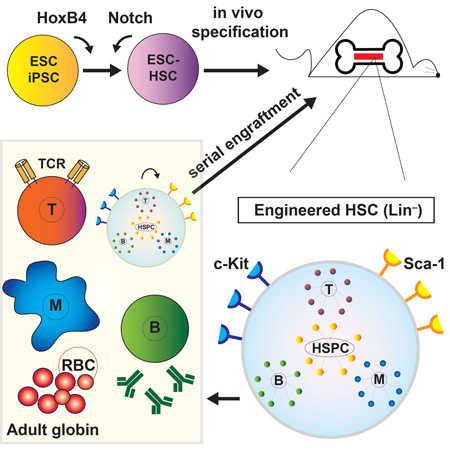
Summary
Hematopoietic stem cell (HSC) transplantation is curative for malignant and genetic blood disorders, but is limited by donor availability and immune-mismatch. Deriving HSCs from patient-matched embryonic/induced-pluripotent stem cells (ESC/iPSCs) could address these limitations. Prior efforts in murine models exploited ectopic HoxB4 expression to drive self-renewal and enable multi-lineage reconstitution, yet fell short in delivering robust lymphoid engraftment. Here, by titrating exposure of HoxB4-ESC-HSC to Notch ligands, we report derivation of engineered HSCs that self-renew, repopulate multi-lineage hematopoiesis in primary and secondary engrafted mice, and endow adaptive immunity in immune-deficient recipients. Single-cell analysis shows that following engraftment in the bone marrow niche, these engineered HSCs further specify to a hybrid cell-type in which distinct gene regulatory networks of hematopoietic stem/progenitors and differentiated hematopoietic lineages are co-expressed. Our work demonstrates engineering of fully functional HSCs via modulation of genetic programs that govern self-renewal and lineage priming.
Graphical Abstract
eTOC Blurb
Embryonic/induced pluripotent stem cells (ESC/iPSCs) represent an unlimited source of hematopoietic stem cells (HSCs) for treating blood diseases. By modulating genetic programs governing self-renewal and lineage-guiding pathways, Lu et al. derive engineered HSCs with robust lymphoid potential and endow adaptive immunity in vivo.
Introduction
Directed differentiation of transplantable and functionally definitive HSCs from ESC/iPSCs has been a long-sought goal, but has not yet been reproducibly demonstrated, presumably due to incomplete understanding of the complex temporal and spatial cues needed to guide cells through immature developmental states to become bona fide adult HSCs. Recent advances in HSC engineering include respecification from committed blood progenitors (Riddell et al., 2014) and trans-differentiation from fibroblasts (Pereira et al., 2013) or endothelial cells (Sandler et al., 2014).
Previously we have derived self-renewing multipotent hematopoietic progenitors from ESCs by culturing pluripotent stem cells as embryoid bodies followed by ectopically expressing HoxB4, a homeobox transcription factor important in early embryonic patterning and HSC self-renewal (Kyba et al., 2002, Wang et al., 2005b). Although HoxB4 overexpression confers long-term engraftment and multi-lineage differentiation potential on ESC- and yolk sac (YS)-derived blood progenitors, which thus qualify as ESC-HSCs, hematopoietic reconstitution is skewed towards the myeloid lineage, and consequently ESC-HSCs do not fully reconstitute the host’s immune system (Kyba et al., 2002, Mckinney-Freeman et al., 2009, Lengerke and Daley, 2010) even when lymphoid fate is modestly boosted by co-expression of Cdx4 (Wang et al., 2005b). Our recent network biology analysis indicated that HoxB4-induced ESC-HSC lack Notch pathway activation (McKinney-Freeman et al., 2012). Thus we set out to determine whether incorporating treatment with Notch ligands into our in vitro differentiation protocols would complement this deficiency and produce more robust ESC-HSCs.
Notch is an evolutionally conserved pathway best known for its role in cell fate decision (Ehebauer et al., 2006) and T cell commitment/lymphopoiesis (Ciofani and Zúñiga-Pflücker, 2005, Radtke et al., 2004). Notch signaling is critically engaged at multiple stages throughout hematopoietic ontogeny. Knockout and chimeric murine studies have shown that Notch1-mediated signaling is autonomously required for the generation of HSC (Hadland, 2004, Kumano et al., 2003). In mice, the earliest HSCs emerge from hemogenic endothelium (HE) of the E10.5 aorta-gonad-mesonephros (AGM) region of the embryo proper (Boisset et al., 2010) and are capable of sustaining the full spectrum of blood lineages (Clements and Traver, 2013, Dzierzak and Speck, 2008). At the E9–10 pre-HSC stage, Notch signaling provided by AGM-derived endothelial cells promotes HSC specification from both HE and HSC precursors (Hadland et al., 2015), and Notch1 signaling promotes the transition from endothelial to hematopoietic fate (Ditadi et al., 2015, Jang et al., 2015, Kim et al., 2013). At the fetal liver stage, Notch is required to sustain HSC survival (Hadland et al., 2015, Gerhardt et al., 2014). Furthermore, ex vivo Notch activation in mouse and human HSPCs by immobilized Delta-like 1 (DL1) extracellular domain fused to the Fc domain of human IgG (DL1-Fc) has resulted in substantial cell expansion that enhances short-term engraftment in patients following myeloablative conditioning in the context of cord blood transplantation (Varnum-Finney et al., 2003, Delaney et al., 2010). Although not required to maintain the HSC state during homeostasis in adult marrow (Maillard et al., 2008), Notch does play a role in regulating the rate of marrow engraftment and types of progenitors generated (Ohishi et al., 2002). Taken together, these observations suggest a successive requirement of Notch signaling during the development of HSCs.
Here, using the myeloid-skewed HoxB4-modified ESC-HSCs as a starting point, we have systematically tested the effects of Notch pathway activation by an inducible form of the intracellular domain of Notch1 (ICN) (Jang et al., 2015), by co-culture with DL1-expressing OP9 stroma, or by exposure to immobilized DL1-Fc in a stromal-free culture system, and have observed that titrated Notch induction significantly affects cell fate and lineage propensity of ESC-HSCs. After optimized exposures, Notch-activated ESC-HSCs established functional adaptive immunity in immune-deficient NOD/scid/γ(c)-null (NSG) recipients. Coordinating approaches of in vitro factor/pathway complementation and in vivo specification, we report a modular route to building physiologically relevant and self-renewing HSCs from ESC/iPSCs.
Results
Notch-activated ESC-HSCs Manifest Enhanced Lymphoid Potential
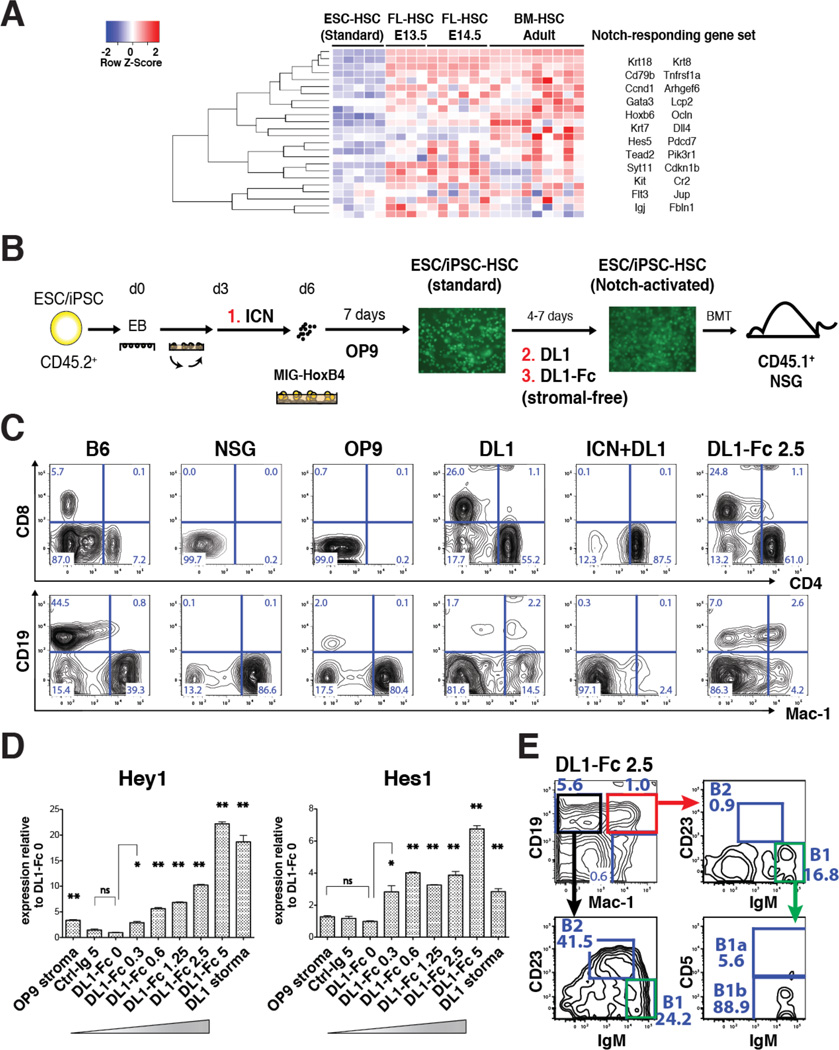
To derive ESC-HSCs, embryoid bodies (EBs) at day 6 of differentiation are transduced with a bi-cistronic murine stem cell virus (MSCV) retroviral construct carrying HoxB4 and eGFP, and expanded on hemato-supportive OP9 stroma (Nakano et al., 1994). The resulting hematopoietic colonies are capable of long-term in vivo reconstitution of irradiated recipient mice (Kyba et al., 2002, Wang et al., 2005b, Mckinney-Freeman et al., 2009). However, a network biology-based approach comparing global gene expression profiles of ESC-HSCs and embryo-derived HSCs throughout ontogeny indicates that ESC-HSCs closely resemble the definitive HSCs at late-fetal liver and adult bone marrow (BM) stages, but lack expression of genes activated in response to Notch pathway stimulation (McKinney-Freeman et al., 2012). To investigate whether complementing Notch signals would improve the lymphoid-deficient phenotype of ESC-HSC, we employed a differentiation strategy that provides Notch induction by cell-intrinsic activation of a doxycycline (dox)-inducible ICN transgene using the iICN ESC (Jang et al., 2015), with or without additional Notch ligand exposure. Earlier attempts using overtly activated iICN cells led to poorly differentiated T cell lymphoma and lethality at 3–7 weeks post-transplant (data not shown). On the other hand, transient ICN induction during d3-5 EB was not oncogenic and enhanced the endothelial to hematopoietic transition (Jang et al, 2015). Hence, we tested the effect of d3-5 ICN induction on hematopoietic specification in the context of HoxB4-driven hematopoiesis (ICN, Fig.1B). To interrogate the effect of Notch at later differentiation stages after ESC-HSCs are formed, we expanded the HoxB4-transduced hematopoietic precursors on DL1 ligand-expressing OP9 stroma (DL1, Fig.1B), or on immobilized DL1-Fc in a stromal-free system (DL1-Fc, Fig.1B). After 4–7 days of ligand exposure with lymphoid-promoting cytokines, we transplanted the resulting Notch-activated ESC-HSCs into sublethally irradiated NSGs and evaluated engraftability, lineage propensity, and long-term repopulating potential. In the reconstituted mice, donor-derived hematopoietic contribution can be distinguished from endogenous host cells by the expression of GFP and the CD45.2 allelic variant. Using this approach, we were able to derive engraftable ESC-HSCs from 6 different ESC lines, as well as 3 iPSC lines reprogrammed from murine embryonic fibroblasts (iPSC-HSC images shown in Fig.1B). Consistent with previous findings, our in vivo reconstitution data showed that ESC-HSCs were T cell-deficient (OP9, Fig.1C), whereas ESC-HSCs co-cultured on DL1-stroma were profoundly T cell-biased (DL1, Fig.1C). We also observed that the lymphoid propensity correlated with the timing and signal strength of Notch induction: ESC-HSCs subjected to a superimposed dose of ICN induction during d3-5 EB and DL1-stroma stimulation reconstituted almost exclusively CD4+ T cells (ICN+DL1, Fig.1C). ESC-HSCs exposed to an intermediate dose (DL1) reconstituted T cells while maintaining differentiation into Mac-1+ myeloid cells. When ESC-HSCs were expanded on a lower dose of immobilized DL1-Fc that corresponds to half the dose of DL1 stromal conditions according to quantifications of downstream Hey1 and Hes1 Notch targets, we observed multi-lineage peripheral reconstitution of T, myeloid and B cells containing both conventional B-2 (CD19mid IgMlow CD23+ Mac-1−) and B-1 cells (CD19hi Mac-1+ IgMhi CD23−) (Baumgarth, 2011, Yoshimoto et al., 2011). The refined dose of Notch activation enhanced B-2 potential, whereas the standard ESC-HSC-OP9 reconstituted mostly B-1 cells and were hence restricted to the peritoneum and spleen (Fig.2A & S1–S2). Our data suggests that Notch pathway activation confers lymphoid fate on ESC-HSCs and that the signal timing and strength of Notch plays a pivotal role in balancing multi-lineage potential. Transient ligand-mediated Notch activation for 4–7 days was the optimum for producing multipotent ESC-HSCs, whereas transient ICN induction at early specification stages (ICN+DL1) shifted the reconstitution to exclusively CD4 T cells.
Figure 1. Notch-activated ESC-HSCs Manifest Enhanced Lymphoid Potential.
Heatmap of gene sets up-regulated by Notch signaling in the standard ESC-HSCs, FL-HSCs and BM-HSCs. (B) Schematic diagram of HoxB4-modified ESC-HSC derivation and methods of Notch pathway activation. ESC/iPSC differentiation was initiated by hanging-drop aggregation and rotational culture. At day 6, EBs were transduced with GFP-tagged HoxB4 retrovirus (MIG-HoxB4) and expanded on OP9 stroma with cytokines. The resulting colonies (CD45.2+) were transplanted into irradiated NSG mice (CD45.1+). Notch pathway can be activated cell-intrinsically by adding dox during d3-5 EB to the Notch-inducible iICN culture (#1, ICN). Further Notch activation can be achieved by transferring ESC-HSCs to DL1-expressing OP9 stroma (#2, DL1), or by expanding ESC-HSCs on immobilized DL1-Fc in a stromal-free system (#3, DL1-Fc). Fluorescent images of iPSC-HSC are shown. (C) Donor-derived peripheral reconstitution of representative NSGs transplanted with standard (OP9) and Notch-activated ESC-HSCs stimulated by DL1 stroma co-culture (DL1), d3-5 ICN induction and DL1 stroma co-culture (ICN+DL1), or by immobilized DL1-Fc at 2.5 µg/mL (DL1-Fc 2.5) using iICN at 11 wks post-transplant. (D) Quantification of Notch downstream targets in CD41+ ESC-HSCs, 4–5 days post-Notch activation. Results of three independent iICN derivations are shown (* p ≤ 0.05, ** p ≤ 0.01). See also Fig.S1–2. (E) B-1 phenotype of peripheral CD19+ Mac-1+ B cells of a representative recipient of DL1-Fc 2.5-activated ESC-HSC at 31 weeks post-transplant. B-1 cells (green gate) were mostly of B-1b (CD5−) subtype.
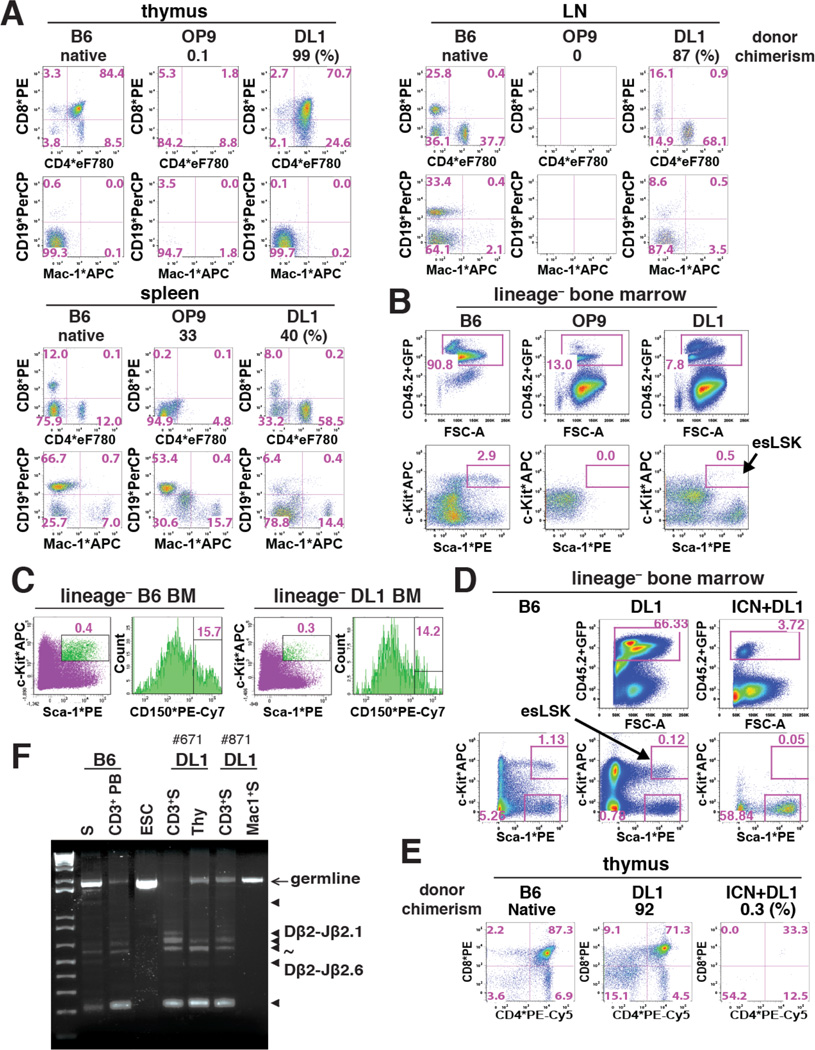
Figure 2. Notch-activated ESC-HSC-DL1 Specifies to esLSKs Upon Engraftment.
(A) Donor-derived reconstitution of standard (OP9) and Notch-activated (DL1) AV15 ESC-HSCs in the thymus, lymph nodes (LN) and spleen of representative NSGs at 12 wks post-transplant. In each panel, percent donor chimerism (CD45.2+ and GFP+), donor-derived CD4/CD8 (middle) and CD19/Mac-1 (bottom) lineages are shown. (B–C) LSK-CD150 profile of BM-engrafted AV15 ESC-HSCs. esLSKs are indicated by arrow. (D–E) BM and thymic engraftment of DL1- and ICN+DL1-activated iICN ESC-HSCs at 16 wks post-transplant. Arrow indicates esLSKs. (F) PCR analysis of TCRβ-DJ rearrangement in sorted lineages of two representative iICN ESC-HSC-DL1 recipients at 16 wks post-transplant. Arrow: germline amplicons; triangles: Dβ2-Jβ2.1 to Dβ2-Jβ2.6 amplicons. Lane#1-2: B6 genomic DNA of splenocytes (S) and peripheral CD3+ T cells (CD3+ PB); #3: ESC; #4-7: CD3/Mac-1+ splenocytes and total thymocytes. See also Fig.S3.
In contrast to HoxB4-modified ESC-HSCs, which failed to reconstitute the T cell compartment in vivo, we found that ESC-HSC-DL1 reconstituted NSG thymus, which were enriched in the CD4+CD8+ double-positive population, as well as the mesenteric/inguinal lymph nodes and the spleen, which were composed of mostly mature single-positive T cells with some CD19+ B cells (Fig.2A). These data suggest that ESC-HSC-DL1-derived HSPCs follow the native path of lymphoid development and contribute to the formation of primary and secondary lymphoid organs in NSG mice. Notably, we found that ESC-HSC-DL1 further acquired the Lin− Sca-1+ c-Kit+ CD150+ (LSK-CD150+) phenotype upon engraftment, whereas the standard ESC-HSCs did not reconstitute LSK in vivo (Fig.2B–C). The maturation of these engrafted ESC-HSC-DL1-derived LSKs, i.e., the esLSKs, indicates that Notch-activated ESC-HSC can be further specified through additional developmental cues provided by the BM microenvironment. Notch-activation enhanced the LSK population in vitro (Fig.S3A). By performing limiting dilution transplantation assay (LDA), we estimated reconstitution frequencies to be 1 per 22 adult BM-LSK, 1 per 9.7 × 104 standard ESC-HSC-LSK (in vitro), and 1 per 5.8 × 104 Notch-activated ESC-HSC-LSK (in vitro), thus indicating that Notch activation modestly improved the reconstitution frequency; however, these cells remain less robust in reconstituting irradiated recipients than native bone marrow-derived HSCs (Fig.S3B).
In an independent cohort, we compared the reconstitution outcomes of ESC-HSCs with intermediate (DL1) or high dose (ICN+DL1) Notch pathway activation, and verified that ESC-HSC-DL1 further matured into esLSK in the BM and showed long-term T cell reconstitution in the thymus (Fig.2D–E), whereas high dose Notch led to CD4-restricted progeny (Fig.1B) and no thymic reconstitution (Fig.2E). BM repopulation was achieved without apparent tumor formation up to 16 weeks post-transplant, the end point of our experiments, with Sca-1+ c-Kit− lymphoid progenitors, but not esLSKs representing the majority population (Fig.2D). These results demonstrate that Notch pathway manipulation superimposed upon the derivation protocols for HoxB4-modified ESC-HSC retains multi-potentiality while enhancing lymphoid potential.
To examine whether T cells derived from ESC-HSC-DL1 had effectively rearranged genes encoding the T cell receptor (TCR), we sorted CD3+ T cells from engrafted NSGs and assessed the D-J recombination status of the TCR β-locus with primers specific to DH β2.1and JH β2.7 (Schmitt and Zuniga-Pflucker, 2002). Similar to native B6 T cells, ESC-HSC-DL1-derived T cells and thymocytes exhibited a diverse pool of polyclonal TCR rearrangements, whereas donor-derived Mac-1+ and ESC controls showed only the germline amplicon (Fig.2F). These data indicate that T cells derived from ESC-HSC-DL1 mature in the thymus, circulate in blood, home to spleen and lymph nodes, and possess functional recombination machinery sufficient to rearrange the TCR-β locus.
Notch-activated ESC-HSCs Restore Functional Adaptive Immunity
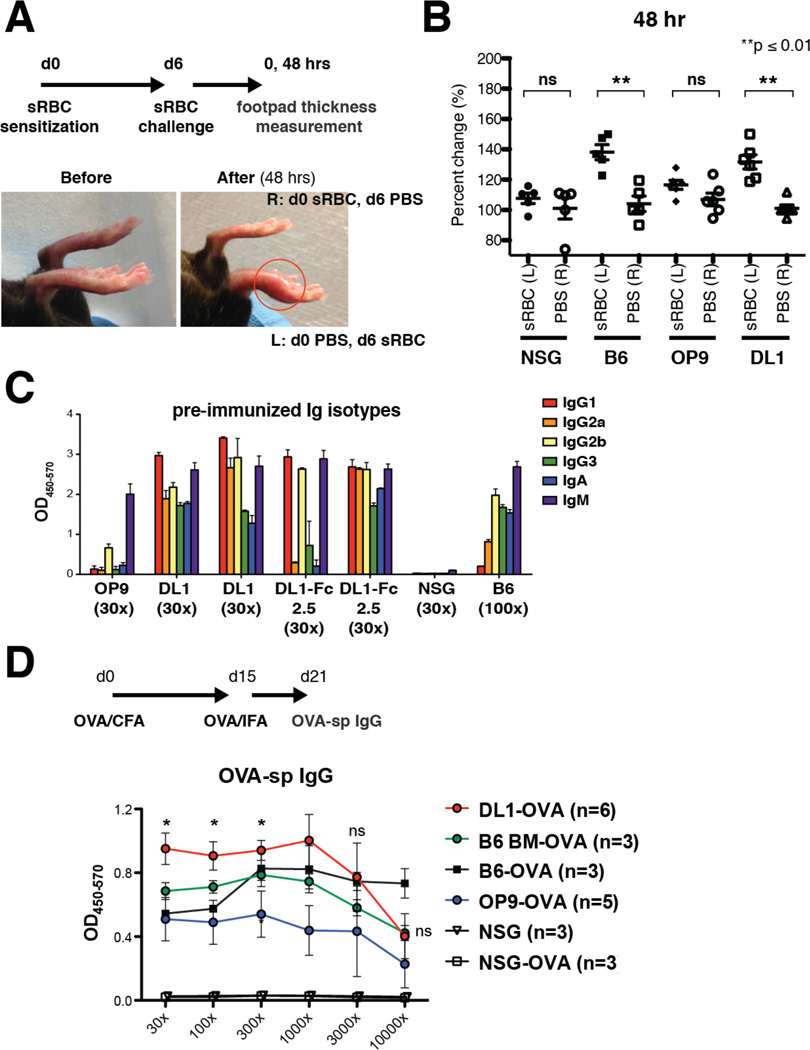
Adaptive immunity refers to antigen-specific cellular and humoral immune responses mediated by T and B cells, respectively. As we have demonstrated enhanced lymphoid reconstitution of Notch-activated ESC-HSCs, we sought to evaluate functional reconstitution by measuring acquired T and B cell-mediated responses in immune-deficient NSG recipients. We adopted a classical Th1 cell-mediated delayed type hypersensitivity (DTH) test to assess T cell function, in which reconstituted NSGs are sensitized with a foreign antigen, sheep red blood cells (sRBC), and challenged intra-dermally with sRBC in one footpad and with PBS in the control footpad. Local erythema and induration consequently reflects recruitment of antigen-specific T cells to the challenged site (Huber et al., 1976). In normal B6 mice, foot pad swelling peaked around 48 hours post-challenge (Fig.3A), whereas control NSGs showed no meaningful difference between the two footpads, consistent with the immune deficiency of this strain. In NSGs reconstituted with ESC-HSC-DL1, we found significant footpad swelling in the challenged site versus control, whereas no difference was observed in NSGs reconstituted with ESC-HSC, which lacked T cells (Fig.3B).
Figure 3. Notch-activated ESC-HSCs Reconstitute Adaptive Immunity.
(A) Protocol DTH model. sRBC-sensitized B6, NSG and iICN ESC-HSC-reconstituted NSGs were challenged with sRBC in the left footpad (L) and with PBS in the right footpad (R) at day 6 post-sensitization. Thickness of each footpad was measured at 0 and 48 hrs post-challenge. Representative images of footpad swelling (red circle) before and 48 hrs post-challenge are show. (B) Percent changes of footpad thickness relative to 0 hour measurement at 48 hrs post-challenge. (C) Pattern of Ig isotype production of reconstituted NSGs prior to immunization, ≥ 8 wks post-transplantation. Sample dilution factors are as indicated. (D) Immunization protocol for eliciting antigen-specific B cell responses. Groups of 5–6 reconstituted mice were immunized with OVA/CFA and boosted with OVA/IFA emulsions. Averaged curves (right) of OVA-sp IgG a week after boosting are shown. Naïve and immunized (−OVA) NSG and B6, as well as B6 BM-engrafted NSG controls (B6 BM) are included. Asterisks indicate statistically significant (p ≤ 0.05) differences between immunized ESC-HSC-OP9 and –DL1 groups.
Another canonical T cell feature is facilitation of class-switch recombination in B cells. We analyzed circulating levels of immunoglobulin (Ig) isotypes, and found evidence of IgM, IgA, and IgG in the serum of normal B6 controls and NSGs reconstituted with Notch-activated ESC-HSCs. As expected, isotype levels of normal NSGs were negligible due to the absence of Ig-producing B cells, and pre-immune sera of NSGs reconstituted with ESC-HSC consisted of predominantly IgM, which is the default isotype before switching occurs (Fig.3C). Specific patterns of Ig isotype production can provide insights into the type of immune response; e.g., Th1 drives the production of IgG2a, IgG2b, and IgG3, whereas Th2 drives IgG1 and IgE (Snapper and Paul, 1987, Toellner et al., 1998). Our isotype analysis suggests that the derived T population contains both Th1 and Th2 cells that are capable of eliciting a spectrum of Ig-production by the derived B cells.
To examine antigen-specific B cell responses, we immunized reconstituted NSGs with ovalbumin (OVA), monitored the production of OVA-specific IgG one week after antigen boosting, and detected high levels of antigen-specific IgG in both Notch-activated and standard ESC-HSC groups. B cells derived from ESC-HSC-DL1 produced significantly higher levels of antigen-specific IgG than the standard ESC-HSC group, and the antibody level was comparable to immunized B6 and B6 BM-reconstituted NSG controls (Fig.3D). These results indicate that engraftment of ESC-HSC-DL1 restored canonical B cell functions such as the ability to perform isotype-switching and produce antigen-specific IgG. Collectively, our data indicate that engraftment of Notch-activated ESC-HSCs restores adaptive immunity in NSG mice.
Notch-activated ESC-HSCs Reconstitute Definitive Erythroid Lineages
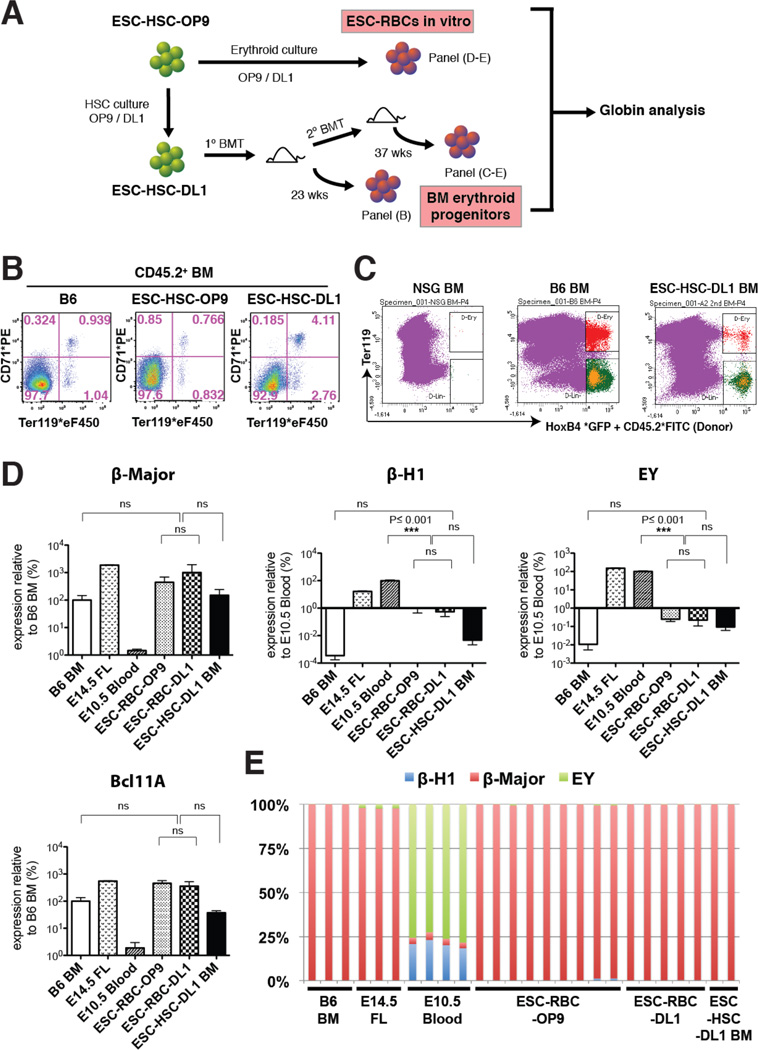
Along with the formation of definitive functional T and B cells, formation of adult, beta-globin expressing erythrocytes is an essential marker of definitive hematopoietic potential. In addition to reconstitution with lymphoid and myeloid lineages, we found erythroid reconstitution in BM of ESC-HSC-engrafted NSG recipients. As erythroid cells enucleate and lose CD45 expression during maturation, we focused on GFP+ BM progenitors that retained CD45.2 expression, and examined the presence of donor-derived erythroid cells by Ter-119 and CD71 surface phenotype (Zhang et al., 2003). As RBCs have a long circulatory half-life (around 50 days in mice), we performed erythroid analysis only in long-term engrafted mice to exclude the possibility of detecting persisting in vitro erythroid colonies or short-term engrafting erythroid progenitors (Fig.4A). Mice engrafted with ESC-HSC-DL1 showed donor-derived erythroid cells from the early basophilic stage and beyond, suggesting that Notch pathway manipulation did not compromise the erythroid potential in ESC-HSCs (Fig.4B). In addition, ESC-HSC-DL1-derived erythropoiesis was detected in serially transplanted NSGs, suggesting that these erythroid BMs originated from a self-renewing cell source (Fig.4C).
Figure 4. Notch-activated ESC-HSCs Reconstitute Definitive Erythroid Lineages.
Derivation of ESC-RBCs and BM erythroid progenitors from iICN ESC-HSCs. (B) Erythroid reconstitution of donor CD45.2+ BMs from NSGs transplanted with ESC-HSC-OP9/DL1 at 23 wks post-transplant. (C) Sorting scheme of ESC-HSC-DL1-derived erythroid BM progenitors (red) at 37 wks post-secondary transplant. (D–E) Transcript levels of β-Major, β-H1, ΕY, Bcl11a, as well as globin composition of each biological replicate of B6 BM and E14.5 FL (Ter119+), E10.5 blood (CD71+ Ter119+), ESC-RBC-OP9/DL1 (GFP+ Ter119+) and engrafted ESC-HSC-DL1-derived erythroid progenitors. See also Fig.S4.
When derived in vitro, ESC-HSC typically lack expression of erythroid genes, but when switched to culture conditions favoring erythroid differentiation, give rise to benzidine+ erythroblasts (ESC-RBC) (Fig.4A & S4). Remarkably, in vitro-derived ESC-RBCs and donor-derived erythroid progenitors recovered from the BM of ESC-HSC-engrafted mice expressed levels of adult β-Major globins comparable to erythroid progenitors recovered from native B6 erythroid BM (Fig.4D). Furthermore, we detected high levels of Bcl11a, a master regulator of adult globin switching, in both ESC-RBCs and engrafted erythroid progenitors, suggesting that the canonical globin switching machinery is preserved in ESC-HSCs. The pattern of hemoglobin expression showed a complete embryonic to adult globin switch in all ESC-derived erythroid cells examined, confirming their definitive hematopoietic potential (Fig.4E).
Notch-activated ESC-HSC Clones Serially Reconstitute Multilineage Hematopoiesis
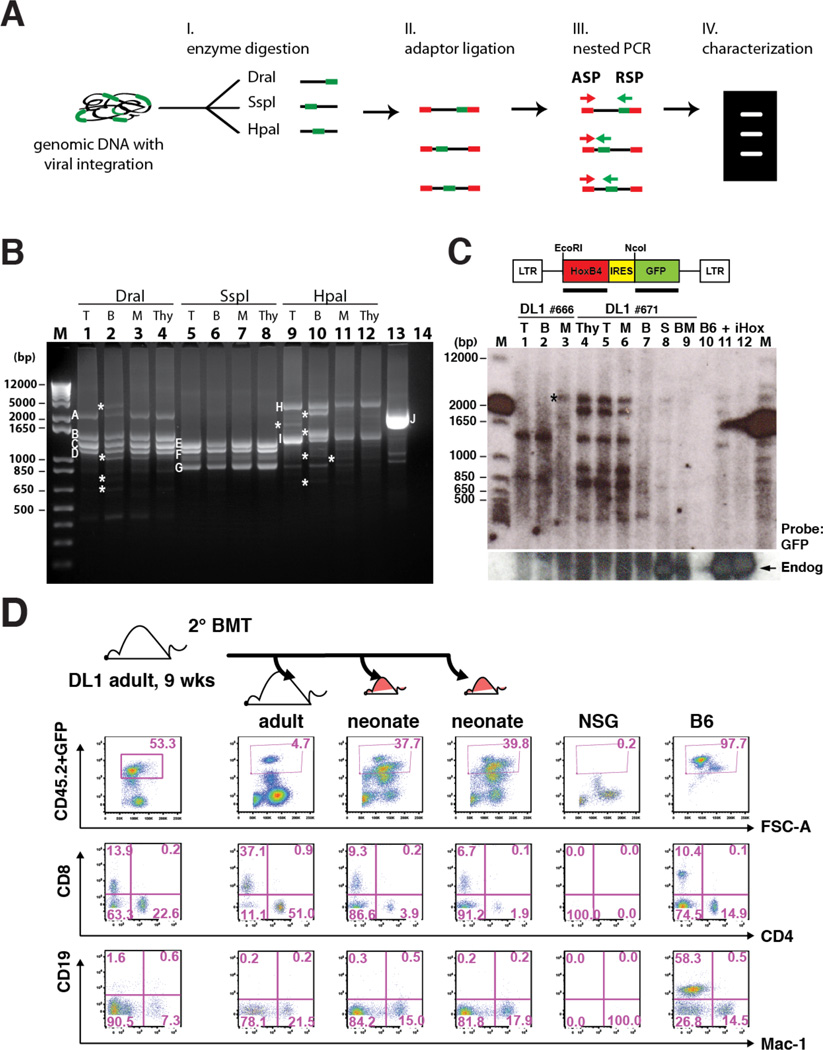
To address whether the multi-lineage hematopoietic reconstitution from Notch-activated ESC-HSC represent clonal, self-renewing HSCs, we performed retroviral integration site analysis of purified lineages by linker adaptor-mediated PCR (LAM-PCR) (Fig.5A). These data confirmed identical retroviral integration sites among multiple donor-derived T, B and myeloid isolates from a representative NSG recipient of ESC-HSC-DL1 (Fig.5B, S5 & Table S1). In one animal, we identified an integration site at the intergenic region near Lmo2, an integration hotspot associated with T cell leukemia (Hacein-Bey-Abina et al., 2008). However, we do not believe that up-regulation of Lmo2 is essential for our system, as 1) Lmo2 was not over-expressed in all ESC-HSC and esLSK examined; and 2) multi-lineage reconstitution can be achieved by using an inducible HoxB4 derivation platform without retroviral transgene delivery, indicating that the enhanced T cell potential and engraftment phenotype were independent of Lmo2 or other insertional mutagenesis (Fig.S6 & Table S2). We independently confirmed the clonal origin of ESC-HSC-DL1-derived blood lineages by Southern blotting analysis in another two representative NSG recipients. By using a provirus-specific GFP probe and restriction digestions that indicate unique proviral insertion sites, we detected comparably sized provirus-containing genomic DNA fragments in multiple independent lineages (Fig.5C). Together, our LAM-PCR and Southern blotting analyses demonstrate that distinct ESC-HSC-DL1-derived lymphoid and myeloid lineages are clonally related. Although most dominant clones exhibited multipotentiality for T, B and myeloid lineages, we observed the presence of clones that were predominant in one/two lineages, suggesting that ESC-HSC-DL1 also gave rise to self-renewing uni- or bipotential lineage-committed progenitors (asterisks, Fig.5B & C).
Figure 5. Notch-activated ESC-HSC Clones Serially Reconstitute Multilineage Hematopoiesis.
(A) Schematic diagram of retroviral integration site analysis by LAM-PCR. Genomic DNAs with proviral integrations (green) were digested with three sets of restriction enzyme (DraI, SspI and HpaI) and ligated with adaptors (red). Major amplicons generated by nested PCR with adaptor-specific primer (ASP) and retrovirus-specific primer (RSP) were sequenced and mapped for integration sites. (B) LAM-PCR of splenic donor-derived lineages of a representative iICN ESC-HSC-DL1 recipient at 16 wks post-transplant. Major bands across lineages (A–I) and an assay control of human genomic DNA containing a known retroviral integration at chromosome 17 near RSAD1 gene (J) were individually sequenced (Fig.S5 & Table S1). T: CD3+ T cells; B: B220+ B cells; M: Mac-1+ cells; Thy: thymocytes; #14: no template control; asterisks indicate amplicons predominant in one/two lineages. (C) Southern blotting analysis of common integration sites of two independent recipients (#666 and #671) engrafted with iICN ESC-HSC-DL1. S: splenocytes; BM: bone marrows; B6: B6 splenocytes; +: total #671 splenocyte; iHox: inducible HoxB4 ESC. Top: GFP probed blot; bottom: HoxB4 probed blot. Arrow: endogenous HoxB4; asterisk indicates the clone predominantly detected in one lineage. (D) At 9 wks post-transplant, BMs of a representative iICN ESC-HSC-DL1 recipient were serially transplanted into one adult and two neonatal secondary recipients; lineage analysis of donor-derived reconstitution at 8 wks post-secondary transplant is shown. See also Fig.S5–6 & Table S1.
Next, we examined the self-renewal capacity of ESC-HSC-DL1 by serial transplantation into adult or neonatal NSGs, and found that ESC-HSC-DL1 were able to engraft secondary adults with robust T cell potential (Fig.5D). As neonatal niches provide a more permissive microenvironment for embryo-derived pre-HSCs (Yoder et al., 1996), we observed a significant increase of donor-chimerism in neonatal recipients; however, the lymphoid reconstitution was reduced for unclear reasons. Our data suggest that Notch-activated ESC-HSCs functionally recapitulate embryo-derived HSCs in terms of the capacity to serially reconstitute multi-lineages, and indicate that ESC-HSC-DL1 favors the adult over neonatal niches in terms of nurturing T cell-potentiality.
In Vivo Specification of esLSK
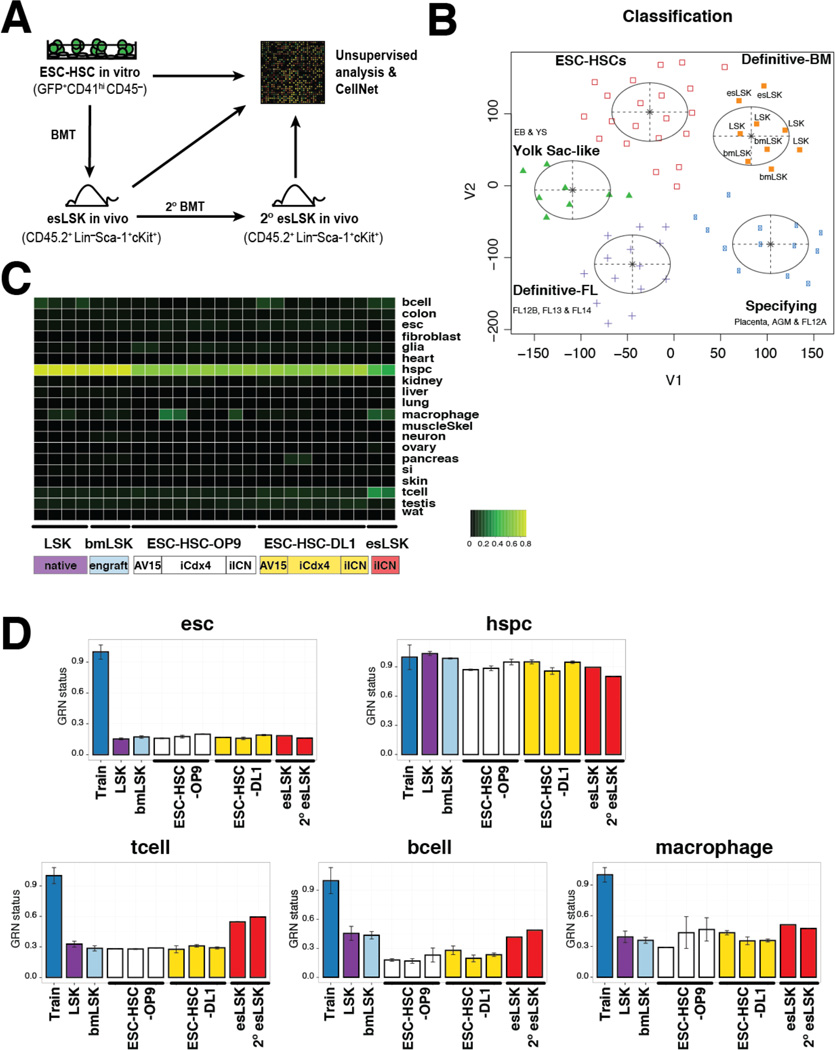
To further investigate the underlying molecular identity of ESC-HSC, we applied unsupervised clustering analysis to expression profiles of ESC-HSCs (GFP+CD41hiCD45−) cultured in vitro, the long-term-reconstituted esLSK (CD45.2+Lin−Sca-1+cKit+) from both primary and secondary recipients at 18/37 weeks post-transplant, and the hematopoietic progenitors at various stages throughout ontogeny (McKinney-Freeman et al., 2012) (Fig.6A). Compared with our previous analysis, which found that embryo-derived HSCs exist in three predominant transcriptional states (McKinney-Freeman et al., 2012), we found that both standard and Notch-activated ESC-HSCs in culture fell into a distinct “ESC-HSC” cluster. Interestingly, Notch-activated ESC-HSC isolated after engraftment in BM showed further specification as esLSK, which manifest a transcriptional profile that clustered with the native B6 LSK (LSK) and NSG BM-engrafted B6 LSK (bmLSK) (Fig.6B). These data indicate that Notch-activated ESC-HSC can be further specified by the BM niche into cells whose global transcriptional profile closely resembles native BM-derived LSK HSCs.
Figure 6. Activation of Lineage-specific GRNs in esLSKs.
(A) Schematic diagram of ESC-HSC processing for unsupervised transcriptome and CellNet analyses. ESC-HSCs in vitro are GFP+ CD41hi CD45− sorted; reconstituted primary and secondary esLSKs are CD45.2+ Lin− Sca-1+ c-Kit+ sorted at 18 and 37 wks post-transplant, respectively. (B) t-SNE analysis of embryo- and ESC-derived hematopoietic progenies. Tissue classification heatmap (C) and GRN scores (D) of native B6 LSK (purple), NSG BM-engrafted B6 LSK (bmLSK, teal), standard and Notch-activated ESC-HSCs (OP9 and DL1 in white and yellow, respectively) of three ESC backgrounds (AV15, iCdx4 and iICN), and iICN esLSKs (red) recovered from primary and secondary (2°) recipients analyzed by CellNet.
We next examined the gene regulatory network (GRN) status of ESC-HSCs in culture and esLSKs, and compared those with native B6 LSK and engrafted bmLSK using CellNet, a network biology-based platform that assesses fidelity of engineered cell types relative to their in vivo targets by assessing GRNs as an indicator of cell identity (Cahan et al., 2014, Morris et al., 2014). CellNet revealed that in vitro-derived ESC-HSCs and in vivo-reconstituted esLSKs possessed GRNs that highly resemble HSPCs and are distinct from their ESC origin. In analyzing 17 in vitro ESC-HSC, we did not observe apparent heterogeneity among ESC lines, rather we noticed batch variations such that some standard ESC-HSCs manifest higher macrophage signature than others, likely reflecting their stronger tendency for myeloid-skewed differentiation (Fig.6C). Differentiation properties of pluripotent lines used in each experiment are summarized in Table S3–4. Notably, relative to their in vitro predecessors, esLSKs exhibited higher expression of lineage-specific GRNs (T, B and macrophage lineages, Fig 6D). After in vivo specification, esLSKs restored B cell- and macrophage-related GRNs to levels comparable to the native or engrafted B6 LSK controls. Different from BM-derived LSKs, esLSKs displayed a strong T cell signature, which was reflective of their robust lymphoid-potential. The expression of lineage-specific GRNs within flow sorted LSK populations suggests enhanced lineage-priming at the multi-potential progenitor level in these engineered cells.
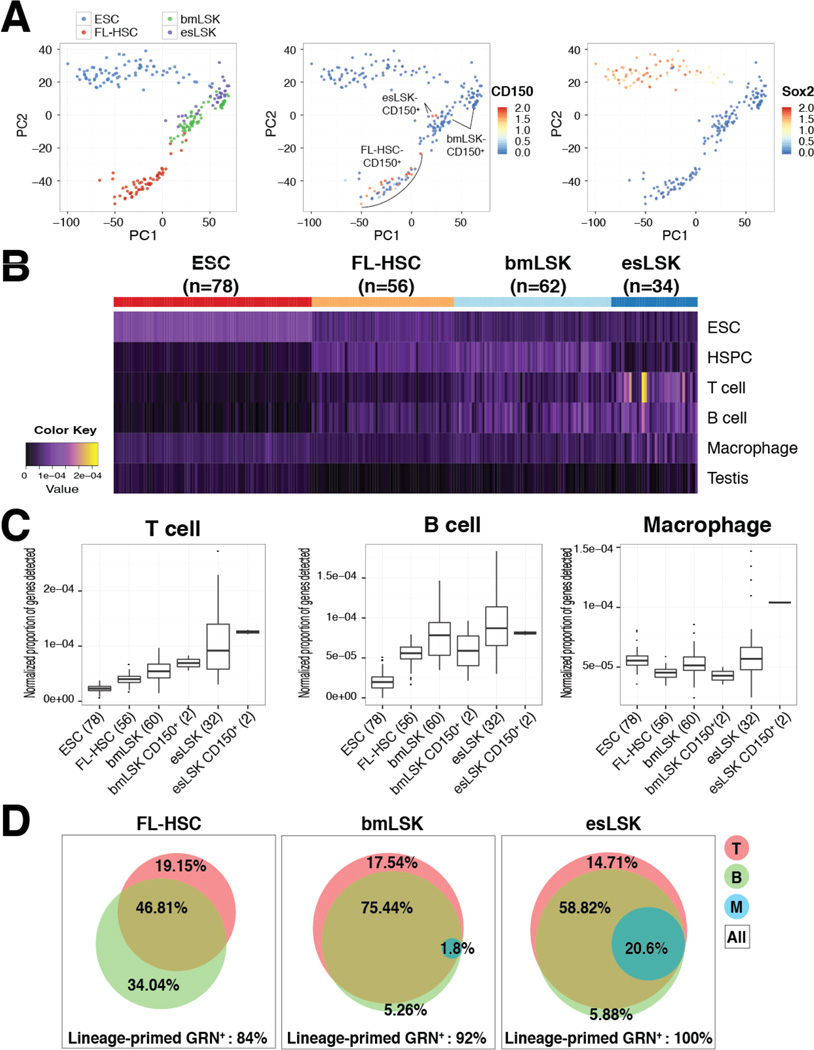
Lastly, we interrogated the esLSK population by microfluidic single-cell RNA-sequencing (scRNA-seq) to discern whether this unique signature of lineage-priming within LSK occurred at the single-cell or the population level. After performing stringent quality control analysis, we were able to analyze 78 ESC, 56 FL-HSC (E13.5 B6 embryos, LSK-CD150+ CD48−), 62 engrafted B6 bmLSK, and 34 engrafted esLSK single-cell libraries, with average reads ranging from 1 x 106 (esLSK) to 3 x 106 (FL-HSC) per cell (Fig.S7 & Supplemental Experimental Procedures). Principle component analysis (PCA) showed major separation between ESC and hematopoietic populations, and tight clustering between the engrafted esLSK and the adult bmLSK. We further applied RaceID analysis to identify the LSK-CD150+ HSCs and to verify the lack of pluripotent gene expression, such as Sox2, within each blood population (Fig.7A). To facilitate the comprehensive assessment of the co-expression of distinct lineages in single-cells, we developed a lineage score that reflects the proportion of lineage-specific genes that are detected in an individual cell (Supplemental Experimental Procedures). This approach compensates for both the relatively low sensitivity of scRNA-Seq and biologically normal stochastic transcription that would confound gene-by-gene analyses. We generated a heatmap of the ESC, HSPC, T cell, B cell, macrophage, and testis lineage scores for all single cells (Fig.7B). As expected, individual ESCs had high ESC lineages scores, FL-HSC and bmLSK had high HSPC scores, and all cells had low testis lineage scores. Supporting the notion of lineage priming, individual esLSKs had high HSPC and T cell, B cell, or macrophage scores, even after normalizing for varying read depths across cells. Next, we compared these lineage scores across groups of cells. Distinct from the expression pattern of FL-HSC or bmLSK, we observed major enrichment of T cell, B cell and macrophage signatures in the esLSK and esLSK-CD150+ populations, of which the enhanced proportion of lineage-primed genes per single-cell transcriptome are shown in Fig.7C. Notably, we detected a 11.4-fold increase in the frequency of multipotent progenitors expressing a hybrid GRN of T cell, B cell and macrophage in the esLSK population (20.6% in esLSK versus 1.8% in bmLSK); whereas we did not observe the presence of such a population in any FL-HSC analyzed (Fig.7D). These data suggest that the multi-lineage priming observed in esLSK occurs within individual cells in the population, and does not result from mixed populations of lineage-restricted cells. Together, our data argue in favor of the lineage-priming hypothesis; esLSK appears to contain a class of HSC-like cells that co-express lineage-specific GRNs.
Figure 7. Multi-lineage-priming Within esLSK at Single-cell Level.
(A) PCA of ESC, FL-HSC, bmLSK and esLSK (left) and RaceID identification of CD150+ (middle) and Sox2+ (right) single cells. (B) Heatmap of genes most critical to the CellNet classifier for ESC, HSPC, T cell, B cell, macrophage and Testis. Each column represents a single-cell transcriptome of indicated sample groups. (C) Proportion of lineage-specific genes detected within indicated single-cell transcriptomes. (D) Area-proportional Venn diagram of percent GRN+ (T, B and M) single-cells over total cells analyzed (All). See also Fig.S7.
Discussion
By complementing the Notch signaling pathway deficiency in our previously defined prototypic ESC-HSC cells, we obtained enhanced populations of ESC-derived hematopoietic progenitors that durably reconstitute multi-lineage hematopoiesis and adaptive immunity in irradiated mice. Incorporation into the native niches of adult BM further promotes developmental maturation of Notch-enhanced ESC-HSCs into esLSKs, which by functional and molecular criteria, closely resemble bona fide HSCs. Furthermore, we have demonstrated shared clonal origins of distinct blood lineages through genome-wide detection of retroviral integration sites in terminally differentiated cells, as well as single-cell quantification of lineage-primed GRNs within esLSKs. Our data reveal a route to establishing functional multipotent HSCs that exploits aspects of in vitro differentiation, transcription factor re-specification with HoxB4 to enhance self-renewal, and Notch-mediated lineage specification. Such a cell engineering platform which exploits aspects of both morphogen-stimulated directed differentiation and enforced expression of a transcription factor represents a distinct strategy that does not rely entirely on recapitulating native patterns and programs of embryonic development. Indeed, functional HSCs might be derived from human pluripotent stem cells using comparable strategies encompassing developmental and synthetic biology.
Although the mechanism through which HoxB4 promotes definitive hematopoietic conversion remains elusive, HoxB4 acts as a master regulatory node for several hematopoietic transcription factors that play key roles in HSCs (Fli1, Meis1, Runx1 and Scl) (Fan et al., 2012) and HSC identity can be achieved by transgene manipulation. Induced HSC (iHSC) can be derived through the respecification of common myeloid progenitors (CMP) and B cell progenitors by ectopic expression of eight transcription factors (Riddell et al., 2014). HSPC-like cells can be transdifferentiated from fibroblasts by the over-expression of Gata2, Gfi1b, cFos, and Etv6 (Pereira et al., 2013), and human umbilical vein endothelial cells/dermal endothelial cells can be converted to HSPCs via the overexpression of FOSB, GFI1, RUNX1, SPI1 (Sandler et al., 2014). With the exception of Gata2 and Meis1, the majority of these fate-altering factors are not directly involved in HoxB4 or Notch pathways, suggesting that HoxB4/Notch-directed conversion takes a different route to achieve HSC-like function. The myeloid-bias in HoxB4-modified ESC-HSC could result from the direct HoxB4-mediated silencing of lymphoid genes and the epigenetic state of these cells (Fan et al., 2012). Supported by a recent finding that Notch pathway activation induces large-scale changes in H3K56 acetylation across Notch-regulated loci and consequently promotes transcription by modifying chromatin state, we hypothesize that Notch activity may enable greater chromatin accessibility to establish multi-lineage-priming in ESC-HSCs (Skalska et al., 2015). Alternatively, Notch is known to inhibit myeloid differentiation and enhance self-renewal of multipotent progenitors, while directing T cell differentiation at higher signal strength (Ohishi et al., 2003). Additional optimization will be necessary to achieve balanced hematopoietic reconstitution, if desired, while for applications in T cell engineering from ESC/iPSCs, lymphoid bias may be preferred.
Whether HoxB4 effectively reprograms human ESC-HSC remains inconclusive. It has been reported that ectopic HoxB4 expression in human ESC enhances myelo-erythroid differentiation and colony forming potential (Bowles et al., 2006); however, contradictory data argues that HoxB4 has no effect on human HSC function (Wang et al., 2005a). The Hox clusters are evolutionarily conserved and possess functional redundancy among different Hox paralogs (Mallo et al., 2010). We have previously investigated 9 HSC-specific transcriptional regulators in conferring self-renewal of human ESC-derived progenitors (Doulatov et al., 2013), and among these factors are several Hox family members: HoxA5, HoxA9 and HoxA10, suggesting that these genes may play comparable roles to HoxB4 in driving self-renewal in human ESC-HSC. Notch signaling has lately drawn increasing attention in driving the endothelial to hematopoietic transition from HE during human pluripotent stem cell differentiation (Ditadi et al., 2015, Kim et al., 2013, Jang et al., 2015). A major goal in the field of BM and stem cell transplantation is understanding how to reprogram available sources of human pluripotent stem cells into HSC fate. Our approach of building multipotentiality by up-regulating lineage-specific GRNs with Notch pathway activation in self-renewing HSPC suggests similar strategies of synthetic biology might prove effective in engineering functional HSCs from human ESC/iPSC-derived HE or early blood progenitors.
Experimental Procedures
ESC culture and EB differentiation
AV15 (Kyba et al., 2002), iCdx4 (Wang et al., 2005b), iICN (Jang et al., 2015) murine ES cells were maintained on MEFs in DMEM with 15% inactivated fetal serum (IFS, HyClone), 1000 U/ml LIF, 0.1 mM nonessential amino acids, 2 mM penicillin/streptomycin/glutamate, and 100 µM β-mercaptoethanol at 37 °C/5% CO2. ES cells were differentiated into EBs after removing MEFs and cultured in IMDM with 15% FCS (Stem Cell Technologies, SCT), 200 µg/ml holo-transferrin, 50 µg/ml ascorbic acid, 2 mM penicillin/streptomycin/glutamate, and 450 µM monothioglycerol as described previously (McKinney-Freeman et al., 2008).
ESC-HSC Derivation
ESC-HSC-OP9 were derived according to protocol published previously (McKinney-Freeman et al., 2008). Briefly, dissociated d6 EBs were transduced with MIG-HoxB4 retrovirus and expanded on OP9 stroma with 10% IMDM containing 100 ng/mL human FMS-like tyrosine kinase 3 ligand (hFlt3L), 100 ng/mL mouse stem cell factor (mSCF), 40 ng/mL human thrombopoietin (hTPO), and 40 ng/mL mouse vascular endothelial growth factor (mVEGF). Emerging ESC-HSCs were further expanded on DL1 storma or retronectin-coated plates with immobilized DL1-Fc (kindly provided by Dr. Irwin Bernstein) in lymphoid-differentiation media of 10% IMDM with 100 ng/mL hFlt3L, 100 ng/mL mSCF, 40 ng/mL hTPO, and 40 ng/mL mVEGF, 100 ng/mL mIL-6, 10 ng/mL mIL7, and 10 ng/mL mIL-11. All cytokines were purchased from PeproTech.
ESC-RBC Derivation
Confluent ESC-HSCs were transferred to fresh OP9 or DL1 stromas in erythroid-differentiation media of 10% IMDM with 10 U/mL mEpo, 10 ng/mL mSCF, 100 ng/mL mIGF-1, 1µM dexamethasone and 20% BIT9500 (SCT) for 6 days before globin analysis. All cytokines were purchased from PeproTech.
Retroviral integration site analysis by LAM-PCR
Genomic DNAs of reconstituted lineages were subjected to genomic amplification using REPLI-g Mini Kit (Qiagen) and LAM-PCR using Retro-X™ Integration Site Analysis Kit (Clonetech). Major amplicons were sequenced for retroviral integration site analysis.
Southern blotting
Genomic DNAs of reconstituted lineages were digested with EcoRI and NcoI, and transferred to nylon membranes after electrophoretic separation. The blots were hybridized with a GFP probe (Lu et al., 2012) to detect individual proviral clones, and with a HoxB4 probe to detect both endogenous and transgene HoxB4 (Wang et al., 2005b).
Delayed Type Hypersensitivity
Mice were sensitized with sRBC (109/mL, 50 µL per site, Rockland Immunochemicals) through subcutaneous (s.c.) and intradermal injections in the lower back and right footpad. At day 6 following sensitization, these mice were challenged with sRBC in the left footpad at 2x109/mL, 50 µL per site, and an equal volume of PBS in the right footpad. Hind footpad thickness at 48 hours after challenge was measured with a micro-caliper. Percent change was normalized to pre-challenged thickness of each footpad at day6.
Pre-immune Ig detection
Pre-immune Ig isotypes was determined by mouse Ig isotyping kit (eBioscience).
Antigen-specific IgG detection
Mice were sensitized with OVA (Sigma)/complete freund’s adjuvant (CFA, Sigma) emulsions at the dose of 100 µg antigen per mouse through s.c. injection. Two weeks following initial priming, mice were boosted with same dose of OVA/incomplete freund’s adjuvant emulsions (IFA, Sigma). Plasma level of OVA-specific IgG was determined by ELISA a week after boosting. ELISA plates were coated with 100 uL/well of OVA (20 ug/ml in PBS) overnight at 4°C and blocked with 5% BSA in PBS for 2 h at room temperature. Samples were incubated overnight at 4°C, followed by addition of anti-mouse IgG*HRP (GE Healthcare) and tetramethylbenzidine-based detection method at absorbance of 450 nm.
Supplementary Material
Highlights.
Engineered HSCs can be made by co-expressing self-renewal and lineage-guiding factors.
Temporal and dosage control of Notch confers lymphoid potential in engineered HSCs.
Engineered HSCs reconstitute functional adaptive immune responses in vivo.
Individual engineered HSC possess hybrid GRNs reflective of lineage priming.
Acknowledgments
We thank members of the Daley laboratory for critical discussions, the flow cytometry core at Boston Children’s Hospital for key analytic resources, the Biopolymers facility at Harvard Medical School for Single cell RNA-seq service and Coriell Institute for microarray processing. G.Q.D. is supported by grants from the NIH (Progenitor Cell Biology Consortium UO1-HL100001 and R24DK092760), and the Boston Children’s Hospital Stem Cell Program. P.C. received support from NIH (NIDDK K01DK096013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Y-F.L. and P.C. designed, performed, analyzed and interpreted experiments. S.R., J.S., P.M.S., E.S. and A.N.E. assisted with experiments. I.D.B., B.K.H. and W.C. provided experimental materials. Y-F. L., P.C. and G.Q.D. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Accession Numbers
The accession numbers for array: GSE71793; for RNA-Seq: GSE71794; for array and RNA-Seq (the suer-series): GSE71796. Flow datasets are available at https://flowrepository.org/id/RvFrNTAaAX2EGmIlCrST8mSEBJETbyWkGFiWkVU6ZjI5BNG0FUSOeikTSJcGsCID.
References
- Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature reviews. Immunology. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- Boisset J-CC, Van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Bowles KM, Vallier L, Smith JR, Alexander MR, Pedersen RA. HOXB4 overexpression promotes hematopoietic development by human embryonic stem cells. Stem cells (Dayton, Ohio) 2006;24:1359–1369. doi: 10.1634/stemcells.2005-0210. [DOI] [PubMed] [Google Scholar]
- Cahan P, Li H, Morris SA, Lummertz Da Rocha E, Daley GQ, Collins JJ. CellNet: network biology applied to stem cell engineering. Cell. 2014;158:903–915. doi: 10.1016/j.cell.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, ZÚÑIga-PflÜCker JC. Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- Clements WK, Traver D. Signalling pathways that control vertebrate haematopoietic stem cell specification. Nature reviews. Immunology. 2013;13:336–348. doi: 10.1038/nri3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nature medicine. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditadi A, Sturgeon CM, Tober J, Awong G, Kennedy M, Yzaguirre AD, Azzola L, Ng ES, Stanley EG, French DL, Cheng X, Gadue P, Speck NA, Elefanty AG, Keller G. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nature cell biology. 2015;17:580–591. doi: 10.1038/ncb3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulatov S, Vo LT, Chou SS, Kim PG, Arora N, Li H, Hadland BK, Bernstein ID, Collins JJ, Zon LI, Daley GQ. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell stem cell. 2013;13:459–470. doi: 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nature immunology. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehebauer M, Hayward P, Arias AM. Notch, a Universal Arbiter of Cell Fate Decisions. Science. 2006 doi: 10.1126/science.1134042. [DOI] [PubMed] [Google Scholar]
- Fan R, Bonde S, Gao P, Sotomayor B, Chen C, Mouw T, Zavazava N, Tan K. Dynamic HoxB4-regulatory network during embryonic stem cell differentiation to hematopoietic cells. Blood. 2012;119:e139–e147. doi: 10.1182/blood-2011-12-396754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt DM, Pajcini KV, D’Altri T, Tu L, Jain R, Xu L, Chen MJ, Rentschler S, Shestova O, Wertheim GB, Tobias JW, Kluk M, Wood AW, Aster JC, Gimotty PA, Epstein JA, Speck N, Bigas A, Pear WS. The Notch1 transcriptional activation domain is required for development and reveals a novel role for Notch1 signaling in fetal hematopoietic stem cells. Genes & development. 2014;28:576–593. doi: 10.1101/gad.227496.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, Macintyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland BK. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104:3097–3105. doi: 10.1182/blood-2004-03-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland BK, Varnum-Finney B, Poulos MG, Moon RT, Butler JM, Rafii S, Bernstein ID. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. The Journal of clinical investigation. 2015;125:2032–2045. doi: 10.1172/JCI80137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B, Devinsky O, Gershon RK, Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. The Journal of experimental medicine. 1976;143:1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IH, Lu YF, Zhao L, Wenzel PL, Kume T, Datta SM, Arora N, Guiu J, Lagha M, Kim PG, Do EK, Kim JH, Schlaeger TM, Zon LI, Bigas A, Burns CE, Daley GQ. Notch1 acts via Foxc2 to promote definitive hematopoiesis via effects on hemogenic endothelium. Blood. 2015;125:1418–1426. doi: 10.1182/blood-2014-04-568170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PG, Albacker CE, Lu Y-FF, Jang I-HH, Lim Y, Heffner GC, Arora N, Bowman TV, Lin MI, Lensch MW, De Los Angeles A, Zon LI, Loewer S, Daley GQ. Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:50. doi: 10.1073/pnas.1214361110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RCR, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lengerke C, Daley GQ. Autologous blood cell therapies from pluripotent stem cells. Blood reviews. 2010;24:27–37. doi: 10.1016/j.blre.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-FF, Gavrilescu LC, Betancur M, Lazarides K, Klingemann H, Van Etten RA. Distinct graft-versus-leukemic stem cell effects of early or delayed donor leukocyte infusions in a mouse chronic myeloid leukemia model. Blood. 2012;119:273–284. doi: 10.1182/blood-2011-01-331009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, Pear WS. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell stem cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo M, Wellik DM, Deschamps J. Hox genes and regional patterning of the vertebrate body plan. Developmental biology. 2010;344:7–15. doi: 10.1016/j.ydbio.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckinney-Freeman S, Cahan P, Li H, Lacadie S, Huang H-T, Curran M, Loewer S, Naveiras O, Kathrein K, Konantz M, Langdon E, Lengerke C, Zon L, Collins J, Daley G. The transcriptional landscape of hematopoietic stem cell ontogeny. Cell Stem Cell. 2012;11:701–714. doi: 10.1016/j.stem.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckinney-Freeman SL, Naveiras O, Daley GQ. Isolation of hematopoietic stem cells from mouse embryonic stem cells. Current protocols in stem cell biology. 2008 doi: 10.1002/9780470151808.sc01f03s4. Chapter 1. [DOI] [PubMed] [Google Scholar]
- Mckinney-Freeman SL, Naveiras O, Yates F, Loewer S, Philitas M, Curran M, Park PJ, Daley GQ. Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood. 2009;114:268–278. doi: 10.1182/blood-2008-12-193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Cahan P, Li H, Zhao AM, San Roman AK, Shivdasani RA, Collins JJ, Daley GQ. Dissecting engineered cell types and enhancing cell fate conversion via CellNet. Cell. 2014;158:889–902. doi: 10.1016/j.cell.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- Ohishi K, Katayama N, Shiku H, Varnum-Finney B, Bernstein ID. Notch signalling in hematopoiesis. Seminars in cell & developmental biology. 2003;14:143–150. doi: 10.1016/s1084-9521(02)00183-0. [DOI] [PubMed] [Google Scholar]
- Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(−) cord blood cells. The Journal of clinical investigation. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C-FF, Chang B, Qiu J, Niu X, Papatsenko D, Hendry CE, Clark NR, Nomura-Kitabayashi A, Kovacic JC, Ma’Ayan A, Schaniel C, Lemischka IR, Moore K. Induction of a hemogenic program in mouse fibroblasts. Cell stem cell. 2013;13:205–218. doi: 10.1016/j.stem.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Mancini SJ, Macdonald HR. Notch regulation of lymphocyte development and function. Nature immunology. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- Riddell J, Gazit R, Garrison BS, Guo G, Saadatpour A, Mandal PK, Ebina W, Volchkov P, Yuan GC, Orkin SH, Rossi DJ. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 2014;157:549–564. doi: 10.1016/j.cell.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler VM, Lis R, Liu Y, Kedem A, James D, Elemento O, Butler JM, Scandura JM, Rafii S. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511:312–318. doi: 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- Skalska L, Stojnic R, Li J, Fischer B, Cerda-Moya G, Sakai H, Tajbakhsh S, Russell S, Adryan B, Bray SJ. Chromatin signatures at Notch-regulated enhancers reveal large-scale changes in H3K56ac upon activation. EMBO J. 2015;34:1889–1904. doi: 10.15252/embj.201489923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Toellner KM, Luther SA, Sze DM, Choy RK, Taylor DR, Maclennan IC, Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VArnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE, Cerdan C, Levac K, Bhatia M. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. The Journal of experimental medicine. 2005a;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA. 2005b;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, Cumming JG, Hiatt K, Mukherjee P, Williams DA. A novel method of myeloablation to enhance engraftment of adult bone marrow cells in newborn mice. Biol Blood Marrow Transplant. 1996;2:59–67. [PubMed] [Google Scholar]
- Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.