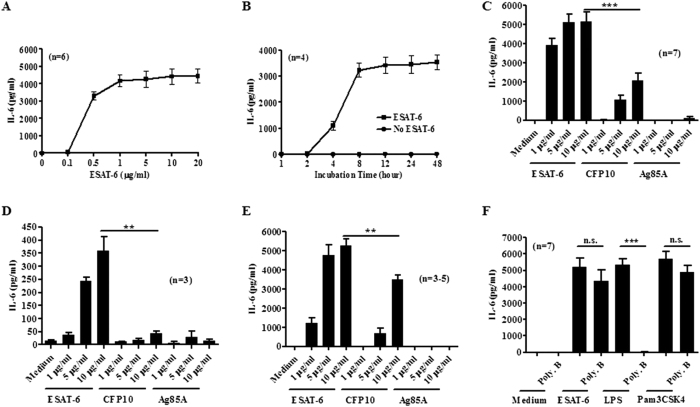
Although IL-6 is required for protective immunity, elevated IL-6 production correlates with disease severity of TB patients24. Excess IL-6 production may lead to suppressed Th1 responses21 and failed IFN-γ driven anti-Mtb responses of macrophages22. Therefore, we determined whether ESAT-6 stimulates IL-6 production by macrophages. ESAT-6 induced IL-6 production by BMDMs in a dose dependent manner (Fig. 1A), started to induce IL-6 at as low as 0.5 μg/ml (80 nM) and peaked at 1 μg/ml (160 nM) after 24 h stimulation. As for the temporal effect, we incubated BMDMs with 1 μg/ml ESAT-6 for different time points. ESAT-6 induced production of IL-6 as early as 1 h after stimulation from nondetectable level without ESAT-6 to 9.3 ± 1.3 pg/ml, at 2 h from 1.5 ± 1.5 pg/ml without ESAT-6 to 19.5 ± 6.8 pg/ml, peaked at 8 h and plateaued thereafter (Fig. 1B). As controls, we used CFP10 and Ag85A prepared as ESAT-6 in our laboratory. Although CFP10 at 5 and 10 μg/ml induced IL-6 production by macrophages, which are significantly less than that stimulated by ESAT-6 at same concentrations, and Ag85A did not stimulate any IL-6 production by macrophages at all three concentrations (Fig. 1C). We also checked whether ESAT-6 induces IL-6 production by primary alveolar macrophages and alveolar macrophage like cell line, RAW264.7 cells. Though IL-6 levels were lower than that by BMDMs, ESAT-6 stimulated significantly elevated IL-6 production by alveolar macrophages in a dose dependent manner compared to the cells with CPF10, Ag85A or with medium alone (Fig. 1D). Similarly, ESAT-6 also stimulated significant amount of IL-6 by RAW 264.7 cells (Fig. 1E). Although CFP10 at 10 μg/ml induced IL-6 production by RAW264.7 cells, which was significantly less than that by same concentration of ESAT-6 and Ag85A did not induce IL-6 production by these cell types (Fig. 1D and E). These data indicate that ESAT-6 stimulates IL-6 production by macrophages including alveolar macrophages.
Figure 1. ESAT-6 stimulates IL-6 production by macrophages.
Mouse BMDMs (A) were treated with or without ESAT-6 as indicated and IL-6 levels in the culture supernatants were determined after 24 h incubation. (B) BMDMs were incubated with or without ESAT-6 at 1 μg/ml for various times and IL-6 levels were determined. BMDMs (C), mouse alveolar macrophages (D) and RAW 264.7 cells (E) were incubated with indicated concentrations of proteins and IL-6 levels after 24 h incubation were determined. (F) BMDMs treated with or without 10 μg/ml polymyxin B (Poly. B) for 1 h prior to incubation with medium alone, ESAT-6 (1 μg/ml), LPS (0.1 μg/ml) or Pam3CSK4 (0.1 μg/ml) for 24 h and IL-6 levels were determined. For all panels, data are expressed as means ± SEM, *P < 0.05, **P < 0.01 or ***P < 0.001. n.s., not significant.
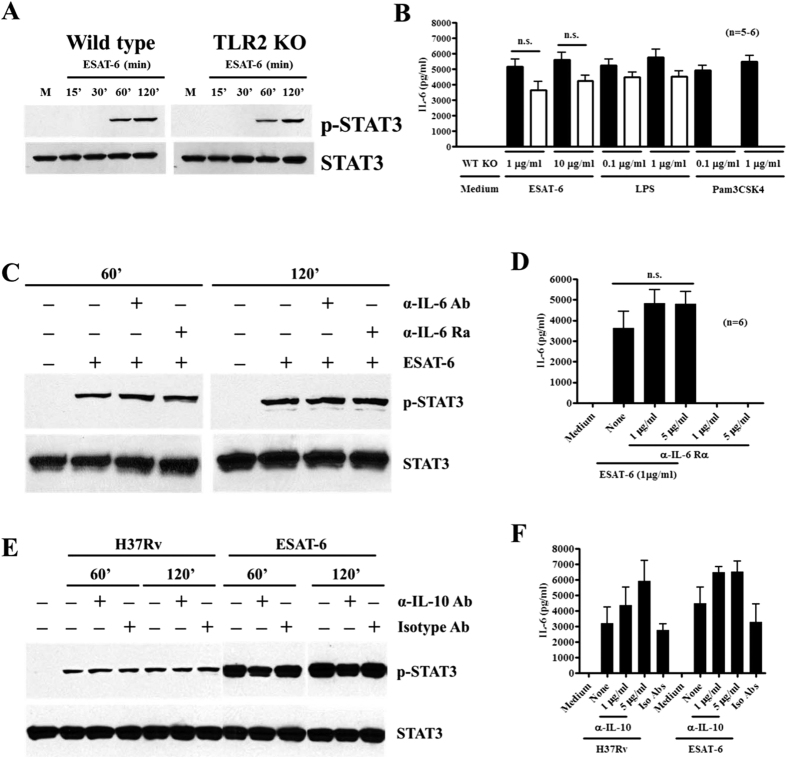
To test whether the trace amounts of LPS in ESAT-6 was the cause for IL-6 induction by macrophages, we used polymyxin B, a neutralizer of LPS25. Polymyxin B at 10 μg/ml did not affect 1 μg/ml ESAT-6 stimulated macrophage IL-6 production and completely blocked that by 100 ng/ml LPS, which is over 1,000 fold higher in LPS content than that in ESAT-6 at 1 μg/ml. The effect of polymyxin B was specific, as it did not affect IL-6 stimulation by TLR2 agonist Pam3CSK4 (Fig. 1F). These results suggest that ESAT-6 stimulated IL-6 production by macrophages is due less likely to LPS contamination.
ESAT-6 activates STAT3 in macrophages
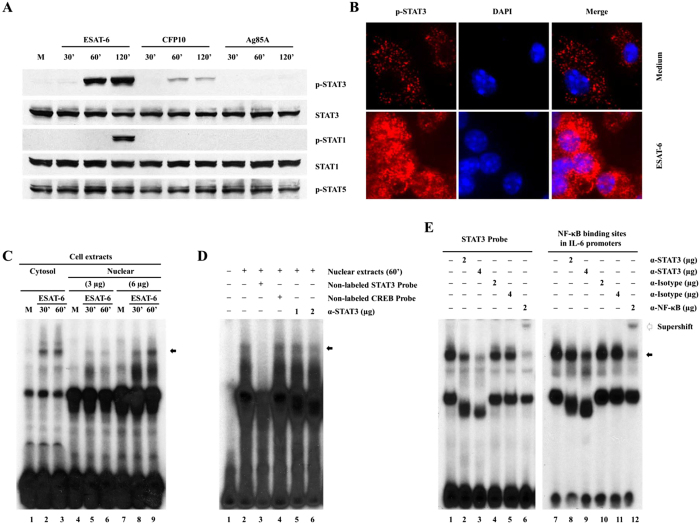
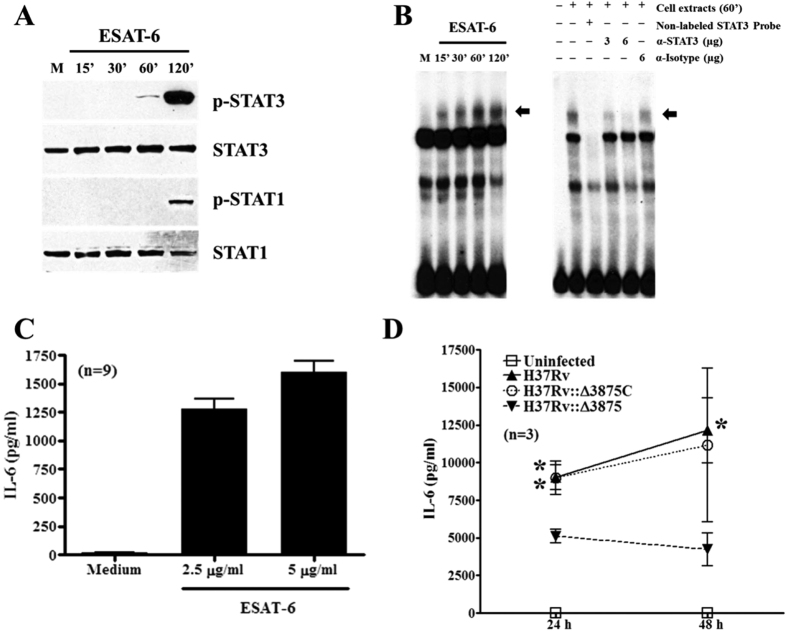
To elucidate the signaling pathways, we focused on STAT3 as it plays critical role in IL-6 production in cancer and infectious diseases26,27 and STAT3 and its regulator suppressor of cytokine signaling (SOCS)3 are critical in TB28,29. To this end, we evaluated phosphorylation of STAT3 in macrophages stimulated with ESAT-6. ESAT-6 at 1 μg/ml induced STAT3 phosphorylation at Tyr-705 in a time-dependent manner (Fig. 2A) compared to the cells without ESAT-6. In contrast, 1 μg/ml CFP10 induced STAT3 phosphorylation which was significantly less than that induced by ESAT-6. Ag85A did not induce STAT3 phosphorylation at all three time points tested. We examined the phosphorylation of STAT3 at Ser-727 and acetylation, but we could not detect any such modifications of STAT3 in ESAT-6 stimulated macrophages (results not shown). We also determined STAT1 phosphorylation in this process. ESAT-6 induced STAT1 phosphorylation only at 120 min, which was later than ESAT-6-induced STAT3 phosphorylation and was probably due to a secondary effect of ESAT-6-induced type I IFNs by macrophages30. CFP10 and Ag85A did not induce STAT1 phosphorylation at any of the time points. None of these proteins affected phosphorylation of STAT-5 in macrophages. These were not due to differential protein loading as comparable total STAT3 and STAT1 were detected across the lanes in the same membrane after stripping.
Figure 2. ESAT-6 activates STAT3 in macrophages.
(A) BMDMs were incubated with 1 μg/ml ESAT-6, CFP10 or Ag85A for different periods and the expression of the proteins was determined by Western blotting. One representative result of five independent experiments is shown. (B) BMDMs on cover slides were incubated with 1 μg/ml ESAT-6 for 60 min, fixed and stained for phospho-STAT3 and the cell nucleus was visualized with DAPI. One representative result from five different experiments is shown. (C) BMDMs were incubated with 1 μg/ml ESAT-6 for different time points and the DNA binding activities in nuclear and cytosolic protein extracts were evaluated by EMSA using labeled STAT3-binding site as probe. The arrow shows the specific protein-DNA complex. (D) STAT3 specific DNA binding was tested by EMSA as in (C) using nuclear extracts of BMDMs with ESAT-6 for 60 min. Non-labeled STAT3 (lane 3) and CREB binding site (lane 4) were used as cold competitors. STAT3 mAb were used at two different concentrations (lanes 5 and 6). (E) EMSA was performed as in (C), with or without different Abs as indicated using total protein extracts of BMDMs treated with ESAT-6 for 60 min and labeled STAT3-binding site and IL-6 proximal promoter as probes. For panels C, D, and E, one representative result from three independent experiments is shown.
To demonstrate ESAT-6 activation of STAT3 in macrophages by an alternative method, we performed immunofluorescence staining and confocal microscopy analysis. Stimulation of macrophages with ESAT-6 at 1 μg/ml for 60 min induced STAT3 phosphorylation. The phosphorylated STAT3 was located both in the nucleus and cytoplasm though with dominance in the cytoplasm (Fig. 2B), consistent with a previous report that activated STAT3 is not only localized in the nucleus but also in the cytoplasm, probably in mitochondria31.
To test the functional significance of ESAT-6 activated STAT3, we examined DNA binding activity in the protein extracts of ESAT-6-treated macrophages by EMSA using a labeled STAT3 binding site as probe. ESAT-6 induced STAT3 binding activities in both the cytoplasmic and nuclear protein extracts of macrophages in a time dependent manner compared to the cells without ESAT-6 (Fig. 2C), consistent with our confocal microscopy data, indicating activation of STAT3 in both cell cytoplasm and nuclear. The STAT3 binding activity is specific since the presence of excess unlabeled STAT3 but not CREB binding site blocked the formation of DNA-protein complex and the presence of anti-STAT3 antibody also blocked this interaction in a concentration dependent manner (Fig. 2D, lanes 5 and 6).
To determine the identities of the STAT3-binding proteins induced by ESAT-6, we performed EMSA supershift assays by incubating the labeled STAT-3 binding site or the proximal promoter of IL-6 as probes with total protin extracts of macrophages treated with ESAT-6 for 60 min followed by incubation with Abs. ESAT-6 induced a strong high molecular weight protein-DNA complex with either STAT3 binding site or the proximal promoter of IL-6 (Fig. 2E, lane 1 and 7 for STAT3 binding site and IL-6 promoter, respectively), and this was disrupted by anti-STAT3 Ab dose dependently (Fig. 2E, lanes 2 and 3 for STAT3 binding site and lanes 8 and 9 for IL-6 promoter) and supershifted by anti-NF-κBp65 (Fig. 2E, lanes 6 and 12) but not affected by control IgG (Fig. 2E, lanes 4 and 5 and, 10 and 11). These results demonstrated that ESAT-6 activates both STAT3 and NF-κB in macrophages and both of these may participate IL-6 gene transcription through the proximal promoter of IL-6 as a complex. Taken together, the results demonstrated that ESAT-6 indeed stimulates activation of STAT3 and may induce transcription of IL-6.
Pharmacological inhibition of STAT3 blocks ESAT-6-induced IL-6 production
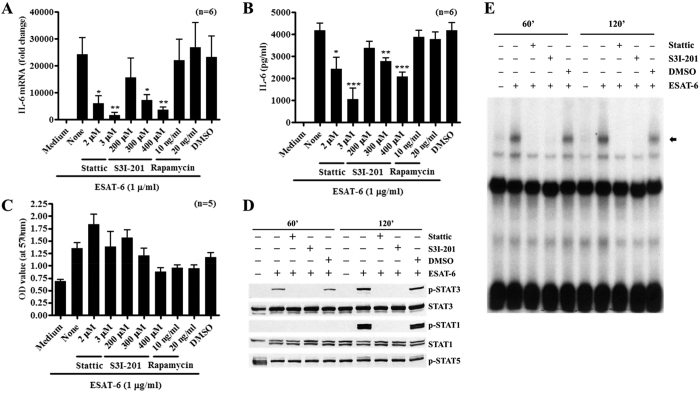
To verify the role of STAT3 in ESAT-6-induced IL-6 production by macrophages, we applied Stattic and S3I-201, the chemical inhibitors of STAT3. Pretreatment of macrophages with Stattic or S3I-201, reduced ESAT-6 induced IL-6 mRNA expression (Fig. 3A) and IL-6 secretion (Fig. 3B) by macrophages in a dose dependent manner compared to the cells without inhibitors or with DMSO, vehicle control. In contrast, pretreatment of macrophages with mTOR inhibitor, rapamycin, did not affect ESAT-6 stimulated IL-6 production, implying a specific effect of STAT3 inhibitors. To verify this was not due to cytotoxicity of the chemicals, we evaluated cell viability using MTT assay. ESAT-6 induced elevated metabolic activity of the macrophages compared to the cells without ESAT-6 and STAT3 inhibitors did not affect cell viability except for S3I-201at 400 μM. Rapamycin at both concentrations slightly reduced the cell viability compared to the cells with ESAT-6 alone (Fig. 3C). These together argue against the cytotoxicity as the cause for the reduced ESAT-6 induced IL-6 production by macrophages in the presence of STAT3 inhibitors. To further examine this in detail, we tested the effect of STAT3 inhibitors on ESAT-6 induced activation of STAT3. Again, ESAT-6 induced phosphorylation of STAT3 at 60 and 120 min and phosphorylation of STAT1 at 120 min compared to the cells without ESAT-6 (Fig. 3D), and this was completely blocked by STAT3 inhibitors but not DMSO. Consistent with this, ESAT-6 induced the high molecular weight protein-DNA complex at both 60 and 120 min after stimulation and this was completely disrupted by STAT3 inhibitors but not by DMSO (Fig. 3E), implying reduced phosphorylation of STAT3 by its inhibitors in macrophages stimulated with ESAT-6. These results clearly showed that ESAT-6 induces expression and secretion of IL-6 by macrophages through activation of STAT3.
Figure 3. Inhibition of STAT3 abrogates ESAT-6 induced macrophage IL-6 expression and STAT3 activation.
BMDMs were incubated with different inhibitors as indicated for one hour prior to incubation with ESAT-6. IL-6 mRNA expression was determined by qPCR after 4 h incubation (A), IL-6 was determined in the culture supernatants (B) and the viability of the cells was determined by MTT assay (C) after 24 h incubation. BMDMs were treated with STAT3 inhibitors Static (3 μM) and S31-201 (400 μM) or DMSO for one h before incubation with 1 μg/ml ESAT-6 for 60 and 120 min. The activation of STAT proteins was determined by Western blotting (D) and the DNA binding was determined by EMSA (E) in the total cell protein extracts. For panels A–C, data expressed as Mean ± SEM, *P < 0.05, **P < 0.01 or ***P < 0.001. For panels D and E, one representative result of 4 independent experiments is shown.
Silencing STAT3 in macrophages leads to reduced IL-6 stimulation by ESAT-6
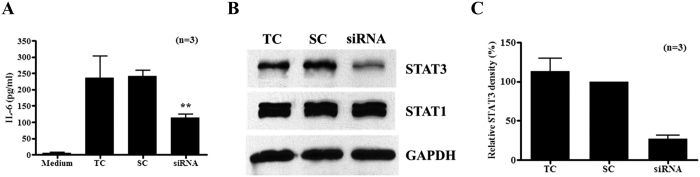
Although chemical inhibitors are convenient and critical tools for understanding the specific functions of proteins of interest, they may have off-target effects. To confirm the role of STAT3 in ESAT-6 induced IL-6 production by macrophages, we applied RNAi silencing technology. Although overall IL-6 levels were lower than the previous experiments probably because of transfection reagent treatment and extended incubation times, the mock or scrambled siRNA transfected BMDM responded with elevated IL-6 production to ESAT-6 compared to the cells without ESAT-6, and this was reduced significantly by STAT3 siRNA transfection (Fig. 4A) and the silencing efficiency was confirmed to be over 80% by Western blotting followed by densitometry analysis (Fig. 4B and C). The results from both biochemical inhibitors and genetic silencing studies support a role for STAT3 in ESAT-6 stimulated IL-6 production by macrophages.
Figure 4. Silencing STAT3 in macrophages reduces ESAT-6 stimulated IL-6 production.
BMDMs were transfected with transfection reagent only (TC), scrambled (SC) and STAT3 siRNA for 48 h. The cells were stimulated with ESAT-6 at 1 μg/ml for 24 h and IL-6 levels were determined in the culture supernatants (A). The silencing efficiency was determined by Western blotting for STAT3 in the cellular lysates after 48 h transfection. As controls, STAT1 and GAPDH were blotted in the same membrane after stripping. One representative result of three experiments (B) is shown. The Means and SEM of relative densities of STAT3 bands were calculated using the density of STAT3 in cells with scrambled siRNA after normalized by densities of respective STAT1 bands from three experiments (C).
Neither TLR-2 nor autocrine effect of IL-6 or IL-10 is involved in ESAT-6-induced STAT3 activation and IL-6 production by macrophages
It was reported that ESAT-6 regulates macrophage cytokine production through TLR232,33,34. Therefore, we tested this using BMDMs from both wild-type and TLR2KO mice. ESAT-6 at both concentrations stimulated IL-6 production by wild-type macrophages at comparable levels to that induced by both Pam3CSK4 and LPS, TLR2 and TLR4 agonists, respectively. With TLR2KO macrophages, ESAT-6 stimulated IL-6 production to the same extent as LPS though overall IL-6 responses were slightly lower than wild-type macrophages, and as expected Pam3CSK4 at both concentrations did not stimulate any detectable levels of IL-6, (Fig. 5B). TLR2KO macrophages responded with phosphorylation of STAT3 at the same level as wild type macrophages did upon ESAT-6 stimulation (Fig. 5A). These results clearly argue against the role of TLR2 in ESAT-6-induced STAT3 phosphorylation and IL-6 production by macrophages. Since cytokines, such as IL-6 and IL-10 are major activators of STAT335 and indeed IL-10 was shown to be responsible for STAT3 phosphorylation in macrophages infected with Mtb36, we examined the effect of these two cytokines on ESAT-6 and H37Rv induced phosphorylation of STAT3 and IL-6 production by macrophages. Blocking IL-6 or IL-6R did not affect ESAT-6 induced activation of STAT3 (Fig. 5C) and production of IL-6 by macrophages (Fig. 5D). Neutralizing IL-10 did not affect the level of STAT3 phosphorylation in macrophages stimulated by ESAT-6 or infected with H37Rv (Fig. 5E). Neutralizing IL-10 resulted in elevated IL-6 production by macrophages in response to either ESAT-6 or H37Rv (Fig. 5F), consistent with previous report37 and confirming the neutralizing activity of anti-IL-10 used. These results together argue against the potential autocrine or secondary effects of IL-6 and IL-10 in STAT3 activation and IL-6 production by macrophages in response to ESAT-6 stimulation and Mtb infection.
Figure 5. ESAT-6 stimulates STAT3 phosphorylation and IL-6 production by macrophages independent of TLR2 or autocrine effects of IL-6 and IL-10.
BMDMs from three wild-type or TLR2−/− mice were incubated with ESAT-6 for different time points and the phosphorylation of STAT3 was determined by Western blotting followed by blotting for total STAT3 in the same membrane after stripping (A). BMDMs from 5 wild-type (filled bars) or 6 TLR2−/− (open bars) mice were incubated with medium alone or indicated concentrations of ESAT-6, LPS or Pam3CSK4 and IL-6 levels were determined 24 h later (B). Data are expressed as means ± SEM. BMDMs from wild-type mice were incubated with either anti-IL-6 or anti-IL-6R for one hour prior to addition of ESAT-6 at 1 ug/ml for different time points. The phosphorylation of STAT3 and total STAT3 (C) was determine as in (A) and IL-6 production (D) was determined as in (B). BMDMs were incubated with anti-IL-10 at 5 μg/ml for one hour prior to stimulation with ESAT-6 or infection with H37Rv. Activation of STAT3 (E) and production of IL-6 (F) were determined as in (A and B), respectively. One representative result of three independent experiments is shown for (A,C and E). The data are expressed as means ± SEM for (B,D and F).
Mtb lacking ESAT-6 induces less IL-6 production and weak STAT3 activation in macrophages
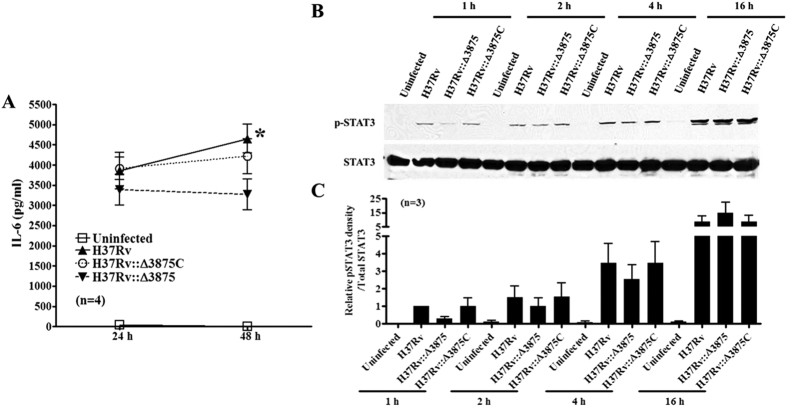
To determine whether our findings from recombinant ESAT-6 studies are also applicable to live Mtb infection, we infected BMDMs with Mtb strain H37Rv, its esat-6 deletion mutant H37Rv::Δ3875 or esat-6 complemented strain H37Rv::Δ3875C38 and determined the activation of STAT3 and production of IL-6. Although all three strains induced IL-6 production by BMDMs compared to uninfected control cells (medium), H37Rv::Δ3875 induced less IL-6 production by macrophages at 24 h and 48 h with significant reduction at 48 h post infection compared to H37Rv (Fig. 6A). In contrast, esat-6 complemented strain restored the capacity of IL-6 stimulation by macrophages. Similarly, esat-6 deletion mutant induced less STAT3 phosphorylation in macrophages than both wild type and esat-6 complemented strains at 1 (70%), 2 (33.5%) and 4 h (26.2%) post infection (Fig. 6B and C). These indicate that ESAT-6 may contribute to STAT3 activation and IL-6 production by macrophages infected with Mtb.
Figure 6. Mtb with esat-6 deletion is less potent in STAT3 activation and IL-6 production by macrophages.
BMDMs were infected with Mtb wild type strain (H37Rv), its esat-6 deletion mutant (H37Rv::Δ3875) or esat-6 complemented strain (H37Rv::Δ3875C) at 10 multiplicity of infection and IL-6 levels in the culture supernatants were determined by ELISA (A) and expressed as means ± SEM. Some cells were collected at 1, 2, 4 and 16 h post infection and phospho-STAT3 was determined by Western blotting followed by blotting for total STAT3 after stripping (B). One representative result of 3 independent experiments is shown (B). Means and SEM of relative densities of phospho-STAT3 were calculated using the densities of phospho-STAT3 with H37Rv at 1 h as 1 and after normalized by the respective densities of total STAT3 from three different experiments (C).
ESAT-6 stimulates activation of STAT3 and production of IL-6 by human macrophages
To confirm our findings from mouse macrophages, we performed experiments with human peripheral blood monocyte derived macrophages. The results show that ESAT-6 at 1 μg/ml induced phosphorylation of STAT3 and STAT1 with the same pattern as in murine BMDMs (Fig. 7A) and induced strong STAT3 binding activity and this was suppressed by anti-STAT-3 antibody but not by isotype control IgG (Fig. 7B). Consistent with this, ESAT-6 also induced strong IL-6 production by macrophages (Fig. 7C). Infection of human monocyte derived macrophages with wild-type and esat-6 complemented strain of H37Rv induced strong IL-6 production compared with the cells with esat-6 deletion mutant H37Rv::Δ3875 (Fig. 7D), further supporting the data from mouse macrophages.
Figure 7. ESAT-6 stimulates STAT3 activation and IL-6 production by human macrophages.
Human monocyte derived macrophages were incubated with 1 μg/ml ESAT-6 for different periods and phospho-STAT3 and phospho-STAT1 were determined by Western blotting followed by blotting for total STAT3 and STAT1 after stripping (A) in cellular protein extracts. The DNA binding activity in the cellular protein extracts was determined by EMSA using labeled STAT3 as probe. The specificity of binding and identity of STAT3 was confirmed by competition and supershift EMSA as indicated (B). The arrow indicates specific DNA-protein complex. Some cells were stimulated with ESAT-6 at indicated concentrations and IL-6 levels were determined in 24 h culture supernatants (C). Human macrophages were infected with different strains of Mtb as described in Fig. 6A and IL-6 levels were determined at 24 and 48 h later (D).
Infection of Mtb and administration of ESAT-6 induces phosphorylation of STAT3 and expression of IL-6 in mouse lungs
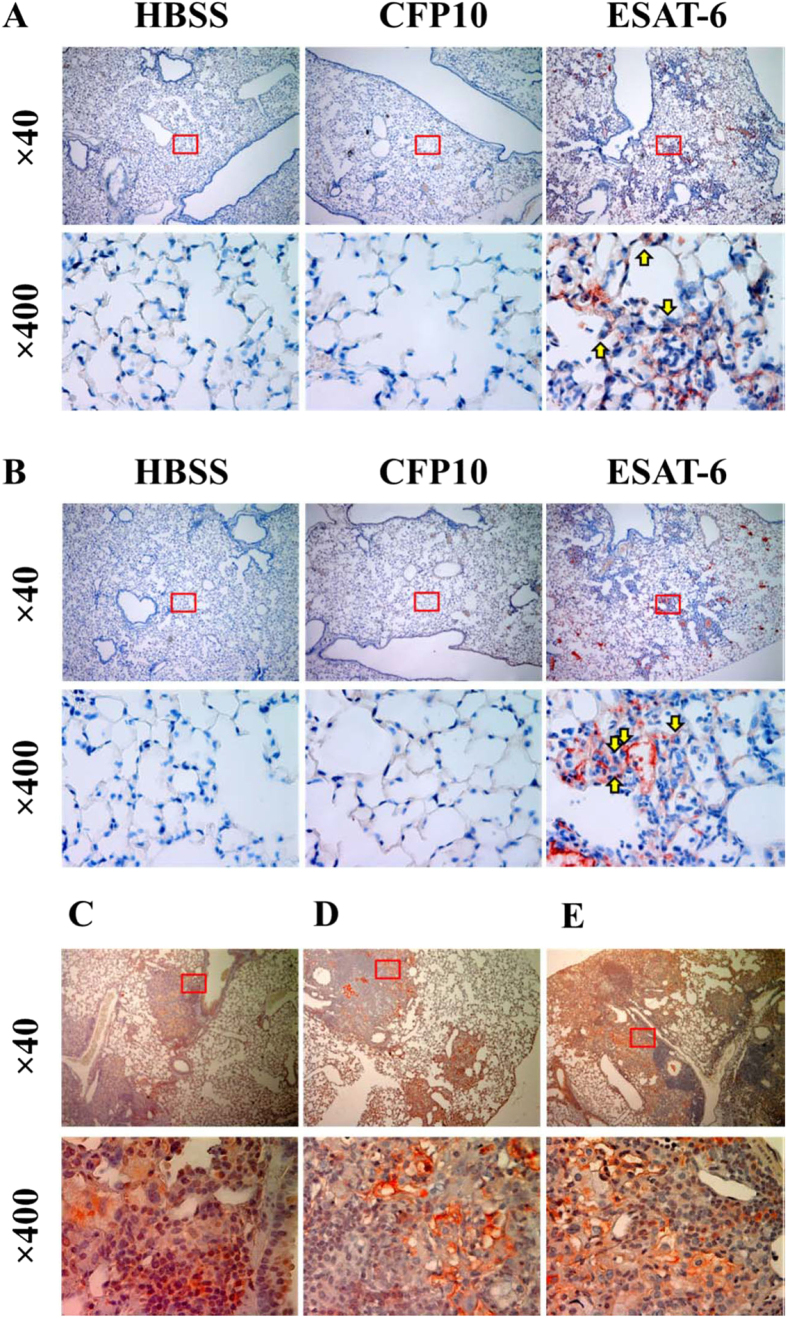
To confirm our in vitro findings in live animals, we administered ESAT-6, CFP10 or HBSS (vehicle control) intranasally into the mouse for 3 days and evaluated the production of IL-6 and the activation of STAT3 in the mouse lungs by immunohistochemistry. Compared to CFP10 or HBSS, ESAT-6 induced infiltration and aggregation of inflammatory cells in the lungs consisting of mostly macrophages. ESAT-6, but not CFP-10 or HBSS, significantly induced IL-6 production (Fig. 8A) and STAT3 phosphorylation in mouse lungs (Fig. 8B), as indicated by red brown color staining, which was mostly located in the alveolar macrophages (Fig. 8B, arrow in lower panels). This indicated that ESAT-6 indeed induces activation of STAT3 and production of IL-6 in vivo. To confirm the expression of ESAT-6, production of IL-6 and activation of STAT3 in live Mtb infection, we examined these in the lung sections of mice that had been infected with Mtb Erdman for 120 days39 by immunohistochemistry. As reported by others40, the lungs of mice infected with Mtb had strong staining for ESAT-6 which was predominantly distributed in macrophages (Fig. 8E), suggesting active secretion of ESAT-6 by Mtb in the mouse lung macrophages. We also observed positive staining for IL-6 (Fig. 8C) which was distributed diffusely through large macrophage/lymphocyte aggregates that are typical of CBA/J mice after 120 days of Mtb infection. Phosphorylated STAT3 (Fig. 8D) was evident within and at the periphery of large macrophage/lymphocyte aggregates and also at high levels within small intra-lesional clusters of macrophages. Surrounding lung tissues were negative for IL-6, phosphorylated STAT3 and ESAT-6, suggesting that ESAT-6 production during live Mtb infection may also activate STAT3 to stimulate IL-6 production in the local granuloma. Of note, p-STAT3 was also observed in macrophage rich areas within granulomas from IL-10 KO mice (not shown).
Figure 8. Administration of ESAT-6 but not CFP10 or infection of Mtb induces expression of ESAT-6, activation of STAT3 and production of IL-6 in the mouse lungs.
C57BL/6 mice were given ESAT-6 or CFP10 at 40 μg/mouse or equal volume of HBSS (vehicle) intranasally. Three days after treatment, production of IL-6 (A) and phosphorylation of STAT3 (B) in the mouse lungs were examined by immunohistochemistry. One representative result from three mice is shown. Lung tissues from M.tb strain Erdman infected CBA/J mice were analyzed for expression of IL-6 (C), phospho-STAT3 (D) and ESAT-6 (E) after immunohistochemistry staining. One representative result from five mice is shown. For all panels, ×400 images are magnification of the insets in the corresponding ×40 images above.
Discussion
Secretion of ESAT-6 by Mtb through ESX-1 secretion system plays a critical role in Mtb pathogenesis. Although ESAT-6 mediated cell damage and Mtb escape from phagolysosomes contribute to pathogenesis, modulation of host immune responses by ESAT-6 may provide additional mechanism for Mtb pathogenesis. In support of this, we showed that ESAT-6 stimulates IL-6 transcription and production by macrophages through activation of STAT3 which is independent of TLR2, IL-6 and IL-10 signaling. Both chemical inhibition and genetic silencing of STAT3 in macrophages inhibited ESAT-6-induced IL-6 production. This was supported by data from the macrophages infected with Mtb with esat-6 deletion and intranasal administration of ESAT-6 into mouse lungs and immunohistochemical examination of Mtb infected mouse lungs for expression of ESAT-6, phospho-STAT3 and IL-6. Thus, this study describes a novel role for ESAT-6 in the initiation and amplification of inflammation at the early stages and in suppression of Th1 immune responses at the chronic phase41 of Mtb infection.
Understanding IL-6 production in TB infection is critical for deciphering TB pathology. Although IL-6 is required for early protective immunity against Mtb infection42,43, excess IL-6 production during chronic TB infection is associated with pathology. Because IL-6 levels in TB patients are associated with pathological changes and manifestation of clinical symptoms, such as thrombocytosis and C reactive protein response44, elevated serum IgG content45,46 and suppressed Th1 responses which is protective against TB infection21,22,41. The concentrations of serum IL-6 from TB patients are correlated with disease severity and changes in clinical parameters44 and spontaneous production of elevated IL-6 together with other inflammatory cytokines is also correlated with development of the lung lesions and their severity in patients with pulmonary TB47,48. Interestingly, compared to other inflammatory cytokines, the concentration of IL-6 together with IL-1β and IL-8 was the most abundant in the bronchial alveolar lavage of TB patients with extensive lung disease and correlated with disease severity as shown by high resolution computed tomography of affected lung areas of TB patients48. Infection of macrophages by Mtb stimulates IL-6 and other inflammatory cytokines and this has been attributed to cell wall components of Mtb, such as, muramyl dipeptide49 and lipoarabinomannan50. This may explain why BCG is capable of stimulating IL-6 production by macrophages21 despite its deficiency in ESAT-6 and its secretion machinery. In addition to cell wall components, the culture filtrate proteins of Mtb were also shown to induce IL-6 production by macrophages but without clear protein identity. This study adds ESAT-6 as another major component of Mtb that stimulates macrophage IL-6 production. This is supported by data from macrophages infected with Mtb H37Rv and its esat-6 deletion mutant. Infection with H37Rv induced significantly higher amounts of IL-6 by both murine and human macrophages than its esat-6 deletion mutant. Surprisingly we also noticed that CFP10 at higher concentration also induced macrophage IL-6 production though significantly less than that stimulated by ESAT-6. This is probably due to CFP10 interaction with macrophages, as we showed previously with human macrophages7. These together support the notion that ESAT-6 serves as an additional factor that contributes to Mtb infection induced IL-6 production by macrophages.
Trace amounts of contaminants may have been the contributing factors to ESAT-6 stimulation of cytokine production including IL-6 by macrophages. Although LPS is a potent IL-6 inducer, and contamination of trace amounts of LPS in ESAT-6 preparations may contribute to the production of IL-6 and other cytokines by macrophages as we have used recombinant proteins purified from the E. coli lysate. However, our results with polymyxin B (Fig. 1F) demonstrated that this is less likely. Although polymyxin B is less efficient in overall blocking of LPS, it has been shown to block efficiently LPS of E. coli25. Consistent with this, polymyxin B at10 μg/ml completely blocked IL-6 production by macrophages stimulated by 100 ng/ml LPS with an E. coli origin from Sigma. Contamination of muramyl dipeptide could not be an issue as these proteins were not purified from Mtb strains. Although peptidoglycan may be another contributing factor, it was not detectable in ESAT-6 by mass spectrometry analysis10. Hence our results clearly demonstrated that ESAT-6 stimulates IL-6 production by macrophages.
ESAT-6 is a major secreted protein of Mtb as Mtb is believed to reside in the membrane bound phagosomes or phagolysosomes of alveolar macrophages, one concern is the availability of free extracellular ESAT-6 for interaction with infected and non-infected bystander macrophages to stimulate STAT3 activation and IL-6 production. However, there is evidence which supports the fact that free ESAT-6 is available for interaction with immune cells during live Mtb infection. As B cells recognize intact protein antigens, recent studies reporting elevated titers of ESAT-6 antibodies in TB patient sera51,52, support the existence of free ESAT-6 in extracellular space of tissues during TB infection. Indeed it was shown recently that dendritic cells infected with Mtb release intact Mtb proteins including ESAT-653. Furthermore, Mtb exists in the extracellular space during live infection in the lungs despite it is an intracellular pathogen54. Therefore, it is reasonable to speculate that free ESAT-6 exists in the extracellular space to interact with macrophages in both autocrine and paracrine manners.
Although we showed that ESAT-6 binds to immune cells including macrophages7, supportingdirect stimulation of macrophages by ESAT-6, the molecular identity of the ESAT-6 interacting components of immune cells remains unclear. Both TLR2 and TLR4 are the most dominant TLRs of macrophages in Mtb infection and play critical roles in macrophage activation and cytokine production. TLR2 was shown to directly bind to C-terminus of ESAT-6 and play a critical role in ESAT-6 regulation of immune responses32,33,34. However, our data with TLR2 knockout mouse macrophages did not support this and lack of TLR2 did not affect ESAT-6 induced STAT3 activation and IL-6 production by macrophages (Fig. 5A and B). Although we could not provide a clear rational for this discrepancy, the existence of other potential ESAT-6 binding proteins may be a contributing factor55,56, and requires further clarification. ESAT-6 activation of STAT3 in macrophages is a surprising finding as STAT family of transcription factors are activated by cytokines and growth factors and both IL-6 and IL-10 have been shown to be the major contributing factors to the activation of STAT3 in macrophages35. However, our studies with neutralizing both of these cytokines did not support their involvement in STAT3 activation by ESAT-6. Although activation of STAT3 in macrophages infected with Mtb was shown to be mediated by IL-1036, the activation of STAT3 was evident only at 3 h post infection later than that in our system even with Mtb infection, which is evident as early as one hour after infection and increased steadily until 16 h post infection (Fig. 6). This continued activation of STAT3 may be due to secondary effects of cytokines including both IL-10 and IL-6. The elevated STAT3 activation and IL-6 and IL-10 production by macrophages in a positive feedback mechanism may contribute to the pathogenesis of Mtb infection.
Regulation of IL-6 is controlled at the transcriptional level by several transcription factors through interaction with its regulatory elements. NF-κB plays a critical role in IL-6 transcription in addition to AP-1, NF-IL-6 and STAT3. STAT3 was shown to be a key signaling molecule in Mtb infected macrophages28,29 and macrophages with activated STAT3 in the peripheral blood of TB patients were shown to be pathogenic associated with TB progression57. Consistent with this, our Western blot results show that ESAT-6 induced STAT3 phosphorylation in macrophages (Fig. 2A), which is supported by confocal microscopy data indicating activation of STAT3 and its localization in both cytoplasm and nucleus of macrophages (Fig. 2B). This is consistent with a previous report showing both mitochondrial and nuclear localization of active STAT3 in the cells31. The EMSA data showed that STAT3 and NF-κB from macrophages stimulated with ESAT-6 bound to both the STAT3 consensus binding site and IL-6 proximal promoter, clearly demonstrating the functional significance of activated STAT3 by ESAT-6 (Fig. 2C–E). This was further supported by the data from STAT3 silencing and chemical inhibition (Figs 3 and 4). One interesting finding was that rapamycin, the inhibitor of mammalian target of rapamycin (mTOR), did not affect ESAT-6 stimulated IL-6 production despite it is shown to enhance LPS stimulated macrophage activation and inflammatory cytokine production by inhibiting IL-1058,59. This might suggest potential differences in the signaling pathways between ESAT-6 and LPS stimulated IL-6 production by macrophages and that the LPS stimulated IL-6 production by macrophages is sensitive to rapamycin. These together suggest that ESAT-6 stimulates IL-6 production by macrophages through activation of STAT3 as well as NF-κB.
Macrophage response by secretion of IL-6 is probably not specific to Mtb infection considering the pathological nature of over produced IL-6 in other chronic infectious diseases. This mechanism is also shared by several other pathogens60,61,62 including HIV63. HSP60 of Helicobacter pylori64 and gp120 of HIV have been shown to stimulate IL-627. Therefore, activation of STAT3 and induction of IL-6 production may be a common mechanism shared by a variety of pathogenic microorganisms including Mtb. Activation of STAT3 and production of IL-6 by Mtb infected macrophages play significant role in host immune responses and pathophysiology of TB29, this study provides a novel evidence that ESAT-6, an essential virulence factor of Mtb, activates STAT3 and stimulates IL-6 production by macrophages. Considering the significance of the IL-6/STAT3 axis in different diseases, detailed signaling pathways of ESAT-6 mediated activation of STAT3 requires further study. Due to the in vitro nature of this study, the findings described here require further examination by animal models of Mtb infection to establish the biological relevance of ESAT-6 induced STAT3 activation and IL-6 production in TB infection.
In conclusion, we have identified a novel mechanism for Mtb infection induced IL-6 production by macrophages through ESAT-6 mediated activation of STAT3. Although detailed signaling pathways for such action of ESAT-6 in macrophages remain to be addressed, this may have significance in understanding the role of ESAT-6 in TB pathology.
Materials and Methods
Antibodies and chemicals
The antibodies for phospho-STAT3 at Y705, STAT3 (124H6), phospho-STAT1 at Y705, STAT1 and phospho-STAT5 at Y694 were from Cell Signaling Technology. Polyclonal rabbit anti-STAT3, anti-NF-κB p65 and isotype matched IgGs for supershift assays were from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated goat anti-rabbit IgG and the goat anti-mouse IgG were from Bio-Rad and Santa Cruz Biotechnology, respectively. Antibodies against mouse IL-6, IL-6 receptor α (gp80) and IL-10 were from R&D systems. STAT3 inhibitors, Stattic and S3I-201 were from Santa Cruz Biotechnology. Rapamycin, Pam3CSK4 and lipopolysaccharide (LPS) were from Sigma.
Mtb strains
Mtb H37Rv, its esat-6 deletion mutant (H37Rv::∆3875) or complemented strain (H37Rv::∆3875 C) were provided by Dr. David Sherman (University of Washington, Seattle, WA). The bacteria were cultured and prepared for infection as previously described9.
Recombinant mycobacterial proteins
Recombinant ESAT-6 and CFP10 were expressed in E. coli and prepared as described earlier7,9. Recombinant plasmid containing Ag85A gene (Rv3804) was obtained from BEI resources and prepared as previously described7. Purity of Ag85A was >95% based on SDS-PAGE and Coomassie Brilliant Blue staining (not shown). The LPS content of recombinant proteins was below 39 pg/mg protein based on QCL-1000 Limulus amebocyte assay (Cambrex). Purified proteins were resuspended at 2 mg/ml in Hanks’ balanced salt solution (HBSS), aliquoted, and stored at −80 °C.
Human monocyte-derived macrophages
Human CD14+ monocytes were purified from the peripheral blood mononuclear cells of 9 QuantiFERON-TB GOLD test negative healthy donors as described before9 after obtained signed consent forms from all the donors. All the experiments with human cells were performed following the NIH guidelines and regulations and the protocols approved by the Institutional Review Board of the University of Texas Health Science Center at Tyler. CD14+ cells were resuspended at 5 × 105 cells/ml in RPMI-1640 medium (Life Technologies) supplemented with 25 ng/ml M-CSF (R&D Systems), 10% heat-inactivated human serum (Atlanta Biologicals), 100 u/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate and 0.1 mM MEM nonessential amino acids and were cultured in 24-well tissue culture plates at 2000 μl/well for 5 days to differentiate.
Mouse bone marrow-derived macrophages
The animal studies were approved by the Animal Care and Use Committee of the University of Texas Health Science Center at Tyler and all the mice experiments were performed in accordance with the NIH guidelines and regulations. Pathogen-free female 6–8-week-old wild-type and TLR2 knockout C57BL/6 mice were purchased from the Jackson Laboratory. Mouse bone marrow-derived macrophages (BMDMs) were prepared as previously described65 with minor modifications. Briefly, bone marrow was flushed out from mouse femur and tibia. After red blood cell lyses, the cells were plated at 4 × 106 cells in a bacteriological petri dish in 10 ml DMEM/F12 medium (Gibco) containing 10% fetal bovine serum (FBS), 100 u/ml penicillin and 100 μg/ml streptomycin, and supplemented with 20% (volume) L-929-conditional media. On day 3, another 5 ml of DMEM/F12 medium with 20% conditional media were added. After 7 days, the cells were harvested for further studies.
Mouse alveolar macrophages
Broncho-alveolar lavage were collected from 6–8-week-old female C57BL/6 mice as described65 with minor modification. Briefly, an 18-gauge cannula inserted into the trachea and the lungs were washed with 1 ml PBS by flushing in and out for several times. The wash solutions were collected into a 15 ml conical tube and centrifuged at 600 × g for 8 min and the cells were resuspended in DMEM containing 10% FBS and 1% antibiotics for further studies.
Mouse alveolar macrophage cell line
Mouse alveolar macrophage cell line RAW 264.7 was purchased from the American Type Culture Collection. The cells were maintained in DMEM containing 10% FBS with antibiotics at 37 °C, 5% CO2 until use.
Determination of IL-6 concentration
IL-6 levels in the culture supernatants were determined by Human IL-6 ELISA MAXTM Standard or mouse IL-6 ELISA MAXTM Standard (both from Biolegend) following the manufacturer’s instructions.
Preparation of total cell, nuclear and cytoplasmic protein extracts
Total cell proteins were prepared as previously described7. The nuclear and cytosolic protein extracts were prepared as previously described66. The concentrations of protein extracts were determined by a BCA assay (Thermo Scientific), aliquoted, and stored at −80 °C.
Western blot
The proteins were separated by 10% SDS-PAGE and electro-blotted to nitrocellulose membrane in Tris-glycine buffer containing 20% methanol. The membrane was blocked with 5% skim milk in TBS for 1 h at room temperature and incubated with a primary antibody in TBS-T (TBS containing 0.05% Tween® 20) with 5% BSA overnight at 4 °C. After washing with TBS-T, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody diluted in TBS-T with 5% skim milk for 45 min at room temperature. After washing four times with TBS-T and once with TBS, antibody-bound proteins were detected by ECL (GE Healthcare).
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assay (EMSA) was performed as previously described66 by incubating 3–6 μg protein extracts with 32P-labeled DNA probes on ice for 30 min. The double-stranded oligonucleotides used as DNA probes were: STAT3, 5′-GATCCTTCTGGGAATTCCTAGATC-3′ (p27); mouse IL-6 proximal promoter (−75 to −55), 5′-TGGGATTTTCCCATGAGTCTC-3′ (28, 29); NF-kB, 5′-AGTTGAGGGGACTTTCCCAGGC-3′66; CREB, 5′-AGAGATTGCCTGACGTCAGAGAGCTAG-3′ (26).
Real-time PCR
The expression of IL-6 mRNA in BMDMs was measured by Real-Time PCR from the total RNA of the cells using glyceraldehyde 3-phosphate dehydrogenase transcripts as internal control and calculating fold changes of IL-6 mRNA in the cells with treatment over that in cells without stimulation as previously described7.
MTT assay
To determine cell viability, the MTT colorimetric assay was performed as previously described7.
Transfection of siRNA
BMDMs with 50% confluence were transfected with either scrambled or STAT3 siRNA (Cell Signaling Technology) using Lipofectamine® 2000 Transfection Reagent (Life Technologies) following the manufacturer’s instructions. The cells were harvested 48 h later, and plated in a 96-well plate at 2 × 105 cells/ml in 200 μl per well DMEM/F12 with 10% FBS. The cells were incubated with or without ESAT-6 at 1 μg/ml for 24 h and IL-6 levels were determined by ELISA. For determination of silencing efficiency, some of the transfected cells were lysed in SDS-PAGE sample buffer, resolved by 10% SDS-PAGE, and transferred to a nitrocellulose membrane for blotting STAT3. The blot was then stripped and reblotted for STAT1 and GAPDH as specificity and loading controls, respectively.
Immunofluorescence staining
Immunofluorescence staining was performed as previously described with minor modification10. Briefly, BMDMs adhered on sterile coverslips were incubated with 1 μg/ml ESAT-6 for 60 min, and then fixed with 1% paraformaldehyde in PBS on ice for 30 min. The cells were washed with PBS containing 5% FBS (staining buffer) and permeablized by incubating with 0.1% Triton X-100 in PBS for 15 min. After washed with staining buffer, the cells were incubated with mouse anti-phospho-STAT3-Y705 or isotype control mouse IgG for 2 h on ice. After washing, the cells were incubated with Alexa Fluo-568-conjugated goat anti-mouse IgG for 45 min. The cells were washed and incubated with DAPI. The coverslips were mounted on glass slides and the images were captured by Nickon Eclipse TE2000-5 inverted fluorescence microscope and analyzed with Ultra-View LCI scanning confocal system (PerkinElmer, Life Sciences).
Immunohistochemistry
Female 6–8-week-old C57BL/6 mice were randomly divided into 3 groups consisting of 3 mice each. The mice were intranasally administered 40 μl of HBSS, 40 μg of CFP10 or ESAT-6 in HBSS10. Three days later the lungs were removed and instilled with 5% formalin in PBS for fixation. The fixed lungs were embedded in paraffin and sectioned and stained for phospho-STAT3 and IL-6. Lung sections from CBA/J mice that had been infected with Mtb strain Erdman (50–70 CFU) for 120 days39 were stained for ESAT-6, phospho-STAT3 and IL-6. For immunohistochemical staining, the lung sections were deparaffinized and rehydrated before antigen retrieval by incubation with sodium citrate buffer. After blocking by serial incubation with hydrogen peroxide, Ultra V block, and 5% goat serum in PBS, the lung sections were incubated with anti-mouse IL-6 mAb (1:25) or anti-phospho-STAT3 (1:100) overnight at 4 °C in humidified chamber. After incubated with biotinylated goat anti-mouse IgG and streptavidin-HRP, the sections were rinsed with PBS and incubated with 3-amino-9-ethylcarbazole, chromogenic HRP substrate, followed by counterstaining with hematoxylin. The images were captured using Ultra Vision Plus Detection System (Thermo Scientific).
Statistical analysis
Data are expressed as mean ± standard error of the mean. Student’s t-test was performed for statistical analysis using GraphPad Prism version 4.0 software (GraphPad Software, Inc.). A P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Jung, B.-G. et al. Early Secreted Antigenic Target of 6-kDa of Mycobacterium tuberculosis Stimulates IL-6 Production by Macrophages through Activation of STAT3. Sci. Rep. 7, 40984; doi: 10.1038/srep40984 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We express our gratitude to Dr. Vijay Boggaram and his assistant for providing technical expertise in histological evaluations of mouse lung sections. We are also grateful to Dr. Amy Tvinnereim for critical reading of the manuscript.
Footnotes
Author Contributions B.J., X.W., N.Y., J.M. and B.S. conceived and performed the experiments, B.J., J.T. and B.S. analyzed the data, B.J., J.T. and B.S. wrote the manuscript.
References
- Comas I. et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nature genetics 45, 1176–1182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samten B., Wang X. & Barnes P. F. Immune regulatory activities of early secreted antigenic target of 6-kD protein of Mycobacterium tuberculosis and implications for tuberculosis vaccine design. Tuberculosis (Edinburgh, Scotland) 91 Suppl 1, S114–118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proceedings of the National Academy of Sciences of the United States of America 100, 12420–12425 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L. Y. et al. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Molecular microbiology 53, 1677–1693 (2004). [DOI] [PubMed] [Google Scholar]
- Derrick S. C. & Morris S. L. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cellular microbiology 9, 1547–1555 (2007). [DOI] [PubMed] [Google Scholar]
- Welin A., Eklund D., Stendahl O. & Lerm M. Human macrophages infected with a high burden of ESAT-6-expressing M. tuberculosis undergo caspase-1- and cathepsin B-independent necrosis. PloS one 6, e20302 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. ESAT-6 inhibits production of IFN-gamma by Mycobacterium tuberculosis-responsive human T cells. J Immunol 182, 3668–3677 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. et al. The Mycobacterium tuberculosis early secreted antigenic target of 6 kDa inhibits T cell interferon-gamma production through the p38 mitogen-activated protein kinase pathway. The Journal of biological chemistry 286, 24508–24518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Early Secreted Antigenic Target of 6-kDa Protein of Mycobacterium tuberculosis Primes Dendritic Cells To Stimulate Th17 and Inhibit Th1 Immune Responses. J Immunol 189, 3092–3103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggaram V., Gottipati K. R., Wang X. & Samten B. Early secreted antigenic target of 6 kDa (ESAT-6) protein of Mycobacterium tuberculosis induces interleukin-8 (IL-8) expression in lung epithelial cells via protein kinase signaling and reactive oxygen species. The Journal of biological chemistry 288, 25500–25511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. & Jung Y. J. Immunity to tuberculosis. Annual review of immunology 22, 599–623 (2004). [DOI] [PubMed] [Google Scholar]
- Cooper A. M. Cell-mediated immune responses in tuberculosis. Annual review of immunology 27, 393–422 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M. & Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma C. L., Sherman D. R. & Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Annual review of microbiology 57, 641–676 (2003). [DOI] [PubMed] [Google Scholar]
- Kishimoto T. IL-6: from its discovery to clinical applications. International immunology 22, 347–352 (2010). [DOI] [PubMed] [Google Scholar]
- Huang W. L. et al. Signal transducer and activator of transcription 3 activation up-regulates interleukin-6 autocrine production: a biochemical and genetic study of established cancer cell lines and clinical isolated human cancer cells. Molecular cancer 9, 309, doi: 10.1186/1476-4598-9-309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges A. H. et al. Determinants of IL-6 levels during HIV infection. Journal of the International AIDS Society 17, 19482 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. et al. Increase in tumor necrosis factor alpha- and interleukin-6-secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infection and immunity 59, 3021–3025 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Ahmady O., Mansour M., Zoeir H. & Mansour O. Elevated concentrations of interleukins and leukotriene in response to Mycobacterium tuberculosis infection. Annals of clinical biochemistry 34 (Pt 2), 160–164 (1997). [DOI] [PubMed] [Google Scholar]
- Hirano T. et al. Human helper T cell factor(s) (ThF). I. Partial purification and characterization. J Immunol 126, 517–522 (1981). [PubMed] [Google Scholar]
- VanHeyningen T. K., Collins H. L. & Russell D. G. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J Immunol 158, 330–337 (1997). [PubMed] [Google Scholar]
- Nagabhushanam V. et al. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J Immunol 171, 4750–4757 (2003). [DOI] [PubMed] [Google Scholar]
- Dutta R. K., Kathania M., Raje M. & Majumdar S. IL-6 inhibits IFN-gamma induced autophagy in Mycobacterium tuberculosis H37Rv infected macrophages. The international journal of biochemistry & cell biology 44, 942–954 (2012). [DOI] [PubMed] [Google Scholar]
- Saunders R., Robson S., Soule S. & Kirsch R. E. Secretion of interleukin-6 by peripheral blood mononuclear cells from patients with pulmonary tuberculosis and their contacts. Journal of clinical & laboratory immunology 40, 19–28 (1993). [PubMed] [Google Scholar]
- Cavaillon J. M. & Haeffner-Cavaillon N. Polymyxin-B inhibition of LPS-induced interleukin-1 secretion by human monocytes is dependent upon the LPS origin. Molecular immunology 23, 965–969 (1986). [DOI] [PubMed] [Google Scholar]
- Chang Q. et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 15, 848–862 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corno M. et al. HIV-1 gp120 activates the STAT3/interleukin-6 axis in primary human monocyte-derived dendritic cells. Journal of virology 88, 11045–11055 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carow B. et al. Critical and independent role for SOCS3 in either myeloid or T cells in resistance to Mycobacterium tuberculosis. PLoS pathogens 9, e1003442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg M. E. & Carow B. SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis. Seminars in immunology 26, 518–532 (2014). [DOI] [PubMed] [Google Scholar]
- Stanley S. A., Johndrow J. E., Manzanillo P. & Cox J. S. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol 178, 3143–3152 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou L. & Too H. P. GDNF family ligand dependent STAT3 activation is mediated by specific alternatively spliced isoforms of GFRalpha2 and RET. Biochimica et biophysica acta 1833, 2789–2802 (2013). [DOI] [PubMed] [Google Scholar]
- Pathak S. K. et al. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nature immunology 8, 610–618 (2007). [DOI] [PubMed] [Google Scholar]
- Chatterjee S. et al. Early Secreted Antigen ESAT-6 of Mycobacterium tuberculosis Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner. PLoS pathogens 7, e1002378 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. et al. The involvement of NADPH oxidase-mediated ROS in cytokine secretion from macrophages induced by Mycobacterium tuberculosis ESAT-6. Inflammation 37, 880–892 (2014). [DOI] [PubMed] [Google Scholar]
- Niemand C. et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol 170, 3263–3272 (2003). [DOI] [PubMed] [Google Scholar]
- Queval C. J. et al. STAT3 Represses Nitric Oxide Synthesis in Human Macrophages upon Mycobacterium tuberculosis Infection. Scientific reports 6, 29297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G. & de Vries J. E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. The Journal of experimental medicine 174, 1209–1220 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn K. M. et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Molecular microbiology 51, 359–370 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyktor J. C. et al. IL-10 inhibits mature fibrotic granuloma formation during Mycobacterium tuberculosis infection. J Immunol 190, 2778–2790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majlessi L. et al. Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. J Immunol 174, 3570–3579 (2005). [DOI] [PubMed] [Google Scholar]
- Dienz O. & Rincon M. The effects of IL-6 on CD4 T cell responses. Clinical immunology 130, 27–33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel C. H. et al. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infection and immunity 65, 4843–4849 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B. M., Frank A. A., Orme I. M. & Cooper A. M. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infection and immunity 68, 3322–3326 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsal E., Aksaray S., Koksal D. & Sipit T. Potential role of interleukin 6 in reactive thrombocytosis and acute phase response in pulmonary tuberculosis. Postgraduate medical journal 81, 604–607 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O. et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. The Journal of experimental medicine 206, 69–78 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahri F. et al. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood 113, 2426–2433 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law K. et al. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. American journal of respiratory and critical care medicine 153, 799–804 (1996). [DOI] [PubMed] [Google Scholar]
- Casarini M. et al. Cytokine levels correlate with a radiologic score in active pulmonary tuberculosis. American journal of respiratory and critical care medicine 159, 143–148 (1999). [DOI] [PubMed] [Google Scholar]
- Huygen K., Vandenbussche P. & Heremans H. Interleukin-6 production in Mycobacterium bovis BCG-infected mice. Cellular immunology 137, 224–231 (1991). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Broser M. & Rom W. N. Activation of the interleukin 6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by nuclear factors NF-IL6 and NF-kappa B. Proceedings of the National Academy of Sciences of the United States of America 91, 2225–2229 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva V. M., Kanaujia G., Gennaro M. L. & Menzies D. Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int J Tuberc Lung Dis 7, 478–484 (2003). [PubMed] [Google Scholar]
- Legesse M. et al. IgA response to ESAT-6/CFP-10 and Rv2031 antigens varies in patients with culture-confirmed pulmonary tuberculosis, healthy Mycobacterium tuberculosis-infected and non-infected individuals in a tuberculosis endemic setting, Ethiopia. Scandinavian journal of immunology 78, 266–274 (2013). [DOI] [PubMed] [Google Scholar]
- Srivastava S. & Ernst J. D. Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming. Cell host & microbe 15, 741–752 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff D. R. et al. Location of intra- and extracellular M. tuberculosis populations in lungs of mice and guinea pigs during disease progression and after drug treatment. PloS one 6, e17550 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreejit G. et al. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (beta2M) affecting antigen presentation function of macrophage. PLoS pathogens 10, e1004446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G. et al. Mycobacterium tuberculosis secreted protein ESAT-6 interacts with the human protein syntenin-1. Central European Journal of Biology 1, 183–202 (2006). [Google Scholar]
- Lastrucci C. et al. Tuberculosis is associated with expansion of a motile, permissive and immunomodulatory CD16(+) monocyte population via the IL-10/STAT3 axis. Cell research 25, 1333–1351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A. K., Wang R., Mackman N. & Luyendyk J. P. Rapamycin enhances LPS induction of tissue factor and tumor necrosis factor-alpha expression in macrophages by reducing IL-10 expression. Molecular immunology 46, 2249–2255 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer V. et al. Role of the mTOR pathway in LPS-activated monocytes: influence of hypertonic saline. The Journal of surgical research 171, 769–776 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L., Moore D. & West D. C. Human cytomegalovirus induces IL-6 and TNF alpha from macrophages and microglial cells: possible role in neurotoxicity. Journal of neurovirology 1, 219–227 (1995). [DOI] [PubMed] [Google Scholar]
- Neveu W. A. et al. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol 183, 1732–1738 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfgott D. C. et al. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol 142, 948–953 (1989). [PubMed] [Google Scholar]
- Birx D. L. et al. Induction of interleukin-6 during human immunodeficiency virus infection. Blood 76, 2303–2310 (1990). [PubMed] [Google Scholar]
- Gobert A. P. et al. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. The Journal of biological chemistry 279, 245–250 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang X., Goncalves R. & Mosser D. M. The isolation and characterization of murine macrophages. Current protocols in immunology/edited by John E. Coligan … [et al.] Chapter 14, Unit 14 11, doi: 10.1002/0471142735.im1401s83 (2008). [DOI] [PMC free article] [PubMed]
- Samten B. et al. Reduced expression of nuclear cyclic adenosine 5′-monophosphate response element-binding proteins and IFN-gamma promoter function in disease due to an intracellular pathogen. J Immunol 168, 3520–3526 (2002). [DOI] [PubMed] [Google Scholar]