Abstract
The most common infections in primary immune deficiency disease (PIDD) patients involve encapsulated bacteria, mainly Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae (pneumococcus). Thus, it is important to know the titers of Hib- and pneumococcus-specific antibodies that are present in immune globulin (Ig) intravenous (IGIV) preparations used to treat PIDD. In this study, seven IGIV preparations were tested by enzyme-linked immunosorbent assay and opsonophagocytic activity for antibody titers to the capsular polysaccharides of Hib and five pneumococcal serotypes. Differences in Hib- and pneumococcus-specific antibody titer were observed among various IGIV preparations, with some products having higher- or lower-than-average titers. Opsonic activity also varied among preparations. As expected, IgG2 was the most active subclass of both binding and opsonic activity except against pneumococcal serotype 6B where IgG3 was the most active. This study determines antibody titers against capsular polysaccharides of Hib and pneumococcus in seven IGIV products that have been shown to be effective in reducing infections in PIDD patients. As donor antibody levels and manufacturing methods continue to change, it may prove useful from a regulatory point of view to reassess IGIV products periodically, to ensure that products maintain antibody levels that are important for the health of IGIV recipients.
Life-threatening infections in primary immune deficiency disease (PIDD) patients are caused chiefly by encapsulated bacteria, especially Streptococcus pneumoniae (pneumococcus) and Haemophilus influenzae type b (Hib) (2, 10, 11, 14, 17, 39). The main virulence factor of these encapsulated bacteria is their polysaccharide (PS) capsule (19). Among the numerous serotypes of Haemophilus influenzae and pneumococcus, only a select few, pneumococcal serotypes, 4, 6B, 9V, 14, and 19F, and Hib cause the majority of disease in persons with PIDD and other susceptible individuals (12, 19). Antibodies produced to the capsule are type specific and have been shown to be protective against invasive Hib (1, 26, 30) and pneumococcal disease (35, 41).
Immune globulin (Ig) therapy provides effective prophylaxis against pneumococcal and Hib infections (28, 35). The survival and health of persons with PIDD has greatly improved since the advent of Ig therapy to prevent these diseases (6, 24, 38). In studies of Apache infants who have a high frequency of Hib and pneumococcal infections, passive immunization with bacterial PS Ig, prepared from plasma of donors immunized with Hib, pneumococcal, and meningococcal capsular PS vaccines, significantly reduced these infections (28, 35). The currently available human Ig intravenous (IGIV) products were licensed based upon their ability to prevent serious infections in clinical trials in the context of an acceptable safety profile. While such trials remain the “gold standard” for proof of efficacy, they are time-consuming and may face recruitment challenges. Although it is unlikely that in vitro surrogate markers of IGIV efficacy could entirely replace clinical trials, these markers may provide useful information in terms of comparing licensed to experimental products early in the development stage, evaluating the potential of manufacturing changes to alter clinically relevant specific antibody levels, monitoring stability, and ensuring production consistency. Food and Drug Administration (FDA) regulations, which also apply to IGIV, require that all Ig product lots possess a minimum level of antibodies to measles, diphtheria, and polio (12a). However, Hib and pneumococci cause most infections in persons with PIDD. The objective of the current study was therefore to define the current range of antipneumococcal and anti-Hib antibody levels, specific IgG subclass concentrations, and functional antibody levels among licensed IGIV products.
Specific antibody concentrations were determined by enzyme-linked immunosorbent assay (ELISA) and compared with a reference serum containing known antipneumococcal and anti-Hib antibody concentrations. Opsonophagocytosis assays were used to measure the functional ability of the total IGIVs and of IgG subclasses against pneumococcus. The results demonstrated very little lot-to-lot variation but revealed some differences among products. Many manufacturing methods that were used in the past, often in an effort to decrease aggregate-related side effects, have diminished specific antibody levels and/or function (15, 18, 23, 31, 40). IGIV manufacturing methods continue to evolve because of efforts to enhance yield and to increase safety assurance with regard to nonenveloped viruses and other pathogens. If a new product or novel manufacturing method resulted in substantially lower titers of anti-capsular PS antibodies, it could be an early indication of potential efficacy concerns. To our knowledge, this is the first report comparing anti-capsular PS antibodies among the current U.S. licensed products. As such, it may provide a useful basis for comparing them with new products and products for which major manufacturing changes are proposed.
MATERIALS AND METHODS
IGIV.
Five lots each of seven commercial IGIV preparations (identified here as A to G) were analyzed in this study. The IGIV products A to F were purified according to a modified method of Cohn-Oncley (36), and product G was fractionated according to the Kistler-Nitschmann method (36). The IGIV purification methods were supplemented with different final treatment steps: product A by solvent detergent and polyethylene glycol-bentonite fractionation, product B by heat treatment, products C and D by solvent detergent treatment, product E by polyethylene glycol trypsin treatment, product F by incubation at pH 4.2 and solvent detergent treatment, and product G by pH 4.0 pepsin treatment (3, 36). All products were manufactured from source plasma except for products D and G, which was produced from recovered plasma. For all assays using total IGIV, the samples were initially diluted to 10 mg of IgG/ml (1%).
IgG subclasses.
IgG subclasses were separated from IGIV preparations using a column of recombinant protein A-conjugated Sepharose beads (rProtein A-Sepharose Fast Flow; Amersham Pharmacia Biotech AB, Uppsala, Sweden) and fast-performance liquid chromatography (FPLC) according to a previously described method (29). Briefly, IGIV (0.25 g/run at a concentration of 50 mg/ml) was applied to the column after dialysis into McIlvaine's citrate-phosphate buffer, pH 6.5 (0.2 M Na2HPO4 titrated to the desired pH with 0.1 M citric acid and preserved with 0.1% sodium azide). Fractions of 8 ml were collected when the A280 (0.5-cm path length) was greater than 0.1. Typically, a two-step gradient was run by programming the admixture of two buffers at pHs 6.5 and 3.5. IgG3 does not bind to protein A and was therefore present in the flowthrough; IgG2 was eluted at pH 4.70 to 4.55, and the majority of IgG1 was eluted at pH 4.50 to 3.70. To determine the enrichment of the individual fractions, as well as the final pooled fractions, an ELISA for each subclass was performed in accordance with the manufacturer's instructions by using a human IgG subclass detection kit (Central Laboratory of The Netherlands Red Cross Blood Transfusion Service; obtained through Accurate Chemical, Westbury, N.Y.). Pooled fractions were concentrated by ultrafiltration (Millipore, Bedford, Mass.) with stirred cells. The subclass-enriched preparations were dialyzed against phosphate-buffered saline (PBS; 10 mM, pH 7.4), and protein concentrations were determined by measuring the A280. The preparation enrichments were ≥84% IgG1, ≥96% IgG2, and ≥94% IgG3.
ELISA.
Antibodies to Hib were detected by an ELISA (25). Immulon 1B plates (Dynatech Laboratories, Chantilly, Va.) were coated with 100 μl per well of a mixture of methylated human serum albumin and 2.5- and 5-μg/ml Hib PS (Praxis Biologics, Rochester, N.Y.) in coating buffer (10 mM PBS, pH 7.4). After an overnight incubation at room temperature, the plates were rinsed with wash buffer (10 mM PBS, pH 7.4, and 0.05% Tween 20). IGIV samples, Hib reference standard 1983 (Center for Biologics Evaluation and Research, FDA, Bethesda, Md.), and quality control serum 532A were added to wells in triplicate in twofold serial dilutions. After an overnight incubation at 4°C, the plates were washed and a goat anti-human IgG (γ-chain specific)-alkaline phosphatase-conjugated antibody (Sigma-Aldrich Corp., St. Louis, Mo.) was added at a 1:2,000 dilution. After a 2-h incubation, the plates were washed, and 100 μl of nitrophenol phosphate (Sigma-Aldrich Corp.) dissolved at 1.0 mg/ml in substrate buffer (1.0 mM Tris and 0.3 mM MgCl2, pH 9.8) was added. When the appropriate color intensity was reached, the A405 was determined. Antibody concentrations were calculated by using a weighted log-logit ELISA program (7). The limit of quantitation (sensitivity) of the Hib ELISA is 0.01 μg of IgG/mg.
S. pneumoniae antibodies were also detected by an ELISA as previously described (8). Briefly, Immulon 1B 96-well plates (Dynatech) were coated with the PSs of pneumococcal serotype 4, 6B, 9V, 14, or 19F (American Type Culture Collection, Manassas, Va.). After an overnight incubation at room temperature, the plates were washed and appropriate dilutions of IGIV, standard serum 89SF (Center for Biologics Evaluation and Research, FDA, Bethesda, Md.), and quality control sera were adsorbed with C PS (State Serum Institute, Copenhagen, Denmark) and 22F PS (American Type Culture Collection), added in triplicate to the starting wells, and diluted serially twofold. Following an overnight incubation at 4°C, the plates were washed and incubated with a 1:2,000 dilution of alkaline phosphatase-labeled anti-human IgG secondary antibody (Sigma-Aldrich Corporation). The plates were developed and the A405 was determined. Antibody concentrations were calculated as for the Hib assay. The limit of quantitation (sensitivity) of the pneumococcal ELISA varies some between serogroups, but on average is about 0.005-μg/mg IgG.
OPA.
For the opsonophagocytosis assay (OPA), the starting concentration for the purified IgG1 and IgG2 samples was adjusted to 2 mg/ml and IgG3 was adjusted to 1 mg/ml in Hanks balanced salt solution containing 10% bovine serum albumin, pH 7.4. The OPA was done as described by Romero-Steiner et al. (27), except that we used human polymorphonuclear neutrophils from multiple donors received from the National Institutes of Health Blood Bank, Bethesda, Md., and further purified by using Polymorphprep (Axis-Shield, Oslo, Norway). The initial Ig dilution was 1:8 in the final opsonization mixture.
Statistical analysis.
All statistical analyses were performed with GraphPad Prism version 3 software. Calculations of P values were performed with the unpaired t test provided in the software.
RESULTS
Specific antibody levels against Hib.
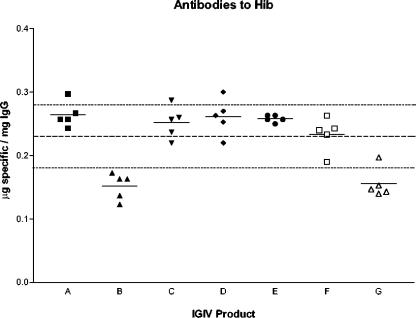
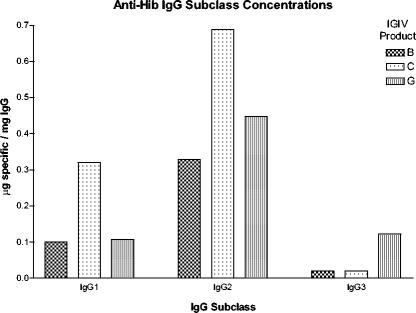
Seven products, five lots each, were tested for anti-Hib antibodies by ELISA. To compare the anti-Hib levels among IGIV products, the mean anti-Hib level of all products was calculated as indicated by the dashed line in Fig. 1. Two products, B and G, had specific antibody concentrations that were significantly different from the mean anti-Hib concentration of all products (P ≤ 0.0032 and P ≤ 0.0053, respectively) (Fig. 1). Representative lots of three products, C (higher anti-Hib level) and B and G (lower anti-Hib levels), were selected and separated by FPLC into IgG subclasses (IgG1, IgG2, and IgG3), and IgG subclass concentrations to Hib were measured by ELISA (Fig. 2). IgG2 was the predominant anti-Hib IgG subclass. Product C had the highest level of anti-Hib IgG1 and IgG2. Product G had the highest anti-Hib IgG3 content. The nonspecific IgG subclass profile of each product was similar (37).
FIG. 1.
Anti-Hib concentrations in IGIV products. Seven licensed IGIV products (five lots each) were tested for anti-Hib antibody concentrations, indicated as micrograms of specific antibody per milligram of total IgG, by ELISA. Each point on the graph represents an IGIV lot, and the mean anti-Hib concentration is represented by a horizontal solid line. The mean anti-Hib concentration of all the products is represented by the dashed line across the graph, while the dotted lines above and below indicate 1 standard deviation (0.23 ± 0.05 μg/mg of IgG).
FIG. 2.
Anti-Hib IgG subclass concentrations. Three IGIV products, B, C, and G, were selected for analysis, and IgG subclasses were separated with a protein A column and FPLC. Anti-Hib IgG subclass levels were measured with an ELISA.
Specific antibody levels against selected pneumococcal serotypes.
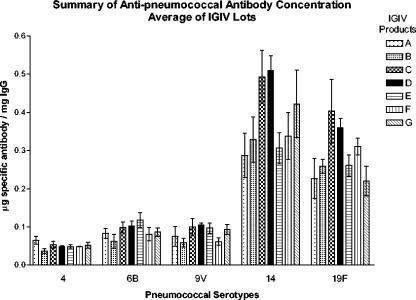
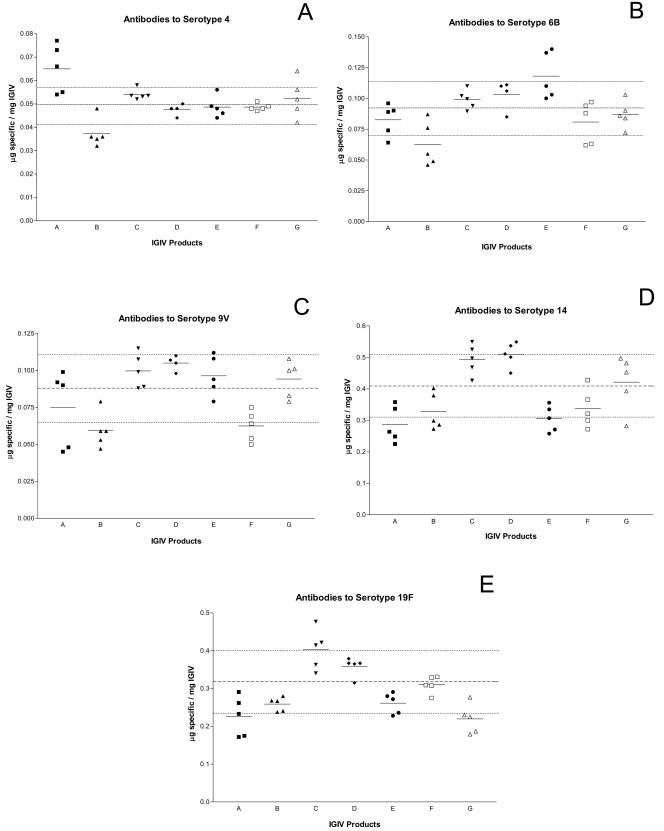
Antibodies to five pneumococcal serotypes, 4, 6B, 9V, 14, and 19F, were measured by ELISA in all 35 IGIV lots. The results of the ELISA are summarized in Fig. 3. In general, antibodies were more abundant against serotypes 14 and 19F than the other serotypes tested. Overall, product B had the lowest content of antibodies to the specific PSs tested, whereas products C and D had the highest anti-PS antibody concentration. Antibodies to serotype 4 were at the highest concentration in product A, whereas product B had anti- 4 concentrations, which were lower than the mean of all products (Fig. 4A). Anti-6B was at highest concentrations in products A, F, and G, whereas product B was again the lowest (Fig. 4B). Products C, D, and E had the highest anti-9V concentrations, while products B and F had the lowest (Fig. 4C). Product C and D had highest anti-14 and anti-19F antibodies (Fig. 4D and E). Interestingly, product E, which had similar anti-4, anti-6B, and anti-9V levels to those in products C and D, had lower anti-14 and anti-19F levels (Fig. 3 and 4D and E).
FIG. 3.
Summary of antipneumococcal concentrations. The means of the antipneumococcal serotype concentrations of all lots were calculated and are summarized in a bar graph. Each bar is the mean of five IGIV lots of the same product. The error bars indicate standard deviations.
FIG. 4.
Antibody concentrations to pneumococcal serotypes 4, 6B, 9V, 14, and 19F (panels A to E, respectively). Antibodies to pneumococcal serotypes were measured for the same IGIV lots as in Fig. 3A. The mean antiserotype concentration for all the lots is indicated by the dashed middle line, whereas the standard deviation is indicated by the dashed lines above and below the mean. The solid line indicates the mean concentration within the same product.
IgG subclasses from three IGIV products were also measured for antipneumococcal antibody concentrations (Table 1). IgG2 was the predominant binding subclass measured. Product C had the highest antipneumococcal IgG1 and IgG2 concentrations, while product G had the highest antipneumococcal IgG3 concentrations (Table 1). As in the case of Hib, very little lot-to-lot variation was observed, suggesting both similarity in donor antibody profiles and manufacturing consistency across IGIV lots for a given manufacturer.
TABLE 1.
Antipneumococcal IgG subclass concentrations as measured by ELISA
| IgG subclass | IGIV product | IgG concn (μg/mg) For pneumococcal serotypea:
|
||||
|---|---|---|---|---|---|---|
| 4 | 6B | 9V | 14 | 19F | ||
| IgG1 | B | 0.01 | 0.07 | 0.03 | 0.09 | 0.11 |
| C | 0.03 | 0.11 | 0.06 | 0.35 | 0.22 | |
| G | 0.02 | 0.07 | 0.04 | 0.13 | 0.13 | |
| IgG2 | B | 0.08 | 0.30 | 0.18 | 0.53 | 0.55 |
| C | 0.18 | 0.46 | 0.19 | 1.17 | 0.81 | |
| G | 0.10 | 0.34 | 0.22 | 0.83 | 0.56 | |
| IgG3 | B | 0.02 | 0.03 | 0.01 | 0.06 | 0.12 |
| C | 0.02 | 0.03 | 0.41 | 0.13 | 0.07 | |
| G | 0.05 | 0.25 | 0.18 | 0.32 | 0.32 | |
Results are expressed in micrograms of specific IgG per milligram of IgG subclass. Samples were measured in triplicate.
Opsonophagocytosis of S. pneumoniae.
In order to measure the functional activity of the antipneumococcal antibodies in IGIV products, OPAs were performed. The OPA was performed using a series of eight twofold dilutions of IGIV. For total IGIV, the Ig concentration in the first dilution (1:8) was 1.25 mg/ml. The opsonophagocytic capability of antipneumococcal antibodies was designated as the maximum dilution of IGIV at which 50% of the pneumococcal CFU are killed. In general, the ability to opsonize bacteria by IGIV depended on the pneumococcal serotype being killed (Table 2). Noticeably, products A and B had the highest titers for serotype 6B, whereas product B had the lowest titer for serotype 4.
TABLE 2.
Opsonophagocytic capacity of total IGIV
| IGIV product | Opsonophagocytic capacity against pneumococcal serotypea:
|
||||
|---|---|---|---|---|---|
| 4 | 6B | 9V | 14 | 19F | |
| A | 32 | 512 | 125 | 128 | 64 |
| B | <8 | >1,024 | 256 | 128 | 64 |
| C | 64 | 64 | 256 | 128 | 64 |
| D | 16 | 256 | 256 | 256 | 256 |
| E | 64 | 128 | 64 | 64 | 32 |
| F | 32 | 128 | 64 | 128 | 128 |
| G | 16 | 128 | 64 | 256 | 128 |
The Ig concentration in the first dilution (1:8) was 1.25 mg/ml. Capacity represents OPA titer − maximum dilution of IGIV at which 50% of the pneumococcal CFU are killed.
Three products (B, C, and G) were chosen to measure the opsonophagocytosis capability of IgG subclasses (Table 3). The OPA was performed as for total IGIV, except that the results are reported as the mean OPA titer of the three products. In general, opsonic activity of antipneumococcal antibodies in IGIV was highest in the IgG2 subclass, with the exception of serotype 6B, where IgG3 was the most active.
TABLE 3.
Opsonophagocytic activity of IgG subclasses against different pneumococcal serotypes in three different IGIV products
| IgG subclass | Opsonophagocytic activity against pneumococcal serotypea:
|
||||
|---|---|---|---|---|---|
| 4 | 6B | 9V | 14 | 19F | |
| IgG1 | 37 | 19 | 27 | 13 | 9 |
| IgG2 | 362 | 75 | 34 | 107 | 19 |
| IgG3 | 67 | 107 | <8 | <8 | <8 |
Each value represents the mean OPA titer for three IGIV products (B, C, and G).
DISCUSSION
In persons with primary and acquired antibody deficiencies, replacement therapy with IGIV is effective in preventing severe acute bacterial infections (24, 38). Using ELISA and OPAs, we compared anti-Hib and antipneumococcal antibody levels in current U.S.-licensed IGIV products. IGIV purification methods are manufacturer specific and unique with respect to specific conditions during fractionation and the combination of methods used to decrease aggregate formation, remove proteins associated with adverse events, and provide viral clearance (21, 36). Another difference among these products may be titers of antibodies in the starting plasma pool. Some products are derived from recovered plasma, harvested from whole-blood donations of volunteer donors. Other products are manufactured from source plasma, obtained by plasmapheresis of paid donors. The age and vaccination status of these donors may differ, thereby influencing the antibody profile. Additionally, plasma and blood collections from different regions may have different antibody profiles because of disease prevalence or vaccination coverage.
In order to examine the differences in antibody levels to pathogens that are important for PIDD patients, we tested seven IGIV products, five lots each for anti-Hib and antipneumococcal antibody concentrations. We also separated three IGIV products into IgG subclasses and measured the pathogen-specific subclass concentration. Finally, we assessed the functional activity of the total IgG and its subclasses against pneumococci using the OPA.
Differences in Hib antibody concentrations, as measured by ELISA, were found among products. Among all products, there was less than a twofold difference. Products B and G had somewhat lower anti-Hib levels than the mean of all products. Product C had the highest specific IgG1 and IgG2 anti-Hib titers, consistent with the overall finding for total IgG. The manufacturing of product B includes a heat treatment step to inactivate potential virus (36). According to one study, heat treatment at a low pH and low sodium concentration was found not to have any detrimental effects on IgG structure (5). However, a study on the thermal stability of Ig indicated that IgG may aggregate following heat treatment, and the Fab portion of IgG is most sensitive to heat (40). Product G is manufactured by using the Kistler-Nitschmann method and includes a low pH and trace pepsin treatment step for virus inactivation (36). It would be difficult to implicate any of these methods in the absence of direct experimentation, since differences in the starting plasma pool titers could be responsible for the observations. In any case, given a typical infusion of 300 to 400 mg/kg, anti-Hib levels should achieve the putative protective level of 0.15 μg/ml for all products (16, 26). It may be anticipated that anti-Hib titers in IGIV will increase over time as a result of childhood vaccination recommendations.
Differences were found in antipneumococcal antibody levels among pneumococcal serotypes and IGIV products. The relative antibody concentration against the five pneumococcal types in the seven different IGIV products reflects differences in normal antibody concentrations in adults (9). Antibody levels to serotypes 14 and 19F are normally severalfold higher than those to types 4, 6B, and 9V, which is consistent with our study. In general, variation among products was at most two- to threefold, and lot-to-lot variation within products was much less. No product had consistently highest or lowest specific antibody levels when all serotypes were considered. Antibodies to serotype 4 were 0.049 ± 0.008 μg/mg of IgG. Most of the IGIV products were in that range, except product A, which had the highest anti-type 4 concentration (0.065 ± 0.010 μg/mg of IgG). Most of the products had a specific antibody concentration to serotypes 6B and 9V within the mean of 0.092 ± 0.022 and 0.088 ± 0.023 μg/mg of IgG, respectively. Antibody profiles to serotypes 14 and 19F were similar, averaging 0.41 ± 0.10 and 0.32 ± 0.08 μg/mg of IgG, respectively. Interestingly, product E, which had specific antibody concentrations similar to those of products C and D for the other serotypes, had lower anti-14 and anti-19F levels. Product E is known to have slightly low IgG3 concentrations, due to the use of small amounts of trypsin during manufacturing. Both IgG1 and IgG3 are susceptible to digestion by trypsin (33), potentially explaining the lower levels of antibody to serotypes 14 and 19F observed for this product.
In order to address the distribution of specific antibodies within IgG subclasses, we selected three IGIV products, B, C, and G. According to the literature, the predominant subclass to Hib, pneumococcal, and other bacterial PS antigens in adults is IgG2 (35). The ability to respond to the Hib vaccine when given in combination with the pertussis vaccine has been shown to coincide with maturation of the antibody response and secretion of IgG2 (32). Also, a direct correlation between IgG2 levels in adults and antibody responses to PS antigens has been observed (34). In accordance with these reports, we found that IgG2 contained the highest proportion of anti-Hib and antipneumococcal antibodies relative to the other IgG subclasses, although both IgG1 and IgG3 antibodies were also represented (Fig. 2 and Table 1). In addition, IgG2 had the best overall antipneumococcal opsonophagocytic activity of the three subclasses (Table 3). On a per-milligram-of-IgG-subclass basis, IgG1 contains nearly as much antipneumococcal antibody as IgG2 (Table 1). Product C had the highest specific IgG1 and IgG2 concentration against all serotypes of pneumococcus. Interestingly, product G tended to have more antipneumococcal and anti-Hib antibody content in IgG3 when this was compared with IgG3 from other products.
Our results contrast with those of Hamill et al. (13). These authors performed a similar study with similar methods on four IGIV products, using ELISA. They found the predecessors of product G to have low titers to serotype 4 and of product F to have much higher titers against serotype 19F compared to the other products tested, whereas our studies demonstrate a near equivalence. As in our study, there was little lot-to-lot variation within manufacturers. The most likely explanation is that changes have occurred both in manufacturing methods and possibly in donor epidemiology in the intervening 12 years since the prior study. Consistent with our results, similar lot-to-lot antipneumococcal antibody levels were observed among a limited number of lots of three U.S. products in 2002 (20).
There was not a strong correlation in our study between the pneumococcal ELISA and OPA results, which is consistent with another study (4). The lack of correlation may be attributable to differences in antibody avidity or complement fixation activity, which were not examined in this study. In addition, the OPA can have high intra- and/or interassay variability. While a functional test such as the OPA is desirable, more research needs to be performed for assay optimization.
The minimum concentration of pneumococcus-specific antibody associated with long-term protection against invasive disease in vaccinated infants is typically between 0.15 and 0.5 μg/ml (4). The average trough levels that would be achieved for a 20-kg child administered 400 mg of IGIV/kg as a one-time first dose, estimating a volume of distribution of 100 ml/kg, would be 0.10, 0.18, 0.17, 0.77, and 0.58 μg/ml for serotypes 4, 6B, 9V, 14, and 19F, respectively. While the average trough level for serotype 4 is less than 0.15 μg/ml, the IGIV preparations may still be effective, as it is not known whether the estimated protective level is the true lowest possible protective antipneumococcal concentration. In addition, trough levels of antibodies rise if regular infusions (every 3 to 4 weeks) are given, so that actual trough levels in a regularly infused patient are likely to be greater than the calculations above reflect (22). A comparison of the protective antibacterial IgG concentrations measured by others and the levels of specific IgG measured in our ELISA suggests there is sufficient antibody, even in the IGIV with the lowest specific antibody concentrations, to provide protection against invasive pneumococcal disease.
Although we found differences in specific antibody levels among preparations, all of these products have been licensed based upon their ability to prevent bacterial infections in PIDD patients. As donor epidemiology and manufacturing methods continue to change, it may prove useful from a regulatory point of view to reassess IGIV products periodically, to ensure that products maintain antibody levels that are important for the health of IGIV recipients.
ADDENDUM IN PROOF
Since the inception of this study, three additional IGIV products have been licensed.
Acknowledgments
We thank Orit Scharf, Nancy Eller, and Oxana Munoz for technical assistance and helpful discussions. We also thank Frejya Lynn and John Finlayson for review of the manuscript.
REFERENCES
- 1.Anderson, P., R. B. Johnston, Jr., and D. H. Smith. 1972. Human serum activities against Hemophilus influenzae, type b. J. Clin. Investig. 51:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asherson, G. L., and A. D. B. Webster (ed.). 1980. Diagnosis and treatment of immunodeficiency diseases. Blackwell, Oxford, United Kingdom.
- 3.Ballow, M. 2002. Intravenous immunoglobulins: clinical experience and viral safety. J. Am. Pharm. Assoc. 42:449-458; quiz, 458-459. [DOI] [PubMed] [Google Scholar]
- 4.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 5.Bridonneau, P., H. Marcilly, M. Vernois-Martin, P. Goigoux, V. Bourdel, A. Laulan, F. X. Deramoudt, M. Desmadril, M. Sitbon, B. Basuyaux, M. Steinbuch, and R. Schmitthaeusler. 1996. Liquid pasteurization of an immunoglobulin preparation without stabilizer: effects on its biological and biochemical properties. Vox Sang. 70:203-209. [DOI] [PubMed] [Google Scholar]
- 6.Busse, P. J., S. Razvi, and C. Cunningham-Rundles. 2002. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 109:1001-1004. [DOI] [PubMed] [Google Scholar]
- 7.Concepcion, N., and C. E. Frasch. 1998. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin. Diagn. Lab. Immunol. 5:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coughlin, R. T., A. C. White, C. A. Anderson, G. M. Carlone, D. L. Klein, and J. Treanor. 1998. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine 16:1761-1767. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham-Rundles, C. 1989. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J. Clin. Immunol. 9:22-33. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham-Rundles, C., and C. Bodian. 1999. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin. Immunol. 92:34-48. [DOI] [PubMed] [Google Scholar]
- 12.Eskola, J., and H. Kayhty. 1997. Pneumococcal vaccines. Clin. Paediatr. 5:101-120. [Google Scholar]
- 12a.Food and Drug Administration. 2004. 21 CFR, part 640.104. Potency. Food and Drug Administration, Rockville, Md.
- 13.Hamill, R. J., D. M. Musher, J. E. Groover, P. J. Zavell, and D. A. Watson. 1992. IgG antibody reactive with five serotypes of Streptococcus pneumoniae in commercial intravenous immunoglobulin preparations. J. Infect. Dis. 166:38-42. [DOI] [PubMed] [Google Scholar]
- 14.Hermaszewski, R. A., and A. D. Webster. 1993. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q. J. Med. 86:31-42. [PubMed] [Google Scholar]
- 15.Hiemstra, P. S., J. Brands-Tajouiti, and R. van Furth. 1994. Comparison of antibody activity against various microorganisms in intravenous immunoglobulin preparations determined by ELISA and opsonic assay. J. Lab. Clin. Med. 123:241-246. [PubMed] [Google Scholar]
- 16.Kayhty, H., H. Peltola, V. Karanko, and P. H. Makela. 1983. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis. 147:1100. [DOI] [PubMed] [Google Scholar]
- 17.Lederman, H. M., and J. A. Winkelstein. 1985. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine 64:145-156. [PubMed] [Google Scholar]
- 18.Le Moli, S., R. Nisini, A. Fattorossi, P. M. Matricardi, and R. D'Amelio. 1989. Intravenous immunoglobulin preparations: a comparative in vitro study of Fc mediated functions. J. Clin. Lab. Immunol. 29:79-84. [PubMed] [Google Scholar]
- 19.Lindberg, A. A. 1999. Polyosides (encapsulated bacteria). C. R. Acad. Sci. III 322:925-932. [DOI] [PubMed] [Google Scholar]
- 20.Matejtschuk, P., K. Chidwick, A. Prince, J. E. More, and D. Goldblatt. 2002. A direct comparison of the antigen-specific antibody profiles of intravenous immunoglobulins derived from US and UK donor plasma. Vox Sang. 83:17-22. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen, H. 1994. Immunoglobulin preparations for intravenous administration. A review of their biologic activities and comparison of various preparation methods. Allergy 49:69-73. [DOI] [PubMed] [Google Scholar]
- 22.Ochs, H. D., S. H. Fischer, R. J. Wedgwood, D. W. Wara, M. J. Cowan, A. J. Ammann, A. Saxon, M. D. Budinger, R. U. Allred, and R. H. Rousell. 1984. Comparison of high-dose and low-dose intravenous immunoglobulin therapy in patients with primary immunodeficiency diseases. Am. J. Med. 76:78-82. [DOI] [PubMed] [Google Scholar]
- 23.Page, M., C. Ling, P. Dilger, M. Bentley, T. Forsey, C. Longstaff, and R. Thorpe. 1995. Fragmentation of therapeutic human immunoglobulin preparations. Vox Sang. 69:183-194. [DOI] [PubMed] [Google Scholar]
- 24.Quartier, P., M. Debre, J. De Blic, R. de Sauverzac, N. Sayegh, N. Jabado, E. Haddad, S. Blanche, J. L. Casanova, C. I. Smith, F. Le Deist, G. de Saint Basile, and A. Fischer. 1999. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J. Pediatr. 134:589-596. [DOI] [PubMed] [Google Scholar]
- 25.Read, J. S., C. E. Frasch, K. Rich, G. A. Fitzgerald, J. D. Clemens, J. Pitt, S. I. Pelton, I. C. Hanson, E. Handelsman, C. Diaz, M. G. Fowler et al. 1998. The immunogenicity of Haemophilus influenzae type b conjugate vaccines in children born to human immunodeficiency virus-infected women. Pediatr. Infect. Dis. J. 17:391-397. [DOI] [PubMed] [Google Scholar]
- 26.Robbins, J. B., J. C. Parke, Jr., R. Schneerson, and J. K. Whisnant. 1973. Quantitative measurement of “natural” and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr. Res. 7:103-110. [DOI] [PubMed] [Google Scholar]
- 27.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santosham, M., R. Reid, D. M. Ambrosino, M. C. Wolff, J. Almeido-Hill, C. Priehs, K. M. Aspery, S. Garrett, L. Croll, S. Foster et al. 1987. Prevention of Haemophilus influenzae type b infections in high-risk infants treated with bacterial polysaccharide immune globulin. N. Engl. J. Med. 317:923-929. [DOI] [PubMed] [Google Scholar]
- 29.Scharf, O., H. Golding, L. R. King, N. Eller, D. Frazier, B. Golding, and D. E. Scott. 2001. Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J. Virol. 75:6558-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneerson, R., L. P. Rodrigues, J. C. Parke, Jr., and J. B. Robbins. 1971. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of “natural,” infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J. Immunol. 107:1081-1089. [PubMed] [Google Scholar]
- 31.Schroeder, D. D., and M. L. Dumas. 1984. A preparation of modified immune serum globulin (human) suitable for intravenous administration. Further characterization and comparison with pepsin-treated intravenous gamma globulin. Am. J. Med. 76:33-39. [DOI] [PubMed] [Google Scholar]
- 32.Shackelford, P. G., D. M. Granoff, M. H. Nahm, M. G. Scott, B. Suarez, and S. J. Nelson. 1985. Correlation of serum immunoglobulin subclass concentrations with antibody responses of children to immunization with Haemophilus influenzae type b polysaccharide-pertussis vaccine. J. Clin. Immunol. 5:390-395. [DOI] [PubMed] [Google Scholar]
- 33.Shakib, F., and D. R. Stanworth. 1980. Human IgG subclasses in health and disease. A review. Part II. Ric. Clin. Lab. 10:561-580. [DOI] [PubMed] [Google Scholar]
- 34.Siber, G. R., P. H. Schur, A. C. Aisenberg, S. A. Weitzman, and G. Schiffman. 1980. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N. Engl. J. Med. 303:178-182. [DOI] [PubMed] [Google Scholar]
- 35.Siber, G. R., C. Thompson, G. R. Reid, J. Almeido-Hill, B. Zacher, M. Wolff, and M. Santosham. 1992. Evaluation of bacterial polysaccharide immune globulin for the treatment or prevention of Haemophilus influenzae type b and pneumococcal disease. J. Infect. Dis. 165:S129-S133. [DOI] [PubMed] [Google Scholar]
- 36.Siegel, J. 2001. Intravenous immune globulins: therapeutic, pharmaceutical, & cost considerations. Pharm. Pract. News 28:11-13. [Google Scholar]
- 37.Siegel, J. 2004. Intravenous immune globulins: therapeutic, pharmaceutical, and cost considerations. Pharm. Pract. News 2004:39-43.
- 38.Skull, S., and A. Kemp. 1996. Treatment of hypogammaglobulinaemia with intravenous immunoglobulin, 1973-93. Arch. Dis. Child. 74:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiehm, E. R., T. W. Chin, A. Haas, and A. G. Peerless. 1986. Infectious complications of the primary immunodeficiencies. Clin. Immunol. Immunopathol. 40:69-86. [DOI] [PubMed] [Google Scholar]
- 40.Vermeer, A. W., and W. Norde. 2000. The thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein. Biophys. J. 78:394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson, D. A., D. M. Musher, and J. Verhoef. 1995. Pneumococcal virulence factors and host immune responses to them. Eur. J. Clin. Microbiol. Infect. Dis. 14:479-490. [DOI] [PubMed] [Google Scholar]