Abstract
Neurofilaments are synthesized in the cell body of neurons and transported outward along the axon via slow axonal transport. Direct observation of neurofilaments trafficking in live cells suggests that the slow outward rate of transport is due to the net effects of anterograde and retrograde microtubule motors pulling in opposition. Previous studies have suggested that cytoplasmic dynein is required for efficient neurofilament transport. In this study, we examine the interaction of neurofilaments with cytoplasmic dynein. We used fluid tapping mode atomic force microscopy to visualize single neurofilaments, microtubules, dynein/dynactin, and physical interactions between these neuronal components. AFM images suggest that neurofilaments act as cargo for dynein, associating with the base of the motor complex. Yeast two-hybrid and affinity chromatography assays confirm this hypothesis, indicating that neurofilament subunit M binds directly to dynein IC. This interaction is blocked by monoclonal antibodies directed either to NF-M or to dynein. Together these data suggest that a specific interaction between neurofilament subunit M and cytoplasmic dynein is involved in the saltatory bidirectional motility of neurofilaments undergoing axonal transport in the neuron.
INTRODUCTION
Neurofilaments (NFs) are neuron-specific intermediate filaments composed of three subunits: neurofilament light chain (NF-L), medium chain (NF-M), and heavy chain (NF-H). These subunits assemble as heteropolymers that provide structural support to the axon. NFs are synthesized in neuronal cell bodies and are then moved outward along the axon via slow transport along microtubules, moving at rates of ∼0.1-3 mm/d. Direct observations of the motility of labeled neurofilaments moving along the axons of cultured neurons indicate that this overall slow axonal transport is the net effect of rapid, intermittent, and highly asynchronous bidirectional motility interrupted by prolonged pauses (Prahlad et al., 2000; Roy et al., 2000; Wang et al., 2000). Although both the outward and inward movements of the neurofilaments are intermittent and of short duration, the rates of anterograde and retrograde motility are consistent with the transport rates of the microtubule motor proteins kinesin and cytoplasmic dynein (Roy et al., 2000; Wang et al., 2000). Therefore, the fast axonal transport motors kinesin and dynein are believed to be involved in transporting neurofilaments as heteropolymers (reviewed in Shea and Flanagan, 2001). Defects in this transport may contribute to the aggregations of neurofilaments observed in several genetically unrelated neurodegenerative diseases, including ALS and Charcot-Marie-Tooth disease (reviewed in Al-chalabi and Miller, 2003).
The bidirectional motility of neurofilaments along microtubules has been reconstituted in vitro (Shah et al., 2000). Both conventional (Prahlad et al., 2000; Xia et al., 2003) and unconventional kinesins (Shah et al., 2000) may drive the anterograde motility of neurofilaments. The mechanism by which kinesin or a kinesin-like protein interacts with neurofilaments has not yet been examined.
Several observations indicate that cytoplasmic dynein is the motor for the minus-end directed motility of neurofilaments along microtubules observed both in vivo and in vitro. Neurofilaments accumulate in the axons of transgenic mice with a targeted disruption of the dynein-activator complex, dynactin, suggesting that retrograde trafficking mediated by dynein is critical for neurofilament transport (LaMonte et al., 2002). Further, dynein accumulates in neurofilament-rich aggregates formed in axonal swellings after administration of beta, beta′-iminodipropionitrile (IDPN; Toyoshima et al., 1998). Cytoplasmic dynein copurifies with neurofilaments from spinal cord, and antibodies directed against dynein IC (DIC) decorate purified native neurofilament polymers. Antidynein antibodies also were found to inhibit the motility of neurofilaments along microtubules in vitro (Shah et al., 2000).
Here we analyze the interaction between cytoplasmic dynein and dynactin, neurofilaments, and microtubules in greater detail. We used atomic force microscopy to image the microtubule-neurofilament and dynein/dynactin-neurofilament interactions in unfixed, unstained specimens, in comparison to electron micrographs of similar preparations. We also examined the dynein-neurofilament interaction biochemically and identified a direct interaction between neurofilament subunit M and the IC of cytoplasmic dynein. Together, these results support a role for cytoplasmic dynein in the short-duration retrograde movement of neurofilaments undergoing slow anterograde transport along the microtubules of the axon.
MATERIALS AND METHODS
Protein Purification and Labeling
NFs were isolated from bovine spinal cords as previously described (Leterrier et al., 1996). The purity of neurofilament preparations was routinely checked by densitometry of Coomassie-stained gels after SDS-PAGE. For the preparations used in these studies the neurofilament triplet subunits (NF-H, NF-M, and NF-L) accounted for 95% of the total protein (see Results). The neurofilament suspension was stored in aliquots at -70°C after rapid freezing in liquid nitrogen; protein concentrations were determined using the Lowry assay (Lowry et al., 1951). Neurofilaments were fragmented for some studies using a Sonic Dismembrator 60 (Fisher Scientific, Pittsburgh, PA) on diluted neurofilament samples (15-30 μg/ml) in reassembly buffer (RB: 0.1 M Mes, pH 6.8, 1 mM MgCl2, 1 mM EGTA) on ice, using a 1-s burst at a setting of 5 W for 3-5 s total. Neurofilaments were dephosphorylated for some studies by incubation with Escherichia coli alkaline phosphatase (Sigma, St. Louis, MO) at 3 U/mg NF for 72 h under dialysis at 4°C against RB buffer with 0.8 M sucrose and 1 mM PMSF (phenylmethylsulfonyl fluoride).
The dynein/dynactin complex was isolated from rat brain by microtubule affinity and ATP extraction, followed by purification by sedimentation through a sucrose gradient (Karki and Holzbaur, 1995). The peak 19-20S fractions containing both dynein and dynactin were used for these experiments. Purified tubulin was prepared from bovine brain microtubules and estimated to be ∼99% pure as determined by densitometric scanning of Coomassie-stained gels (Hyman et al., 1991); alternatively, purified tubulin was purchased from Cytoskeleton (Denver, CO). The polymerized microtubules were stabilized by the addition of taxol (paclitaxel; Cytoskeleton) to 10 μM. An mAb to the cytoplasmic dynein IC was purchased from Chemicon (Temecula, CA).
Atomic Force Microscopy
Atomic force microscopy (AFM) imaging was done with a Bioscope IIIa instrument (Digital Instruments, Santa Barbara, CA). The sample (300 μl) containing the diluted proteins was transferred to freshly cleaved mica double taped on a 35 × 10-mm plastic dish. Imaging was performed in fluid tapping mode using DNP-S oxide-sharpened silicon nitride probes (Digital Instruments) with a spring constant of 0.32 N/m at a scanning frequency of 0.8-1.2 Hz. Images were processed using the Nanoscope (R) IIIa software (v. 5.12; Digital Instruments), and the WSxM freeware (v. 3.0; Nanotec, Madrid, Spain) was used for height measurements, flattening, 3D conversion, and inserting scale bars. The G-scanner was calibrated using a standard grid. All buffers were filtered using a sterile 0.22-μm mesh-size filter.
To obtain a statistical analysis of the frequency with which dynein/dynactin bound to neurofilaments, a scan was made longitudinally along the middle of the NF contour and plots of height vs. distance along the NF contour were calculated. The height scale was first normalized to the mica surface by making a height scan perpendicular to the contour of a NF that was lying on a clean surface far from other filaments. Typically this measurement yielded a value between 8 and 10 nm as the height of the NF on the surface. Then a scan of multiple filaments was made longitudinally, along the center of their contour. For each condition, 35 μm of NF contour was scanned with ∼2000 height measurements made along this length. Plots of height vs. contour for a 35-μm virtual filament composed of separate measurements of multiple filaments were used to derive two quantities: an average filament height in the absence or presence of dynein/dynactin or antibodies and the frequency with which individual height measurements were significantly higher than the average expected for a NF without bound dynein/dynactin. The threshold for significant increases in height was selected as slightly greater than the sun of the heights of NFs and dynein/dynactin measured separately. The average height of a control NF was 8 nm and the height of isolated dynein/dynactin was ∼6 nm. Therefore, local filament height measurements greater than 15 nm were taken as evidence for dynein/dynactin complex bound to the surface of the NF. This conservative threshold value may underestimate the number of motors bound to the NF but was necessary because height fluctuations of the control NFs were significant, probably because of the apparent periodic packing of intermediate filament subunits (Heins et al., 1993). The average height of dephospho NFs was less than that of control NFs, and height fluctuations were larger, preventing statistically significant measures of possible motor binding to dephospho NFs.
Electron Microscopy of Neurofilament-Microtubule Complexes
Purified NFs were incubated for 30 min with assembly competent pure tubulin (isolated from twice-cycled microtubules by phosphocellulose chromatography) in RB with 1 mM GTP. Samples were loaded on glow-discharged formvar-carbon-coated 300 mesh copper grids, stained by 1% uranyl acetate in H2O for 20 s, dried in air after side adsorption of the drop, and observed in a Jeol 100 electron microscope (Peabody, MA) at 80 kV.
Yeast Two-Hybrid Interaction Screen
We used the LexA yeast two-hybrid system to screen a random-primed human brain library (Stratagene, La Jolla, CA) for proteins interacting with DIC. The majority of clones isolated were found to encode p150Glued, so we further screened positives by Southern blot to identify novel clones, one of which was identified by database searching as encoding a fragment of NFM-spanning residues 122-428. Full-length clones encoding NF-M and NF-L were generously provided by Dr. Virginia Lee of the University of Pennsylvania. A subclone of NF-L-spanning residues 100-420 was generated by restriction digests and PCR. Both the full-length and partial clones of NF-M and NF-L were tested in the yeast two-hybrid system for direct interactions with DIC; the results were scored qualitatively in comparison to both positive and negative controls.
Affinity Chromatography and Cosedimentation Binding Assays
Affinity matrices were generated by covalently coupling purified recombinant dynein IC to Sepharose beads, as previously described (Karki and Holzbaur, 1995). A recombinant DIC construct (pET15brDIC) was expressed in the BL21 strain of E. coli, and the His-tagged protein was purified by nickel sulfate chromatography. The purified protein was covalently coupled to activated CH-Sepharose 4B beads (Amersham Pharmacia Biotech, Piscataway, NJ). The TNT T7 Quick coupled transcription/translation system (Promega, Madison WI) was used to express full-length cDNAs encoding NF-M and NF-L, and subclones spanning residues 122-428 of NF-M and residues 100-420 NF-L in vitro. The resulting proteins were then loaded onto the rDIC affinity matrix and batch-bound with gentle shaking at 25°C for 10 min. After extensive washing with phosphate-buffered saline and 0.2% Triton X-100, the columns were eluted by boiling the beads in sample buffer for 5 min and collecting the supernatant. The resulting fractions were analyzed by SDS-PAGE and autoradiography.
The binding of endogenous neurofilaments to DIC was examined by incubating a rat brain homogenate and clarified by centrifugation at 39,000 × g, batchwise with rotation with either DIC or BSA-bound Sepharose beads. After extensive washes with HEM buffer (100 mM HEPES, 1 mM EGTA, 1 mM MgSO4, pH 7.3) with 25 mM NaCl and 0.1% Triton X-100, bound proteins were eluted with HEM buffer with 2 M NaCl. Fractions were analyzed by SDS-PAGE and Western blot.
Cosedimentation experiments were performed with sonicated neurofilaments, both phosphorylated and dephosphorylated, dynein/dynactin, and microtubules stabilized with taxol. After a 30-min incubation at room temperature, binding reactions were centrifuged for 20 min at 17,000 × g. To test the nucleotide dependence of the association, MgATP was added to some reactions at 5 mM at the start of the incubation, and an additional 5 mM MgATP was added before centrifugation to replenish loss due to the activity of the dynein ATPase. Gel samples from the resulting supernatant and pellet fractions were analyzed by SDS-PAGE and Western blot.
RESULTS
The bidirectional transport of neurofilaments along microtubules observed by Wang et al. (2000) and Roy et al. (2000) suggests that neurofilaments are actively transported by both plus- and minus-end-directed microtubule motors. This bidirectional transport of neurofilaments along microtubules has been reconstituted in vitro (Shah et al., 2000). Cytoplasmic dynein copurifies with neurofilaments, and antibodies to cytoplasmic dynein were found to inhibit the minus-end directed motility of neurofilaments along microtubules. To look further at the mechanism of dynein-mediated motility of neurofilaments, we examined the interactions in vitro between purified dynein and neurofilaments or microtubules.
Structural Details of Neurofilaments, Microtubules, and Their Interactions
Fluid phase tapping mode AFM was used to visualize single neurofilaments, microtubules, and their interactions. Analysis of purified neurofilaments by AFM reveals long and entangled filaments adsorbed on the surface from solutions of moderately high concentrations (0.1 mg/ml; Figure 1a). At lower concentrations, (<10 ng/ml, Figure 1b) single, highly flexible filaments were observed, consistent with the expectation for filaments undergoing Brownian motion being deposited from solution onto an adhesive surface (Wagner et al., 2003; Dogic et al., 2004). Microtubules, in contrast, are easily differentiated from NFs because they are wider and much stiffer than neurofilaments (Figure 1, c-f). Height measurements reveal an average NF height of 8-10 nm compared with 25 nm for MTs (Figure 1g).
Figure 1.
Fluid phase tapping mode AFM was used to visualize single neurofilaments, microtubules, and their interactions. (a) AFM imaging of purified neurofilaments reveals long and entangled filaments adsorbed on the surface from solutions of moderately high concentrations (0.1 mg/ml). Average filament length was 1.8 ± 0.8 μm (n = 212). (b) Single, highly flexible filaments (17% form loops, n = 212) can be seen at lower concentrations (<10 ng/ml). (c) Microtubules are wider and stiffer than neurofilaments. (d) Long flexible neurofilaments appear to drape over wider, stiffer microtubules. (e and f) Gaps are seen between adjacent neurofilaments and microtubules. Height data shown in panel e were converted to a 3D image shown in f. (g) Height measurements (data from the line scan shown in f) reveal an average neurofilament height of 11.5 nm (±2 nm, n = 50) compared with 27 nm (±3 nm, n = 34) for microtubules. Additional hydration in the AFM image as compared with dried TEM samples are likely to account for the slightly higher diameters.
When mixtures of microtubules and neurofilaments are imaged (Figure 1, c-f), there are numerous interactions between the polymers, but it is not possible to discriminate biochemical bonds from steric interactions between the filaments. Frequently observed structural features include neurofilaments that appear to be draped over the larger microtubules (small arrow in Figure 1d) and sites where neurofilaments appear to be adjacent to but not in contact with microtubules. The latter feature is seen in more detail in Figure 1, e and f, which shows that even with the added apparent width of the filaments due to the finite size of the AFM tip, there is a clear separation between the two filament cores, possibly mediated by the neurofilament sidearms, which we cannot resolve by AFM in fluid measurements. Such close contacts for long distances between neurofilaments and microtubules are observed instead by negative staining electron microscopy of microtubules polymerized from pure tubulin in the presence of neurofilaments (Figure 2). The constant spacing between neurofilament and microtubule polymers under these conditions is ∼4-7 nm. The occurrence of cross-bridges between the two polymers is suggested by the direct binding of neurofilament extensions to the microtubule wall (Figure 2b).
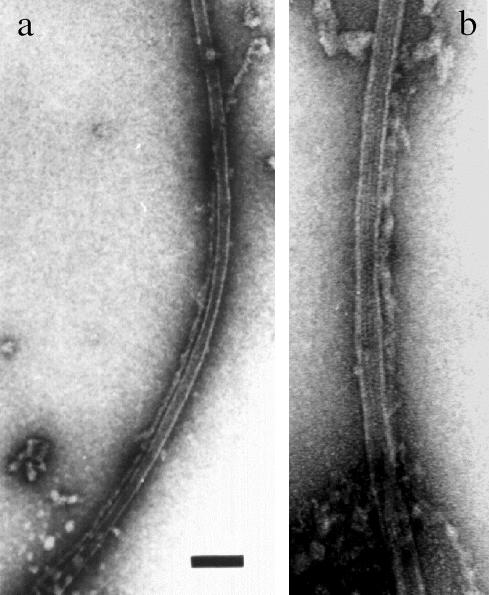
Figure 2.
Negative staining electron microscopy of neurofilament-microtubule interactions. (a and b) Microtubule polymers obtained by tubulin assembly in the presence of neurofilaments are associated with neurofilaments for long distances (>1 μm in a), with an average distance between neurofilaments and the microtubule wall of 4-7 nm. Regular densities along the neurofilament length seem to mediate direct contacts between the two polymers (b).
Cytoplasmic Dynein/Dynactin Binds to Neurofilaments
Cytoplasmic dynein and dynactin, isolated from rat brain by microtubule affinity and ATP-release (Karki and Holzbaur, 1995), was imaged by AFM. As shown in Figure 3a, we noted a heterogeneous population. Dynactin is most easily identified in these unfixed, unstained populations due to the distinctive structure of the Arp1 filament at the base of the molecule (Figure 3, b and c; Schafer et al., 1994). The p150Glued sidearm of dynactin is also clearly visible. The orientation and morphology of cytoplasmic dynein appears to be more variable in AFM images (Figure 3d), suggesting that the molecule may be more flexible in solution and therefore may not uniformly orient on the mica surface. However, large bilobed structures are apparent, consistent with the morphology and dimensions of cytoplasmic dynein as determined by STEM (Vallee et al., 1988). Some images are consistent with the overall morphology of a dynein/dynactin cocomplex (Figure 3e).
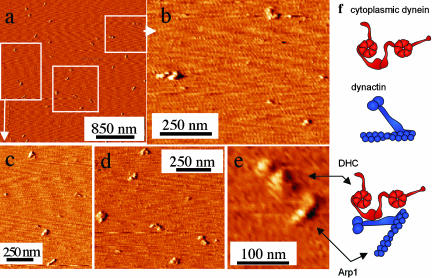
Figure 3.
AFM images of cytoplasmic dynein/dynactin complex. (a) AFM images of a dynein/dynactin reveal a heterogeneous population. (b and c) Images of dynactin reveal the distinctive structure of the Arp1 filament at the base of the molecule as well as the p150Glued sidearm. (d) Images of cytoplasmic dynein reveal a bilobed structure, but the orientation of the protein on the mica surface appears more variable, suggesting that the molecule may be more flexible in solution. (e) AFM image of a dynein/dynactin cocomplex. (f) Schematic representations of cytoplasmic dynein, dynactin, and the cocomplex.
To examine the interaction of dynein and dynactin with neurofilaments, AFM was used to determine if binding of dynein/dynactin to purified neurofilaments could be visualized and if the motor complex increased the association of neurofilaments with microtubules. When added to purified neurofilaments, dynein/dynactin decorated the neurofilaments along their contour (Figure 4, a and b) often forming thin projections of length 50-100 nm extending from the neurofilament core. The contour of the neurofilament becomes highly nonuniform, as shown by the height map of the neurofilament in Figure 4e. The additional mass on the neurofilaments and the structures extending away from their cores are likely due to binding of dynein/dynactin to multiple sites along the neurofilament. This binding was not observed if the dynein/dynactin was first incubated with an antibody to the dynein IC (Figure 4, c and d). After addition of the anti-DIC antibody, the neurofilament contours were again smooth, despite the abundance of material presumed to be dynein/antibody complexes in the background. Close inspection of these images shows that the zone within 50-100 nm of the NF core is depleted of the protein complexes that are visible farther away (Figure 4c), consistent with the model proposed by Kumar et al. (2002a, 2002b) that the neurofilament sidearms, not visible in fluid phase AFM, manifest their presence by excluding other macromolecules from the volume they occupy around the NF core. When sonicated neurofilament fragments were added to purified microtubules together with dynein/dynactin, numerous contacts were observed between the neurofilaments and the microtubules (Figure 4, f and g).
Figure 4.
AFM images of cytoplasmic dynein/dynactin complex bound to neurofilaments. (a) Dynein/dynactin decorate neurofilaments along their lengths, often forming thin projections of length 50-100 nm extending from the neurofilament core. (b) The binding of dynein/dynactin to neurofilaments occurs primarily (80%) with globular domains projecting away from the backbone of the NF, connected via a filamentous structure, probably the dynein-stem (white arrows in b). (c and d) No decoration of neurofilaments is observed in the presence of an antibody to the dynein IC. A clear exclusion zone is seen along the NF-backbone in lower resolution images (c). (e) Dynein/dynactin decorate neurofilaments along their lengths, so that the contour of the neurofilament becomes highly nonuniform, as shown by height maps. (f and g) Sonicated neurofilament fragments bind along the side of microtubules in the presence of dynein/dynactin In the absence of dynein/dynactin less binding of sonicated NF fragments to microtubules is seen (our unpublished results).
We performed a statistical analysis comparing height fluctuation of AFM scans of neurofilaments and neurofilaments with added dynein and dynactin. Figure 5a shows a typical comparison of height traces for NFs incubated either with dynein/dynactin or dynein/dynactin plus an antibody to dynein IC. While there are frequent height fluctuations above 15 nm in the filaments treated with dynein/dynactin (black trace), addition of anti-DIC strongly reduces the number of such fluctuations (red trace) but does not change the most frequent height measurements that are indistinguishable from those of control neurofilaments. Figure 5b shows the number of peaks per 35 μm contour length that are greater than 15 nm high and therefore are likely to be a bound dynactin/dynactin complex. The number of bound dynein/dynactin is strongly reduced by antibodies to either DIC or NF-M (SMI 31) but only partly decreased by antibodies to NF-L or p150Glued, consistent with the hypothesis that NF-M is the primary ligand for the motor and that the movement of neurofilaments on microtubules is disrupted by the anti-DIC antibody (Shah et al., 2000).
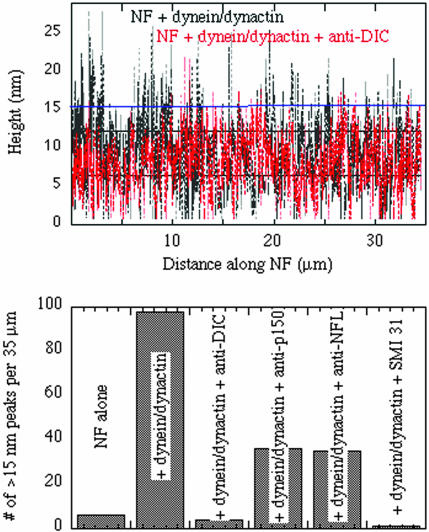
Figure 5.
Fluctuations in filament height from binding of dynein/dynactin complex. (a) Apparent height of neurofilament derived from AFM measurement along 35 μm of filament contour derived from measurements of multiple neurofilaments in different fields of the AFM image for two representative NF preparations: NF with added dynein/dynactin complex or the same preparation in the presence of an anti-DIC antibody. (b) Comparison of the number of height fluctuations per 35 μm NF contour length greater than 15 nm for neurofilaments alone, neurofilaments with added dynein/dynactin or neurofilaments with dynein/dynactin and antibodies against dynein IC (DIC), the p150Glued subunit of dynactin, NF-L, or NF-M/NF-H (SMI 31).
Purified dynein/dynactin also bound to microtubules, decorating their surface in AFM images (Figure 6). The appearance of the structures that decorate the microtubules differ from those decorating neurofilaments in that the dynein/dynactin appear as a more compact mass on microtubules rather than one that extends a projection away from the neurofilament core (Figure 6, a-c). No binding of dynein/dynactin to microtubules was observed in the presence of MgATP (Figure 6d).
Figure 6.
AFM images of cytoplasmic dynein/dynactin complex bound to microtubules. In AFM images of dynein bound along the sides of microtubules, the mass of the motor complex is observed to associate more closely with the filament than is seen in images of dynein/dynactin binding to neurofilaments. (a) A height image; (b) the corresponding amplitude image; (c) a 3D conversion of the black square shown in panel a. (d) No binding of dynein/dynactin to microtubules was observed in the presence of Mg-ATP.
Cytoplasmic Dynein Binds Directly to Neurofilament Subunit M
The observations of neurofilament motility along microtubules observed by Shah et al. (2000) as well as the AFM images shown above suggested to us that neurofilaments are a cargo for cytoplasmic dynein. We hypothesized that neurofilaments bind to the cargo-binding domain at the base of the motor complex. This association is likely to involve the IC of cytoplasmic dynein, because incubation of neurofilaments with anti-DIC antibodies significantly depletes bound dynein from neurofilaments (Shah et al., 2000; and Figure 4, c and d). The association could either be a direct interaction, or an indirect interaction via dynactin, which binds to DIC (Karki et al., 1995), or other neurofilament-associated proteins such as NUDEL (Nguyen et al., 2004).
To examine the possibility of a direct interaction, we used a yeast two-hybrid assay to identify DIC-binding proteins from a human brain cDNA library. In the screen, we isolated a subclone of NF-M, suggesting that NF-M is a dynein-binding protein. The subclone spans residues 122-428, encoding part of the neurofilament rod domain (Figure 7). To confirm this interaction, we expressed the NF-M fragment in an in vitro transcription/translation assay and examined the association of the protein with recombinant dynein IC covalently bound to Sepharose beads using affinity chromatography. As shown in Figure 7, we observed a robust association of this domain of NF-M with DIC. In parallel experiments with full-length NF-M, we did not observe as robust binding to dynein either in yeast two-hybrid or affinity chromatography assays (Figure 7). This observation suggested to us that there may be epitope-masking in the full-length NF-M polypeptide that blocks the dynein-binding site.
Figure 7.
Yeast two-hybrid and affinity chromatography assays identify a direct binding interaction between DIC and NF-M. A fragment from the rod domain of NF-M spanning amino acid residues 122-428, identified as a DIC-binding protein in a yeast two-hybrid screen, was specifically bound to a DIC affinity matrix. A similar fragment from NF-L as well as full-length NF-M and NF-L polypeptides, did not bind significantly to DIC in either the yeast two-hybrid or affinity chromatography assays. For affinity chromatography, in vitro-translated proteins (L) were loaded in parallel onto DIC or BSA affinity columns, and the flowthrough (F), wash (W), and elution (E) fractions were analyzed by SDS-PAGE and autoradiography. Interactions were also assayed pairwise in the yeast two-hybrid assay and scored vs. positive (p150Glued) and negative (vector only) controls.
To test the specificity of the interaction detected between NF-M and dynein, we subcloned the homologous region from NF-L and tested the binding of this domain (residues 100-420) as well as of full-length NF-L to dynein IC using both the yeast two-hybrid assay and affinity chromatography. As shown in Figure 7, we observed no significant interaction between the NF-L subfragment and DIC and only a minor interaction between full-length NF-L and DIC in both of these assays. However, we have noted direct interactions between NF-L and cytoplasmic dynein in blot overlay assays (our unpublished results), suggesting that while the interaction between dynein and NF-M is most robust, dynein may interact directly with assembled neurofilaments via multiple binding determinants.
Binding of Cytoplasmic Dynein to Assembled Neurofilaments
Both the yeast two-hybrid and affinity chromatography assays demonstrated direct binding of dynein IC to a fragment of NF-M, but not to full-length NF-M. This result suggests that the dynein binding site may be masked in the full-length protein. However, AFM images reveal the association of dynein with native neurofilament polymers and in vitro studies have shown dynein-dependent motility of neurofilaments along microtubules. Therefore we sought to test whether the observed epitope masking is relieved when NF-M becomes incorporated into polymer. To test this, we examined the binding of endogenous neurofilaments to recombinant dynein IC bound to Sepharose beads. As shown in Figure 8, when a soluble rat brain extract was incubated with DIC-bound beads, we observed the binding of NF-M to the affinity matrix. Both NF-H and NF-L were also bound to the DIC beads, although the binding of NF-H appeared less robust than that of NF-M and NF-L. In contrast, no significant binding was observed to BSA-bound beads, demonstrating that the interactions were specific and not due to trapping of neurofilaments on the matrix. These results suggest that the assembly of NF-M into polymer may unmask the binding site for cytoplasmic dynein.
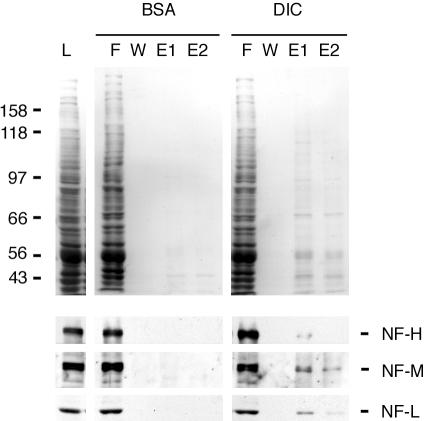
Figure 8.
Rat brain neurofilaments bind to a dynein IC affinity column. Soluble rat brain extract (L) was mixed with either DIC-bound Sepharose beads or BSA control beads. Flowthrough (F), wash (W), and eluate fractions (E1 and E2) were analyzed by SDS-PAGE (top panel is stained for total protein) and immunoblot with antibodies to NF-H, NF-M, and NF-L (bottom panels). All three neurofilament polypeptides were specifically bound to the DIC beads in comparison to BSA control beads.
To further investigate the role of dynein in mediating interactions between neurofilaments and microtubules, we incubated sonicated neurofilaments with microtubules in the absence and presence of exogenous dynein and then examined the cosedimentation of neurofilaments with microtubules. We performed this experiment with neurofilaments isolated from an adult animal, which are extensively phosphorylated, and also with neurofilaments that had been dephosphorylated in vitro. As shown in Figure 9A, we did not observe any significant association of phosphorylated neurofilaments with microtubules in this assay. The dephosphorylated neurofilaments also remained in the supernatant fraction in the absence or presence of exogenous dynein and dynactin. However, in the presence of microtubules we observed the specific cosedimentation of dephosphorylated neurofilaments with microtubules. A similar dephosphorylation-induced interaction between neurofilaments and microtubules was observed by Hisanaga and Hirokawa (1990) and attributed to the direct binding of dephosphorylated NF-H sidearms to the microtubule. However, as shown in Figure 9B, the association of the sonicated and dephosphorylated neurofilaments with microtubules was ATP-dependent—no significant cosedimentation was observed in the presence of MgATP. The nucleotide dependence of this interaction suggests that endogenous microtubule motors may also be mediating the association between neurofilaments and microtubules. Addition of exogenous purified dynein/dynactin marginally enhanced the cosedimentation. The limited enhancement observed may be due to the low molarity of the purified dynein/dynactin (0.03 mg/ml), in contrast to the higher concentrations of neurofilaments (0.25 mg/ml) used in this assay. Alternatively, a high-affinity interaction between the motor complex and neurofilaments may require additional factors such as NUDEL (Nguyen et al., 2004).
Figure 9.
ATP-induced dissociation of the neurofilament-dynein/dynactin-microtubule interaction. (A) Phosphorylated neurofilaments do not cosediment with microtubules even upon addition of exogenous purified dynein/dynactin complex. (B) Dephosphorylated neurofilaments and cytoplasmic dynein/dynactin cosediment with microtubules in the absence but not in the presence of MgATP. Sonicated neurofilaments, either phosphorylated or dephosphorylated, were incubated with dynein/dynactin (D), microtubules (MT), dynein/dynactin and microtubules (D-MT), or dynein/dynactin and microtubules in the presence of 10 mM MgATP (D-MT-ATP), as noted, and microtubule-bound proteins were isolated by sedimentation. Supernatant (S) and pellet (P) fractions were fractionated by SDS-PAGE and stained for total protein (top panels), or for dynein and dynactin using antibodies to DIC and dynamitin. Alkaline phosphatase, used to dephosphorylate the neurofilaments is present in the NF preparation shown in B (alk. phos.). Dephosphorylation of NF-H leads to a decrease in staining intensity with Coomassie because of increased heterogeneity in size (Aranda-Espinoza et al., 2002).
DISCUSSION
Direct observations of neurofilament motility within neurons has shown that the characteristic slow anterograde movement of these filaments is the net result of rapid movements in both the anterograde and retrograde direction, interrupted by pauses (Roy et al., 2000; Wang et al., 2000). The rates of active movement in the anterograde and retrograde directions are consistent with the known motility rates of kinesin and cytoplasmic dynein, respectively.
In vitro, purified neurofilaments are translocated bidirectionally in an ATP-dependent manner along microtubules at rates of ∼0.5 μm/s; microtubule minus end-directed movements are inhibited by antidynein antibodies (Shah et al., 2000).
To further investigate the role of cytoplasmic dynein and its accessory complex dynactin in mediating the motility of neurofilaments along microtubules, we examined interactions among these proteins using EM and AFM. Both approaches suggest that there are distinct points of contact between neurofilaments and microtubules. We hypothesize that microtubule motors may mediate some of these lateral contacts, allowing for the movement of neurofilaments along axonal microtubules. In vitro binding studies support this hypothesis, because we noted the ATP-dependent cosedimentation of sonicated neurofilaments with microtubules. Cosedimentation in the absence of nucleotide was enhanced by the dephosphorylation of neurofilaments, as previously noted (Hisanaga and Hirokawa, 1990). Both motor-mediated and NF-H sidearm-mediated cross-bridging may be more effective at lower levels of neurofilament phosphorylation. In vivo, the interaction of neurofilaments with microtubules is likely to be more complex, involving structural MAPs as well (Heimann et al., 1985, Hirokawa et al., 1988). Potentially, the phosphorylation-dependent NF-H sidearm-mediated interactions between neurofilaments and microtubules may be involved in the intermittent pausing noted between bidirectional motor-mediated excursions observed for neurofilaments undergoing axonal transport in situ (Roy et al., 2000; Wang et al., 2000).
AFM images suggest that most of the mass of dynein bound along neurofilaments is projecting away from the polymer. Cytoplasmic dynein is composed of two large globular heads, each ∼13 nm in diameter, which bind in an ATP-dependent manner to microtubules via stalk domains (Gee et al., 1997). The large heads are each connected via relatively slender stems ∼25 nm long to a common base (Burgess et al., 2003). Although the dynein heads are required for motor function, the base of the molecule is involved in attachment to cargo. Because neurofilaments act as cargo for dynein, we hypothesized that they are associated with the DIC at the base of the motor. Dynein intermediate and light chains form the cargo binding domain and have been shown to interact either directly or indirectly with specific cargos (reviewed in Karki and Holzbaur, 1999).
Here we demonstrate that neurofilaments bind directly to dynein, via an interaction between NF-M and DIC. Both yeast two-hybrid and affinity chromatography experiments demonstrate that there is a binding site for dynein in the rod domain of a neurofilament subunit. The mapping studies we have done identify the key binding site for cytoplasmic dynein within amino acid residues 122-428 of NF-M. This observation of the binding of dynein to the central domain of NF-M rather than to the tail domain is consistent with the results of Rao et al. (2003), who noted that knock-in mice expressing a tail-deleted construct of NF-M showed no alteration in rates of axonal transport of neurofilaments. Interestingly, in vitro binding studies indicate that the tail domain may actually block dynein binding to unassembled neurofilaments, because we did not observe significant binding of DIC to full-length NF-M in the absence of other neurofilament subunits. However, we did observe binding when NF-M is copolymerized with NF-L and NF-H into native neurofilaments (Figure 8), suggesting that when NF-M becomes incorporated into polymer the binding site for dynein is exposed. Although these data suggest that NF-M is a key determinant for dynein binding, we cannot rule out additional interactions between neurofilaments and cytoplasmic dynein. Although yeast two-hybrid and affinity chromatography experiments revealed only weak binding between DIC and NF-L, and AFM data indicate that an antibody to NF-M (and NF-H) disrupts dynein binding to neurofilaments more efficiently than an antibody directed to NF-L, we did note evidence for an additional interaction between DIC and NF-L using a blot overlay assay (our unpublished results). There may be multiple binding determinants for the interaction between dynein and neurofilaments in vivo.
The direct interactions that we have noted between NF-M and cytoplasmic DIC also indicate that no intermediary adaptor proteins may be necessary to mediate the binding. The DIC polypeptide is localized at the base of dynein and has been shown to participate directly in some motor-cargo binding interactions. Both casein kinase II (Karki et al., 1997) and β-catenin (Ligon et al., 2000) bind directly to DIC. In contrast, other dynein cargos may associate indirectly with dynein, via dynactin (Holleran et al., 2001). Studies on the role of dynein and dynactin in the intracellular motility of vimentin have suggested that dynactin is key in mediating cargo-binding interactions (Helfand et al., 2002). Dynactin copurifies with neurofilaments from spinal cord (Shah et al., 2000), but is likely to bind independently to neurofilaments because incubation of neurofilaments with antidynein antibodies effectively removes dynein but not dynactin from the filaments (Shah et al., 2000). We cannot rule out a possible role for dynactin in increasing either the affinity or the specificity of the interaction of the motor complex with neurofilaments. However, antidynein antibodies block the dynein/neurofilament association more effectively than antidynactin antibodies (Figures 4 and 5, and our unpublished results).
Our observations that dynein binds directly to neurofilaments and thus is the likely motor providing microtubule minus end-directed force on neurofilaments in the axon is also consistent with in vivo observations. We have noted that neurofilaments accumulate in the axons of transgenic mice with a targeted disruption in dynein/dynactin function (LaMonte et al., 2002). The microtubule plus end-directed motor is likely to be either conventional kinesin or a member of the kinesin superfamily. Although conventional kinesin was not observed to copurify with neurofilament preparations from spinal cord, Shah et al. (2000) did find evidence for the copurification of kinesin-like proteins. However, gene-targeting of KIF5A, which encodes a neuronally expressed isoform of conventional kinesin, was found to lead to the accumulation of NF-H, NF-M, and NF-L within the cell bodies of peripheral sensory neurons (Xia et al., 2003), suggesting that this kinesin may be directly involved in the outward transport of neurofilaments in vivo (Prahlad et al., 2000).
The somewhat unusual tug-of-war mechanism that results in the net slow transport outward movement of neurofilaments may be required because of the intrinsic properties of neurofilament polymers. In vitro studies of the motility of purified neurofilaments along microtubules indicate that the neurofilaments often pack into ball-like aggregates when they are moved uni-directionally along a microtubule. The action of opposing motors in vivo may serve to keep neurofilaments elongated, much like a rubber band will kink up when at rest, but will stretch out when under tension at both ends. In support of this hypothesis is the observation that loss of KIF5A leads to the accumulation of neurofilaments in the cell body in knockout mice (Xia et al., 2003), but inhibition of dynein/dynactin activity leads to the accumulation of neurofilaments in the axons of dynamitin-transgenic mice (LaMonte et al., 2002). Further, the overexpression of neurofilament subunit M was found to actually accelerate the net outward transport of neurofilaments (Xu and Tung, 2000), suggesting that if the excess NF-M protein competes for dynein-binding, thus reducing the opposing force on assembled filaments, they can then move outward at faster rates.
Although neurofilaments in live cells are observed to move bidirectionally along microtubules, there is also evidence that they may move along actin filaments in vivo via myosin Va (Rao et al., 2002). This additional motor interaction may be required to provide the correct spacing or distribution of neurofilaments in axons. It is interesting to note that the combined actions of a kinesin (kinesin II), dynein, and myosin V have also shown to be required to obtain the normal dynamics of melanosome motility in cultured melanophores (Gross et al., 2002).
Given the complex dynamics of neurofilaments in vivo, it is perhaps not surprising that neurofilament aggregation is a hallmark of many neurodegenerative diseases, including ALS. The accumulation or aggregation of neurofilaments along the axon may in turn further impede transport along the axon. The further investigation of the motor proteins involved in the transport of neurofilaments and their regulation may therefore provide further insight into the pathological mechanisms underlying neuronal degeneration and disease.
Acknowledgments
We gratefully acknowledge the contributions of Anish Patel, Bernadette LaMonte, and Nicholas Weber. Dr. Virginia Lee generously provided the cDNA clones of full-length NF-L and NF-M. These studies were supported by National Institutes of Health (NIH) grant GM56707 to P.A.J. and E.L.F.H., NIH grant AR051174 to E.L.F.H. and P.A.J., and a grant from the Merck/Merial Summer Research Program to support J.A.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-05-0401. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-05-0401.
References
- Al-chalabi, A., and Miller, C.C. J. (2003) Neurofilaments and neurological disease. BioEssays 24, 346-355. [DOI] [PubMed] [Google Scholar]
- Aranda-Espinoza, H., Carl, P., Leterrier, J.F., Janmey, P., and Discher, D.E. (2002). Domain unfolding in neurofilament sidearms: effects of phosphorylation and ATP. FEBS Lett. 531, 397-401. [DOI] [PubMed] [Google Scholar]
- Burgess, S.A., Walker, M.L., Sakaibara, H., Knight, P.J., and Oiwa, K. (2003). Dynein structure and power stroke. Nature 421, 715-718. [DOI] [PubMed] [Google Scholar]
- Dogic, Z. et al. (2004). Elongation and fluctuations of semiflexible polymers in a nematic solvent. Phys. Rev. Lett. 92, 125503-125504. [DOI] [PubMed] [Google Scholar]
- Gee, M.A., Heuser, J.E., and Vallee, R.B. (1997). An extended microtubule-binding structure within the dynein motor domain. Nature 390, 636-639. [DOI] [PubMed] [Google Scholar]
- Gross, S.P., Tuma, M.C., Deacon, S.W., Serpinskaya, A.S., Reilein, A.R., and Gelfand, V.I. (2002). Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 156, 855-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann, R., Shelanski, M.L., and Liem, R.K. (1985). Microtubule-associated proteins bind specifically to the 70-kDa neurofilament protein. J. Biol. Chem. 260, 12160-12166. [PubMed] [Google Scholar]
- Heins, S., Wong, P.C., Muller, S., Goldie, K., Cleveland, D.W., and Aebi, U. (1993). The rod domain of NF-L determines neurofilament architecture, whereas the end domains specify filament assembly and network formation. J. Cell Biol. 123, 1517-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand, B.T., Mikami, A., Vallee, R.B., and Goldman, R.D. (2002). A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J. Cell Biol. 157, 795-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa, N., Hisanaga, S., and Shiomura, Y. (1988). MAP2 is a component of crossbridges between microtubules and neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etch immunoelectron microscopy and reconstitution studies. J. Neurosci. 8, 2769-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga, S., and Hirokawa, N. (1990). Dephosphorylation-induced interactions of neurofilaments with microtubules. J. Biol. Chem. 265, 21852-21858. [PubMed] [Google Scholar]
- Holleran, E.A., Tokito, M.K., LaMonte, B.H., Stankewich, M., Morrow, J., and Holzbaur, E.L.F. (2001). βIII spectrin binds to the Arp1 subunit of dynactin. J. Biol. Chem. 276, 36598-36605. [DOI] [PubMed] [Google Scholar]
- Hyman, A., Drechsel, D., Kellogg, D., Salser, S., Sawin, K., Steffen, P., Wordeman, L., and Mitchison, T. (1991). Preparation of modified tubulins. Methods Enzymol. 196, 478-485. [DOI] [PubMed] [Google Scholar]
- Karki, S., and Holzbaur, E.L.F. (1995). Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 270, 28806-28811. [DOI] [PubMed] [Google Scholar]
- Karki, S., and Holzbaur, E.L.F. (1999). Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 11, 45-53. [DOI] [PubMed] [Google Scholar]
- Karki, S., Tokito, M.K., and Holzbaur, E.L. (1997). Casein kinase II binds to and phosphorylates cytoplasmic dynein. J. Biol. Chem. 272, 5887-5891. [DOI] [PubMed] [Google Scholar]
- Kumar, S., Yin, X., Trapp, B.D., Hoh, J.H., and Paulaitis, M.E. (2002a). Relating interactions between neurofilaments to the structure of axonal neurofilament distributions through polymer brush models. Biophys. J. 82, 2360-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., Yin, X., Trapp, B.D., Paulaitis, M.E., and Hoh, J.H. (2002b). Role of long-range repulsive forces in organizing axonal neurofilament distributions: evidence from mice deficient in myelin-associated glycoprotein. J. Neurosci. Res. 68, 681-690. [DOI] [PubMed] [Google Scholar]
- LaMonte, B. H., Wallace, K. E., Holloway, B. A., Shelly, S. S., Ascano, J., Tokito, M., Van Winkle, T., Howland, D. S. and Holzbaur, E. L. (2002). Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron 34, 715-727. [DOI] [PubMed] [Google Scholar]
- Leterrier, J.F., Kas, J., Hartwig, J., Vegners, R., and Janmey, P.A. (1996). Mechanical effects of neurofilament cross-bridges. Modulation by phosphorylation, lipids, and interactions with F-actin. J. Biol. Chem. 271, 15687-15694. [DOI] [PubMed] [Google Scholar]
- Ligon, L.A., Karki, S., Tokito, M., and Holzbaur, E.L.F. (2002). Dynein binds to β-catenin and may tether microtubules at adherens junctions. Nat. Cell Biol. 3, 913-917. [DOI] [PubMed] [Google Scholar]
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951). Protein measurement using the Folin phenol reagent. J. Biol. Chem. 193, 265-275. [PubMed] [Google Scholar]
- Nguyen, M.D., Shu, T., Sanada, K., Lariviere, R.C., Tseng, H.-C., Park, S.K., Julien, J.-P., and Tsai, L.-H. (2004). A NUDEL-dependent mechanism of neurofilament assembly regulates the integrity of CNS neurons. Nat. Cell Biol. 6, 595-608. [DOI] [PubMed] [Google Scholar]
- Prahlad, V., Helfand, B.T., Langford, G.M., Vale, R.D., and Goldman, R.D. (2000). Fast transport of neurofilament protein along microtubules in squid axoplasm. J. Cell Sci. 113, 3939-3946. [DOI] [PubMed] [Google Scholar]
- Rao, M.V. et al. (2002). Myosin Va binding to neurofilaments is essential for correct myosin Va distribution and transport and neurofilament density. J. Cell Biol. 159, 279-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M.V. et al. (2003). The neuroflament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J. Cell Biol. 163, 1021-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S., Coffee, P., Smith, G., Liem, R.K., Brady, S.T., and Black, M.M. (2000). Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J. Neurosci. 20, 6849-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D.A., Gill, S.R., Cooper, J.A., Heuser, J.E., and Schroer, T.A. (1994). Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J. Cell Biol. 126, 403-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, J.V., Flanagan, L.A., Janmey, P.A., and Leterrier, J.F. (2000). Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol. Biol. Cell 11, 3495-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea, T.B. and Flanagan, L.A. (2001) Kinesin, dynein and neurofilament transport. Trends Neurosci. 24, 644-648. [DOI] [PubMed] [Google Scholar]
- Toyoshima, I., Kato, K., Sugawara, M., Wada, C., and Masamune, O. (1998). Kinesin accumulation in chick spinal axonal swellings with beta, beta′-iminodipropionitrile (IDPN) intoxication. Neurosci. Lett. 249, 103-106. [DOI] [PubMed] [Google Scholar]
- Vallee, R.B., Wall, J.S., Paschal, B.M., and Shpetner, H.S. (1988). Microtubule-associated protein 1C from brain is a two-headed cytosolic dynein. Nature 332, 561-563. [DOI] [PubMed] [Google Scholar]
- Wagner, O.I., Lifshitz, J., Janmey, P.A., Linden, M., McIntosh, T.K., and Leterrier, J.F. (2003). Mechanisms of mitochondria-neurofilament interactions. J. Neurosci. 23, 9046-9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Ho, C.L., Sun, D., Liem, R.K., and Brown, A. (2000). Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat. Cell Biol. 2, 137-141. [DOI] [PubMed] [Google Scholar]
- Xia, C.-H., Roberts, E.A., her, L.-S., Liu, X., Williams, D.S., Cleveland, D.W., and Goldstein, L.S.B. (2003) Abnormal neurofilaments transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 161, 55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., and Tung, V.W. (2000). Overexpression of neurofilament subunit M accelerates axonal transport of neurofilaments. Brain Res. 866, 326-332. [DOI] [PubMed] [Google Scholar]