Summary
Immune checkpoint inhibitors significantly improve clinical outcomes in numerous malignancies, but high-grade immune-related adverse events can occur, particularly with combination immunotherapy. Herein, we report two melanoma patients who developed fatal myocarditis following treatment with ipilimumab and nivolumab. Both patients developed myositis with rhabdomyolysis, early progressive and refractory cardiac electrical instability, and myocarditis with robust T-cell and macrophage infiltrates. Selective clonal T-cell populations infiltrating the myocardium were identical to those present in tumor and skeletal muscle. Pharmacovigilance data revealed that myocarditis occurred in 0.27% of patients treated with ipilimumab/nivolumab, suggesting this is a rare, potentially fatal, T-cell-driven drug reaction.
Keywords: Myocarditis, myositis, PD-1, CTLA-4, nivolumab, ipilimumab, PD-L1, cardiac
Introduction
Immune checkpoint inhibitors have transformed the treatment of several malignancies by releasing restrained anti-tumor immune responses.1 Ipilimumab, an anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibody, and nivolumab, an anti-programmed death-1 (PD-1) antibody have individually improved survival in patients with melanoma, and early results suggest that the combination further enhances anti-tumor activity and survival.2–5 Common side effects of these agents include dermatitis, endocrinopathies, colitis, hepatitis, and pneumonitis, all thought to arise from aberrant activation of autoreactive T cells.6,7 These toxicities are more frequent and severe with combination ipilimumab and nivolumab.4 Here we report two cases of lethal myocarditis accompanied by myositis in patients treated with nivolumab and ipilimumab.
Case Reports
Case 1
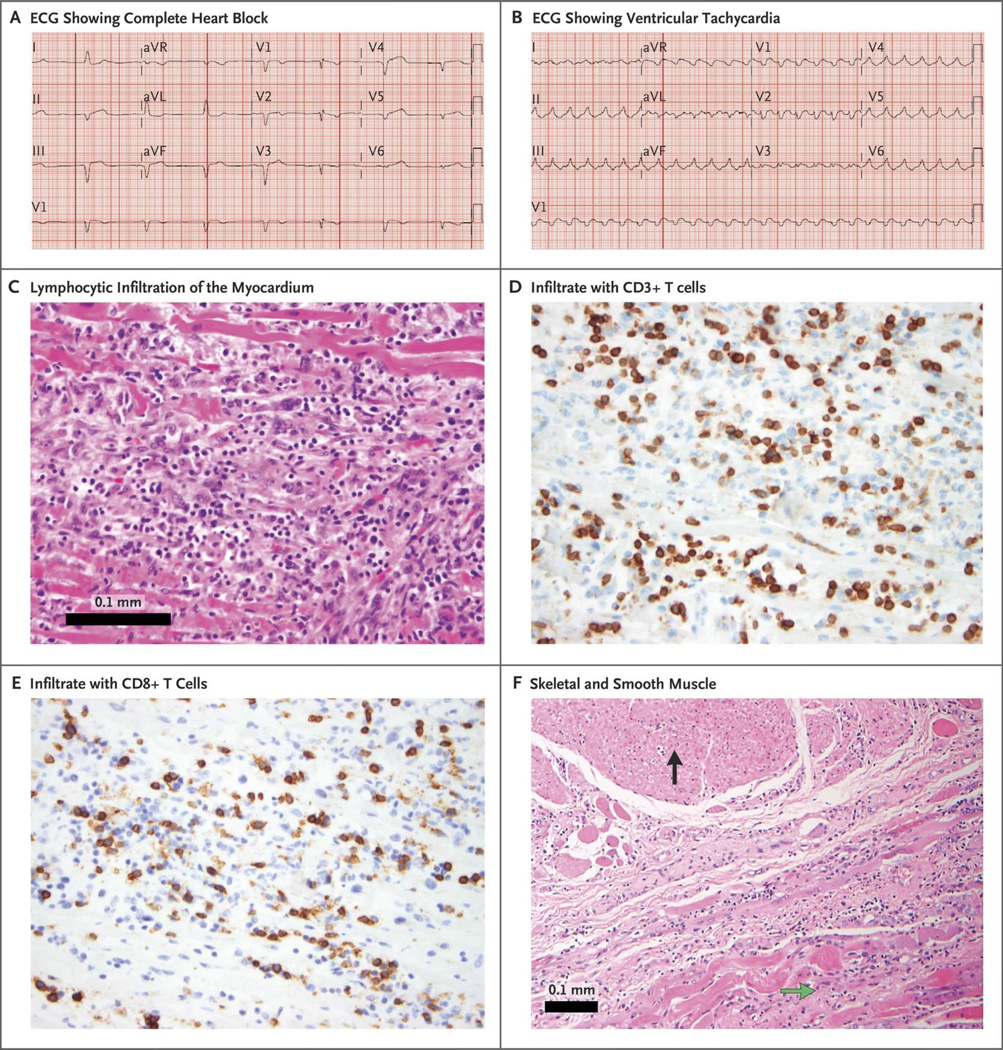
A 65-year-old woman (Patient 1) with metastatic melanoma was admitted to the hospital with atypical chest pain, dyspnea and fatigue 12 days after receiving her first dose of nivolumab (1 mg/kg) and ipilimumab (3 mg/kg). Initial work-up revealed myocarditis and myositis with rhabdomyolysis (CPK 17,720 unit/L [normal range 29–168], CK-MB >600 ng/mL [normal <5.99], troponin I 4.7 increasing to 51.3 ng/mL [normal <0.03]). Electrocardiogram (ECG) demonstrated PR prolongation with normal QRS complexes without evidence of ischemia. Within 24 hours, she developed new intraventricular conduction delay, and later complete heart block (Figure 1A). Serial echocardiograms demonstrated preserved left ventricular systolic function with ejection fraction calculated as 73% (Supplementary Video 1). She was treated with high-dose glucocorticoids (2mg/kg/day IV methylprednisolone) within 24 hours of admission, but nonetheless developed progressive clinical deterioration with multisystem organ failure and refractory ventricular tachycardia (Figure 1B) from which she could not be resuscitated.
Figure 1.
Electrocardiographic and immune effects on cardiac muscle following ipilimumab and nivolumab treatment. Patient 1’s ECG rapidly progressed to complete heart block (Panel A) followed by ventricular tachycardia (Panel B). Autopsy demonstrated lymphocytic infiltration in myocardium (intraventricular septum pictured, Panel C). Inflammatory infiltrate was comprised of CD3 positive T lymphocytes (Panel D), many of which were positive for CD8 (Panel E). Only cardiac and skeletal muscle were affected; smooth muscle and other tissue were spared (Panel F). The black arrow denotes esophageal smooth muscle without immune infiltration and the green arrow denotes esophageal skeletal muscle, which is heavily infiltrated by immune cells.
Case 2
A 63-year-old male (Patient 2) with metastatic melanoma was admitted to the hospital with fatigue and myalgias 15 days after his initial dose of nivolumab (1mg/kg) and ipilimumab (3mg/kg). Diagnostic workup revealed profound ST segment depression, a new intraventricular conduction delay, myocarditis (troponin I 47 ng/mL, CK-MB 451 ng/mL), and myositis (CPK 20,270 unit/L) (Supplementary Figure 1). Serial echocardiograms revealed low-normal left ventricular systolic function with ejection fraction of 50% (Supplementary Video 2). He was treated with high-dose glucocorticoids (methylprednisolone 1 gram daily for 4 days) and infliximab 5 mg/kg. Despite these measures, he developed complete heart block requiring a temporary pacemaker and later cardiac arrest. Initial return of spontaneous circulation was achieved, but the patient suffered a second cardiac arrest and supportive care was withdrawn.
Results
Both patients had hypertension, but did not have other cardiac risk factors, or history of statin use, prior systemic therapies, radiation, or cardiac metastases, and received ipilimumab and nivolumab on clinical trials (NCT02320058 and NCT02224781). A post-mortem gross and microscopic evaluation of both patients was performed. Cardiac histopathology on patient 1 showed an intense patchy lymphocytic infiltrate within the myocardium also involving the cardiac sinus and atrioventricular nodes (Figure 1C). No eosinophilic granulomas or giant cells were noted. Likewise, skeletal muscle showed lymphocytic destruction of isolated myocytes (Supplementary Figure 2). Infiltrating cells within the myocardium and skeletal muscle were positive for the T-cell marker CD3 (Figure 1D) or the macrophage marker CD68. T-cell infiltrates showed abundant CD4 and CD8 positive T cells (data not shown and Figure 1E). Notably, the cells were negative for CD20 and further immunofluorescence studies showed no antibody deposits (data not shown). Post-mortem histopathology of patient 2 showed similar T-cell and macrophage infiltrates in the myocardium, cardiac conduction system and skeletal muscle indicative of lymphocytic myocarditis and myositis (Supplementary Figure 1 and 2). Importantly, in both patients, immune infiltration was restricted to cardiac and skeletal muscle with no other affected tissues, including adjacent smooth muscle (Figure 1F). Pre-treatment tumor biopsies from each patient demonstrated modest or absent immune infiltrates. By contrast, post-mortem evaluation showed substantially increased, intense lymphocytic infiltrates in metastases from both patients, particularly in patient 1 (Supplementary Figure 2C–F).
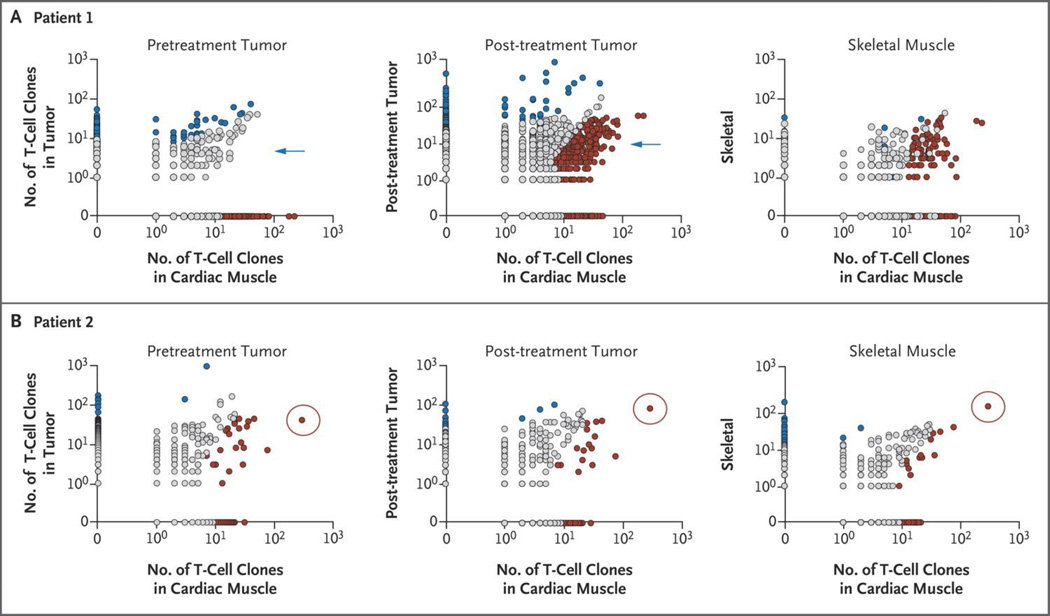
To further characterize the infiltrating lymphocytes in the myocardium, skeletal muscle, and tumor, we performed T-cell receptor next generation sequencing (CDR3 region, the antigen-binding portion of the T-cell receptor beta chain, ImmunoSeq, Adaptive Biotechnologies)8,9 to address the distribution, clonality and diversity of T-cell receptor. Both patients shared high-frequency T-cell receptor sequences among cardiac, skeletal muscle, and tumor infiltrates (Figure 2A, 2B). Specific shared clones were expanded in tumors following checkpoint inhibitor therapy. T-cell clonal expansion was most striking in patient 2, in which the most abundant T-cell receptor in myocardium and skeletal muscle was expanded more than 4-fold in the tumor following treatment (Supplementary Figure 3, 4). By contrast, no one specific clonotype predominated in patient 1. These findings raise the possibility that antigens (epitopes) present in myocardium, skeletal muscle, and perhaps tumor were recognized by the same T-cell clone(s). Whole transcriptome sequencing of the afflicted tissues demonstrated high expression of inflammatory T-cell cytokines as well as considerable expression of muscle-specific transcripts in the tumor (Supplementary Figure 5), potentially supporting the hypothesis of a common (shared) epitope between the tumor and striated muscle.
Figure 2.
T-cell receptor clones in striated muscle and tumor. T-cell receptor sequencing was performed on cardiac and skeletal muscle, and on pre/post-treatment tumors for each patient (CDR3 region of TCR beta chain, ImmunoSeq, Adaptive Biotechnologies), as previously described8,9. T-cell clones prevalence by counts displayed on biaxial plots. Numerous T-cell clones are present in all affected tissues in patient 1 (panel A) and patient 2 (panel B). Red denotes T-cell clones more prevalent in heart tissue and blue denotes more frequent clones in other tissues; for a clonotype to be determined to be differentially present, a P value for each clone in two samples being compared was calculated using a Fisher exact test and adjusted for a positive false discovery rate using the Storey method as described in25. Blue arrow denotes that few prevalent T-cell clones were shared between pre-treatment tumor and heart, but numerous shared clones expanded in the post-treatment tumor. Red circle denotes that the highest frequency T-cell clone in the heart was also highly prevalent in skeletal muscle, and expanded from the pre-treatment to post-treatment tumor.
To investigate potential patient-specific etiologies, we performed four digit class I and II human leukocyte antigen (HLA) typing (ABC DR DQ DP) (Illumina Miseq) on DNA extracted from peripheral blood and formalin-fixed tissue.10 The highly prevalent major histocompatibility complex (MHC) class II allele HLA-DQB1*03:01 was the only HLA allele shared between cases. Viral myocarditis PCR testing on frozen tissue, as well as viral serologies, were negative for adenovirus, cytomegalovirus, parvovirus, respiratory syncytial virus, influenza A, enterovirus, hepatitis C, and HHV-6. To look more broadly for viruses, we used a deep-sequencing target-enrichment system designed to detect the genomes of 472 DNA and RNA viruses known to infect humans (unpublished data, Seidman lab, Harvard Medical School). We detected Herpes virus simplex -1 (HSV-1, 38,583 nucleotides, 25.34% of the viral genome) sequences in heart tissue, but not in skeletal muscle tissue from patient 1. We also detected Epstein Barr virus (EBV, 5,456 nucleotides, 3.18% of the viral genome) sequences in heart tissue only from patient 2. EBV and HSV-1 have been rarely associated with myocarditis.11–13 Given that detection of viral genomes does not necessarily reflect active infection, it is uncertain whether these myocarditis cases are of viral etiology.
We then assessed the expression of PD-L1 from tumor tissue and inflamed cardiac and skeletal muscle. PD-L1 was expressed on the membranous surface of injured myocytes (Supplementary Figure 6) and on infiltrating CD8+ T cells and histiocytes from the inflamed myocardium. By contrast, skeletal muscle and tumor specimens were negative for PD-L1 expression (1% threshold). Similarly, mRNA transcriptional data from patient 2 showed 10-fold more abundant expression of PD-L1 (CD274) in affected cardiac tissue than in non-diseased smooth muscle and 5-fold higher than in affected skeletal muscle (Supplementary Figure 7).
To assess the frequency of myocarditis and myositis in a larger population, Bristol Myers Squibb corporate safety databases were interrogated with a cutoff date of April 2016 to identify these events arising during nivolumab +/− ipilimumab therapy. Among 20,594 patients, 18 drug-related severe adverse events of myocarditis were reported (0.087%). Patients who received combination ipilimumab and nivolumab appeared to experience more frequent and severe myocarditis compared with those who received nivolumab alone (0.27% vs. 0.06%; p<0.001; 5 fatal events vs. 1) (Table 1). Myocarditis with ipilimumab and nivolumab therapy arose at a median of 17 days following first treatment (range 13–64 days) in multiple cancer types (Supplementary Table 1). Severe (grade 3–4) myositis also appeared more frequently with the combination compared with single-agent nivolumab (0.24% vs. 0.02%) (Table 1). No obvious cardiac or cancer-specific clinical features predisposed patients to these severe adverse events (Supplementary Table 1). These events were investigator-dependent reports to Bristol Myers Squibb. No clinical trials involving nivolumab +/− ipilimumab routinely tested for myocarditis either biochemically or via cardiac imaging.
Table 1.
Incidence of myocarditis and myositis in patients receiving nivolumab monotherapy and ipilimumab and nivolumab combination therapy
| Characteristic | Patients receiving nivolumab (N = 17,620) |
Patients receiving nivolumab + ipilimumab (N = 2974) |
|---|---|---|
| Myocarditis* - no. (%) | 10 (0.06%) | 8 (0.27%) |
| Fatal events - no. (%) | 1 (<0.01%) | 5 (0.17%) |
| Myositis - no. (%) | 27 (0.02%) | 7 (0.24%) |
| Fatal events - no. (%) | 2 (0.01%) | 1 (0.03%) |
Includes 6 cases of concurrent myocarditis and myositis and/or rhabdomyolysis.
Discussion
Combined immune checkpoint inhibition with ipilimumab and nivolumab produces frequent and durable anti-tumor responses in patients with advanced melanoma and has demonstrated promising activity in other cancers.14 Immune-related adverse events, however, frequently complicate therapy, requiring cessation of therapy in nearly 40% of patients.4,5 These events are generally manageable with high-dose glucocorticoids although clinically severe, prolonged, and even fatal events rarely occur.4,5 Therefore, characterizing these severe toxicities is a major priority, even for uncommon events.
Myocarditis was rarely reported in early clinical trials with anti-CTLA-4 and anti-PD-1(nivolumab and pembrolizumab), resulting in one death in a patient treated with ipilimumab 10 mg/kg in the adjuvant setting.15–18 Our review of a large safety database suggests that myocarditis is more frequent and severe with the combination of ipilimumab and nivolumab compared with nivolumab monotherapy, but remains rare (<1%) with both regimens. Since cardiac monitoring (e.g. ECG or troponin) is not routinely performed in most immunotherapy trials, the true incidence is unknown.
Clinicians should be vigilant for immune-mediated myocarditis, particularly due to its early onset, non-specific symptomatology and fulminant progression. There are no data as to what monitoring strategy may be of value; in our practice we are obtaining a baseline ECG and weekly troponin levels during weeks 1–3 for patients on combination immunotherapy. In our experience, both patients experienced strikingly elevated troponin levels and refractory conduction system abnormalities with preserved cardiac function. Pathological examination was reminiscent of acute allograft rejection in cardiac transplantation. In this regard, high-dose glucocorticoids appeared to blunt ongoing inflammation and resulted in decreasing CPK and troponin levels but the data are not directive. Other adjunctive, immunosuppressants could also be considered, including infliximab or anti-thymocyte globulin.
We sought to mechanistically characterize these aberrant immune responses. Notably, striated muscle (cardiac and skeletal) and tumor were the only affected tissues. Robust T-cell infiltration, activation and clonal expansion were observed across tissue types with evidence of shared high-frequency T-cell receptors. Possible mechanisms for the observed toxicities include 1) T cells targeting an antigen shared among the tumor, skeletal muscle, and heart, 2) the same T-cell receptor targeting a tumor antigen and a different but homologous muscle antigen, or 3) clonal, high-frequency T-cell receptor sequences across tumor and muscle samples are misleading and distinct T-cell receptor specificities are targeting dissimilar antigens. Consistent with the first possibility, we observed high levels of muscle-specific antigens (desmin and troponin) in tumors from both patients. It is also conceivable that subclinical viral infection could generate T-cell targets, although extensive viral profiling did not reveal a clear etiology. Similarly, we did not identify common HLA alleles between patients, arguing against an HLA/drug hypersensitivity correlation. Ultimately, defining which epitopes are being recognized by these T-cell receptors within the universe of potential antigens is a difficult task. Moreover, only early mechanistic insights for any immune checkpoint inhibitor-derived toxicities have been generated.19,20 Further studies are needed to elucidate causative antigens and molecular mechanisms of these events.
There is biological plausibility for the development of myocarditis from immune checkpoint inhibition. In mouse models, PD-1 plays a role in myocardial immune responses and protects against inflammation and myocyte damage in models of T-cell mediated myocarditis.21 Genetic deletion of PD-1 in mice leads to cardiomyopathy caused by autoantibodies against cardiac troponin I.22,23 We did not observe IgG autoantibody deposition in the affected tissue, arguing against a directly analogous mechanism. Thus, the underlying etiology for T-cell reactivity to myocardial and other striated muscle tissue is not clear, and is certainly not universal across patients. Interestingly, we show increased expression of PD-L1 in the injured myocardium in our cases, consistent with upregulated myocardial PD-L1 in mouse models of T-cell mediated myocarditis.24 PD-L1 upregulation in the myocardium is likely a cytokine-induced cardioprotective mechanism that is abrogated by immune checkpoint blockade. Better understanding of the mechanism of this drug-induced toxicity may provide valuable insight into idiopathic myocarditis in the non-cancer population, as well as the general interaction between the immune system with the myocardium.
Supplementary Material
Acknowledgments
Dr. Johnson is a consultant for Bristol-Myers Squibb and Genoptix. Drs. Reshef, Kola, Plautz, and Deutsch are employees of Bristol-Myers Squibb. Dr. Deering is an employee of Neon Therapeutics. Dr. Diaz is a director and founder of Personal Genome Diagnostics (PGDx) and a founder of PapGene, Inc. and owns stock in both entities. He is a consultant to Merck, Cell Design Laboratories, Illumina and PGDx. PapGene, PGDx and other entities have licensed several patent applications from Johns Hopkins, where Dr. Diaz is an inventor. These relationships are subject to certain restrictions under Johns Hopkins University policy, and the terms of these arrangements are managed by the university in accordance with its conflict-of-interest policies. Dr. Sosman is a consultant for Merck and Array. Dr. Moslehi is a consultant for Novartis, Pfizer, Bristol-Myers Squibb, Takeda, Ariad, Acceleron, Vertex, Incyte, Rgenix, Verastem.
Funding: The authors have received funding from the Bready Family Foundation (DBJ and JMB), NIH/NCI 6R00CA181491 (JMB), VICC ambassadors (JMB and JJM), the Breast Cancer Specialized Program of Research Excellence (SPORE) grant P50 CA098131 (JMB and JJM), and the National Comprehensive Cancer Network Young Investigator Award (DBJ).
Footnotes
Conflicts of interest
No other authors reported conflicts of interest.
References
- 1.Wolchok JD. PD-1 Blockers. Cell. 2015;162:937. doi: 10.1016/j.cell.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 7.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. The New England journal of medicine. 2015;373:288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson CS, Emerson RO, Sherwood AM, et al. Using synthetic templates to design an unbiased multiplex PCR assay. Nature communications. 2013;4:2680. doi: 10.1038/ncomms3680. [DOI] [PubMed] [Google Scholar]
- 9.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham J, Oseroff C, Hinz D, et al. Sequence conservation predicts T cell reactivity against ragweed allergens. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2016 doi: 10.1111/cea.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis--diagnosis, treatment options, and current controversies. Nature reviews Cardiology. 2015;12:670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 12.Cooper LT., Jr Myocarditis. The New England journal of medicine. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin LD, Kearney D, Ni J, et al. Analysis of formalin-fixed and frozen myocardial autopsy samples for viral genome in childhood myocarditis and dilated cardiomyopathy with endocardial fibroelastosis using polymerase chain reaction (PCR) Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 1995;4:3–11. doi: 10.1016/1054-8807(94)00025-m. [DOI] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. The lancet oncology. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 16.Laubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. Journal for immunotherapy of cancer. 2015;3:11. doi: 10.1186/s40425-015-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. The New England journal of medicine. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koelzer VH, Rothschild SI, Zihler D, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. Journal for immunotherapy of cancer. 2016;4:13. doi: 10.1186/s40425-016-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nature communications. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Science translational medicine. 2014;6:230ra45. doi: 10.1126/scitranslmed.3008002. [DOI] [PubMed] [Google Scholar]
- 21.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188:4876–4884. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 24.Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 25.DeWitt WS, Emerson RO, Lindau P, et al. Dynamics of the cytotoxic T cell response to a model of acute viral infection. Journal of virology. 2015;89:4517–4526. doi: 10.1128/JVI.03474-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.