Abstract
Seven species-specific monoclonal antibodies (MAbs) were produced against Encephalitozoon cuniculi and characterized. The MAbs were immunoglobulin G, and when used for indirect microimmunofluorescence microscopy and Western immunoblot assays, they detected E. cuniculi originating from clinical samples. They did not cross-react with other Encephalitozoon species (E. intestinalis and E. hellem) or with a collection of gram-negative bacteria, yeast, and other parasites. The MAbs reacted primarily with 121-, 56-, 45-, 43-, and 41-kDa protein epitopes of E. cuniculi. These epitopes were demonstrated to be E. cuniculi species specific by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. We developed MAbs to strains of E. cuniculi that can be used successfully to distinguish E. cuniculi from other microsporidial species in cultures established from clinical specimens. These MAbs may provide a specific, simple, rapid, and low-cost tool for the identification and diagnosis of infections due to microsporidia.
Encephalitozoon cuniculi is a unicellular, obligately intracellular microsporidian species responsible for emerging opportunistic infections (5, 28, 34). Its genome sequence has been published recently (11). The infective stage of E. cuniculi is the mature spore, which contains a unique extrusion apparatus in the form of a coiled polar tubule for injecting infectious sporoplasms into host cells (7, 31). Spores are highly resistant in the environment because of the presence of a thick, chitin-containing wall (2). E. cuniculi causes hepatitis, peritonitis, encephalitis, intestinal infections, keratoconjunctivitis, sinusitis, rhinitis, respiratory infection, urinary tract infection, and disseminated infection in immunocompromised patients (13, 15, 20, 30, 33) and nonimmunocompromised patients (e.g., the elderly, travelers to the tropics, and residents of the tropics) (6, 8, 9).
Definitive identification of E. cuniculi currently depends on time-consuming and costly transmission electron microscopy to definitively identify spores in clinical samples. In addition, transmission electron microscopy may not be sensitive enough to detect small numbers of organisms. Serological studies for detecting microsporidium-specific antibodies are reliable for antemortem diagnosis in infected laboratory animals (24, 25). Mammalian microsporidian spores do stain with Gram stain, Giemsa stain, Calcofluor, and concentrated trichrome stain (26), but because these organisms are very small, they are difficult to distinguish from bacteria and small yeasts. A few E. cuniculi isolates were recovered from various clinical specimens (27), but PCR assays are currently available in only a few laboratories (20). Identification of microsporidian agents at the species level is important because several new drug therapies are effective in treating infections caused by some, but not all, microsporidia (1). Certain therapeutic agents (e.g., fumagillin, albendazole) are effective in treating urogenital and respiratory infections caused by E. cuniculi (8).
We (14) and others (4, 19) previously demonstrated the usefulness of monoclonal antibodies (MAbs) for the rapid and specific detection of microsporidia, including Encephalitozoon hellem (14) and E. cuniculi (4, 19). It would be advantageous to have species-specific monoclonal antibodies available for the diagnosis of microsporidiosis. In this study, our goal was to develop a diagnostic reagent for the routine identification of E. cuniculi in microsporidian-positive clinical specimens. Here, we describe the characteristics and specificities of seven species-specific monoclonal antibodies that we produced against E. cuniculi.
MATERIALS AND METHODS
Sources of Encephalitozoon spp.
The geographic sources of the Encephalitozoon sp. strains used in the study are listed in Table 1. Encephalitozoon isolates were cocultured with MRC5 cells (human fetal lung fibroblasts) in minimum essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum and 1% glutamine, and the culture medium was replaced every week. Culture media from all T-150 flasks, containing extruded spores and unattached host cells infected with developmental stages of the parasite, were centrifuged at 1,500 × g for 20 min at 4°C, and the supernatant was aspirated. The pellets were put back into the same culture flasks. This facilitated infection of a maximum number of host cells with the respective parasites (>70%), as revealed by using DiffQuik and Weber 2R staining according to the manufacturer's recommendations, followed by microscopic examination (Axioskop 20; Carl Zeiss, Gottingen, Germany) at a magnification of ×1,000. Therefore, spores that were extruded into the culture supernatants from all parasites were harvested by centrifugation as described above, sonicated three times (for 1 min each time), and centrifuged over 25% sucrose at 7,500 × g for 30 min. The parasites were washed twice with sterile phosphate-buffered saline (PBS [pH 7.2]) to remove the sucrose and were then suspended either in sterile deionized water, for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), or in PBS, for microimmunofluorescence (MIF).
TABLE 1.
Strains of Encephalitozoon spp. used for screening and determination of MAb specificity
| Species | Strain | Genotype | Karyotype | Source |
|---|---|---|---|---|
| Encephalitozoon intestinalis | The Netherlands | |||
| Encephalitozoon cuniculi | The Netherlands | |||
| Encephalitozoon hellem | EU1 | 1 | A | North America |
| ASM1 | 1 | A | The Netherlands | |
| PV4 | 1 | B | Italy | |
| PV6 | 1 | B | Italy | |
| PV7 | 1 | B | Italy | |
| PV8 | 1 | B | Italy | |
| PV9 | 1 | B | Italy | |
| PV10 | 1 | B | Italy | |
| PV11 | 1 | B | Italy | |
| PV12 | 1 | B | Italy | |
| PV93 | 1 | B | Italy | |
| PV94 | 1 | B | Italy | |
| PV95 | 1 | B | Italy |
MIF.
The MIF assay (16) was used to screen hybridoma clones and to determine the specificities of the MAbs. Antigens were placed on 24-well microscope slides with a pen nib. The antigens were fixed in acetone for 10 min at room temperature, air dried, and incubated with supernatants in a humidified chamber at 37°C for 30 min. After two washes in PBS (5 min each) and rinsing in sterile distilled water for 1 min, the slides were air dried at 37°C. Following incubation at 37°C for 30 min with dechlorotriazinyl amino fluorescein-conjugated goat anti-mouse immunoglobulin G (IgG) plus IgM (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) diluted 1:200 in PBS with 0.2% Evans blue (Sigma Chemical Co.), the slides were washed as described above and mounted with Fluoprep (Bio-Merieux, Marcy l'Etoile, France) before being observed under an epifluorescence microscope (Axioskop 20; Carl Zeiss) at a magnification of ×400. Serum samples from healthy, uninoculated mice were used as negative controls.
Production of MAbs.
For production of MAbs (10), 6-week-old female BALB/c mice were inoculated intraperitoneally six times with 107 E. cuniculi organisms in 0.5 ml of PBS without adjuvant, at 7-day intervals. One week after the final intraperitoneal inoculation, the mice were injected once through the tail vein with 106 spores suspended in 0.1 ml of PBS. Serum samples from the mice were screened by a MIF assay, and the antibody titer was 1:1,600. Three days later, spleen cells from the mice were fused with SP2/0-Ag14 myeloma cells (10:1) by using 50% polyethylene glycol 6000 (Sigma Chemical Co.). Fusion cells were grown in hybridoma medium (Seromed, Berlin, Germany) with 17% heat-inactivated fetal bovine serum (Gibco BRL, Eggenstein, Germany) and hypoxanthine-aminopterin-thymidine selective medium (Sigma Chemical Co.) at 37°C under a humidified atmosphere supplemented with 5% CO2. The supernatants were screened for antibodies to the antigens of E. cuniculi, Encephalitozoon intestinalis, E. hellem EU1, and E. hellem PV6 by MIF, and positive hybridomas were subcloned three times by limited dilution. Isotypes of MAbs were determined by using ImmunoType Mouse IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 assays (Sigma Chemical Co.) according to the manufacturer's recommendations. The specificities of the MAbs were tested by Western immunoblotting and MIF.
SDS-PAGE and Western immunoblotting.
Antigens were suspended in an equal volume of sample buffer (0.0625 M Tris hydrochloride [pH 8.0], 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, 0.02% bromophenol blue) (12) and separated electrophoretically in 10% resolving gels with 5% stacking gels at a constant current of 8 to 10 mA per gel for 3 to 4 h in running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS) in a Mini Protean II apparatus (Bio-Rad, Richmond, Calif.). Prestained SDS-PAGE standards (low range [112, 81, 49.9, 36.2, 29.2, and 21.3 kDa]; Bio-Rad) were used as a reference. The separated antigens were transferred to 0.45-μm-pore-size nitrocellulose membranes (Hybond-C; Amersham, Little Chalfont, Buckinghamshire, United Kingdom) at 100 V for 1 h at 4°C in an electrophoretic transfer cell (Mini Trans-Blot; Bio-Rad). After transfer, the nitrocellulose membranes were incubated overnight in PBS with 5% nonfat dry milk to block nonspecific binding sites. After three 10-min washes in PBS, the membranes were air dried, cut into strips, incubated with MAbs diluted to 1:100 or higher in PBS containing 5% nonfat dry milk at room temperature for 1 h, and washed twice (10 min each time). After incubation at room temperature for 1 h with peroxidase-conjugated goat anti-mouse IgG F(ab′)2 fragment (heavy and light chains) (AffiniPure; Jackson ImmunoResearch) diluted 1:200 in PBS with 5% nonfat dry milk and three washes in PBS as described above, color was developed with a coloring buffer containing 0.015% 4-chloro-1-naphthol and 0.015% hydrogen peroxide in 16.7% methanol in PBS.
Cross-reactivity studies.
The reactivities of the MAbs were assessed by MIF. Blind testing of MAbs by MIF was carried out with E. cuniculi, E. intestinalis, 13 strains of E. hellem isolates from patients in Italy, The Netherlands, and North America, Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Shigella dysenteriae, Proteus vulgaris, Salmonella enterica, and Aspergillus fumigatus.
RESULTS
MAb production.
Antisera from immunized BALB/c mice were screened for E. cuniculi-specific antibody responses 7 days after each injection before a final booster. All mice began to produce antibodies after the second intraperitoneal injection. The highest antibody response was raised after the sixth injection and produced a titer of 1:1,600. One mouse (whose spleen was three times bigger than those of nonimmunized mice) was then selected for the fusion protocol. A total of 1,248 wells were seeded with fused SP2/O myeloma cells. On the 5th day after fusion, viable clones were observed in about 80% of the wells. The supernatants began to be screened with whole spores of E. cuniculi, E. hellem EU1, E. hellem PV6, and E. intestinalis on the 10th and 15th days after fusion. After the second screening, 191 antibody secretion hybridomas still reacted against spores of E. cuniculi. Seventy-three hybridomas were selected for subcloning by limiting dilution. After cloning procedures by limiting dilution were performed three times, seven stable hybridomas were selected on the basis of their reactivities with the four antigens and were selected against the second large panel of Encephalitozoon spp. (Table 2). The seven MAbs were expanded in culture.
TABLE 2.
Reactivities of MAb panel elicited by E. cuniculi with different Encephalitozoon sp. antigens
| MAb | Class | Specificity (kDa) | Reactivitya with antigens of:
|
|||
|---|---|---|---|---|---|---|
| E. cuniculi | E. intestinalis |
E. hellem
|
||||
| PV6 | EU1 | |||||
| EC8C12 | IgG3 | 56 | + | − | − | − |
| EC14E10 | IgG2a | 56 | + | − | − | − |
| EC14D10 | IgG1 | 56 | + | − | − | − |
| EC11C5 | IgG3 | 45 | + | − | − | − |
| EC10G4 | IgG3 | 43 | + | − | − | − |
| EC7D5 | IgG3 | 41 | + | − | − | − |
| EC10E7 | IgG3 | 121 | + | − | − | − |
+, positive reactivity; −, no reactivity.
MIF reactivities and isotyping of MAbs.
The immunoglobulin class and subclass of each of the seven MAbs are presented in Table 2. Seven MAbs of subclasses IgG1, IgG2a, and IgG3 (Table 2), produced from subcloned hybridomas, were examined for their reactivities with Encephalitozoon strains. These MAbs were strongly reactive with E. cuniculi strains but displayed no cross-reaction with E. intestinalis or with any strains of E. hellem. The reactivity of each hybridoma is presented in Table 2. The polyclonal antisera generated more background than MAbs in acetone-fixed antigens with a MIF stain.
SDS-PAGE.
The protein profile of E. cuniculi could be divided into three major groups of bands: (i) high-molecular-mass bands greater than 100 kDa, (ii) intermediate-molecular-mass bands of 30 to 80 kDa, and (iii) low-molecular-mass bands of less than 30 kDa. Although the SDS-PAGE profiles of the different species of Encephalitozoon were not the same, 121-, 56-, 45-, 43-, and 41-kDa protein bands appeared to be common to the E. cuniculi isolates under study, and they were also the most prominent bands in the SDS-PAGE profiles.
Western immunoblotting.
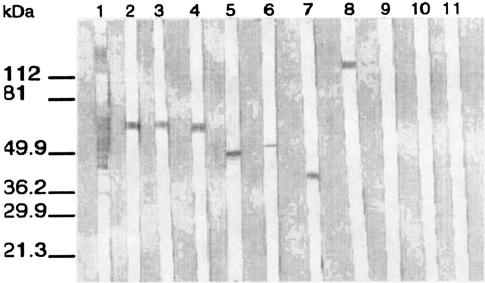
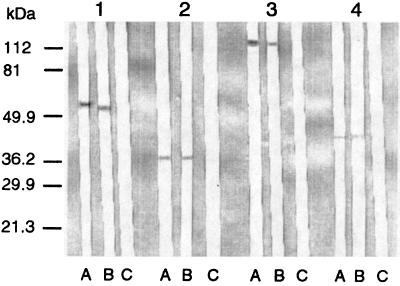
Immunoblotting demonstrated that polyclonal antisera raised against E. cuniculi displayed many bands (Fig. 1, lane 1). Seven MAbs were directed against epitopes of the four previously identified intermediate-molecular-mass proteins and one high-molecular-mass protein (Fig. 1, lanes 2 to 8). Of the seven MAbs, three showed reactivity with a 56-kDa protein, one each showed reactivity with a 45-, a 43-, and a 41-kDa protein, and one showed reactivity with a high-molecular-mass protein (121 kDa), but they did not react with any antigens from the other Encephalitozoon species, E. hellem and E. intestinalis (Fig. 1, lanes 9 to 11). In Western blots of E. cuniculi antigens that had been digested with proteinase K, the MAbs failed to react with protein epitopes. However, heating of the antigens (100°C for 10 min) before immunoblotting did not affect the reactivities of the MAbs (Fig. 2).
FIG. 1.
Western immunoblotting of seven representative MAbs with E. cuniculi and other Encephalitozoon species. Lanes 1 to 8, immunoblotting of murine polyclonal antisera raised against E. cuniculi and MAbs EC8C12, EC14E10, EC11D10, EC10G4, EC11C5, EC7D5, and EC10E7, respectively, with E. cuniculi; lanes 9 to 11, immunoblotting of MAb EC812 with E. intestinalis, E. hellem PV6, and E. hellem EU1, respectively. Molecular mass markers are shown on the left.
FIG. 2.
Western immunoblotting of E. cuniculi antigens treated in different ways with MAbs. Lanes: A, native antigens without treatment; B, antigens heated at 100°C for 10 min; C, antigens treated with proteinase K at 1.5 mg/ml at 37°C for 2 h. Numbers 1 to 4 above lanes correspond to MAbs EC8C12, EC7D5, EC10E7, and EC11C5, respectively. Molecular mass markers are shown on the left.
Cross-reactivity studies.
Blind testing of bacteria was performed with MAbs from supernatants of hybridomas. The MAbs reacted with all the strains of E. cuniculi tested but did not react with the other Encephalitozoon species or with other bacteria and yeasts used in the study.
DISCUSSION
Microsporidian infections are difficult to diagnose, primarily because the organisms are difficult to distinguish from bacteria and small yeasts in clinical samples. Giemsa stain (17, 18) and a modified trichrome stain using chromotrope 2R (29) have been used to detect microsporidia in clinical samples, but with some difficulties. Giesma-stained microsporidia are blue and display a purple-blue nucleus which distinguishes them from bacteria. It is difficult, however, to find microsporidia in clinical samples in which most other organisms also stain blue. The modified trichrome (chromotrope 2R) staining method described by Weber et al. (29) has the advantage that most bacteria counterstain light green, leaving the microsporidia pink. However, microsporidia may be missed if the parasite burden is low or if microsporidia are mixed with mucus. In addition, small yeast and sporulated bacteria in stool also stain pink, which can complicate the interpretation of smears. A Calcofluor staining method utilizing Uvitex 2B (Ciba-Geigy) or a fluorescent brightener also may be useful for detecting microsporidia (26). The microsporidia display relatively thick rings of fluorescence. However, the anterior regions appear concave, because yeasts also stain with Calcofluor.
The use of microsporidian-specific antibodies in MIF procedures appears to overcome some of these difficulties. Recently, polyclonal antisera produced against E. cuniculi and E. hellem were used to diagnose ocular and systemic E. hellem infections (21, 22), and a polyclonal antiserum raised against E. cuniculi in rabbits was used to detect cross-reacting Enterocytozoon bieneusi organisms in deparaffinized tissue sections (32) and in stool (35). We also raised MAbs against E. hellem (14). Currently, identification of human microsporidia at the species level depends on the use of time-consuming electron microscopy and molecular techniques (23, 28). Electron microscopy, however, cannot distinguish E. cuniculi from E. hellem in formalin-fixed tissue sections (20, 23). Because E. cuniculi and E. hellem are morphologically identical, staining and culture methods do not allow for their differentiation. The MAbs and polyclonal antibodies provided different advantages in MIF staining; we therefore used MIF and Western blotting for species identification.
In this report, we describe the production of seven MAbs that may be used as specific diagnostic reagents to identify E. cuniculi in cultures established from clinical samples from patients with microsporidiosis. The MAbs were characterized by using both MIF and Western blotting techniques. They detected acetone-fixed, culture-derived Encephalitozoon species. We found that the polyclonal antisera generated more background in acetone-fixed antigens with the MIF stain. The degree of background depended on the dilution of antiserum, as also described by Weiss et al. (32). The results clearly showed that the MAbs reacted with isolates of E. cuniculi at titers of 1:100 or greater yet showed no reactivity with either E. hellem or E. intestinalis in the MIF test. The murine polyclonal antisera detected many bands by Western blotting. The MAbs produced specific banding patterns by Western blotting with isolates of E. cuniculi. Three of the seven MAbs reacted with proteins of 58 kDa. Four of the seven MAbs reacted with 121-, 45-, 43-, and 41-kDa protein bands, respectively, in immunoblotting with E. cuniculi. This reactivity appeared to be specific for E. cuniculi. Finally, we have shown that these MAbs failed to react with spores of E. intestinalis and E. hellem, as well as with bacteria, yeasts, and other parasites, thus emphasizing the specificity of these MAbs for definitive clinical diagnosis.
Diagnosis of microsporidiosis at the species level is important, because certain therapeutic agents are effective in treating infections caused by some, but not all, microsporidian species. For example, systemic infections caused by E. cuniculi can be successfully treated with fumagillin and albendazole (3, 22). Hence, quick and definitive identification of E. cuniculi, preferably by MIF and Western blotting using our MAbs described here, will be greatly advantageous in the successful diagnosis and treatment of microsporidiosis due to E. cuniculi.
Acknowledgments
We acknowledge the review of the manuscript by Paul Walden, Associate Professor, Director of Urology Research Laboratories, New York University School of Medicine.
This work was supported by the Programme Hospitalier de Recherche Clinique-1997, Assistance Publique-Hopitaux de Marseille.
REFERENCES
- 1.Anwar-Bruni, D. M., S. E. Hogan., and D. A. Schwartz. 1996. Atovaquone is effective treatment for the symptom of gastrointestinal microsporidiosis in HIV-1-infected patients. AIDS 10:619-624. [DOI] [PubMed] [Google Scholar]
- 2.Bigliardi, E., M. G. Selmi, P. Lupetti, S. Corona, S. Gatti, M. Scaglia, and L. Sacchi. 1996. Microsporidian spore wall: ultrastructural findings on Encephalitozoon hellem exospore. J. Eukaryot. Microbiol. 43:181-186. [DOI] [PubMed] [Google Scholar]
- 3.Bryan, R. T., R. Weber, and D. A. Schwartz. 1997. Microsporidiosis in persons without HIV. Clin. Infect. Dis. 24:534-535. [DOI] [PubMed] [Google Scholar]
- 4.Croppo, G. P., G. S. Visvesvara, G. J. Leitch, S. Wallace, and D. A. Schwartz. 1998. Identification of the microsporidian Encephalitozoon hellem using immunoglobulin G monoclonal antibodies. Arch. Pathol. Lab. Med. 122:182-186. [PubMed] [Google Scholar]
- 5.Didier, E. S. 1998. Microsporidiosis. Clin. Infect. Dis. 27:1-7. [DOI] [PubMed] [Google Scholar]
- 6.Didier, E. S., P. J. Didier, K. F. Snowden, and J. A. Shadduck. 2000. Microsporidisis in mammals. Microbes Infect. 2:709. [DOI] [PubMed] [Google Scholar]
- 7.Foucault, C., and M. Drancourt. 2000. Entry of Encephalitozoon intestinalis into the human enterocyte-like cell line Caco-2. Microb. Pathog. 28:51-58. [DOI] [PubMed] [Google Scholar]
- 8.Franzen, C., and A. Muller. 1999. Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia. Clin. Microbiol. Rev. 12:243-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamboa-Dominguez, A., J. De Anda, J. Donis, F. Ruiz-Maza, G. S. Visvesvara, and H. Diliz. 2003. Disseminated Encephalitozoon cuniculi infection in a Mexican kidney transplant recipient. Transplantation 75:1898-1900. [DOI] [PubMed] [Google Scholar]
- 10.Harlow, E., and D. Lane. 1988. Monoclonal antibodies and growing hybridomas, p. 139-182. In E. Harlow and D. Lane (ed.), Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 11.Katinka, M. D., S. Duprat, E. Cornillot, G. Metenier, F. Thomarat, G. Prensier, V. Barbe, E. Peyretaillade, P. Brottier, P. Wincker, F. Delbac, H. El Alaoui, P. Peyret, W. Saurin, M. Gouy, J. Weissenbach, and C. P. Vivares. 2001. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450-453. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 13.Mertens, R. B., E. S. Didier, M. C. Fishbein, D. C. Bertucci, L. B. Rogers, and J. M. Orenstein. 1997. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod. Pathol. 10:68. [PubMed] [Google Scholar]
- 14.Mo, L., and M. Drancourt. 2002. Antigenic diversity of Encephalitozoon hellem demonstrated by subspecies-specific monoclonal antibodies. J. Eukaryot. Microbiol. 49:249-254. [DOI] [PubMed] [Google Scholar]
- 15.Mohindra, A., M. W. Lee, G. Vivesvara, H. Moura, R. Parasuraman, G. J. Leitch, L. Xiao, J. Yee, and R. Del Busto. 2002. Disseminated microsporidiosis in a renal transplant recipient. Transpl. Infect. Dis. 4:102. [DOI] [PubMed] [Google Scholar]
- 16.Niederkorn, J. Y., J. A. Shadduck, and E. Weidner. 1980. Antigenic cross-reactivity among different microsporidian spores as determined by immunofluorescence. J. Parasitol. 66:675-677. [PubMed] [Google Scholar]
- 17.Orenstein, J. M., J. Chiang, W. Steinberg, P. Smith, H. Rotterdam, and D. P. Kotler. 1990. Intestinal microsporidiosis as a cause of diarrhea in human immunodeficiency virus-infected patients. A report of 20 cases. Hum. Pathol. 21:475-481. [DOI] [PubMed] [Google Scholar]
- 18.Orenstein, J. M., W. Zierdt, C. Zierdt, and D. P. Kotler. 1990. Identification of spores of Enterocytozoon bieneusi in stool and duodenal fluid from AIDS patients. Lancet 336:1127-1128. [DOI] [PubMed] [Google Scholar]
- 19.Sak, B., K. Sakova, and O. Ditrich. 2004. Effects of a novel anti-exospore monoclonal antibody on microsporidial development in vitro. Parasitol. Res. 92:74-80. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz, D. A., I. Sobottka, G. J. Leitch, A. Cali, and G. S. Visvesvara. 1996. Pathology of microsporidiosis: emerging parasitic infections in patients with acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 120:173-188. [PubMed] [Google Scholar]
- 21.Schwartz, D. A., R. T. Bryan, K. O. Hewan-Lowe, G. S. Visvesvara, R. Weber, A. Cali, and P. Angritt. 1992. Disseminated microsporidiosis (Encephalitozoon hellem) and acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 116:660-668. [PubMed] [Google Scholar]
- 22.Schwartz, D. A., G. S. Visvesvara, M. C. Diesenhouse, R. Weber, R. L. Font, L. A. Wilson, G. Corrent, O. N. Seradarevic, D. F. Rosberger, P. C. Keenen, H. E. Grossniklaus, K. Hewan-Lowe, and R. T. Bryan. 1993. Pathologic features and immunofluorescence antibody demonstration of ocular microsporidiosis (Encephalitozoon hellem) in seven patients with acquired immunodeficiency syndrome. Am. J. Ophthalmol. 115:285-292. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz, D. A., R. T. Bryan, R. Weber, and G. S. Visvesvara. 1994. Microsporidiosis in HIV positive patients: current methods for diagnosis using biopsy, cytologic, ultrastructural, immunological, and tissue culture techniques. Folia Parasitol. (Prague) 41:101-109. [PubMed] [Google Scholar]
- 24.Shadduck, J. A. 1989. Human microsporidiosis in AIDS. Rev. Infect. Dis. 11:203-207. [DOI] [PubMed] [Google Scholar]
- 25.Shadduck, J. A., and E. Greeley. 1989. Microsporidia and human infections. Clin. Microbiol. Rev. 2:158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Gool, T., F. Snidjers, P. Reiss, J. K. M. Eeftinck-Schattenkerk, M. van den Bergh Weerman, J. F. W. M. Bartelsman, J. J. M. Bruins, E. U. Canning, and J. Dankert. 1993. Diagnosis of intestinal and disseminated microsporidial infections in patents with HIV by a new rapid fluorescence technique. J. Clin. Pathol. 46:694-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visvesvara, G. S. 2002. In vitro cultivation of microsporidia of clinical importance. Clin. Microbiol. Rev. 15:401-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber, R., R. T. Bryan, R. L. Owen, C. M. Wilcox, L. Gorelkin, and G. S. Visvesvara. 1992. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N. Engl. J. Med. 326:161-166. [DOI] [PubMed] [Google Scholar]
- 30.Weber, R., P. Deplazes, M. Fleep, A. Mathis, R. Baumann, B. Sauer, H. Kuster, and R. Luthy. 1997. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. N. Engl. J. Med. 336:474. [DOI] [PubMed] [Google Scholar]
- 31.Weidner, E., W. Byrd, A. Scarborough, J. Pleshinger, and D. Sibley. 1984. Microsporidian spore discharge and the transfer of polaroplast organelle membrane into plasma membrane. J. Protozool. 31:195-198. [Google Scholar]
- 32.Weiss, L. M., A Cali, E. Levee, D. Laplace, H. Tanowitz, D. Simon, and M. Wittner. 1992. Diagnosis of Encephalitozoon cuniculi infection by Western blot and the use of cross-reactive antigens for the possible detection of microsporidiosis in human. Am. J. Trop. Med. Hyg. 47:456-462. [DOI] [PubMed] [Google Scholar]
- 33.Weitzel, T., M. Wolff, J. Dabanch, I. Levy, C. Schmetz, and G. S. Visvesvara. 2001. Dual microsporidial infection with Encephalitozoon cuniculi and Enterocytozoon bieneusi in an HIV-positive patient. Infection 29:237. [DOI] [PubMed] [Google Scholar]
- 34.Wittner, M., and L. M. Weiss. 1999. The microsporidia and microsporidiosis. American Society for Microbiology, Washington, D.C.
- 35.Zierdt, C. H., V. J. Gill, and W. S. Zierdt. 1993. Detection of microsporidian spores in clinical samples by indirect fluorescent-antibody assay using whole-cell antisera to Encephalitozoon cuniculi and Encephalitozoon hellem. J. Clin. Microbiol. 31:3071-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]