Abstract
STAT5 plays a critical role in the development and function of many cell types. Here, we review the role of STAT5 in the development of T lymphocytes in the thymus and its subsequent role in the differentiation of distinct CD4 + helper and regulatory T-cell subsets.
Keywords: STAT5, T-cell development, T-cell function
Introduction
The transcription factor STAT5 is expressed in all lymphocytes and plays a key role in multiple aspects of lymphocyte development and function. STAT5 is a modular transcription factor that consists of an N-terminal domain that allows for homotypic interactions and tetramerization 1, a DNA binding domain, an SH2 domain involved in recruitment to phosphorylated receptors and ultimately homodimerization, and a C-terminal transactivation domain 2. STAT5 was initially identified as a transcription factor activated by prolactin in mammary gland epithelial cells 3, 4. Subsequent studies identified STAT5 binding activity in T cells 5, and it was later established that STAT5 was expressed in multiple cell types and activated by a number of cytokines, including the common gamma chain (γc)-dependent cytokines interleukin 2 (IL2), IL4, IL7, IL13, and IL15 6 as well as a number of γc-independent cytokines, including thymic stromal lymphopoietin (TSLP), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL27 7– 11. Molecular characterization of the Stat5 gene demonstrated that Stat5 was encoded by two closely linked genes that encoded STAT5a and STAT5b 12– 14. These two genes are likely the result of gene duplication and are highly homologous. Initial studies showed that STAT5a and STAT5b bound to a similar DNA core motif, although there were subtle differences in their DNA binding preferences 15. Subsequent chromatin immunoprecipitation followed by massively parallel DNA sequencing (chromatin immunoprecipitation sequencing [ChIP-Seq]) studies suggest that there may be differences in the subsets of genes bound by STAT5A and STAT5B 16, 17. However, these two transcription factors appear to be functionally redundant if expressed at similar levels 18. Substantial work has focused on the role of STAT5 in both lymphocyte development and function. These studies have clearly established a critical role for STAT5 in early T-cell development and pointed to critical functions for STAT5 in distinct T-cell subsets. Here, we will briefly review the role of STAT5 in T-cell development and then focus on advances in our understanding of the role that STAT5 plays in the differentiation of distinct T-cell subsets.
STAT5 in T-cell development
The observation that STAT5 is activated by multiple cytokines in T cells suggested that it might play a critical role in the development or function (or both) of these cells. Disruption of Stat5a or Stat5b genes alone resulted in relatively modest phenotypes; for example, Stat5a -/- mice had defects in mammary gland development and lactation while Stat5b -/- mice had defects in response to growth hormone in male mice and natural killer cell proliferation 19, 20. To determine whether combined deletion of Stat5a and Stat5b might result in more profound immunodeficiencies, subsequent studies deleted the first coding exons of both Stat5a and Stat5b. This intervention resulted in the production of truncated forms of STAT5a and STAT5b that acted as functional hypomorphs. These mice too had surprisingly mild defects in lymphocyte development, although T cells were grossly dysfunctional, as they could no longer proliferate in response to IL2 21, 22. Subsequent studies using mice expressing a constitutively active form of STAT5b suggested that STAT5 might play a more critical role in lymphocytes than suggested by the studies of STAT5 hypomorphs. These mice exhibited significant expansion of progenitor B cells, CD8 + memory T cells, and CD25 + regulatory T (Treg) cells 23. Finally, complete deletion of Stat5a and Stat5b using Cre-LoxP approaches demonstrated that STAT5a and STAT5b are absolutely required for lymphocyte development, as Stat5a/b -/- mice had profound blocks in lymphocyte development, which mimicked that observed in Il7r -/- mice 24, 25. These studies definitively demonstrated that the STAT5 hypomorph mice retained significant STAT5 function. Studies with STAT5 knockout mice demonstrated that STAT5 plays a critical role in the development of γδ T cells, as it regulates T-cell receptor (TCR) γ gene rearrangement 26, 27. Likewise, STAT5 is required for expansion of double-negative thymocytes 25. Finally, IL7R/STAT5 signaling plays an important role in CD8 versus CD4 lineage choice, and increased STAT5 signaling promotes CD8 T-cell differentiation 28. The mechanism by which STAT5 regulates early B- and T-cell development is still somewhat unclear, but there is clearly a key role for STAT5 in driving the expression of the pro-survival gene Mcl1 29. In addition, STAT5 promotes CD8 differentiation by upregulating the transcription factor Runx3 28. Additional work is required to obtain a more complete understanding of the molecular mechanisms by which STAT5 entrains lymphocyte development.
STAT5 promotes development of specific T-cell subsets
The availability of both STAT5 gain-of-function and complete loss-of-function mice allowed for a more refined examination of the role of STAT5 in various T-cell subsets. STAT5 was found to play an important role in the development of T helper type 1 (TH1), TH2, TH9, T helper type GM-CSF (TH GM), and Treg cell subsets.
T helper type 1
TH1 polarization is driven by IL12 signaling and T-bet expression leading to production of TH1 cytokines, such as interferon gamma (IFNγ). Naïve T cells, however, do not express the IL12 receptor β2 subunit (IL12Rβ2) and thus are unable to respond to IL12. Early studies observed that T cells deficient in JAK3, the kinase required for STAT5 activation downstream of γc-containing receptors, failed to produce IFNγ under TH1 polarizing conditions 30. Furthermore, this study observed that IL2 blockade inhibited TH1 differentiation. Subsequent studies revealed that IL2 signaling, via STAT5 activation, potentiates the TH1 fate by inducing IL12Rβ2 and T-bet expression, thereby allowing the cell to respond to IL12 and polarize toward the TH1 fate 31.
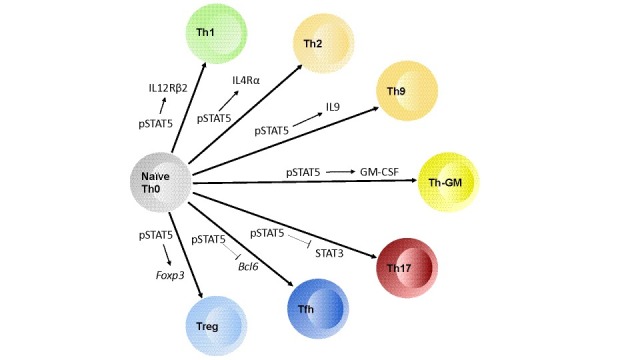
Figure 1. Model outlining how STAT5 activation (pSTAT5) contributes to the differentiation of naïve CD4 T cells into various T helper (TH) subsets.
In TH1 development, STAT5 drives interleukin 12 receptor beta 2 subunit (IL12Rβ2) expression. For TH2, STAT5 drives upregulation of IL4Rα. For TH9, STAT5 activation is required for IL9 production. In T helper type granulocyte-macrophage colony-stimulating factor (TH GM), STAT5 is critical for granulocyte-macrophage colony-stimulating factor (GM-CSF) production. STAT5 opposes the activation of STAT3, which is required for TH17 differentiation. STAT5 downregulates Bcl6 expression to inhibit T follicular helper (TFH) cell differentiation, and in regulatory T cells STAT5 turns on Foxp3 as well as CD25.
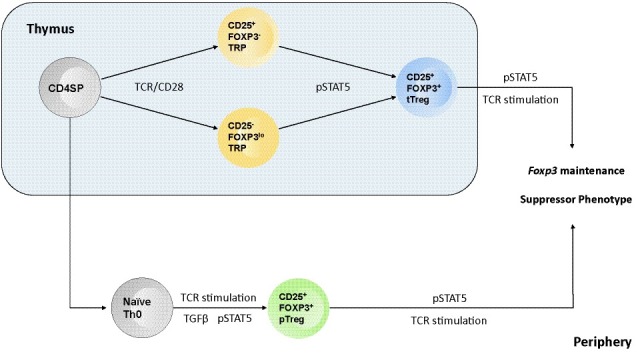
Figure 2. Model outlining the roles STAT5 plays in T regulatory biology in both the thymus and the periphery.
STAT5 activation (pSTAT5) is required to complete the differentiation of thymic regulatory T (tTreg) cells and initiate the differentiation of peripherally induced regulatory T (pTreg) cells. STAT5 is also critical for the maintenance of Foxp3 expression, via binding the Cns2 regulatory region in Foxp3, and the suppressor phenotype of regulatory T cells.
T helper type 2
Similar paradigms have been observed with respect to TH2 polarization, which requires IL4 signaling and GATA3 expression. Early studies hinted at a role for STAT5 in TH2 development as T-cell production of IL4 was diminished without IL2 32, 33. Subsequent studies demonstrated that STAT5 binds to the Il4 locus and drives IL4 production independently of GATA3; however, GATA3 expression is still critical for the adoption of the TH2 fate 34– 37. It was later revealed that STAT5 mediates TCR-induced IL4 receptor alpha (IL4Rα) expression and this role was critical for TH2 induction 38. This latter study suggested that STAT5 was induced by IL2 in differentiating TH2 cells. Additional studies have shown that TSLP-dependent activation of STAT5 can also contribute to proliferation, survival, and function of TH2 cells 39. In a more recent study, another unique role of STAT5 was observed in TH2 polarization. This study indicated that STAT5 activation drove expression of NLRP3, a component of the inflammasome, in T cells. Moreover, this expression of NLRP3 was required for efficient TH2 polarization, an effect that was due to the ability of NLRP3 to form a complex with IRF4, which in turn induced the expression of TH2 cytokines such as IL4, IL5, and IL13 40. Unlike STAT5 deficiency, however, NLRP3 deficiency did not reduce IL4Rα expression. These studies have illustrated that STAT5 plays a unique role in TH2 development and function.
T helper type 9
TH9 T cells, a subset closely related to the TH2 lineage, differentiate in the presence of transforming growth factor beta (TGFβ) and IL4 and are defined by prominent IL9 production. Initially, it was observed that the presence of IL4 inhibits TGFβ-driven induction of FOXP3 via a STAT6/GATA3-dependent mechanism 41, 42. This initial study found that instead of generating suppressive induced Treg cells, the combination of TGFβ and IL4 formed effector cells that produced IL9 and IL10, and thus resembled TH9 T cells. Thus, much like in TH2 cell differentiation, STAT5 plays a key role in TH9 development and function. The idea that STAT5 plays an important role in TH9 development is supported by the fact that TSLP/STAT5 induces IL9 production, which was required for allergic airway inflammation induced by TSLP 43. Consistent with this idea, two recent studies demonstrated that activated STAT5 binds to the Il9 promoter and facilitates Il9 transcription by driving an activated chromatin configuration characterized by reduced H3K9 histone methylation 44, 45. This effect was reversed by IL21-driven induction of BCL6, which also interacts at adjacent locations in the Il9 promoter. Subsequent studies demonstrated that IL6-mediated activation of STAT3 opposes STAT5-driven differentiation of TH9 cells; however, this effect was mediated by inhibition of STAT5 activation through diminished IL2 production and not via induction of BCL6 46. Thus, STAT5 activation and pathways that intersect with STAT5 signaling play important roles in TH9 differentiation.
Whereas the precise mechanisms by which STAT5 contributes to specific T helper subset differentiation are unique, the general mode by which STAT5 acts is very similar. Namely, STAT5 functions to prime T cells such that they are competent to respond to the cytokine milieu and differentiate into a particular T helper subset. This suggests a model whereby appropriately activated T cells, receiving TCR and co-stimulation, upregulate IL2 production and via autocrine signaling activate STAT5. Activated STAT5 then induces the expression of polarizing cytokine receptor genes, such as IL12Rβ2 and IL4Rα, allowing these cells to integrate the local cytokines into an appropriate differentiation decision. A similar mechanism may hold for Treg cell differentiation, as STAT5 can upregulate CD25 expression 47, which is required for efficient Treg cell differentiation. Furthermore, STAT5 acts in all of these T-cell subsets to drive the expression of T helper subset cytokines. Thus, STAT5 activation plays a crucial role in the differentiation and function of TH1, TH2, and TH9 subsets.
T helper type GM-CSF
Recently, another unique T helper subset which produces GM-CSF and IL3 was observed: the TH GM subset. A 2014 study observed that TH GM cells were critical mediators of disease progression in a murine model of autoimmune neuroinflammation: experimental autoimmune encephalomyelitis 48. This article observed that IL7-driven, not IL2-driven, STAT5 activation is required for the formation of these GM-CSF-producing pathogenic T cells. The authors also provide evidence that TH1 and TH17 differentiation cues are inhibitory to the development of TH GM, similar to findings in a human study which observed that IL17 antagonistically regulated GM-CSF-producing T cells that also trafficked to the central nervous system of patients with multiple sclerosis 49. Furthermore, the study by Sheng et al. showed that the TH GM cells are a unique T helper subset, as their expression profile is distinct from those of both TH1 and TH17 cells 48. Interestingly, another study observed that IL2Rα polymorphisms associated with multiple sclerosis potentiated IL2-mediated GM-CSF production in TH cells; however, the production of IFNγ and IL17 was unaffected 50. Thus, STAT5 activation has an important role in the development of TH GM cells and may contribute to their pathogenicity in neuroinflammation.
Regulatory T cells
STAT5 plays a central role in the development and function of Treg cells. Early studies identified CD25, the high-affinity IL2Rα chain, as an accurate marker for suppressor T cells 51. Subsequent studies observed that mice deficient in CD122, the IL2Rβ chain, developed autoimmune disease because they were devoid of functional Treg cells 52. Similar results were observed in mice lacking CD25, the IL2Rα chain 53, 54, and in human patients with loss-of-function mutations in STAT5b 55. These observations suggested that STAT5 activation, downstream of IL2 receptor signaling, was important for Treg cell development or function or both.
STAT5 in the development of regulatory T cells
Successive studies on the differentiation of thymus-derived Treg (tTreg) cells built on the observation that Il2rb -/- mice failed to develop Treg cells. Initial studies demonstrated that STAT5 was the critical downstream effector in IL2Rβ signaling that drove tTreg cell development and that this was due in part to direct targeting of STAT5 to the Foxp3 promoter 56– 58. Follow-up studies proposed a two-step model for tTreg cell development. In step one, high-affinity TCR stimulation drove the expression of CD25 and the development of a CD25 +FOXP3 - Treg progenitor cell. In the second step, Treg progenitor cells competed for a limiting amount of thymic IL2; progenitor cells that competed effectively for IL2 and activated STAT5 then converted into mature Treg cells 59, 60. Consistent with this model, constitutive STAT5 activation was sufficient to drive a subset of conventional thymocytes into the Treg cell lineage 60. A more recent study observed that the degree of TCR stimulation a thymocyte received correlated with the expression of the TNFRSF members GITR, OX40, and TNFR2 61. Upregulation of these TNFRSF members facilitated tTreg cell development by sensitizing thymocytes to IL2 stimulation. Those thymocytes with the highest expression of GITR, OX40, and TNFR2 responded to much lower doses of IL2 by activating STAT5 and thus initiating the final step in Treg cell differentiation. Other recent studies have continued to point to the modulation of STAT5 as a critical factor in tTreg cell differentiation. For example, TRAF3 activation dampens tTreg cell development via inhibition of STAT5 activation 62. It is not yet clear what regulates TRAF3 in Treg progenitor cells, but one possibility is that GITR/OX40/TNFR2 are involved in direct degradation of TRAF3 and thereby promote increased IL2 sensitivity. Likewise, thymocytes deficient in IFNAR fail to readily develop into tTreg cells. This effect was due to IFNα/IFNβ enhancement of STAT5 activation, either directly or indirectly 63. Thus, multiple pathways all impinge on STAT5 in developing Treg cells to regulate the number of Treg cells generated in the thymus.
A distinct type of tTreg progenitor cell population was also recently proposed. These progenitor cells express low levels of FOXP3 but no detectable CD25 (CD4 +CD25 -FOXP3 lo). However, differentiation of this population into mature tTreg cells was still dependent on IL2/STAT5 activation 64. A subsequent study suggested that the formation of CD25 -FOXP3 lo tTreg progenitor cells was more dependent on IL15 than the parallel CD25 +FOXP3 - tTreg progenitor subset 65. Future studies will need to extend these observations and determine whether there are distinct roles for IL2 and IL15 in tTreg progenitor cell formation and determine whether these effects are also dependent on STAT5 activation.
In addition to tTreg cells, there is another well-accepted class of Treg cells that differentiate from naïve CD4 + T cells outside the thymus (peripheral Treg, or pTreg, cells). Multiple studies have established that this class of Treg cells is important for maintaining complete tolerance, particularly at mucosal sites interacting with commensal microbes 66, 67. Conversion of naïve T cells to pTreg cells is driven by TGFβ ligation; however, this conversion is also dependent on STAT5 activation via IL2 signaling 68. IL2/STAT5-dependent signals are required not only for the conversion of naïve T cells into pTreg cells in vitro but also to generate these cells in vivo 69. Further studies indicated that without IL2 the stability of TGFβ-induced Treg cells was greatly diminished 70. A more recent study has provided some mechanistic details on the convergence of TGFβ and STAT5 in controlling pTreg cell differentiation. Specifically, hydrogen sulfide is required to activate TET1 and TET2 demethylases and maintain Treg cell homeostasis 71. Furthermore, these authors observed that activated SMAD3, downstream of TGFβ signaling, and STAT5, downstream of the IL2 receptor, targeted TET1 and TET2 to the Foxp3 locus and initiated a hypomethylated state, which facilitated stable expression of Foxp3 in Treg cells.
STAT5 in regulatory T-cell function
In addition to its role in the differentiation of both tTreg and pTreg cells, a critical role for STAT5 has been observed in Treg cell maintenance and function. For example, Blazar and colleagues demonstrated that in the context of graft-versus-host disease, Treg cells expressing a constitutively active Stat5b transgene provide better protection than wild-type Treg cells 72. One proposed mechanism by which STAT5 enhances Treg cell functionality is via binding sites within the Foxp3 gene locus, functioning to stabilize expression of Foxp3 and thus the suppressor phenotype. More recent studies have provided support for such a function. Specifically, the Cns2 enhancer region, which binds several transcription factors, including STAT5, was shown to be required for the maintenance of Foxp3 expression 73. This study further demonstrated that STAT5 binding to Cns2 enhanced the stability of Treg cells within inflammatory contexts. A subsequent study provided additional evidence that STAT5 plays a central role in maintaining Treg cell homeostasis. First, using histocytometric analysis of whole lymph nodes, the authors observed that Treg cells which contain activated STAT5 are clustered around IL2-producing effector cells that are being stimulated by self-antigen 74. Taking this observation further, the authors demonstrated that Treg cells are unable to properly restrain IL2-deficient effector cells and that the IL2-deficient effector T cells had longer interaction times with dendritic cells. This study also provided data that this suppressive function was dependent on TCR signaling in the Treg cells, a conclusion that was supported by a study by Rudensky and colleagues 75. To further understand the role of STAT5 activation in mature Treg cells, another study used Foxp3-Cre to drive deletion of the IL2Rβ chain in mature Treg cells. Those experiments largely recapitulated the severe autoimmunity observed in Il2rb -/- mice 76, 77. To understand whether this effect was due to an inability to activate STAT5 or another pathway downstream of the IL2 receptor, the authors generated mice in which a Stat5b-CA transgene was integrated into the ROSA26 locus preceded by a loxP flanked Stop cassette. Importantly, the Rosa26-Stat5b-CA transgene was able to rescue the autoimmune symptoms observed in Foxp3-Cre x Il2rb FL/FL mice. Similar to the report by Blazar and colleagues 72, this latter study also observed that Treg cells expressing STAT5b-CA were more potent suppressor cells 76. Interestingly, RNA-Seq studies comparing wild-type and STAT5b-CA-expressing Treg cells revealed that the STAT5b-CA gene signature was unique and not simply an enhancement of the baseline Treg gene profile. Thus, STAT5 plays a multifunctional role in Treg cell biology. Initially, STAT5 acts as a central effector in initiating the differentiation of Treg cells but, in mature Treg cells, drives their suppressive capabilities and maintains FOXP3 expression. Thus, STAT5 acts as a bridge between effector and suppressor responses, via integration with TCR signaling, to prevent effector responses toward self-antigens while permitting responses to non-self-antigens.
STAT5 inhibits the development of other T helper subsets
Although STAT5 is required for the development and function of some T helper subsets, it also plays an important role in blocking the development of other T helper subsets, most notably TH17 and T follicular helper (T FH) cells. TH17 cells can be generated by stimulation with cytokines that activate STAT3, consistent with a role for STAT3 in TH17 generation 78– 80. Subsequent studies demonstrated that the inflammation observed in IL2-deficient mice stemmed from not only a lack of Treg cells but also the fact that IL2 and STAT5 signaling was no longer able to counter the development of inflammatory TH17 cells 81. ChIP-Seq studies of STAT3 and STAT5 in CD4 + T cells showed that these two transcription factors bound to identical sites within the Il17 gene locus and exerted opposite effects on gene transcription 82. Other studies demonstrated that IL2/STAT5 signaling can also affect TH17 development by downregulating expression of the IL6R, which is required to activate STAT3 83. Although the molecular mechanisms by which STAT5 repressed Il17 transcription have not been completely defined, it appeared that at least three mechanisms could exist. First, STAT5 directly competed with STAT3 for DNA binding and thereby prevented STAT3 from directly inducing Il17 transcription. Second, STAT5 binding also correlated with binding of the co-repressor NCOR2 and thus might actively repress gene transcription by altering histone methylation or acetylation 82. Third, STAT5 repressed expression of the IL6R, leading to reduced activation of STAT3 83. Further studies are needed to clarify the mechanisms by which STAT5 represses or prevents gene transcription.
In addition to suppressing TH17 differentiation, STAT5 inhibits the development of T FH cells. T FH cells require STAT3-inducing cytokines, such as IL21 and IL6, for their differentiation 84. In contrast, the STAT5-inducing cytokine IL2 was initially shown to inhibit T FH cell development 85– 87. Moreover, these studies demonstrated that the effect of IL2 required STAT5 activation 18, 86, 87. This effect appears to involve negative regulation of Bcl6, a key transcription factor required for T FH cell differentiation 18, 88– 90. STAT5 binds to the Bcl6 gene promoter and potently blocks Bcl6 transcription 18, 91. The mechanism by which STAT5 prevents Bcl6 gene transcription remains unclear, although it is possible that this once again involves competition between STAT5 and STAT3 for common binding sites in the Bcl6 gene. More recent studies have found that IL7 also plays an important role in T FH cell differentiation. These studies demonstrated that TH1 cells which lack IL2R expression eventually upregulate both the IL6R and the IL7R. This results in a bi-potent state, in which cells that are stimulated with IL7 activate STAT5, block T FH cell differentiation, and preferentially give rise to central memory T cells. In contrast, preferential exposure to IL6 induces Bcl6 transcription via a STAT3-dependent process and promotes T FH cell differentiation 92. A subsequent study demonstrated that this also involves additional feedback loops, as BCL6 has been shown to bind to many STAT5 binding sites (including in the Il7r gene) in T FH cells and inhibit the expression of these STAT5-dependent genes 93.
Future directions
It is now clear that STAT5 plays important roles in both T-cell development and shaping the CD4 + T-cell immune response. However, major gaps remain in our knowledge. First, substantial evidence now supports the idea that STAT5 competes for binding sites with opposing effectors (for example, STAT3 and BCL6), but the molecular mechanisms by which STAT5 alters the epigenome to enhance or repress transcription remain unclear. Second, we know that STAT5 can interact with co-activators or co-repressors 94– 96, but we do not know whether these known interactors are critical for STAT5 function. Moreover, very little is known about what determines whether STAT5 induces or represses transcription at specific gene loci. One study suggested that this may be due to STAT5 binding as a dimer versus a tetramer 97. In contrast, other reports, using STAT5 mutant mice in which STAT5 cannot form tetramers, primarily reported defects in STAT5-dependent gene activation and not repression 98. Thus, key future questions will be to resolve the molecular mechanisms by which STAT5 alters chromatin structure and promotes or represses gene transcription and to establish what determinants result in STAT5 promotion versus repression of gene transcription.
Abbreviations
γc, gamma chain; ChIP-Seq, chromatin immunoprecipitation sequencing; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFNγ, interferon gamma; IL, interleukin; IL4Rα, interleukin 4 receptor alpha; IL12Rβ2, interleukin 12 receptor beta 2 subunit; pTreg, peripheral-induced regulatory T cell; TCR, T-cell receptor; T FH, T follicular helper; TGFβ, transforming growth factor beta; TH, T helper; TH GM, T helper type granulocyte-macrophage colony-stimulating factor; Treg, regulatory T cell; TSLP, thymic stromal lymphopoietin; tTreg, thymus-derived regulatory T cell.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Mark Kaplan, Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN, USA; Department of Pediatrics, Wells Center for Pediatric Research, Indianapolis, IN, USA
Demin Wang, Blood Research Institute, Blood Center of Wisconsin, Milwaukee, WI, USA
John O'Shea, Molecular Immunology and Inflammation Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health, Bethesda, MD, USA
Alejandro Villarino, Molecular Immunology and Inflammation Branch, NIAMS, National Institutes of Health, Bethesda, USA
Thomas Malek, Department of Microbiology and Immunology, Miller School of Medicine, University of Miami, Miami, FL, 33101, USA
Funding Statement
DLO is funded by a National Institutes of Health (NIH) T32 fellowship (T32 AI 007313). MAF is supported by grants from the NIH (R01 AI113138, R01 CA151845, R01 CA154998, and R01 CA185062).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 4 approved]
References
- 1. Ota N, Brett TJ, Murphy TL, et al. : N-domain-dependent nonphosphorylated STAT4 dimers required for cytokine-driven activation. Nat Immunol. 2004;5(2):208–15. 10.1038/ni1032 [DOI] [PubMed] [Google Scholar]
- 2. Grimley PM, Dong F, Rui H: Stat5a and Stat5b: Fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 1999;10(2):131–57. 10.1016/S1359-6101(99)00011-8 [DOI] [PubMed] [Google Scholar]
- 3. Wakao H, Schmitt-Ney M, Groner B: Mammary gland-specific nuclear factor is present in lactating rodent and bovine mammary tissue and composed of a single polypeptide of 89 kDa. J Biol Chem. 1992;267(23):16365–70. [PubMed] [Google Scholar]
- 4. Schmitt-Ney M, Happ B, Hofer P, et al. : Mammary gland-specific nuclear factor activity is positively regulated by lactogenic hormones and negatively by milk stasis. Mol Endocrinol. 1992;6(12):1988–97. 10.1210/mend.6.12.1491685 [DOI] [PubMed] [Google Scholar]
- 5. Beadling C, Guschin D, Witthuhn BA, et al. : Activation of JAK kinases and STAT proteins by interleukin-2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994;13(23):5605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin J, Migone T, Tseng M, et al. : The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2(4):331–9. 10.1016/1074-7613(95)90141-8 [DOI] [PubMed] [Google Scholar]
- 7. Isaksen DE, Baumann H, Trobridge PA, et al. : Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163(11):5971–7. [PubMed] [Google Scholar]
- 8. Mui AL, Wakao H, O'Farrell AM, et al. : Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14(6):1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barahmand-Pour F, Meinke A, Eilers A, et al. : Colony-stimulating factors and interferon-gamma activate a protein related to MGF-Stat 5 to cause formation of the differentiation-induced factor in myeloid cells. FEBS Lett. 1995;360(1):29–33. 10.1016/0014-5793(95)00072-H [DOI] [PubMed] [Google Scholar]
- 10. Gouilleux F, Pallard C, Dusanter-Fourt I, et al. : Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995;14(9):2005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lucas S, Ghilardi N, Li J, et al. : IL-27 regulates IL-12 responsiveness of naive CD4 + T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100(25):15047–52. 10.1073/pnas.2536517100 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Liu X, Robinson GW, Gouilleux F, et al. : Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A. 1995;92(19):8831–5. 10.1073/pnas.92.19.8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin JX, Mietz J, Modi WS, et al. : Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271(18):10738–44. 10.1074/jbc.271.18.10738 [DOI] [PubMed] [Google Scholar]
- 14. Copeland NG, Gilbert DJ, Schindler C, et al. : Distribution of the mammalian Stat gene family in mouse chromosomes. Genomics. 1995;29(1):225–8. 10.1006/geno.1995.1235 [DOI] [PubMed] [Google Scholar]
- 15. Soldaini E, John S, Moro S, et al. : DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol Cell Biol. 2000;20(1):389–401. 10.1128/MCB.20.1.389-401.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liao W, Schones DE, Oh J, et al. : Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9(11):1288–96. 10.1038/ni.1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanai T, Seki S, Jenks JA, et al. : Identification of STAT5A and STAT5B target genes in human T cells. PLoS One. 2014;9(1):e86790. 10.1371/journal.pone.0086790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Villarino A, Laurence A, Robinson GW, et al. : Signal transducer and activator of transcription 5 (STAT5) paralog dose governs T cell effector and regulatory functions. eLife. 2016;5: pii: e08384. 10.7554/elife.08384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imada K, Bloom ET, Nakajima H, et al. : Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188(11):2067–74. 10.1084/jem.188.11.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Robinson GW, Wagner KU, et al. : Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11(2):179–86. 10.1101/gad.11.2.179 [DOI] [PubMed] [Google Scholar]
- 21. Teglund S, McKay C, Schuetz E, et al. : Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93(5):841–50. 10.1016/S0092-8674(00)81444-0 [DOI] [PubMed] [Google Scholar]
- 22. Moriggl R, Topham DJ, Teglund S, et al. : Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10(2):249–59. 10.1016/S1074-7613(00)80025-4 [DOI] [PubMed] [Google Scholar]
- 23. Burchill MA, Goetz CA, Prlic M, et al. : Distinct effects of STAT5 activation on CD4 + and CD8 + T cell homeostasis: development of CD4 +CD25 + regulatory T cells versus CD8 + memory T cells. J Immunol. 2003;171(11):5853–64. 10.4049/jimmunol.171.11.5853 [DOI] [PubMed] [Google Scholar]
- 24. Cui Y, Riedlinger G, Miyoshi K, et al. : Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24(18):8037–47. 10.1128/MCB.24.18.8037-8047.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao Z, Cui Y, Watford WT, et al. : Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci U S A. 2006;103(4):1000–5. 10.1073/pnas.0507350103 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Ye S, Agata Y, Lee H, et al. : The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity. 2001;15(5):813–23. 10.1016/S1074-7613(01)00230-8 [DOI] [PubMed] [Google Scholar]
- 27. Wagatsuma K, Tani-ichi S, Liang B, et al. : STAT5 Orchestrates Local Epigenetic Changes for Chromatin Accessibility and Rearrangements by Direct Binding to the TCRgamma Locus. J Immunol. 2015;195(4):1804–14. 10.4049/jimmunol.1302456 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Park J, Adoro S, Guinter T, et al. : Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11(3):257–64. 10.1038/ni.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Malin S, McManus S, Cobaleda C, et al. : Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11(2):171–9. 10.1038/ni.1827 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Shi M, Lin TH, Appell KC, et al. : Janus-kinase-3-dependent signals induce chromatin remodeling at the Ifng locus during T helper 1 cell differentiation. Immunity. 2008;28(6):763–73. 10.1016/j.immuni.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liao W, Lin JX, Wang L, et al. : Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12(6):551–9. 10.1038/ni.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Le Gros G, Ben-Sasson SZ, Seder R, et al. : Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172(3):921–9. 10.1084/jem.172.3.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ben-Sasson SZ, Le Gros G, Conrad DH, et al. : IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990;145(4):1127–36. [PubMed] [Google Scholar]
- 34. Cote-Sierra J, Foucras G, Guo L, et al. : Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101(11):3880–5. 10.1073/pnas.0400339101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamane H, Zhu J, Paul WE: Independent roles for IL-2 and GATA-3 in stimulating naive CD4 + T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005;202(6):793–804. 10.1084/jem.20051304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu J, Cote-Sierra J, Guo L, et al. : Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19(5):739–48. 10.1016/S1074-7613(03)00292-9 [DOI] [PubMed] [Google Scholar]
- 37. Zhu J, Min B, Hu-Li J, et al. : Conditional deletion of Gata3 shows its essential function in T H1-T H2 responses. Nat Immunol. 2004;5(11):1157–65. 10.1038/ni1128 [DOI] [PubMed] [Google Scholar]
- 38. Liao W, Schones DE, Oh J, et al. : Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9(11):1288–96. 10.1038/ni.1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kitajima M, Lee HC, Nakayama T, et al. : TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41(7):1862–71. 10.1002/eji.201041195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bruchard M, Rebé C, Derangère V, et al. : The receptor NLRP3 is a transcriptional regulator of T H2 differentiation. Nat Immunol. 2015;16(8):859–70. 10.1038/ni.3202 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Veldhoen M, Uyttenhove C, van Snick J, et al. : Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9(12):1341–6. 10.1038/ni.1659 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Dardalhon V, Awasthi A, Kwon H, et al. : IL-4 inhibits TGF-beta-induced Foxp3 + T cells and, together with TGF-beta, generates IL-9 + IL-10 + Foxp3 - effector T cells. Nat Immunol. 2008;9(12):1347–55. 10.1038/ni.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yao W, Zhang Y, Jabeen R, et al. : Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38(2):360–72. 10.1016/j.immuni.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liao W, Spolski R, Li P, et al. : Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc Natl Acad Sci U S A. 2014;111(9):3508–13. 10.1073/pnas.1301138111 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Bassil R, Orent W, Olah M, et al. : BCL6 controls Th9 cell development by repressing Il9 transcription. J Immunol. 2014;193(1):198–207. 10.4049/jimmunol.1303184 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Olson MR, Verdan FF, Hufford MM, et al. : STAT3 Impairs STAT5 Activation in the Development of IL-9-Secreting T Cells. J Immunol. 2016;196(8):3297–304. 10.4049/jimmunol.1501801 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Nakajima H, Liu XW, Wynshaw-Boris A, et al. : An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor alpha chain induction. Immunity. 1997;7(5):691–701. 10.1016/S1074-7613(00)80389-1 [DOI] [PubMed] [Google Scholar]
- 48. Sheng W, Yang F, Zhou Y, et al. : STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 2014;24(12):1387–402. 10.1038/cr.2014.154 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Noster R, Riedel R, Mashreghi MF, et al. : IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med. 2014;6(241):241ra80. 10.1126/scitranslmed.3008706 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Hartmann FJ, Khademi M, Aram J, et al. : Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human T H cells. Nat Commun. 2014;5:5056. 10.1038/ncomms6056 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Sakaguchi S, Sakaguchi N, Asano M, et al. : Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Malek TR, Yu A, Vincek V, et al. : CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17(2):167–78. 10.1016/S1074-7613(02)00367-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. D'Cruz LM, Klein L: Development and function of agonist-induced CD25 +Foxp3 + regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6(11):1152–9. 10.1038/ni1264 [DOI] [PubMed] [Google Scholar]
- 54. Fontenot JD, Rasmussen JP, Gavin MA, et al. : A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–51. 10.1038/ni1263 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Cohen A, Nadeau K, Tu W, et al. : Sa.86. Decreased Generation and Function of CD4 + CD25 Hi T Regulatory Cells in Human STAT5b Deficiency. Clin Immunol. 2006;119:S135 10.1016/j.clim.2006.04.318 [DOI] [PubMed] [Google Scholar]
- 56. Burchill MA, Yang J, Vogtenhuber C, et al. : IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3 + regulatory T cells. J Immunol. 2007;178(1):280–90. 10.4049/jimmunol.178.1.280 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Yao Z, Kanno Y, Kerenyi M, et al. : Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109(10):4368–75. 10.1182/blood-2006-11-055756 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Soper DM, Kasprowicz DJ, Ziegler SF: IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur J Immunol. 2007;37(7):1817–26. 10.1002/eji.200737101 [DOI] [PubMed] [Google Scholar]
- 59. Lio CW, Hsieh CS: A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–11. 10.1016/j.immuni.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Burchill MA, Yang J, Vang KB, et al. : Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28(1):112–21. 10.1016/j.immuni.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mahmud SA, Manlove LS, Schmitz HM, et al. : Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15(5):473–81. 10.1038/ni.2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yi Z, Lin WW, Stunz LL, et al. : The adaptor TRAF3 restrains the lineage determination of thymic regulatory T cells by modulating signaling via the receptor for IL-2. Nat Immunol. 2014;15(9):866–74. 10.1038/ni.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Metidji A, Rieder SA, Glass DD, et al. : IFN-α/β receptor signaling promotes regulatory T cell development and function under stress conditions. J Immunol. 2015;194(9):4265–76. 10.4049/jimmunol.1500036 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Tai X, Erman B, Alag A, et al. : Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38(6):1116–28. 10.1016/j.immuni.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Marshall D, Sinclair C, Tung S, et al. : Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J Immunol. 2014;193(11):5525–33. 10.4049/jimmunol.1402144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haribhai D, Williams JB, Jia S, et al. : A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35(1):109–22. 10.1016/j.immuni.2011.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Lathrop SK, Bloom SM, Rao SM, et al. : Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–4. 10.1038/nature10434 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Zheng SG, Wang J, Wang P, et al. : IL-2 is essential for TGF-beta to convert naive CD4 +CD25 - cells to CD25 +Foxp3 + regulatory T cells and for expansion of these cells. J Immunol. 2007;178(4):2018–27. 10.4049/jimmunol.178.4.2018 [DOI] [PubMed] [Google Scholar]
- 69. Knoechel B, Lohr J, Kahn E, et al. : Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202(10):1375–86. 10.1084/jem.20050855 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Chen Q, Kim YC, Laurence A, et al. : IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3 + T cells in vivo. J Immunol. 2011;186(11):6329–37. 10.4049/jimmunol.1100061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang R, Qu C, Zhou Y, et al. : Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity. 2015;43(2):251–63. 10.1016/j.immuni.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Vogtenhuber C, Bucher C, Highfill SL, et al. : Constitutively active Stat5b in CD4+ T cells inhibits graft-versus-host disease lethality associated with increased regulatory T-cell potency and decreased T effector cell responses. Blood. 2010;116(3):466–74. 10.1182/blood-2009-11-252825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Feng Y, Arvey A, Chinen T, et al. : Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158(4):749–63. 10.1016/j.cell.2014.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu Z, Gerner MY, van Panhuys N, et al. : Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;528(7581):225–30. 10.1038/nature16169 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Levine AG, Arvey A, Jin W, et al. : Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15(11):1070–8. 10.1038/ni.3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chinen T, Kannan AK, Levine AG, et al. : An essential role for the IL-2 receptor in T reg cell function. Nat Immunol. 2016;17(11):1322–33. 10.1038/ni.3540 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Suzuki H, Kundig TM, Furlonger C, et al. : Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268(5216):1472–6. 10.1126/science.7770771 [DOI] [PubMed] [Google Scholar]
- 78. Zhou L, Ivanov II, Spolski R, et al. : IL-6 programs T H-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–74. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Yang XO, Panopoulos AD, Nurieva R, et al. : STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–63. 10.1074/jbc.C600321200 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Mathur AN, Chang H, Zisoulis DG, et al. : Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178(8):4901–7. 10.4049/jimmunol.178.8.4901 [DOI] [PubMed] [Google Scholar]
- 81. Laurence A, Tato CM, Davidson TS, et al. : Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–81. 10.1016/j.immuni.2007.02.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Yang XP, Ghoreschi K, Steward-Tharp SM, et al. : Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–54. 10.1038/ni.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Liao W, Lin JX, Wang L, et al. : Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12(6):551–9. 10.1038/ni.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Nurieva RI, Chung Y, Hwang D, et al. : Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138–49. 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Ballesteros-Tato A, León B, Graf BA, et al. : Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36(5):847–56. 10.1016/j.immuni.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Johnston RJ, Choi YS, Diamond JA, et al. : STAT5 is a potent negative regulator of T FH cell differentiation. J Exp Med. 2012;209(2):243–50. 10.1084/jem.20111174 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Nurieva RI, Podd A, Chen Y, et al. : STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. 2012;287(14):11234–9. 10.1074/jbc.M111.324046 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Johnston RJ, Poholek AC, DiToro D, et al. : Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–10. 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Nurieva RI, Chung Y, Martinez GJ, et al. : Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–5. 10.1126/science.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yu D, Rao S, Tsai LM, et al. : The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–68. 10.1016/j.immuni.2009.07.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Walker SR, Nelson EA, Frank DA: STAT5 represses BCL6 expression by binding to a regulatory region frequently mutated in lymphomas. Oncogene. 2007;26(2):224–33. 10.1038/sj.onc.1209775 [DOI] [PubMed] [Google Scholar]
- 92. McDonald PW, Read KA, Baker CE, et al. : IL-7 signalling represses Bcl-6 and the T FH gene program. Nat Commun. 2016;7: 10285. 10.1038/ncomms10285 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Liu X, Lu H, Chen T, et al. : Genome-wide Analysis Identifies Bcl6-Controlled Regulatory Networks during T Follicular Helper Cell Differentiation. Cell Rep. 2016;14(7):1735–47. 10.1016/j.celrep.2016.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Pfitzner E, Jähne R, Wissler M, et al. : p300/CREB-binding protein enhances the prolactin-mediated transcriptional induction through direct interaction with the transactivation domain of Stat5, but does not participate in the Stat5-mediated suppression of the glucocorticoid response. Mol Endocrinol. 1998;12(10):1582–93. 10.1210/mend.12.10.0180 [DOI] [PubMed] [Google Scholar]
- 95. Zhu M, John S, Berg M, et al. : Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFNgamma-mediated signaling. Cell. 1999;96(1):121–30. 10.1016/S0092-8674(00)80965-4 [DOI] [PubMed] [Google Scholar]
- 96. Nakajima H, Brindle PK, Handa M, et al. : Functional interaction of STAT5 and nuclear receptor co-repressor SMRT: implications in negative regulation of STAT5-dependent transcription. EMBO J. 2001;20(23):6836–44. 10.1093/emboj/20.23.6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mandal M, Powers SE, Maienschein-Cline M, et al. : Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nat Immunol. 2011;12(12):1212–20. 10.1038/ni.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Lin J, Li P, Liu D, et al. : Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity. 2012;36(4):586–99. 10.1016/j.immuni.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation