Abstract
An impressive development has been achieved toward the production of well-defined “smart” inorganic nanoparticles, in which the physicochemical properties can be controlled and predicted to a high degree of accuracy. Nanoparticle design is indeed highly advanced, multimodal and multitargeting being the norm, yet we do not fully understand the obstacles that nanoparticles face when used in vivo. Increased cooperation between chemists and biochemists, immunologists and physicists, has allowed us to think outside the box, and we are slowly starting to understand the interactions that nanoparticles undergo under more realistic situations. Importantly, such an understanding involves awareness about the limitations when assessing the influence of such inorganic nanoparticles on biological entities and vice versa, as well as the development of new validation strategies.
Introduction
The use of nanoparticles (NPs) for drug delivery and imaging is undoubtedly one of the most important areas in biomedicine.1−4 This relatively new field, known as nanomedicine, merges distinct disciplines such as chemistry, pharmacology, immunology, and even electronics for applications such as biomolecular sensing. One of the central features in nanomedicine is the controlled interaction of NPs with target cells,5−7 in such a way that physical and chemical obstacles are overcome, while avoiding undesired toxicity in the long term.8 We are currently seeing a renewed interest in studying how the intrinsic properties of nanomaterials are related to the results we see in vivo.9−11 Consequently, we are asking again all the important questions as to why nanomaterials are failing clinical trials in such high numbers? How do the physicochemical features of NPs change when they are suspended in biological fluids?12 Can cell–NP interactions be predicted if protein corona formation is modulated on demand?13 How do NPs act in flow environments, as compared to nonflowing cell cultures? Is mitochondrial activity a suitable read-out for cell viability?14 Addressing such questions has turned a page in our understanding as to why so many NP formulations fail clinical trials.
We focus this Topical Review specifically on inorganic NPs for a number of reasons. NPs are used for biomedical applications because their small size is favorable for different administration routes and allows delivery of active molecules to subcellular locations via various internalization mechanisms. Additionally, the high surface-to-volume ratio of NPs facilitates the incorporation of multiple moieties, such as antifouling or targeting molecules, toward the assembly of multifunctional NPs. While both inorganic and organic NPs share these size-dependent features, it is mainly inorganic NPs that exhibit novel physical properties at the nanoscale, such as localized plasmon resonances, fluorescence, or superparamagnetism, as compared with their bulk or micron-sized counterparts. These features can be exploited in many potential applications regarding imaging, sensing, and drug delivery. In contrast, there are fewer examples of organic NPs (e.g., perylene based nanocrystals) exhibiting such size dependent physical properties.15,16 In inorganic NPs, physical properties can be tailored on demand by modifying the composition, size, or shape, thereby obtaining “responsive” materials toward external stimuli, including magnetic fields or light. These modifications are not easily achieved with organic nanocrystals. In this context, gold NPs can be produced in various sizes and shapes, which determine their optical response (due to localized plasmon resonances); such NPs have been widely exploited for photoacoustic detection, fluorescence, hyperthermia, or surface-enhanced Raman scattering (SERS).17 Another typical example of inorganic NPs used in nanomedicine is iron oxide NPs which can be used as contrast agents in magnetic resonance imaging (MRI) or heat producers for hyperthermia.18 Iron oxide nanoparticles aside, the presence of inorganic NPs in clinical trials is becoming commonplace and it is clear that other inorganic NPs will likely soon enter the clinic.19 Finally, due to this interest in the use of inorganic NPs for clinical applications, we find ourselves in a situation lacking internal controls relating to cytotoxicity, dosing, administration protocols, and other aspects such as in vitro models.20 Equally important is to understand the fate of internalized inorganic NPs21 (see, for example, a recent study by Wilhem et al. focused on iron oxide NP degradation22) and potentially overlooked allergy formation against inorganic NP core components.23 Herein we thus discuss recent work pointing out the challenges involved in predicting the interactions between inorganic NPs and biological surfaces due to their modifiable physical properties, and the choice of appropriate protocols for in vitro validation on the efficient application of nanomaterials in biomedicine.
Nanoparticles, a Wolf in Sheep’S Clothing? Understanding Unexpected Toxicity and Common Pitfalls
The toxicity of inorganic NPs is largely due to alterations in the physicochemical properties of the NPs in biological fluids,24 and while comparative studies in which changes in NP size, charge, surface chemistry, or the like are investigated, or different inorganic NPs with similar physicochemical properties are compared, discrepancies are continually encountered (see Figure 1).20,25 In addition to a lack of standards in the field, common issues encountered in toxicity testing include attempts to compare unrelated types of inorganic NPs, different administration protocols (a problem that is also often overlooked in in vivo studies), poor choice or differences in chosen cell types (resulting in differences in growth or endocytosis kinetics), and frequent lack of nanomaterial stability testing or poor choice of sterilization methods, both of which are a key aspects to any pharmaceutical product.26 In this context, the toxicity studies by Manshian et al. using quantum dots (QDs) with similar physicochemical characteristics have shown the following: (i) cell-type differences, (ii) differences in the QD agglomeration degree depending on the amount of serum proteins, (iii) differences due to the exact composition of cell culture media, (iv) differences due to varying exposure time, and (v) higher uptake levels not necessarily correlating with higher toxicities.27 The quantification of cellular uptake is a key factor when determining the toxicity of NPs. Many techniques such as flow cytometry and fluorescence microscopy with poor z-resolution cannot differentiate between NPs adsorbed on the cell membrane and those which have been internalized. Furthermore, most techniques rely on quantifying the proportion of labels on the NP, i.e., fluorescent or radioactive markers which are assumed to remain conjugated to the NP. Important concerns include dissociation of the label from the NP,28,29 which may lead to altered biodistribution and/or enhanced toxicity arising from the various individual components. Ironically, often the major concern to the researcher is how to show that the marker remains with the NP, rather than the effectiveness of the NP itself.30 In order to study NP cellular uptake, novel strategies based on mass spectrometry are being applied for accurate quantification of the NP core uptake,31,32 which can be combined with spectral imaging of whole tissues.33−36
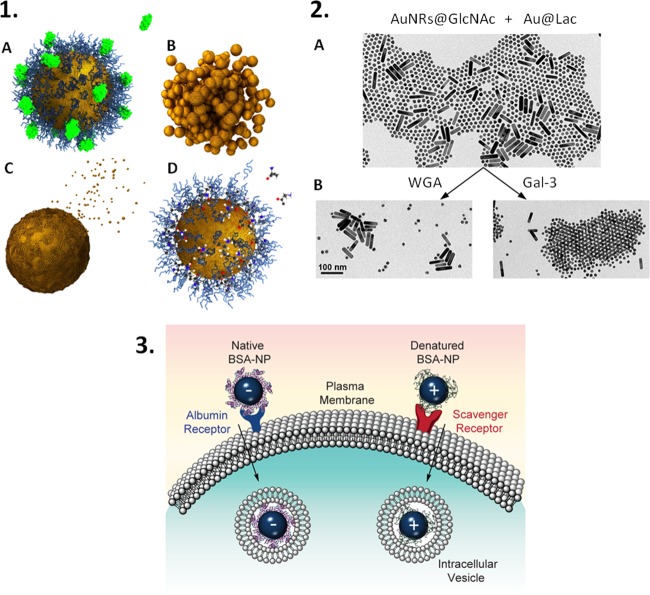
Figure 1.
NPs face significant physicochemical changes upon exposure to biomolecules found in physiological fluids. These effects may be (1) uncontrolled, such as protein corona formation (a), NP aggregation (b), NP dissolution (c), and even removal or exchange of surface ligands (d);24 or (2) highly controlled, as in the case for ligand–receptor mediated agglomeration of spherical NPs coated with the carbohydrate disaccharide lactose (Lac) triggered by the presence of the protein β-galactoside binding lectin galectin-3 (Gal-3).7 Physicochemical changes can affect cellular uptake. For example, differences in protein structure (native vs denatured) within the protein corona affect the cellular internalization pathways as shown in illustration 3.37 Reproduced in part from ref (24) with permission from Elsevier, and from refs (7,37) with permission from the American Chemical Society.
Another often overlooked aspect when assessing toxicity is the correct choice of the toxicity assay; arguably the most commonly used assays when analyzing the effect of NPs in vitro are cell viability assays based on membrane leakage or mitochondrial activity.38 With the increasing variety of NP types, biotech firms have worked hard to produce more varied options for measuring cytotoxicity, in which the inference of the NP itself is reduced. Considering spectroscopic tetrazolium based assays, in addition to the demonstration of direct and indirect inhibition of the assay components,39−41 a vast majority of inorganic NPs absorb in the measurement window of 400–600 nm, meaning that there is a major risk that the observed cytotoxic effect is incorrectly lower due to absorption by the NP.42 It has been reported that carbon nanotubes can quench the fluorescence readout in the resazurin assay,43 and that resazurin can be reduced by molecules such as ascorbic acid which are often used for gold NP synthesis and stabilization. Care must also be taken in understanding what the cell viability assay is truly showing. For example, the Live/Dead fluorescent assay is very useful at providing a visible overview of cell viability based on the membrane integrity of the cells in question. However, cells may have altered membrane integrity yet remain alive, for example, after certain laser treatments.44 This is especially seen when studying cellular redox levels, as while inorganic NPs can be used to determine intracellular levels of reactive oxygen species (ROS), they are also known to cause ROS production and have been shown to interfere in ROS detection.45−49 Cytoskeletal changes, alterations of intracellular signaling pathways, triggering and inhibition of protein fibrillation, and alteration of protein or gene expression are further examples of reported effects mediated by inorganic NPs.50−52 In this context, we know that the phenotype of cells can be affected by the presence of inorganic NPs, without directly affecting cell viability, which can result in altered cellular functions such as triggering signaling cascades or distinct inflammatory functions.53−57 We refer the reader to several review articles which address in detail common pitfalls while assessing NP cytotoxicity, with special reference to intracellular changes.10,58,59
First Come, First Served? Foreseeing Nanoparticle Interactions with the Biological Interface
The importance of NP shape, size, and surface charge for efficient cellular uptake is well-known (see Canton for a critical review),60 and recent works aimed at directly investigating such factors with a high degree of control.61−65 For example, it is well-known that protein binding to NPs may affect their overall size and charge, and situations in which NPs must cross physical barriers such as the blood-brain barrier or the blood vessel endothelium to enter tumors, must be carefully considered. Protein corona formation depends on the nature of the NPs (composition and physicochemical properties), their surface coating and their route of administration into the body or onto a cell culture.7,8,13,66,67 Interestingly, it has been demonstrated that differently charged NPs can affect the molecular structure of the proteins binding to their surface, ultimately affecting cellular uptake (see Figure 1).37 Therefore, one should be aware that heat inactivation of serum, thereby denaturing serum proteins, in cell culture may also have significant effects on NP uptake. It has been recently demonstrated that the suborgan biodistribution of gold NPs is also affected by NP surface charge and their subsequent interactions with biomolecules.36 The authors propose neutral NPs as immunologically stronger NPs due to their accumulation in the immune active white pulp of the spleen, possibly due to interactions of the NP surface with proteins such as IgG or fibronectin, the latter known to bind PEG stabilized NPs.68
In line with the increased interest in 3D cell models (see below) and enhancing NP uptake via nonspecific and relatively simple means, current focus is given to the effects of sedimentation and gradients on uptake. While arguably more complicated to design, experiments comparing inverted cell cultures with more traditional upright cultures have shown that gravity, or sedimentation, plays an important role in enhancing NP uptake (Figure 2).64,69 In cancer cell cultures, the enhanced metabolism of cancer cell lines compared to “healthy” cells can be exploited to achieve higher levels of NP uptake.70−74 In vivo, the simplest method to improve NP localization in cancerous tissues involves taking advantage of the enhanced permeability and retention (EPR) effect; however, without NP ligand-cell receptor targeting, this method does not necessarily result in increased levels of cellular uptake (Figure 3).75,76 The principle downfall in this strategy is, however, the inherent diversity in cancers, ranging from variations in the EPR effect within the same tumor, cancer location, stage progression, and even between patients. Importantly, after leaking into tumor sites, NPs are known to accumulate in the cells they first encounter, thereby limiting their penetration and potential usefulness. A method to overcome this problem has been demonstrated in vitro by Setyawati et al., using endocytosed nanodiamonds to increase the EPR effect by inducing intracellular ROS production within endothelial cells.77 The result was a decrease in the intercellular adherence properties and cytoskeletal remodeling, leading to a further increase of NP penetration and allowing the movement of model cytotoxic drugs across the normally nonleaky endothelial cell barrier (Figure 3). Another interesting method to increase NP cytotoxicity in cancer cell models involves predictable intracellular NP aggregation states. Hu and colleagues produced “smart NPs” that take advantage of the relatively high intracellular glutathione concentration to induce reduction of diselenide bonds within their surface coating, leading to high NP uptake of small NPs, massive intracellular flocculation, ROS production, and apoptosis of MCF-7 and HeLa cancer cells in an in vitro model.73 Intracellular NP aggregation states can also be exploited for imaging or treatment modalities. For example, the photothermal heating efficiency due to plasmon-coupling between gold NPs can be tuned through the size and morphology of NPs, as well as their aggregation state within intracellular endosomal compartments.78 Equally interesting are methods to avoid intracellular NP aggregation, such as the encapsulation with silica79 or amphiphilic polymer80 shells, thereby ensuring that the optical properties of gold NPs remain for applications such as photothermal therapy or imaging.
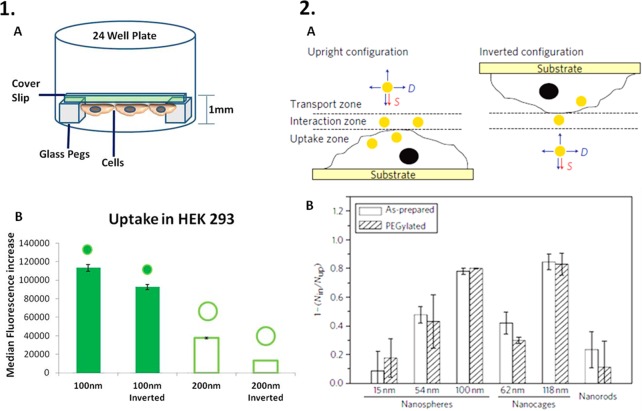
Figure 2.
Effects of NP sedimentation onto the cell surface. (1) Experimental set up (A) used by Agarwal et al. to demonstrate that the orientation of cells affects the rate of uptake of spherical NPs with different sizes (B).69 (2) Schematic representation of the different zones involved in cellular uptake of NPs (A) and two of the factors affecting the uptake process: sedimentation (S) and diffusion (D). Cho et al. demonstrated that the disparity in cellular uptake (B) of NPs with different size and shape did not depend on the surface coating and was higher for the larger NPs.64 Reproduced in part from refs (69) and (64) with permission from Nature Publishing Group.
Figure 3.
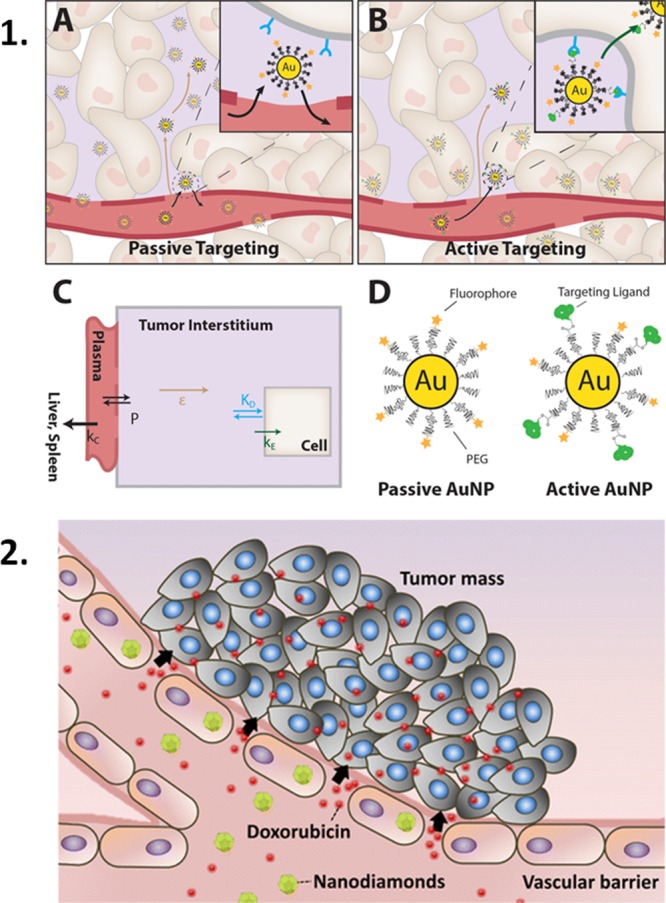
(1) Passive targeting of NPs via the EPR effect leads to relatively high levels of NPs in metabolically active cancerous tissues.81 (2) The EPR effect can be increased using NPs such as nanodiamonds which have been shown to increase intracellular ROS levels, resulting in cytoskeletal remodeling and loss of intercellular connections, thereby allowing NP movement through a subsequent “leaky” endothelial cell barrier.77 Reprinted with permission from (81) and (77).
Moving Forward: 3D Tissue Culture Systems, Microfluidics, and In Silico Simulations
Numerous techniques are under development for the characterization of the interactions of NPs with eukaryotic cells in an environment that resembles the real situation in vivo.82 Such techniques can be divided into two basic groups, those addressing the interactions of cells with each other and with external supports, and those that focus on the flow that is present in vivo to allow the diffusion of both soluble and insoluble factors. Within the first group, current research focuses on moving from 2D to 3D, and from single cell to multiple cell type (co)cultures (Figure 4).82−84 Therefore, the interaction between inorganic NPs and cells is starting to take into consideration (among other facts) the existence of extracellular barriers, multiple cell type interactions, and possible modifications in the cell phenotype due to interactions between cells and their culture dish supports. The simplest models are acellular or cell-seeded gels, which are useful to study NP transport, uptake, and therapeutic efficiency.85−87 However, their use is limited because particles can interact with the gels but cannot easily mimic tissue structures. Interestingly, multicellular spheroids, which do not require an external scaffold for cell packing, or multilayer cell cultures, are more advanced systems to study NP tissue penetration, targeting, and toxicity.88−92 Several studies have aimed to compare the penetration, toxicity, and targeting of various kinds of inorganic NPs between multicellular spheroids and animal models.93,94 There appears to be a good correlation between in vitro 3D and in vivo models, showing the usefulness of such 3D coculture models to comply with the 3R’s and overcome ethical issues related to animal testing. A recent study using stem cell spheroids and magnetic NPs has elegantly shown the long-term effects of both NPs on cells, and vice versa, i.e., what is the extent of endosomal iron oxide NP degradation and how does free iron affect gene expression.22 Commercially available 3D spheroids can help to standardize protocols to study NP–cell interactions.95 However, such models have limitations mainly related to the nature of the spheroid itself, which cannot match the level of complexity of true tissues as well as vascularization. Notwithstanding, technologies based on stem cells have opened new opportunities for in vitro experimentation.96 It is now possible to fabricate miniaturized organs, so-called organoids, that can replace in some cases ex vivo systems and open up new horizons in human biology research, overcoming some of the limitations involved in using animal models.97−99 Such promising 3D coculture models include organoids mimicking the intestine, breast, brain, kidney, heart, and lungs. They are essentially aggregates of several cell types fabricated from pluripotent stem cells, adipose-derived stem cells and tissue progenitors from animal or human sources that are able to form some of the complex structures of organs, self-organize, self-renew, and perform cellular functions typical from in vivo tissues. Therefore, they can be used to study nutrient transport, tissue replacement therapy, disease diagnosis, drug screening, or toxicity and be applied for personalized medicine.100 Interestingly, they are bridging the gap between in vitro and in vivo experimentation. Recently, two detailed protocols describing the production of liver, kidney, and pancreas organoids have been published,101,102 which may be crucial in the field of nanomedicine to assess NP cytotoxicity or drug release, for example. In this context, Astashkina et al. studied the cytotoxicity and cytokine production of gold and polymeric NPs in a 3D kidney organoid, observing that only the organic NPs were able to penetrate the organoid and produce indicators of toxicity.103 While we are convinced that this exciting and emerging research field will raise the number of studies assessing inorganic NP–cell interactions and NP therapeutic efficiency,104 a lack of standards and “teething” issues such as the lack of nutrients and oxygen at the center of the organoid, resulting in cellular necrosis, limits current use for NP validation.105
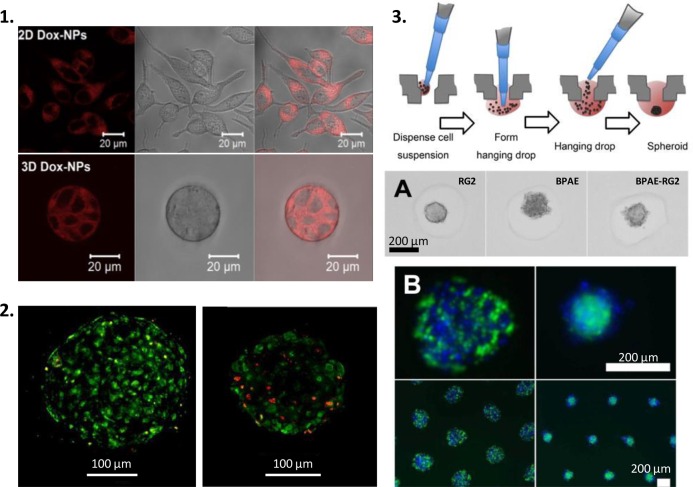
Figure 4.
2D to 3D, single to multicellular cultures; the use of more realistic cell culture models to show NP effects in vitro. (1) Classical 2D vs 3D tumoroid cell culture model showing similar cytoplasmic doxorubicin staining when delivered to cells using NPs.86 (2) Live/Dead staining of human liver microtissue control (A) or previously exposed to PVP-coated silver NPs (B).92 (3) Diagram showing spheroid production;106 bright field (A) and fluorescence (B) images of single and multicell spheroids.88 Reproduced in part from ref (86) with permission from Elsevier, from refs (88,92) and ref (106) with permission from Nature Publishing Group.
All the previously mentioned 3D coculture models lack vascularization and do not consider that NPs in vivo are immersed in a fluid flow. Such flows affect many aspects of cell–NP interactions, ranging from tissue biodistribution through stress forces at the cell surface–NP level.107 In the most simple context, gravity affects NP sedimentation rates and subsequently NP uptake by cells.64 Microfabrication and microfluidic systems have the potential to contribute greatly in this field.108,109 Basic perfusable 3D culture models exist that allow control over flow rate, pH, or drug concentration, in some cases permitting in situ visualization.110 Ng and Pun studied and compared the penetration and uptake of fluorescent NPs of two different sizes under interstitial fluid flow in a perfusable 3D monoculture of cells. It was possible not only to image the penetration in situ but also to detach the cells from the scaffold and study the NP uptake using flow cytometry. Results were similar to those obtained with multicellular spheroids under static conditions. Indeed, the most advanced perfusable 3D cell culture systems to study cell–NP interactions, NP penetration, and drug delivery are currently based on tissue-on-a-chip platforms,111,112 and the more advanced organ-on-a-chip technology is being used for drug screening.113,114 In parallel to the development of microfluidic devices including multi-cell type cultures for in situ imaging, a pool of experimental data derived from animal models will be essential for comparison (Figure 5). This will shed light on the open question of whether it will be possible to truly correlate results obtained with in vivo animal models and advanced in vitro models.109
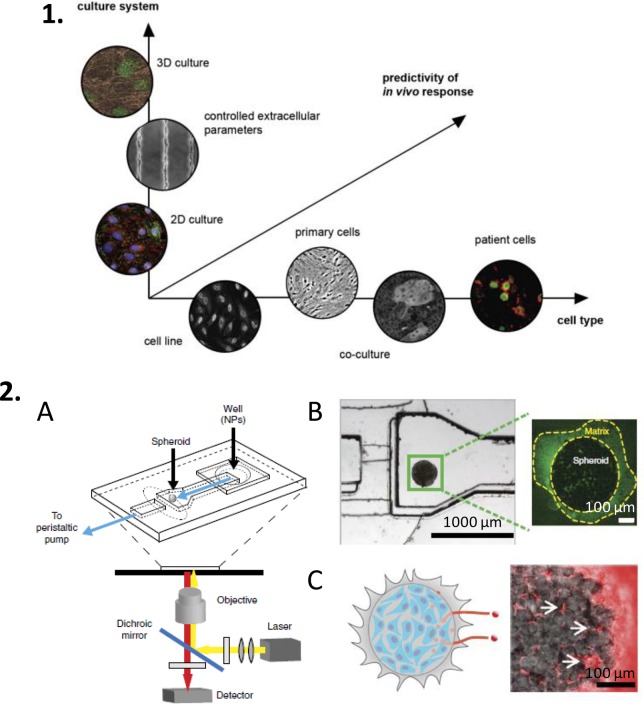
Figure 5.
Current methods to predict in vivo responses rely on the toolbox of available in vitro techniques, ranging from 2D to 3D cultures, involving single or multiple cell types (1).109 An example of a perfusable 3D cell culture designed as a spheroid-on-a-chip.112 The microfluidic device (A) contains a cancer cell spheroid embedded in an extracellular matrix (B), which permits real-time optical analysis of the penetration of NPs with different sizes (C). Reproduced in part from ref (109) with permission from Elsevier, and from ref (112) with permission from Nature Publishing Group.
In silico simulations that can predict in vitro inorganic NP uptake and intracellular distribution, as well as in vivo NP fate and drug delivery efficiency, are not yet commonplace.115,116 We however expect that they will become increasingly important toward the optimal design of drug carriers, prediction of NP uptake, pharmacokinetics, toxicity, and determination of biodistribution within cells of targeted tissues.81,117,118 For example, computational fluidic dynamics is used to understand which aerodynamic and physicochemical factors of NPs affect their circulation in blood.119,120 In addition, simulations have also provided information regarding the way NPs extravase out of blood vessels, diffuse, and reach targeted cancer cells. Combination of this research with experimental data will be a powerful tool for the implementation of nanomedicine based on “safe-by-design” production of NPs. However, collaborative efforts between scientists are required to face the challenges that exist in modeling NPs in biological environments in a more realistic manner.121
Conclusions
The future of NPs in drug delivery and imaging will rely on the ability to produce standardized protocols by which we can compare our findings on aspects ranging from physicochemical properties and stability to in vitro and in vivo performance. Unfortunately, the wide variety of inorganic NP cores and surface coatings used to produce drug delivery or imaging systems complicates this aspiration. Although we are increasingly able to better understand why NPs behave as they do, the time and effort required for the validation of a new NP formulation is not yet efficient; essentially, new methods for the prediction of NP interaction with living organisms are required. Like most pharmaceutical products, inorganic NPs are not perfectly stable, especially in biological fluids, and their aggregation state and surface properties including the formation of protein corona make their characterization complicated. While methods emerge to standardize in vitro testing and to fabricate systems better resembling the in vivo environment, additional efforts to provide standards and comparative methods in NP production are required. We should remember that nanomedicine is a relatively new field involving cross-disciplinary scientists, and while a large number of inorganic NPs have advanced from the lab to the clinic,19 collaboration is key.122 New technologies for in vitro testing and new computational methods to realistically predict the interaction of NPs with living organisms starting from cellular models will be very important. We also predict an increase in collaborations between materials scientists and medical practitioners, thereby addressing from the start what are the important pharmaceutical goals and the current flaws.
Acknowledgments
Funding is acknowledged from the European Research Council (ERC Advanced Grant #267867 Plasmaquo) and MINECO (project MAT2013-46101-R).
The authors declare no competing financial interest.
References
- Park K. (2015) Drug delivery of the future: Chasing the invisible gorilla. J. Controlled Release 240, 2–8. 10.1016/j.jconrel.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.; Moon J. J. (2015) Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines 3, 662–85. 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Montes A. B.; Langer J.; Henriksen-Lacey M.; Jimenez de Aberasturi D.; Solís D. M.; Taboada J. M.; Obelleiro F.; Sentosun K.; Bals S.; Bekdemir; et al. (2016) Gold nanostar-coated polystyrene beads as multifunctional nanoprobes for SERS bioimaging. J. Phys. Chem. C 120, 20860–20868. 10.1021/acs.jpcc.6b02282. [DOI] [Google Scholar]
- Bodelón G.; Montes-García V.; Fernández-López C.; Pastoriza-Santos I.; Pérez-Juste J.; Liz-Marzán L. M. (2015) Au@pNIPAM SERRS tags for multiplex immunophenotyping cellular receptors and imaging tumor cells. Small 11, 4149–4157. 10.1002/smll.201500269. [DOI] [PubMed] [Google Scholar]
- Gao X.; Cui Y.; Levenson R. M.; Chung L. W. K.; Nie S. (2004) In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976. 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- Qian X.; Peng X.-H.; Ansari D. O.; Yin-Goen Q.; Chen G. Z.; Shin D. M.; Yang L.; Young A. N.; Wang M. D.; Nie S. (2007) In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 26, 83–90. 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- García I.; Sánchez-Iglesias A.; Henriksen-Lacey M.; Grzelczak M.; Penadés S.; Liz-Marzán L. M. (2015) Glycans as biofunctional ligands for gold nanorods: stability and targeting in protein-rich media. J. Am. Chem. Soc. 137, 3686–3692. 10.1021/jacs.5b01001. [DOI] [PubMed] [Google Scholar]
- Nel A. E.; Mädler L.; Velegol D.; Xia T.; Hoek E. M. V; Somasundaran P.; Klaessig F.; Castranova V.; Thompson M. (2009) Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557. 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- del Pino P.; Yang F.; Pelaz B.; Zhang Q.; Kantner K.; Hartmann R.; Martinez de Baroja N.; Gallego M.; Möller M.; Manshian B. B.; et al. (2016) Basic physicochemical properties of polyethylene glycol coated gold nanoparticles that determine their interaction with cells. Angew. Chem., Int. Ed. 55, 5483–5487. 10.1002/anie.201511733. [DOI] [PubMed] [Google Scholar]
- Soenen S. J.; Parak W. J.; Rejman J.; Manshian B. (2015) Intra)cellular stability of inorganic nanoparticles: Effects on cytotoxicity, particle functionality, and biomedical applications. Chem. Rev. 115, 2109–2135. 10.1021/cr400714j. [DOI] [PubMed] [Google Scholar]
- Soenen S. J.; Manshian B. B.; Himmelreich U.; Demeester J.; Braeckmans K.; De Smedt S. C. (2014) The performance of gradient alloy quantum dots in cell labeling. Biomaterials 35, 7249–7258. 10.1016/j.biomaterials.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Chanana M.; Rivera-Gil P.; Correa-Duarte M. A.; Liz-Marzán L. M.; Parak W. J. (2013) Physicochemical properties of protein-coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion. Angew. Chem., Int. Ed. 52, 4179–4183. 10.1002/anie.201208019. [DOI] [PubMed] [Google Scholar]
- Arvizo R. R.; Giri K.; Moyano D.; Miranda O. R.; Madden B.; McCormick D. J.; Bhattacharya R.; Rotello V. M.; Kocher J.-P.; Mukherjee P. (2012) Identifying new therapeutic targets via modulation of protein corona formation by engineered nanoparticles. PLoS One 7, e33650. 10.1371/journal.pone.0033650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soenen S. J. H.; Illyes E.; Vercauteren D.; Braeckmans K.; Majer Z.; De Smedt S. C.; De Cuyper M. (2009) The role of nanoparticle concentration-dependent induction of cellular stress in the internalization of non-toxic cationic magnetoliposomes. Biomaterials 30, 6803–6813. 10.1016/j.biomaterials.2009.08.050. [DOI] [PubMed] [Google Scholar]
- Jana A.; Nguyen K. T.; Li X.; Zhu P.; Tan N. S.; Ågren H.; Zhao Y. (2014) Perylene-Derived Single-Component Organic Nanoparticles with Tunable Emission: Efficient Anticancer Drug Carriers with Real-Time Monitoring of Drug Release. ACS Nano 8, 5939–5952. 10.1021/nn501073x. [DOI] [PubMed] [Google Scholar]
- Gesquiere A. J.; Uwada T.; Asahi T.; Masuhara H.; Barbara P. F. (2005) Single Molecule Spectroscopy of Organic Dye Nanoparticles. Nano Lett. 5, 1321–1325. 10.1021/nl050567j. [DOI] [PubMed] [Google Scholar]
- Boisselier E.; Astruc D. (2009) Gold nanoparticles in nanomedicine: preparations{,} imaging{,} diagnostics{,} therapies and toxicity. Chem. Soc. Rev. 38, 1759–1782. 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- Colombo M.; Carregal-Romero S.; Casula M. F.; Gutierrez L.; Morales M. P.; Bohm I. B.; Heverhagen J. T.; Prosperi D.; Parak W. J. (2012) Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 41, 4306–4334. 10.1039/c2cs15337h. [DOI] [PubMed] [Google Scholar]
- Anselmo A. C.; Mitragotri S. (2015) A review of clinical translation of inorganic nanoparticles. AAPS J. 17, 1041–54. 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Amtenbrink M.; Grainger D. W.; Hofmann H. (2015) Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomedicine 11, 1689–1694. 10.1016/j.nano.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Feliu N.; Docter D.; Heine M.; Del Pino P.; Ashraf S.; Kolosnjaj-Tabi J.; Macchiarini P.; Nielsen P.; Alloyeau D.; Gazeau F.; et al. (2016) In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 45, 2440–57. 10.1039/C5CS00699F. [DOI] [PubMed] [Google Scholar]
- Mazuel F.; Espinosa A.; Luciani N.; Reffay M.; Le Borgne R.; Motte L.; Desboeufs K.; Michel A.; Pellegrino T.; Lalatonne Y.; et al. (2016) Massive Intracellular Biodegradation of Iron Oxide Nanoparticles Evidenced Magnetically at Single-Endosome and Tissue Levels. ACS Nano 10, 7627–7638. 10.1021/acsnano.6b02876. [DOI] [PubMed] [Google Scholar]
- Hirai T.; Yoshioka Y.; Izumi N.; Ichihashi K.; Handa T.; Nishijima N.; Uemura E.; Sagami K.; Takahashi H.; Yamaguchi M.; et al. (2016) Metal nanoparticles in the presence of lipopolysaccharides trigger the onset of metal allergy in mice. Nat. Nanotechnol. 11, 808–816. 10.1038/nnano.2016.88. [DOI] [PubMed] [Google Scholar]
- Urban D. A.; Rodriguez-Lorenzo L.; Balog S.; Kinnear C.; Rothen-Rutishauser B.; Petri-Fink A. (2016) Plasmonic nanoparticles and their characterization in physiological fluids. Colloids Surf., B 137, 39–49. 10.1016/j.colsurfb.2015.05.053. [DOI] [PubMed] [Google Scholar]
- Rivera-Gil P.; Jimenez De Aberasturi D.; Wulf V.; Pelaz B.; Del Pino P.; Zhao Y.; De La Fuente J. M.; Ruiz De Larramendi I.; Rojo T.; Liang X. J.; et al. (2013) The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Acc. Chem. Res. 46, 743–749. 10.1021/ar300039j. [DOI] [PubMed] [Google Scholar]
- França Á.; Pelaz B.; Moros M.; Sánchez-Espinel C.; Hernández A.; Fernández-López C.; Grazú V.; de la Fuente J. M.; Pastoriza-Santos I.; Liz-Marzán L. M.; et al. (2010) Sterilization matters: Consequences of different sterilization techniques on gold nanoparticles. Small 6, 89–95. 10.1002/smll.200901006. [DOI] [PubMed] [Google Scholar]
- Manshian B. B.; Soenen S. J.; Al-Ali A.; Brown A.; Hondow N.; Wills J.; Jenkins G. J. S.; Doak S. H. (2015) Cell type-dependent changes in cdse/ZnS quantum dot uptake and toxic endpoints. Toxicol. Sci. 144, 246–258. 10.1093/toxsci/kfv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Zou P.; Connarn J.; Paholak H.; Sun D. (2012) Intracellular dissociation of a polymer coating from nanoparticles. Nano Res. 5, 815–825. 10.1007/s12274-012-0265-7. [DOI] [Google Scholar]
- Kreyling W. G.; Abdelmonem A. M.; Ali Z.; Alves F.; Geiser M.; Haberl N.; Hartmann R.; Hirn S.; de Aberasturi D. J.; Kantner K.; et al. (2015) In vivo integrity of polymer-coated gold nanoparticles. Nat. Nanotechnol. 10, 619–623. 10.1038/nnano.2015.111. [DOI] [PubMed] [Google Scholar]
- Shang L.; Yang L.; Wang H.; Nienhaus G. U. (2016) In Situ monitoring of the intracellular stability of nanoparticles by using fluorescence lifetime imaging. Small 12, 868–873. 10.1002/smll.201503316. [DOI] [PubMed] [Google Scholar]
- Hou S.; Sikora K. N.; Tang R.; Liu Y.; Lee Y.-W.; Kim S. T.; Jiang Z.; Vachet R. W.; Rotello V. M. (2016) Quantitative differentiation of cell surface-bound and internalized cationic gold nanoparticles using mass spectrometry. ACS Nano 10, 6731–6736. 10.1021/acsnano.6b02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyuzin M. V.; Honold T.; Carregal-Romero S.; Kantner K.; Karg M.; Parak W. J. (2016) Influence of temperature on the colloidal stability of polymer-coated gold nanoparticles in cell culture media. Small 12, 1723–1731. 10.1002/smll.201503232. [DOI] [PubMed] [Google Scholar]
- Legin A. A.; Schintlmeister A.; Jakupec M. A.; Galanski M.; Lichtscheidl I.; Wagner M.; Keppler B. K. (2014) NanoSIMS combined with fluorescence microscopy as a tool for subcellular imaging of isotopically labeled platinum-based anticancer drugs. Chem. Sci. 5, 3135. 10.1039/c3sc53426j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetto M. T.; Anderton C. R.; Hu D.; Szymanski C. J.; Zhu Z.; Joseph P.; Kammeyer J.; Nilewski L. G.; Rush A. M.; Bell N. C.; et al. (2016) Cellular delivery of nanoparticles revealed with combined optical and isotopic nanoscopy. ACS Nano 10, 4046–4054. 10.1021/acsnano.5b06477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Xiong C.; Liu H.; Wan Q.; Hou J.; He Q.; Badu-Tawiah A.; Nie Z. (2015) Mass spectrometry imaging reveals the sub-organ distribution of carbon nanomaterials. Nat. Nanotechnol. 10, 176–182. 10.1038/nnano.2014.282. [DOI] [PubMed] [Google Scholar]
- Elci S. G.; Jiang Y.; Yan B.; Kim S. T.; Saha K.; Moyano D. F.; Yesilbag Tonga G.; Jackson L. C.; Rotello V. M.; Vachet R. W. (2016) Surface charge controls the sub-organ biodistributions of gold nanoparticles. ACS Nano 10, 5536–5542. 10.1021/acsnano.6b02086. [DOI] [PubMed] [Google Scholar]
- Fleischer C. C.; Payne C. K. (2014) Nanoparticle – cell interactions: molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 47, 2651–2659. 10.1021/ar500190q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Riviere N. A.; Inman A. O.; Zhang L. W. (2009) Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharmacol. 234, 222–235. 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Stepanenko A. A.; Dmitrenko V. V. (2015) Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 574, 193–203. 10.1016/j.gene.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Lupu A. R.; Popescu T. (2013) The noncellular reduction of MTT tetrazolium salt by TiO2 nanoparticles and its implications for cytotoxicity assays. Toxicol. In Vitro 27, 1445–1450. 10.1016/j.tiv.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Darolles C.; Sage N.; Armengaud J.; Malard V. (2013) In vitro assessment of cobalt oxide particle toxicity: Identifying and circumventing interference. Toxicol. In Vitro 27, 1699–1710. 10.1016/j.tiv.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Ong K. J.; MacCormack T. J.; Clark R. J.; Ede J. D.; Ortega V. a.; Felix L. C.; Dang M. K. M.; Ma G.; Fenniri H.; Veinot J. G. C. (2014) Widespread nanoparticle-assay interference: Implications for nanotoxicity testing. PLoS One 9, e90650. 10.1371/journal.pone.0090650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznan D.; Das D.; MacKinnon-Roy C.; Simard B.; Kumarathasan P.; Vincent R. (2015) Non-specific interaction of carbon nanotubes with the resazurin assay reagent: Impact on in vitro assessment of nanoparticle cytotoxicity. Toxicol. In Vitro 29, 142–147. 10.1016/j.tiv.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Nakashima N.; Sada T.; Fujigaya T. (2014) Manipulation of cell membrane using carbon nanotube scaffold as a photoresponsive stimuli generator. Sci. Technol. Adv. Mater. 15, 45002. 10.1088/1468-6996/15/4/045002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. J.; Kim H.; Liu Y.; Han H. K.; Kwon K.; Chang K. H.; Park K.; Kim Y.; Shim K.; An S. S. a; et al. (2014) Incompatibility of silver nanoparticles with lactate dehydrogenase leakage assay for cellular viability test is attributed to protein binding and reactive oxygen species generation. Toxicol. Lett. 225, 422–432. 10.1016/j.toxlet.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Tournebize J.; Boudier A.; Joubert O.; Eidi H.; Bartosz G.; Maincent P.; Leroy P.; Sapin-Minet A. (2012) Impact of gold nanoparticle coating on redox homeostasis. Int. J. Pharm. 438, 107–116. 10.1016/j.ijpharm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Tournebize J.; Sapin-Minet A.; Bartosz G.; Leroy P.; Boudier A. (2013) Pitfalls of assays devoted to evaluation of oxidative stress induced by inorganic nanoparticles. Talanta 116, 753–763. 10.1016/j.talanta.2013.07.077. [DOI] [PubMed] [Google Scholar]
- Waiskopf N.; Ben-Shahar Y.; Galchenko M.; Carmel I.; Moshitzky G.; Soreq H.; Banin U. (2016) Photocatalytic reactive oxygen species formation by semiconductor–metal hybrid nanoparticles. Toward light-induced modulation of biological processes. Nano Lett. 16, 4266–4273. 10.1021/acs.nanolett.6b01298. [DOI] [PubMed] [Google Scholar]
- Tee J. K.; Ong C. N.; Bay B. H.; Ho H. K.; Leong D. T. (2016) Oxidative stress by inorganic nanoparticles. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 8, 414–438. 10.1002/wnan.1374. [DOI] [PubMed] [Google Scholar]
- Soenen S. J.; Rivera-Gil P.; Montenegro J. M.; Parak W. J.; De Smedt S. C.; Braeckmans K. (2011) Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6, 446–465. 10.1016/j.nantod.2011.08.001. [DOI] [Google Scholar]
- Arvizo R. R.; Saha S.; Wang E.; Robertson J. D.; Bhattacharya R.; Mukherjee P. (2013) Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl. Acad. Sci. U. S. A. 110, 6700–6705. 10.1073/pnas.1214547110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández C.; González-Rubio G.; Langer J.; Tardajos G.; Liz-Marzán L. M.; Giraldo R.; Guerrero-Martínez A. (2016) Nucleation of amyloid oligomers by RepA-WH1-prionoid-functionalized gold nanorods. Angew. Chem., Int. Ed. 55, 11237–11241. 10.1002/anie.201604970. [DOI] [PubMed] [Google Scholar]
- Saghiri M. A.; Asatourian A.; Orangi J.; Sorenson C. M.; Sheibani N. (2015) Functional role of inorganic trace elements in angiogenesis-Part I: N, Fe, Se, P, Au, and Ca. Crit. Rev. Oncol. Hematol. 96, 129–142. 10.1016/j.critrevonc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Hussain S.; Garantziotis S.; Rodrigues-Lima F.; Dupret J.-M.; Baeza-Squiban A.; Boland S. (2014) Intracellular signal modulation by nanomaterials. Adv. Exp. Med. Biol. 811, 111–134. 10.1007/978-94-017-8739-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.; Wu J.; Sun J. (2012) Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol. Lett. 214, 91–98. 10.1016/j.toxlet.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Tung Y.-C.; Hsiao A. Y.; Allen S. G.; Torisawa Y.; Ho M.; Takayama S. (2011) High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 136, 473–8. 10.1039/C0AN00609B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.; Kim B.; Shin J.; Ryu S.; Noh M.; Woo J.; Park J.; Lee Y.; Lee N.; Hyeon T.; et al. (2015) Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 9, 2805–2819. 10.1021/nn506732n. [DOI] [PubMed] [Google Scholar]
- Joris F.; Manshian B. B.; Peynshaert K.; De Smedt S. C.; Braeckmans K.; Soenen S. J. (2013) Assessing nanoparticle toxicity in cell-based assays: influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap. Chem. Soc. Rev. 42, 8339–59. 10.1039/c3cs60145e. [DOI] [PubMed] [Google Scholar]
- Azhdarzadeh M.; Saei A. A.; Sharifi S.; Hajipour M. J.; Alkilany A. M.; Sharifzadeh M.; Ramazani F.; Laurent S.; Mashaghi A.; Mahmoudi M. (2015) Nanotoxicology: advances and pitfalls in research methodology. Nanomedicine (London, U. K.) 10, 2931–2952. 10.2217/nnm.15.130. [DOI] [PubMed] [Google Scholar]
- Canton I.; Battaglia G. (2012) Endocytosis at the nanoscale. Chem. Soc. Rev. 41, 2718–2739. 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- He Y.; Park K. (2016) Effects of the microparticle shape on cellular uptake. Mol. Pharmaceutics 13, 2164–2171. 10.1021/acs.molpharmaceut.5b00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hühn D.; Kantner K.; Geidel C.; Brandholt S.; De Cock I.; Soenen S. J. H.; Rivera-Gil P.; Montenegro J. M.; Braeckmans K.; Müllen K.; et al. (2013) Polymer-coated nanoparticles interacting with proteins and cells: Focusing on the sign of the net charge. ACS Nano 7, 3253–3263. 10.1021/nn3059295. [DOI] [PubMed] [Google Scholar]
- Saha K.; Rahimi M.; Yazdani M.; Kim S. T.; Moyano D.; Hou S.; Das R.; Mout R.; Rezaee F.; Mahmoudi M.; et al. (2016) Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona. ACS Nano 10, 4421–4430. 10.1021/acsnano.6b00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E. C.; Zhang Q.; Xia Y. (2011) The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 6, 385–391. 10.1038/nnano.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halamoda-Kenzaoui B.; Ceridono M.; Colpo P.; Valsesia A.; Urbán P.; Ojea-Jiménez I.; Gioria S.; Gilliland D.; Rossi F.; Kinsner-Ovaskainen A. (2015) Dispersion behaviour of silica nanoparticles in biological media and its influence on cellular uptake. PLoS One 10, e0141593. 10.1371/journal.pone.0141593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monopoli M. P.; Walczyk D.; Campbell A.; Elia G.; Lynch I.; Baldelli Bombelli F.; Dawson K. A. (2011) Physical–chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 133, 2525–2534. 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- Gref R.; Lück M.; Quellec P.; Marchand M.; Dellacherie E.; Harnisch S.; Blunk T.; Müller R. H. (2000) Stealth” corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf., B 18, 301–313. 10.1016/S0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- Pelaz B.; Del Pino P.; Maffre P.; Hartmann R.; Gallego M.; Rivera-Fernández S.; De la Fuente J. M.; Nienhaus G. U.; Parak W. J. (2015) Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano 9, 6996–7008. 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- Agarwal R.; Singh V.; Jurney P.; Shi L.; Sreenivasan S. V.; Roy K. (2013) Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc. Natl. Acad. Sci. U. S. A. 110, 17247–17252. 10.1073/pnas.1305000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Volsi A.; Jimenez de Aberasturi D.; Henriksen-Lacey M.; Giammona G.; Licciardi M.; Liz-Marzán L. M. (2016) Inulin coated plasmonic gold nanoparticles as a tumor-selective tool for cancer therapy. J. Mater. Chem. B 4, 1150–1155. 10.1039/C5TB01810B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Butler E. B.; Tan M. (2013) Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 4, e532. 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann L.; Erbes T.; Halbach S.; Brummer T.; Jäger M.; Hirschfeld M.; Fehm T.; Neubauer H.; Stickeler E.; Kammerer B. (2015) Exometabolom analysis of breast cancer cell lines: Metabolic signature. Sci. Rep. 5, 13374. 10.1038/srep13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Lin W.; Pei Q.; Hu X.; Xie Z.; Jing X. (2016) Redox-hypersensitive organic nanoparticles for selective treatment of cancer cells. Chem. Mater. 28, 4440–4446. 10.1021/acs.chemmater.6b01641. [DOI] [Google Scholar]
- Hu X.-Y.; Liu X.; Zhang W.; Qin S.; Yao C.; Li Y.; Cao D.; Peng L.; Wang L. (2016) Controllable construction of biocompatible supramolecular micelles and vesicles by water-soluble phosphate pillar[5,6]arenes for selective anti-cancer drug delivery. Chem. Mater. 28, 3778–3788. 10.1021/acs.chemmater.6b00691. [DOI] [Google Scholar]
- Barua S.; Mitragotri S. (2014) Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 9, 223–243. 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. (2010) Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjugate Chem. 21, 797–802. 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- Setyawati M. I.; Mochalin V. N.; Leong D. T. (2016) Tuning endothelial permeability with functionalized nanodiamonds. ACS Nano 10, 1170–1181. 10.1021/acsnano.5b06487. [DOI] [PubMed] [Google Scholar]
- Espinosa A.; Silva A. K. A.; Sánchez-Iglesias A.; Grzelczak M.; Péchoux C.; Desboeufs K.; Liz-Marzán L. M.; Wilhelm C. (2016) Cancer cell internalization of gold nanostars impacts their photothermal efficiency in vitro and in vivo: Toward a plasmonic thermal fingerprint in tumoral environment. Adv. Healthcare Mater. 5, 1040–1048. 10.1002/adhm.201501035. [DOI] [PubMed] [Google Scholar]
- Comenge J.; Fragueiro O.; Sharkey J.; Taylor A.; Held M.; Burton N. C.; Park B. K.; Wilm B.; Murray P.; Brust M.; et al. (2016) Preventing plasmon coupling between gold nanorods improves the sensitivity of photoacoustic detection of labeled stem cells in vivo. ACS Nano 10, 7106–7116. 10.1021/acsnano.6b03246. [DOI] [PubMed] [Google Scholar]
- Jimenez de Aberasturi D.; Serrano-Montes A.; Langer J.; Henriksen-Lacey M.; Parak W.; Liz-Marzán L. (2016) (28AD) Surface enhanced raman scattering encoded gold nanostars for multiplexed cell discrimination. Chem. Mater. 28, 6779–6790. 10.1021/acs.chemmater.6b03349. [DOI] [Google Scholar]
- Sykes E. A.; Chen J.; Zheng G.; Chan W. C. W. (2014) Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano 8, 5696–5706. 10.1021/nn500299p. [DOI] [PubMed] [Google Scholar]
- Goodman T. T.; Ng C. P.; Pun S. H. (2008) 3-D tissue culture systems for the evaluation and optimization of nanoparticle-based drug carriers. Bioconjugate Chem. 19, 1951–9. 10.1021/bc800233a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampaloni F.; Reynaud E. G.; Stelzer E. H. K. (2007) The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8, 839–845. 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- Lee D.; Weitz D. A. (2009) Nonspherical colloidosomes with multiple compartments from double emulsions. Small 5, 1932–1935. 10.1002/smll.200900357. [DOI] [PubMed] [Google Scholar]
- Child H. W.; Del Pino P. a.; De La Fuente J. M.; Hursthouse A. S.; Stirling D.; Mullen M.; McPhee G. M.; Nixon C.; Jayawarna V.; Berry C. C. (2011) Working together: the combined application of a magnetic field and penetratin for the delivery of magnetic nanoparticles to cells in 3D. ACS Nano 5, 7910–7919. 10.1021/nn202163v. [DOI] [PubMed] [Google Scholar]
- Xu X.; Sabanayagam C. R.; Harrington D. a.; Farach-Carson M. C.; Jia X. (2014) A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics. Biomaterials 35, 3319–3330. 10.1016/j.biomaterials.2013.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald C.; Friedman G.; Alamia J.; Barbee K.; Polyak B. (2010) Time-varied magnetic field enhances transport of magnetic nanoparticles in viscous gel. Nanomedicine (London, U. K.) 5, 65–76. 10.2217/nnm.09.97. [DOI] [PubMed] [Google Scholar]
- Ho D. N.; Kohler N.; Sigdel A.; Kalluri R.; Morgan J. R.; Xu C.; Sun S. (2012) Penetration of endothelial cell coated multicellular tumor spheroids by iron oxide nanoparticles. Theranostics 2, 66–75. 10.7150/thno.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.; Kievit F. M.; Florczyk S. J.; Stephen Z. R.; Zhang M. (2015) 3D porous chitosan-alginate scaffolds as an in vitro model for evaluating nanoparticle-mediated tumor targeting and gene delivery to prostate cancer. Biomacromolecules 16, 3362–3372. 10.1021/acs.biomac.5b01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy M.; Lavrentieva A.; Walter J.; Sambale F.; Green M.; Stahl F.; Scheper T. (2016) Evaluation of CdTe/CdS/ZnS core/shell/shell quantum dot toxicity on three-dimensional spheroid cultures. Toxicol. Res. 5, 126–135. 10.1039/C5TX00236B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann A.; Zwierzina M.; Gamerith G.; Bitsche M.; Huber J. M.; Vogel G. F.; Blumer M.; Koeck S.; Pechriggl E. J.; Kelm J. M.; et al. (2014) Development of an innovative 3D cell culture system to study tumour - Stroma interactions in non-small cell lung cancer cells. PLoS One 9, e92511. 10.1371/journal.pone.0092511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermanizadeh A.; L hr M.; Roursgaard M.; Messner S.; Gunness P.; Kelm J. M.; Møller P.; Stone V.; Loft S. (2014) Hepatic toxicology following single and multiple exposure of engineered nanomaterials utilising a novel primary human 3D liver microtissue model. Part. Fibre Toxicol. 11, 1–15. 10.1186/s12989-014-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.; Ma H.; Liu J.; Huo S.; Kumar A.; Wei T.; Zhang X.; Jin S.; Gan Y.; Wang P. C.; et al. (2012) Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 6, 4483–4493. 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Li J.; Li X.; Mu H.; Zhang X.; Shi Y.; Chu Y.; Wang A.; Wu Z.; Sun K. (2016) Magnetically and pH dual responsive dendrosomes for tumor accumulation enhanced folate-targeted hybrid drug delivery. J. Controlled Release 232, 161–174. 10.1016/j.jconrel.2016.04.015. [DOI] [PubMed] [Google Scholar]
- InSphero. 3D InSight Products http://www.insphero.com/products-services.
- Wu J.; Izpisua Belmonte J. C. (2016) Stem cells: A renaissance in human biology research. Cell 165, 1572–1585. 10.1016/j.cell.2016.05.043. [DOI] [PubMed] [Google Scholar]
- Sato T.; Vries R. G.; Snippert H. J.; van de Wetering M.; Barker N.; Stange D. E.; van Es J. H.; Abo A.; Kujala P.; Peters P. J.; et al. (2009) Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Grabinger T.; Luks L.; Kostadinova F.; Zimberlin C.; Medema J. P.; Leist M.; Brunner T. (2014) Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 5, e1228. 10.1038/cddis.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinaris C.; Brizi V.; Remuzzi G. (2015) Organoid models and applications in biomedical research. Nephron 130, 191–9. 10.1159/000433566. [DOI] [PubMed] [Google Scholar]
- Fatehullah A.; Tan S. H.; Barker N. (2016) Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–54. 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- Broutier L.; Andersson-Rolf A.; Hindley C. J.; Boj S. F.; Clevers H.; Koo B.-K.; Huch M. (2016) Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743. 10.1038/nprot.2016.097. [DOI] [PubMed] [Google Scholar]
- Takasato M.; Er P. X.; Chiu H. S.; Little M. H. (2016) Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 11, 1681–1692. 10.1038/nprot.2016.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astashkina A. I.; Jones C. F.; Thiagarajan G.; Kurtzeborn K.; Ghandehari H.; Brooks B. D.; Grainger D. W. (2014) Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. Biomaterials 35, 6323–6331. 10.1016/j.biomaterials.2014.04.060. [DOI] [PubMed] [Google Scholar]
- Parvani J. G.; Gujrati M. D.; Mack M. A.; Schiemann W. P.; Lu Z.-R. (2015) Silencing β3 integrin by targeted ECO/siRNA nanoparticles inhibits EMT and metastasis of triple-negative breast cancer. Cancer Res. 75, 2316–2325. 10.1158/0008-5472.CAN-14-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astashkina A.; Grainger D. W. (2014) Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv. Drug Delivery Rev. 69–70, 1–18. 10.1016/j.addr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Horman S. R., To J., Orth A. P., Slawny N., Cuddihy M. J., and Caracino D. (2013) High-content analysis of three-dimensional tumor spheroids: Investigating signaling pathways using small hairpin RNA. Nat. Methods 10. [Google Scholar]
- Kang T.; Park C.; Choi J.-S.; Cui J.-H.; Lee B.-J. (2016) Effects of shear stress on the cellular distribution of polystyrene nanoparticles in a biomimetic microfluidic system. J. Drug Delivery Sci. Technol. 31, 130–136. 10.1016/j.jddst.2015.12.001. [DOI] [Google Scholar]
- Valencia P. M.; Farokhzad O. C.; Karnik R.; Langer R. (2012) Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 7, 623–629. 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkanson M.; Cukierman E.; Charnley M. (2014) Miniaturized pre-clinical cancer models as research and diagnostic tools. Adv. Drug Delivery Rev. 69–70, 52–66. 10.1016/j.addr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C. P.; Pun S. H. (2008) A perfusable 3D cell-matrix tissue culture chamber for in situ evaluation of nanoparticle vehicle penetration and transport. Biotechnol. Bioeng. 99, 1490–1501. 10.1002/bit.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervantonakis I. K.; Arvanitis C. D. (2016) Controlled drug release and chemotherapy response in a novel acoustofluidic 3D tumor platform. Small 12, 2616–2626. 10.1002/smll.201503342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A.; Lam A. K.; Sykes E. A.; Rocheleau J. V.; Chan W. C. W. (2013) Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat. Commun. 4, 2718. 10.1038/ncomms3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer M. J.; Ng C. P.; Lanz H. L.; Vulto P.; Suter-Dick L.; Masereeuw R. (2016) Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 34, 156–170. 10.1016/j.tibtech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Bhatia S. N.; Ingber D. E. (2014) Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772. 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- Lynch I.; Ahluwalia A.; Boraschi D.; Byrne H. J.; Fadeel B.; Gehr P.; Gutleb A. C.; Kendall M.; Papadopoulos M. G. (2013) The bio-nano-interface in predicting nanoparticle fate and behaviour in living organisms: towards grouping and categorising nanomaterials and ensuring nanosafety by design. BioNanoMaterials 14, 195–216. 10.1515/bnm-2013-0011. [DOI] [Google Scholar]
- Dobay M. P. D., Alberola A. P., Mendoza E. R., and Rädler J. O. (2012) Modeling nanoparticle uptake and intracellular distribution using stochastic process algebras. J. Nanopart. Res. 14, DOI: 10.1007/s11051-012-0821-9 [DOI] [Google Scholar]
- Siepmann J. (2013) In-silico simulations of advanced drug delivery systems: What will the future offer?. Int. J. Pharm. 454, 512–516. 10.1016/j.ijpharm.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Hauert S.; Bhatia S. N. (2014) Mechanisms of cooperation in cancer nanomedicine: Towards systems nanotechnology. Trends Biotechnol. 32, 448–455. 10.1016/j.tibtech.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullstone G.; Wood J.; Holcombe M.; Battaglia G. (2015) Modelling the transport of nanoparticles under blood flow using an agent-based approach. Sci. Rep. 5, 10649. 10.1038/srep10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.; Shah S.; Thomas A.; Ou-Yang H. D.; Liu Y. (2013) The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid. Nanofluid. 14, 77–87. 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachau R. E.; Gonzalez-Nilo F. D.; Ventura O. N.; Fritts M. J. (2007) In-silico nanobio-design. A new frontier in computational biology. Curr. Top. Med. Chem. 7, 1537–1540. 10.2174/156802607782194680. [DOI] [PubMed] [Google Scholar]
- Henriksen-Lacey M.; Giner-Casares J. J. (2015) Nice to know you. Science 349, 1254. 10.1126/science.349.6253.1254. [DOI] [PubMed] [Google Scholar]