Abstract
Immunodiagnostic methods based on the detection of antibodies continue to be the most effective and practical methods for the diagnosis of imported schistosomiasis. Schistosoma bovis is a species whose final natural hosts are bovines, ovines, caprines, and small wild ruminants. Different studies have demonstrated the analogies existing between S. bovis and other Schistosoma species which affect humans. The objective of this work was to evaluate the utility of S. bovis adult worm antigens (AWA) for the diagnosis of imported human schistosomiasis by enzyme-linked immunosorbent assay (ELISA) and electroimmunotransfer blotting (EITB) techniques. By detecting eggs, the ELISA for S. bovis AWA was able to definitively detect imported cases with a sensitivity of 94%. The specificity of the ELISA for S. bovis AWA was 97%. There were no differences between the results of the S. bovis AWA ELISA for patients infected with Schistosoma mansoni and those infected with Schistosoma haematobium. The EITB technique showed bands of 85, 37, and 20 kDa, which are characteristic of infections with Schistosoma spp. Specific bands to indicate infection by different species of Schistosoma have not been detected. The combined use of the ELISA for S. bovis AWA and EITB increased the global sensitivity of the study to 97%. Our findings suggest that the ELISA for S. bovis AWA is a useful test for the immunodiagnosis of imported schistosomiasis and that EITB for detecting S. bovis AWA permits the confirmation of diagnosis when the ELISA for S. bovis AWA is positive.
Human schistosomiasis is endemic in 76 developing countries. More than 190 million people living in these countries suffer from the illness, and about 650 million are at risk (32). In recent years, immigration and the increase in travel from high-risk areas have led to an increase in the number of imported cases. Some studies indicate that 15% of immigrants and 2.5% of the travelers from areas where schistosomiasis is endemic have schistosomiasis (26). The species most commonly implicated are Schistosoma mansoni, Schistosoma haematobium, and Schistosoma intercalatum.
Definitive diagnosis of schistosomiasis is carried out by the detection of eggs in feces and/or urine. Nevertheless, the parasitological methods of diagnosis have low sensitivities, especially with patients with the acute phase of the illness or with low-intensity infection (8, 13).
The detection of circulating antigens and copro-antigens allows the diagnosis of active schistosomiasis. The most frequently used methods have been those using cathodic and anodic circulating antigens. These circulating antigens have been correlated clinically with the intensity of the infection and with morbidity (14). Moreover, techniques using these antigens have demonstrated their utility in posttreatment monitoring (15, 30). Their sensitivities lie between 65 and 95%, according to different studies (10, 31). However, these methods are not readily available, and the use of specific monoclonal antibodies limits their clinical use. Techniques of molecular diagnosis by PCR have also recently demonstrated their utility in the diagnosis of infection with S. mansoni (23), although no data are available for other Schistosoma spp.
Immunodiagnostic methods based on the detection of antibodies continue to be the most effective and practical methods for the diagnosis of imported schistosomiasis (29). Different methods have been used, with enzyme-linked immunosorbent assay (ELISA) being the most widely developed (13). The use of recombinant and purified antigens does not appear to have important advantages in comparison with the use of complex antigens. These antigens from adult worms and eggs from different species of Schistosoma are still the most widely used for the diagnosis of schistosomiasis (3, 13).
Schistosoma bovis is a species whose final natural hosts are bovines, ovines, and caprines and whose secondary hosts are small wild ruminants. It is distributed throughout Africa, Southwest Asia, and Mediterranean Europe. Different studies have demonstrated the analogies existing between S. bovis and other Schistosoma species which affect humans. The similarities include morphological, ecological, physiological, and genetic aspects (6, 7, 12). Furthermore, a high degree of cross-reactivity among S. bovis, S. mansoni, and S. haematobium has been demonstrated (2, 4). In spite of all these homologies, human infection with S. bovis has not been reported.
The objective of this work was to evaluate the utility of S. bovis adult worm antigen (AWA) for the diagnosis of imported human schistosomiasis using ELISA and electroimmunotransfer blotting (EITB) techniques.
MATERIALS AND METHODS
Patients.
Two hundred nineteen samples of human sera from immigrants and travelers presenting at the Departments of Tropical Medicine of Hospital Insular (Las Palmas, Gran Canaria, Spain) and Hospital Ramón y Cajal (Madrid, Spain) from 1997 to 2003 were collected. The sera were divided into five groups as described below.
Group 1.
Thirty-five sera were collected from patients with definitive diagnoses of schistosomiasis; 20 of these sera were from patients infected with S. haematobium, 12 were from patients infected with S. mansoni, 2 were from patients infected with S. intercalatum, and 1 was from a patient infected with both S. haematobium and S. mansoni. The diagnoses were made by the detection of parasite eggs in stool, urine, and/or biopsy specimens.
Group 2.
Forty-one sera were collected from healthy controls from areas of sub-Saharan Africa where schistosomiasis is endemic. Sera from individuals with schistosomiasis or other parasitic infections were excluded based on parasitological tests.
Group 3.
Fifty-two sera from healthy Spanish blood donors were used as controls.
Group 4.
Forty-five sera were collected from patients with other isolated helminthic infections, namely, infections with Fasciola hepatica (nine specimens), Echinococcus granulosus (three specimens), Taenia sp. (three specimens), Cisticercus cellulosae (two specimens), Onchocerca volvulus (two specimens), Loa loa (two specimens), Mansonella perstans (six specimens), Ascaris lumbricoides (two specimens), Trichuris trichiura (three specimens), Ancylostoma duodenale (four specimens), Strongyloides stercolaris (two specimens), Trichostrongylus sp. (one specimen), Toxocara canis (two specimens), Trichinella spiralis (two specimens), and Gnathostoma sp. (two specimens).
Group 5.
Twenty-seven sera were collected from patients with protozoal infections, namely, infections with Plasmodium falciparum (13 specimens), Plasmodium malariae (1 specimen), Plasmodium ovale (1 specimen), Trypanosoma cruzi (2 specimens), Microsporidium sp. (1 specimen), Entamoeba histolytica (4 specimens), Entamoeba coli (1 specimen), Giardia lamblia (3 specimens), and Isospora belli (1 specimen).
Group 6.
Nineteen sera were collected from patients with bacterial or viral infections, namely, infections with Rickettsia typhi (four specimens), Leptospira interrogans (one specimen), Bartonella henselae (one specimen), Nocardia asteroides (one specimen), Borrelia burgdorferi (one specimen), Aeromona sp. (two specimens), human immunodeficiency virus (HIV) (four specimens), hepatitis A virus (three specimens), hepatitis B virus (one specimen), and hepatitis C virus (one specimen).
Antigens.
S. bovis AWA was obtained as described previously by Abán et al. (1). The worms were suspended in sterile phosphate-buffered saline (PBS) at a concentration of 20 worms/ml with 1 mM phenylmethylsulfonyl fluoride, homogenized with a Ten Broeck tissue grinder, frozen and thawed three times, and then sonicated with three cycles at 70 kHz for 1 min each. The suspension was centrifuged at 5,000 × g for 30 min at 4°C. The protein concentration of the supernatant was determined using the Micro-BCA protein assay reagent kit (Pierce).
Whole-worm egg antigen (WWE) of S. mansoni was provided by G. V. Hillyer, University of Puerto Rico (25).
ELISA with S. bovis and S. mansoni antigens.
Polystyrene microtiter plates (Costar) were coated with 100 μl of S. bovis AWA per well at a previously determined protein concentration of 5 μg/ml diluted in carbonate buffer (pH 9.6). Serum at a dilution of 1:100 was added to the wells and incubated for 1 h at 37°C. Horseradish peroxidase goat anti-human immunoglobulin G (Sigma, St. Louis, Mo.) at a dilution of 1:2,000 was added. Washes were performed three times with 200 μl of PBS-Tween 20 per well. After incubation for 1 h at 37°C, substrate solution (ortho-phenylene diamine plus H2O2) was added, and the reaction was stopped at 10 min with 3 N H2SO4. This test was performed with all sera described above.
The ELISA for S. mansoni WWE was performed as described above with modifications in order to correlate the results with those of the ELISA for S. bovis AWA. The S. mansoni WWE ELISA was carried out using 46 sera: 28 sera from patients with schistosomiasis (group 1), 7 sera from healthy controls (group 3), and 11 sera from patients with other infections (groups 4, 5, and 6). All the sera were analyzed in triplicate.
EITB with S. bovis AWA.
Proteins of S. bovis AWA were separated by electrophoresis under reducing conditions by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis, as described by Laemmli (16). One hundred thirty micrograms of S. bovis AWA was boiled for 3 min and applied to the 15% polyacrylamide gel with a Mini Protean III apparatus (Bio-Rad). The separated proteins were electrotransferred to nitrocellulose filters using a Trans Blot transfer cell apparatus (Bio-Rad), and 5-mm-wide strips were cut. The transference was verified with Ponceau solution. Nonspecific sites were blocked with a solution of PBS-0.05% Tween and 5% bovine serum albumin and incubated for 30 min at room temperature. Serum diluted 1:50 in a solution containing PBS-0.05% Tween and 5% bovine serum albumin was added and left overnight at 4°C. Horseradish peroxidase goat anti-human immunoglobulin G (Sigma) was added at a dilution of 1:500 and incubated for 2 h at room temperature. After being washed, the bands were visualized with substrate solution (methanol plus 4-chloro-1-naphthol plus H2O2). The reaction was stopped by washing the strips in distilled water. This test was performed on all sera from positive controls, all sera shown by ELISA to be positive for S. bovis AWA, 31 randomly selected sera from patients with other infections, and 12 sera from healthy controls.
Statistical analysis.
Statistical tests were carried out using the SPSS 11.5 statistical package. The level of significance accepted was a P value of <0.05. The results are expressed as means and standard deviations. The analysis of variance test and SDS post hoc test were used for comparison of the means of the groups. The Kruskal-Wallis test was used if the variances were not homogenous. The receiver operating characteristic curve was used for establishment of the cutoff of the ELISA. In order to compare the mean optical densities (ODs) of different species of Schistosoma, the Mann-Whitney U test was used. Wilcoxon's matched-pair signed-rank test and the Pearson correlation coefficient were used to compare the results for S. bovis AWA and S. mansoni AWA. The kappa test was used for the establishment of reliability.
RESULTS
ELISA for S. bovis AWA.
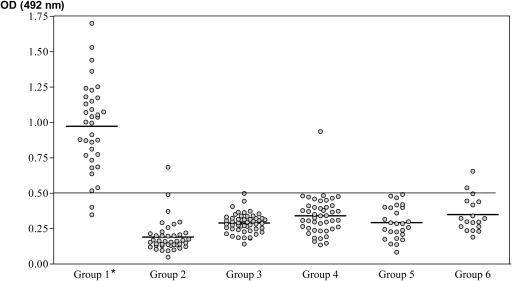
The ODs of the 218 sera analyzed are presented in Fig. 1. The mean OD ± standard deviation for the group of patients with confirmed schistosomiasis was the highest (0.972 ± 0.307) of those calculated. The remaining groups presented lower mean ODs: 0.195 ± 0.119 for healthy patients from zones of endemicity, 0.289 ± 0.06 for healthy Spanish blood donors, 0.338 ± 0.133 for patients with other isolated helminthic diseases, 0.293 ± 0.112 for patients with protozoan infections, and 0.347 ± 0.121 for patients with other infections. Comparative analysis of the mean ODs detected significant differences between values for group 1 and those for the remaining groups (P < 0.0001). A receiver operating characteristic curve was established from these results to define the sensitivities and specificities at different cutoff OD values. A cutoff of 0.500 with a 94% sensitivity and a 97% specificity was established. The positive predictive value was 89%, and the negative predictive value was 99%. None of the sera from healthy Spanish donors or from patients with protozoan infections presented ODs higher than this cutoff value.
FIG. 1.
Values of OD at 492 nm determined by the S. bovis AWA ELISA with sera from patients included in this study. We included sera from patients diagnosed with schistosomiasis by the detection of eggs (group 1), sera from healthy controls from areas of schistosomiasis endemicity (group 2), sera from healthy Spanish blood donors (group 3), sera from patients with other helminth infections (group 4), sera from patients with protozoan infections (group 5), and sera from patients with viral and bacterial infections (group 6). The horizontal line represents the cutoff value. *, significant differences were found between group 1 and the other groups.
Only one serum specimen from the group of healthy patients from an area of endemicity presented an OD higher than the cutoff value. Among the sera from patients with helminthiasis, only the serum from one patient (of nine sera analyzed) with Fasciola hepatica infection had an OD higher than the cutoff value. Among the sera of patients with other infections, only that of one patient with HIV and another with Nocardia infection had ODs higher than the cutoff value.
There were no significant differences between the results of the ELISA for S. bovis AWA with the sera of patients infected with S. mansoni and S. haematobium. We analyzed the reliability of the test on 45 sera, finding a kappa index of 0.86.
Correlation between the S. bovis AWA ELISA and the S. mansoni WWE ELISA.
The sera from the patients with a definite diagnosis of schistosomiasis exhibited, in the S. bovis AWA ELISA, a higher mean OD than the sera used in the S. mansoni WWE ELISA (1.04 ± 0.347 versus 0.793 ± 0.244). This difference was statistically significant (P < 0.001).
The remainder of the sera analyzed presented a mean OD in the S. bovis AWA ELISA lower than that obtained in the S. mansoni WWE ELISA (0.284 ± 0.104 versus 0.382 ± 0.039), and the difference was statistically significant (P = 0.028).
We detected a strong correlation (r = 0.856) between the ODs of the sera analyzed with the two antigens (Fig. 2).
FIG. 2.
Correlation among OD values of the sera analyzed with the S. bovis AWA (AWA Sb) and S. mansoni WWE (WWE Sm) ELISA using 46 sera: 28 sera from patients with schistosomiasis (group 1), 7 sera from healthy controls (group 3), and 11 sera from patients with other infections (groups 4, 5, and 6).
EITB with S. bovis AWA.
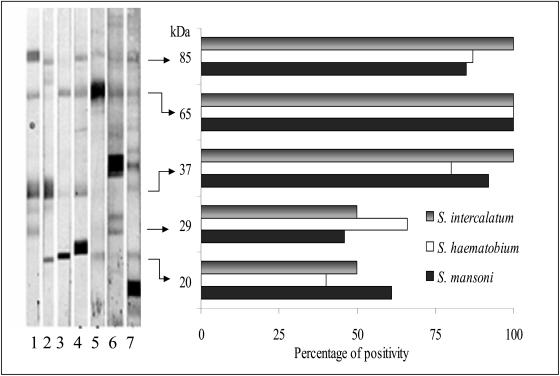
The patterns of bands of antigenic recognition of sera from patients infected with S. mansoni, S. haematobium, and S. intercalatum and the percentages of sera that exhibited each band are shown in Fig. 3. The five principal protein clusters recognized were 85, 65, 37, 29, and 20 kDa. Of the sera analyzed, 86% exhibited the band of 85 kDa, 96% exhibited the band of 65 kDa, 83% exhibited the band of 37 kDa, 55% exhibited the band of 29 kDa, and 44% exhibited the band of 20 kDa. No differences were detected in the profiles of bands among the different species of Schistosoma.
FIG. 3.
EITB of sera from patients diagnosed with schistosomiasis, including one to three sera from patients with infections with S. mansoni, four to five sera from patients with infections with S. haematobium, and six to seven sera from patients with infections with S. intercalatum. The percentages of positivity of the principal bands detected in the sera of patients infected with S. intercalatum, S. haematobium, and S. mansoni are shown.
Sera from 38 patients with a negative ELISA result for S. bovis AWA were analyzed with EITB (Fig. 4). Only one serum sample, from a patient infected with P. falciparum, exhibited a band close to 85 kDa. No sera exhibited bands in the regions of 20 and 37 kDa. Moreover, 31% of the sera that were S. bovis AWA ELISA negative exhibited bands in the region of the 65-kDa cluster, and 26% exhibited bands in the region of 29 kDa. Only two of the four sera that were S. bovis AWA ELISA positive from HIV patients and healthy individuals from an area of endemicity exhibited bands of 85, 65, 29, and 20 kDa.
FIG. 4.
Percentages of positivity of the bands detected by EITB with S. bovis AWA. Group 1 consisted of sera from patients with a definitive diagnosis of schistosomiasis by detection of eggs, groups 2 and 3 consisted of sera from healthy patients from areas of endemicity and nonendemicity, group 4 consisted of sera from patients with isolated helminthic infections, group 5 consisted of sera from patients with protozoan infections, and group 6 consisted of sera from patients with viral and bacterial infections. Numbers in parentheses indicate the number of sera in each group.
Estimation of the sensitivities of the ELISA and EITB for S. bovis antigen.
Considering the sera that exhibited the band of 85, 37, or 20 kDa, we found that EITB presented sensitivities of 94% for S. haematobium and 100% for S. mansoni and S. intercalatum. The utilization of ELISA and Western blotting in parallel increased the global sensitivity of the study to 97% for the patients with schistosomiasis, as confirmed by the detection of eggs (Table 1).
TABLE 1.
Sensitivity of ELISA and EITB with S. bovis AWA
| Organism in or characteristic of serum | No. of serum samples positive/total no. tested (%) by:
|
||
|---|---|---|---|
| ELISA | EITB | ELISA plus EITB | |
| S. mansoni | 11/12 (91.6) | 11/11 (100) | 12/12 (100) |
| S. haematobium | 19/20 (95) | 18/19 (94.7) | 19/20 (95) |
| S. intercalatum | 2/2 (100) | 2/2 (100) | 2/2 (100) |
| All from patients with definitive schistosomiasis | 33/35 (94.2) | 32/33 (96.9) | 34/35 (97.1) |
DISCUSSION
The diagnosis of imported schistosomiasis is habitually carried out by detecting eggs of Schistosoma in samples of feces and urine. Nevertheless, the low and fluctuating excretion of eggs makes the diagnosis of many cases difficult. The method of immunodiagnosis based on the detection of antibodies increases the detection of cases and constitutes an especially useful method for the study of imported schistosomiasis. Although different techniques, such as those utilizing hemagglutination and immunofluorescence, and the circumoval precipitin test have been used, ELISA has been the technique most widely developed. A great variety of antigens have been employed.
The crude antigens from adult worms and eggs have a high sensitivity for the diagnosis of infection (22); however, they present a specificity lower than 80%, giving false positives for infections with intestinal nematodes and especially with hookworms (21). Moreover, it is common to find polyparasitism in patients from areas of endemicity (27). The fundamental advantage of using complex antigens from adult worms over using antigens from eggs is their greater facility and their high yield of antigenic material (13). Tests using the purified antigens, such as CEF6 (11), cathodic circulating antigen (24), and the recombinant antigens rTEG of Schistosoma japonicum (17) and r22 kDa of S. mansoni, have demonstrated acceptable sensitivities, although they are less than those of tests using the crude antigens from adult worms and eggs.
Heterologous antigens have been used in the immunodiagnosis of different parasite infections. Their clinical yield has been demonstrated using antigens of Strongyloides ratti in the diagnosis of human strongyloidiasis (20) or antigens of Dirofilaria immitis in the diagnosis of tropical filariasis (18). In this study, we used complete S. bovis AWA for the detection of antibodies by ELISA and EITB in sera from patients with a definitive diagnosis of schistosomiasis. Using the S. bovis AWA ELISA, we detected 94% of the cases of confirmed schistosomiasis. Moreover, the specificity achieved was 97%. Of the 185 sera analyzed, only 4 for which a diagnosis of schistosomiasis was discounted were positive by the S. bovis AWA ELISA. Two of these patients, infected with Nocardia asteroides and Fasciola hepatica (5), may represent cases of cross-reactivity. The other two patients with positive ELISA results for S. bovis AWA came from an area of endemicity, and it is probable that these test results occurred in both cases due to cryptic schistosomiasis, given the high prevalence of infection with Schistosoma in immigrants from areas of endemicity.
The purified antigens of adult worms have allowed the obtainment of microsomal antigens of S. mansoni (MAMA) and of S. haematobium with sensitivities of 96 and 98% for the diagnosis of schistosomiasis due to S. mansoni and S. haematobium, respectively (3, 19). Nonetheless, the diagnostic sensitivity for cases produced by other species is lower. The ELISA for MAMA permits the diagnosis of only 55 to 83% of the cases caused by S. haematobium (3, 19). This fact limits the potential of using only one of these ELISAs for the diagnosis of imported schistosomiasis, especially among travelers and immigrants coming from Africa (9, 26). In contrast with ELISAs using microsomal antigens, the ELISA in our study revealed no differences in the diagnostic results for patients infected with S. mansoni and S. haematobium.
The performance of an ELISA using sera from patients both with and without schistosomiasis and using the complete S. bovis AWA and the S. mansoni WWE allowed us to prove a high degree of correlation between the two tests. This assay further supports the utility of the S. bovis AWA ELISA in the diagnosis of human schistosomiasis.
EITB has been used as a method for confirming infections by Schistosoma (22, 29). Performing the ELISA and EITB in parallel can increase the sensitivity to 97%. The use of EITB with microsomal antigens allows a species diagnosis. The detection of the 30-kDa band in MAMA EITB is highly specific for infection with S. mansoni, and the detection of the 23-kDa band in EITB for microsomal antigens of S. haematobium is specific for infection with S. haematobium (3, 28).
In this study, we analyzed sera of patients from the different groups by EITB with S. bovis AWA. We found that the sera of patients with confirmed schistosomiasis exhibited a pattern of five principal protein clusters. The bands of 85, 37, and 20 kDa were characteristic of infection with Schistosoma. Only two S. bovis AWA ELISA-positive sera from patients from areas of endemicity exhibited a pattern of antigenic recognition similar to that of sera from patients with confirmed schistosomiasis. This fact supported the suspicion of cryptic schistosomiasis. In our study, in contrast with what was found by EITB with microsomal antigens, we did not detect specific bands of infection for different species of Schistosoma.
In summary, we can conclude that the S. bovis AWA ELISA is a useful test for the immunodiagnosis of imported schistosomiasis, comparable with other habitually used techniques which permit the diagnosis of different species of Schistosoma. Moreover, the utilization of EITB with S. bovis AWA permits the confirmation of diagnosis when the ELISA for S. bovis AWA is positive.
Acknowledgments
This work has been supported in part by grants from the Red de Centros de Investigación en Medicina Tropical (FIS reference no. C03/04), the Fondo de Investigación Sanitaria (FIS reference no. 01/0685), and Comunidad Canaria (PI 2003/024).
We thank A. M. Martín-Sanchez and R. Elcuaz for the study of coproparasitological samples and Miguel Cordero Sánchez for the critical review of the manuscript.
REFERENCES
- 1.Abán, J. L., V. Ramajo, J. L. Pérez Arellano, A. Oleaga, G. V. Hillyer, and A. Muro. 1999. A fatty acid binding protein from Fasciola hepatica induced protection in C57/BL mice from challenge infection with Schistosoma bovis. Vet. Parasitol. 83:107-121. [DOI] [PubMed] [Google Scholar]
- 2.Agnew, A. M., H. M. Murare, S. B. Lucas, and M. J. Doenhoff. 1989. Schistosoma bovis as an immunological analogue of S. haematobium. Parasite Immunol. 11:329-340. [DOI] [PubMed] [Google Scholar]
- 3.Al-Sherbiny, M. M., A. M. Osman, K. Hancock, A. M. Deelder, and V. C. Tsang. 1999. Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings. Am. J. Trop. Med. Hyg. 60:960-966. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 1972. Observations on cross-immunity to Ornithobilharzia turkestanicum, Schistosoma bovis, S. mansoni, and S. haematobium in mice, sheep, and cattle in Iran. Bull. W. H. O. 47:591-600. [PMC free article] [PubMed] [Google Scholar]
- 5.Azab, M. E.-S., and E. A. el Zayat. 1996. Evaluation of purified antigens in haemagglutination test (IHA) for determination of cross reactivities in diagnosis of fascioliasis and schistosomiasis. J. Egypt. Soc. Parasitol. 26:677-685. [PubMed] [Google Scholar]
- 6.Barber, K. E., G. M. Mkoji, and E. S. Loker. 2000. PCR-RFLP analysis of the ITS2 region to identify Schistosoma haematobium and S. bovis from Kenya. Am. J. Trop. Med. Hyg. 62:434-440. [DOI] [PubMed] [Google Scholar]
- 7.Camacho, M., and A. Agnew. 1995. Glucose uptake rates by Schistosoma mansoni, S. haematobium, and S. bovis adults using a flow in vitro culture system. J. Parasitol. 81:637-640. [PubMed] [Google Scholar]
- 8.Corachan, M. 2002. Schistosomiasis and international travel. Clin. Infect. Dis. 35:446-450. [DOI] [PubMed] [Google Scholar]
- 9.Day, J. H., A. D. Grant, J. F. Doherty, P. L. Chiodini, and S. G. Wright. 1996. Schistosomiasis in travellers returning from sub-Saharan Africa. BMJ 313:268-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jonge, N., A. Rabello, F. W. Krijger, P. G. Kremsner, R. S. Rocha, N. Katz, and A. M. Deelder. 1991. Levels of the schistosome circulating anodic and cathodic antigens in serum of schistosomiasis patients from Brazil. Trans. R. Soc. Trop. Med. Hyg. 85:756-759. [DOI] [PubMed] [Google Scholar]
- 11.Ghandour, A. M., K. Tricker, M. J. Doenhoff, A. A. al-Robai, and A. A. Banaja. 1997. An enzyme-linked immunosorbent assay using Schistosoma mansoni purified egg antigen for the diagnosis of schistosomiasis in Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 91:287-289. [DOI] [PubMed] [Google Scholar]
- 12.Hamburger, J., He-Na, I. Abbasi, R. M. Ramzy, J. Jourdane, and A. Ruppel. 2001. Polymerase chain reaction assay based on a highly repeated sequence of Schistosoma haematobium: a potential tool for monitoring schistosome-infested water. Am. J. Trop. Med. Hyg. 65:907-911. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton, J. V., M. Klinkert, and M. J. Doenhoff. 1998. Diagnosis of schistosomiasis: antibody detection, with notes on parasitological and antigen detection methods. Parasitology 117(Suppl.):S41-S45. [DOI] [PubMed] [Google Scholar]
- 14.Hassan, M. M., M. H. Hegab, S. Z. Soliman, O. A. Gaber, M. M. Shalaby, and F. M. Kamel. 1999. Relationship between circulating antigen level and morbidity in Schistosoma mansoni-infected children evaluated by ultrasonography. Am. J. Trop. Med. Hyg. 61:635-638. [DOI] [PubMed] [Google Scholar]
- 15.Kremsner, P. G., P. Enyong, F. W. Krijger, N. De Jonge, G. M. Zotter, F. Thalhammer, F. Muhlschlegel, U. Bienzle, H. Feldmeier, and A. M. Deelder. 1994. Circulating anodic and cathodic antigen in serum and urine from Schistosoma haematobium-infected Cameroonian children receiving praziquantel: a longitudinal study. Clin. Infect. Dis. 18:408-413. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y. S., A. G. Ross, A. C. Sleigh, Y. Li, G. J. Waine, G. J. Williams, M. Tanner, and D. P. McManus. 1999. Antibody isotype responses, infection and re-infection for Schistosoma japonicum in a marshland area of China. Acta Trop. 73:79-92. [DOI] [PubMed] [Google Scholar]
- 18.Libman, M. D., J. D. MacLean, and T. W. Gyorkos. 1993. Screening for schistosomiasis, filariasis and strongyloidiasis among expatriates returning from the tropics. Clin. Infect. Dis. 17:353-359. [DOI] [PubMed] [Google Scholar]
- 19.Maddison, S. E., S. B. Slemenda, V. C. Tsang, and R. A. Pollard. 1985. Serodiagnosis of Schistosoma mansoni with microsomal adult worm antigen in an enzyme-linked immunosorbent assay using a standard curve developed with a reference serum pool. Am. J. Trop. Med. Hyg. 34:484-494. [DOI] [PubMed] [Google Scholar]
- 20.Neva, F. A., A. A. Gam, and J. Burke. 1981. Comparison of larval antigens in an enzyme-linked immunosorbent assay for strongyloidiasis in humans. J. Infect. Dis. 144:427-432. [DOI] [PubMed] [Google Scholar]
- 21.Noya, O., B. Alarcon de Noya, S. Losada, C. Colmenares, C. Guzman, M. A. Lorenzo, and H. Bermudez. 2002. Laboratory diagnosis of schistosomiasis in areas of low transmission: a review of a line of research. Mem. Inst. Oswaldo Cruz 97(Suppl. 1):167-169. [DOI] [PubMed] [Google Scholar]
- 22.Noya, O., Z. Fermin, B. Alarcón de Noya, S. Losada, C. Colmenares, and T. Hermoso. 1995. Humoral immune response of children with schistosomiasis. Isotype recognition of adult worm antigens. Parasite Immunol. 17:319-328. [DOI] [PubMed] [Google Scholar]
- 23.Pontes, L. A., E. Dias-Neto, and A. Rabello. 2002. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am. J. Trop. Med. Hyg. 66:157-162. [DOI] [PubMed] [Google Scholar]
- 24.Qian, Z. L., and A. M. Deelder. 1993. Dot immunoassay with biotinylated antigen for determination of antibodies against the circulating cathodic antigen (CCA) in schistosomiasis japonica. Am. J. Trop. Med. Hyg. 49:777-782. [DOI] [PubMed] [Google Scholar]
- 25.Rivera-Marrero, C. A, and G. V. Hillyer. 1985. Isolation and partial characterization of shared antigens of Biomphalaria glabrata and Schistosoma mansoni and their evaluation by the ELISA and the EITB. J. Parasitol. 71:547-555. [PubMed] [Google Scholar]
- 26.Roca, C., X. Balanzo, J. Gascon, J. L. Fernandez-Roure, T. Vinuesa, M. E. Valls, G. Sauca, and M. Corachan. 2002. Comparative, clinico-epidemiologic study of Schistosoma mansoni infections in travellers and immigrants in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 21:219-223. [DOI] [PubMed] [Google Scholar]
- 27.Tchuem Tchuente, L. A., J. M. Behnke, F. S. Gilbert, V. R. Southgate, and J. Vercruysse. 2003. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop. Med. Int. Health 8:975-986. [DOI] [PubMed] [Google Scholar]
- 28.Tsang, V. C., K. Hancock, S. E. Maddison, A. L. Beatty, and D. M. Moss. 1984. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA). J. Immunol. 132:2607-2613. [PubMed] [Google Scholar]
- 29.Tsang, V. C., and P. P. Wilkins. 1997. Immunodiagnosis of schistosomiasis. Immunol. Investig. 26:175-188. [DOI] [PubMed] [Google Scholar]
- 30.van Lieshout, L., N. de Jonge, N. el-Masry, M. M. Mansour, S. Bassily, F. W. Krijger, and A. M. Deelder. 1994. Monitoring the efficacy of different doses of praziquantel by quantification of circulating antigens in serum and urine of schistosomiasis patients. Parasitology 108:519-526. [DOI] [PubMed] [Google Scholar]
- 31.Van Lieshout, L., N. De Jonge, N. A. el Masry, M. M. Mansour, F. W. Krijger, and A. M. Deelder. 1992. Improved diagnostic performance of the circulating antigen assay in human schistosomiasis by parallel testing for circulating anodic and cathodic antigens in serum and urine. Am. J. Trop. Med. Hyg. 47:463-469. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. 1998. Report of the WHO informal consultation on schistosomiasis control. [Online.] www.who.int/wormcontrol/documents/publications/en/99_2en.pdf.