Abstract
Fetal brown adipocytes are insulin-like growth factor-I (IGF-I) target cells. To assess the importance of the IGF-I receptor (IGF-IR) in brown adipocytes during fetal life, we have generated immortalized brown adipocyte cell lines from the IGF-IR-/- mice. Using this experimental model, we demonstrate that the lack of IGF-IR in fetal brown adipocytes increased the susceptibility to apoptosis induced by serum withdrawal. Culture of cells in the absence of serum and growth factors produced rapid DNA fragmentation (4 h) in IGF-IR-/- brown adipocytes, compared with the wild type (16 h). Consequently, cell viability was decreased more rapidly in fetal brown adipocytes in the absence of IGF-IR. Furthermore, caspase-3 activity was induced much earlier in cells lacking IGF-IR. At the molecular level, IGF-IR deficiency in fetal brown adipocytes altered the balance of the expression of several proapoptotic (Bcl-xS and Bim) and antiapoptotic (Bcl-2 and Bcl-xL) members of the Bcl-2 family. This imbalance was irreversible even though in IGF-IR-reconstituted cells. Likewise, cytosolic cytochrome c levels increased rapidly in IGF-IR-deficient cells compared with the wild type. A rapid entry of Foxo1 into the nucleus accompanied by a rapid exit from the cytosol and an earlier activation of caspase-8 were observed in brown adipocytes lacking IGF-IR upon serum deprivation. Activation of caspase-8 was inhibited by 50% in both cell types by neutralizing anti-Fas-ligand antibody. Adenoviral infection of wild-type brown adipocytes with constitutively active Foxol (ADA) increased the expression of antiapoptotic genes, decreased Bcl-xL and induced caspase-8 and -3 activities, with the final outcome of DNA fragmentation. Up-regulation of uncoupling protein-1 (UCP-1) expression in IGF-IR-deficient cells by transduction with PGC-1α or UCP-1 ameliorated caspase-3 activation, thereby retarding apoptosis. Finally, insulin treatment prevented apoptosis in both cell types. However, the survival effect of insulin on IGF-IR-/- brown adipocytes was elicited even in the absence of phosphatidylinositol 3-kinase/Akt signaling. Thus, our results demonstrate for the first time the unique role of IGF-IR in maintaining the balance of death and survival in fetal brown adipocytes.
INTRODUCTION
Type I insulin-like growth factor (IGF-I) receptor (IGF-IR) belongs to the tyrosine kinase growth factor receptor family (Ullrich et al., 1986; Dupont and LeRoith, 2001). Binding of IGF-I results in the tyrosine phosphorylation of multiple cytosolic docking proteins (i.e., IRS-1,-2,-3, and -4 and SHC) that consequently activate multiple signaling cascades, including the phosphatidylinositol 3-kinase (PI 3-kinase)/Akt and the mitogen-activated protein kinase (MAPK), responsible for the biological effects of IGF-I (for review, see White and Kahn, 1994; White, 2002; Clemmons and Maile, 2003). These include regulation of proliferation and differentiation and suppression of apoptosis (Benito et al., 1996; Peruzzi et al., 1999).
The importance of IGF-I and IGF-IR during fetal development in vivo has been demonstrated in mice with null mutations in the genes encoding these receptors or their ligands (White, 2002; Clemmons and Maile, 2003; Powell-Braxton et al., 1993). These animals present growth retardation, with birth weights that were 30-65% of normal control mice. Moreover, IGF-IR knockout mice died even before birth due to pulmonary complications. More recently, a β-cell tissue-specific IGF-IR knockout has been generated (Kulkarni et al., 2002). Surprisingly, this model suggests that IGF-IR is essential in β-cell differentiation rather that in cell growth. The creation of transgenic mice overexpressing IGF-I has furthered the understanding of IGF-I actions in postnatal life. These transgenic animals display an enhanced body weight (30% over control mice) with a dramatic increase in brain size (Mathews et al., 1988).
The IGF-IR has a well documented role in cancer development and progression (Baserga, 1999). Signals from the IGF-IR can enhance tumor cell survival and growth and increase the expression of genes that mediate invasion and metastasis (Blakesley et al., 1997). The dependence of tumor cells on IGF-IR is supported by the observations that inhibition of IGF-IR function by antibodies, triple helix formation, or antisense strategies (Shapiro et al., 1994; Rininsland et al., 1997; Resnicoff, 1998) can reverse the transformed phenotype and lead to cell death. In a number of human tumors, elevated IGF-IR levels have been noted (i.e., lung, breast, colon; for review, see Moschos and Mantzoros, 2002), and this is often associated with increased levels of circulating IGF-I. For many tumors, IGF-I acts as a mitogen in vitro (Nakanishi et al., 1988), suggesting the involvement of IGF-IR in autocrine and paracrine signaling loops in tumors in vivo (Yee et al., 1989; LeRoith et al., 1995).
Brown adipose tissue (BAT), the main tissue involved in adaptive thermogenesis in newborn animals, is an IGF-I target tissue. In fact, BAT expresses both IGF-I and IGF-IR mRNAs through late fetal development in the rat (Teruel et al., 1995). Moreover, fetal brown adipocytes bear a high number of high-afinity IGF-I binding sites (Lorenzo et al., 1993). This has prompted us to study the role of IGF-I and its signaling cascade in the balance of biological processes such as proliferation (Lorenzo et al., 1993; Valverde et al., 1995), differentiation (Valverde et al., 1997; Teruel et al., 1998), and survival (Navarro et al., 1998) in these cells. Thus, IGF-I, in an autocrine/paracrine level, may be a major signal involved in driving BAT to differentiate before birth (Teruel et al., 1995; Benito et al., 1996).
To further assess the role of IGF-I in the biology of the brown adipocyte, we have recently generated immortalized brown adipocyte cell lines from the IGF-IR-/- mice, as well as from the wild type. IGF-IR-deficient cells maintain the phenotypical features of brown adipocytes. Surprisingly, compared with wild-type brown adipocytes, these cells display increased insulin sensitivity based on activation of the IRS-1/Grb-2/Ras/MAPK signaling pathway that leads to increased mitogenesis (Mur et al., 2002). However, IGF-IR-deficient brown adipocytes did not respond to insulin by inducing brown adipocyte differentiation-related genes such as the uncoupling protein-1 (UCP-1) and fatty acid synthase (FAS). This was due to a lack of expression of transcription factors that up-regulate these genes in response to insulin (Mur et al., 2003). In the present article, we demonstrate that IGF-IR deficiency in brown adipocytes confers increased susceptibility to programmed cell death after serum withdrawal, compared with the wild type. The increase in apoptosis is the result of multiple events, including an altered mitochondrial integrity, differences in the expression of several members of the Bcl-2 family, rapid activation of caspases, and a faster nuclear translocation of Foxo1 (previously known as FKHR), which activates the death receptor pathway through Fas ligand (FasL). Furthermore, exogenous expression of the nuclear coactivator PGC-1α or UCP-1 in IGF-IR-deficient brown adipocytes decreases the susceptibility to apoptosis in these cells. Finally, we have investigated distinct signaling pathways by which insulin rescues both wild-type and IGF-IR-/- brown adipocytes from serum withdrawal-induced apoptosis.
MATERIALS AND METHODS
Reagents
Fetal calf serum (FS) and culture media were obtained from Invitrogen (Carlsbad, CA). Insulin, dibutiryl cAMP (dbcAMP), hygromycin, and the anti-β-actin antibody were from Sigma-Aldrich (St. Louis, MO). PD098059, LY294002, and IGF-I were from Calbiochem (San Diego, CA). The anti-Bim (Ref. 556499), anti-Bcl-X (Ref. 559685), and anti-cytochrome c (Ref. 556433) antibodies were from BD Biosciences PharMingen (San Diego, CA). The anti-Foxo1 (FKHR) antibody (Ref. 9462) was from Cell Signaling Technology (Beverly, MA). The anti-Bid antibody (Ref. AF860) was from R & D Systems (Minneapolis, MN). The anti-Bcl-2 antibody (Ref. 06-474) and the anti-p85α (Ref. 06-195) antibodies were from Upstate Biotechnology (Lake Placid, NY). The anti-PGC-1α (sc-5816), anti-IGF-IR (sc-713), and anti-TyrP (Py20) (sc-508) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-UCP-1 antibody (Ref. AB3038) was from Chemicon International (Temecula, CA). The anti-FasL neutralizing antibody (clone FLIM58, Ref. D057-3) was from MBL International (Watertown, MA). The anti-cytochrome c oxidase subunit I antibody (Ref. A-6403) was from Molecular Probes (Eugene, OR). The anti-p53 antibody (Ref. OP03) was from Calbiochem. Caspase-3 substrate (Ac-DEVD-AFC) was from BD Biosciences Clontech (Palo Alto, CA). All other reagents used were of the purest grade available.
Generation of Cell Lines
Brown adipocytes were obtained from interscapular brown adipose tissue of 17.5-18.5 fetuses from two to three pregnant mice of normal genotype (IGF-IR+/+), or from a pool of tissue of fetuses with body weight <0.5 g (IGF-IR-/-) from two to three pregnant mice IGF-IR+/- mated with males IGF-IR+/-. Brown adipocyte primary cultures were performed as described previously (Lorenzo et al., 1993). Viral Bosc-23 packaging cells were transfected at 70% confluence by calcium phosphate coprecipitation with 3 μg/6-cm dish of the puromycin-resistance retroviral vector pBabe encoding SV-40 Large T antigen (kindly provided by J. deCaprio, Dana-Farber Cancer Institute, Boston, MA). Then, brown adipocytes (wild type and IGF-IR-/-) were infected at 60% confluence with polybrene (4 μg/ml)-supplemented virus for 48 h and maintained in culture medium for 72 h, before selection with puromycin (1 μg/ml) for 1 wk. These pools of stable cells were further cultured in DMEM supplemented with 10% FS and puromycin. The pBabe/hygro PGC-1α viral expression vector was a generous gift from Drs. P. Puiserverg and B. Spiegelman (Danna-Farber Cancer Institute). Brown adipocyte IGF-IR-deficient cells were infected with this vector as described above. Selection with 200 μg/ml hygromycin was started 48 h after infection to select stable cell lines.
IGF-IR-deficient brown adipocytes were cultured for 24 h in the presence of 10% FS and then, when 60-70% confluence was reached, cells were transfected according to the calcium phosphate-mediated protocol with the plasmid construct pcDNA3.1-IGF-IRzeo kindly provided by R.W. Furlanetto (National Institutes of Health, Bethesda, MD). DNA (10 μg) was added to each 6-cm dish. After 4-6 h of incubation, cells were shocked with 3 ml of 15% glycerol for 2 min, washed, and then fed with DMEM-10% FS. Twenty-four hours after transfection, zeocyn (250 μg/ml) was added to select stable transfectants. Several zeocyn-resistant cell lines were obtained and the expression of IGF-IR was assessed by Western blot.
Transduction of Brown Adipocytes by Adenoviral Infection
Immortalized wild-type or IGF-IR-/- brown adipocytes were infected with 1 or 10 multiplicity of infection (MOI or viral particles per cell) of constitutively active Foxo1 (ADA), PGC-1α, UCP-1, and mock (β-gal) adenoviruses. Cells (80-90% confluence) were routinely infected for 24-48 h. Then, cells were collected and subsequently used for analysis of DNA fragmentation, caspase-3, and caspase-8 activities and gene expression.
Extraction of Nuclear Proteins
Cells were resuspended at 4°C in 10 mM HEPES-KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 0.75 μg/ml leupeptin, 0.75 μg/ml aprotinin (buffer A); allowed to swell on ice for 10 min; and then vortexed for 10 s. Samples were centrifuged and the supernatant containing the cytosolic fraction was stored at -70°C. The pellet was resuspended in cold buffer C (20 mM HEPES-KOH, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF, 0.75 μg/ml leupeptin, 0.75 μg/ml aprotinin) and incubated on ice for 20 min for high salt extraction. Cellular debris was removed by centrifugation for 2 min at 4°C, and the supernatant fraction was stored at -70°C.
Isolation of Mitochondrial Protein
At the end of the culture time, cells were scrapped off, collected by centrifugation at 2500 × g for 5 min at 4°C, and resuspended in hipotonic isolation buffer (1 mM EDTA, 10 mM HEPES, 50 mM sucrose, pH 7.6). Then, cells were incubated at 37°C for 5 min and homogenized under a Teflon pestle (Overhead Stirrer; Wheaton Instruments, Milville, NJ). Hipertonic isolation buffer (1 mM EDTA, 10 mM HEPES, 450 mM sucrose, pH 7.6) was added to balance the buffer's tonicity. Samples were centrifuged at 10,000 × g for 10 min and the pellets, containing the mitochondrial fraction, were resuspended in lysis buffer. The supernatants contained the cytosolic protein fraction. Cytochrome c was analyzed by Western blotting after eletrophoresis separation of 50 μg of mitochondrial or cytosolic proteins in 15% polyacrilamide-SDS gels.
Protein Determination
Protein determination was performed by the Bradford dye method, by using the Bio-Rad reagent and bovine serum albumin (BSA) as the standard.
Western Blotting
To obtain total cell lysates, cells from supernatants were collected by centrifugation at 2000 × g for 5 min at 4°C. Attached cells were scraped off in ice-cold phosphate-buffered saline (PBS), pelleted by centrifugation at 4000 × g for 10 min at 4°C, and resuspended in lysis buffer (25 mM HEPES, 2.5 nM EDTA, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin). Samples were sonicated 30 s at 1.5 mA, and lysates were clarified by centrifugation at 12,000 × g for 10 min. After SDS-PAGE, gels were transferred to Immobilon membranes and were blocked using 5% nonfat dried milk or 3% BSA in 10 mM Tris-HCl, 150 mM NaCl, pH 7.5, and incubated overnight with several antibodies as indicated in 0.05% Tween 20, 10 mM Tris-HCl, 150 mM NaCl, pH 7.5. Immunoreactive bands were visualized using the ECL Western blotting protocol (Amersham Biosciences, Piscataway, NJ).
Analysis of Mitochondrial Transmembrane Potential
The fluorescent probe tetramethyl-rodamine (TMRM) was used to analyze the changes in mitochondrial potential (Δψm) by flow cytometry. After serum deprivation for 4 h, cells were collected by centrifugation at 2500 × g for 5 min and then resuspended in PBS. The cellular fluorescence intensity was measured after 30-min incubation of the cells with 0.5 μM TMRM. A FACScan flow cytometer (Becton Dickinson, Fullerton, CA) was used (λ excitation, 549 nm; λ emission, 573 nm). For each analysis, 10,000 events were recorded.
Analysis of DNA Laddering
Cells were washed twice with ice-cold PBS and then scraped and pelleted at 4°C. Cells were resuspended in 500 μl of buffer containing 10 mM EDTA, 0.25% Triton X-100, 2.5 mM Tris-HCl, pH 8, and stored at 4°C for 15 min. Intact nuclei were pelleted and eliminated by centrifugation at 500 × g at 4°C for 30 min, and the supernatant was centrifuged at 25,000 × g at 4°C for 15 min. DNA in the supernatant was precipitated at -80°C after the addition of 2 volumes of ethanol (70% final concentration), pelleted by microcentrifugation at 4°C for 15 min, dried, resuspended in 200 μl of 10 mM Tris-HCl, 1 mM EDTA, pH 8 (TE buffer), and incubated at 37°C for 30 min with 0.1 mg/ml RNAse A and for 2-3 h with 0.24 mg/ml proteinase K. DNA was purified by phenol-chloroform extraction and precipitated at -70°C after adding 1/10 volume of 3 M sodium acetate, pH 5.3, and 2 volumes of ethanol. Precipitated DNA was dissolved in TE buffer containing 30% glycerol, 1 μg/ml ethidium bromide and electrophoresed in a 1.5% agarose gel. Gel was visualized and photographed under transmitted UV light with a Polaroid camera.
Analysis of Cell Viability
After cell incubation in the absence of serum and growth factors for 2-24 h, the medium was discarded and the remaining viable adherent cells were stained with crystal violet (0.2% in 2% ethanol) for 20 min. After this time, plates were rinsed with water, allowed to dry, and 1% SDS was added. Absorbance of each plate was read at 560 nm. Remnant viable cells were calculated as percentage of absorbance with respect to control cells (incubated in the presence of 10% FS).
Analysis of PI 3-Kinase Activity
Quiescent cells were stimulated with 100 nM insulin for 5 min or pretreated with 10 μM LY294002 for 20 min followed by 5-min insulin stimulation. PI 3-kinase activity was measured in antiphosphotyrosine immunoprecipitates as described previously (Valverde et al.,1997).
Analysis of Caspase-3 Activity
Cells were scraped off, collected by centrifugation at 2500 × g for 5 min, and lysed at 4°C in 5 mM Tris-HCl, pH 8, 20 mM EDTA, 0.5% Triton X-100. Lysates were clarified by centrifugation at 13,000 × g for 10 min. Reaction mixture contained 25 μl of cellular lysates, 325 μl of assay buffer (20 mM HEPES pH 7.5, 10% glycerol, 2 mM dithiothreitol), and 20 μM caspase-3 substrate (Ac-DEVD-AMC). After 2-h incubation in the dark, enzymatic activity was measured in a luminiscence spectrophotometer (LS-50; PerkinElmer Life and Analytical Sciences, Boston, MA) (λ excitation, 380 nm; λ emission, 440 nm). We define a unit of caspase-3 activity as the amount of active enzyme necessary to produce an increase in 1 arbitrary unit in the fluorimeter after 2-h incubation with the reaction mixture. Then, protein concentration of cell lysates was determined, and final expression of the results is presented as caspase-3 activity per microgram of total protein.
Analysis of Caspase-8 Activity
Caspase-8 activity was measured with the ApoAlert caspase-8 fluorescent assay kit (Ref. K2028; BD Biosciences Clontech) accordingly with the manufacturer's instructions by using IETD-AFC as a substrate. Then, protein concentration of cell lysates was determined, and final expression of the results is presented as caspase-8 activity per microgram of total protein.
Transfection with the Foxo1-Green Fluorescent Protein (GFP) Construct and Confocal Immunofluorescence
Cells were grown to 80% confluence and then transiently transfected with 16 μg/6-cm dish of Foxo1-GFP cDNA construct accordingly with the calcium phosphate-mediated protocol (Stratagene, La Jolla, CA). Twenty-four hours after transfection, cells were serum starved for 4 and 8 h or maintained under growing conditions. Then, cells were washed twice with PBS, fixed in methanol (-20°C) for 2 min, and examined with a MRC-1024 confocal microscope (Bio-Rad, Hemel Hempstead, United Kingdom) adapted to an inverted Nikon Eclipse TE 300 microscope.
RESULTS
Increased Susceptibility of IGF-IR-deficient Brown Adipocytes to Undergo Apoptosis in Response to Serum Withdrawal
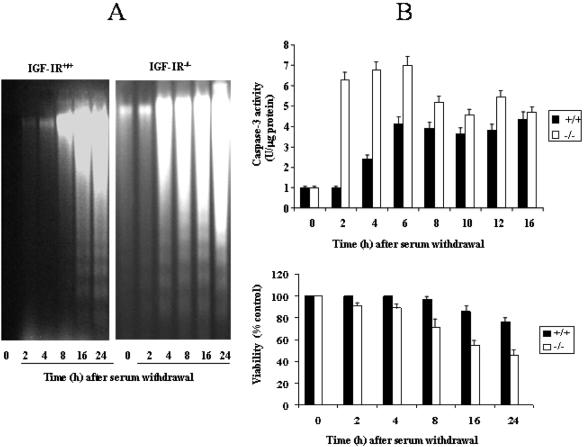
Immortalized fetal brown adipocytes derived from the wild-type (IGF-IR+/+) and the IGF-IR-deficient mice (IGFIR-/-) were deprived of serum and growth factors for various periods of time. Subsequently, DNA cleavage (180-base pair ladder) was analyzed by electrophoresis of extranuclear DNA. Wild-type mouse brown adipocytes are resistant to serum withdrawal-induced apoptosis, as has been previously described in rat primary cultures (Porras et al., 1997). After 8 h of serum deprivation, control cells did not reveal DNA fragmentation, requiring longer periods (16-24 h) to induce DNA cleavage (Figure 1A). In contrast, IGF-IR-deficient brown adipocytes displayed DNA fragmentation after only 4 h of serum withdrawal, indicating that these cells are more susceptible to undergo apoptosis.
Figure 1.
Increased susceptibility to undergo apoptosis in IGF-IR-deficient brown adipocytes after serum withdrawal. (A) IGF-IR+/+ and IGF-IR-/- brown adipocytes cultured in 10% FS were serum deprived for 2, 4, 8, 16, and 24 h. Then, cells were scraped and subjected to extranuclear DNA extraction. Purified DNA was electrophoresed and visualized by UV fluorescence after staining with ethidium bromide. A representative experiment out of three is shown. (B) Top, after incubation of wild-type and IGF-IR-/- brown adipocytes in serum-free medium for several time periods, cells were collected and lysed. Total protein content was determined and caspase-3 activity was measured as described under Materials and Methods. Results are expressed as arbitrary units per microgram of total protein and are means ± SE from three independent experiments with duplicate dishes. Bottom, cells were cultured as described above. At the end of the culture time, medium was removed, and viable cell number was analyzed by crystal violet staining as described under Materials and Methods. Results are expressed as percentage of absorbance with respect to control cells (10% FS) and are means ± SE from three independent experiments with duplicate dishes.
Caspases seem to be general executioners of apoptosis and are activated by serum deprivation in a number of cell types. To assess whether DNA fragmentation observed in serum-free cultures of fetal mouse brown adipocytes was dependent on caspases activation, we next measured caspase-3 activity under these experimental conditions. Serum deprivation resulted in a gradual increase in caspase-3 activity in wild-type brown adipocytes, reaching the maximal value (4-fold) after 6 h (Figure 1B). However, absence of serum and growth factors provoked a rapid and dramatic increase (6-fold) in caspase-3 enzymatic activity in IGF-IR-/- cells, reaching a maximum after 2 h. Interestingly, in parallel with caspase-3 activation and DNA fragmentation, the lack of IGF-IR in brown adipocytes accelerated the loss of cellular viability induced by serum withdrawal (Figure 1B).
Inhibition of Antiapoptotic Genes Bcl-xL and Bcl-2 and Rapid Accumulation of Proapoptotic Genes Bim and BclxS and Cytosolic Cytochrome c in IGF-IR-/- Brown Adipocytes after Serum Withdrawal
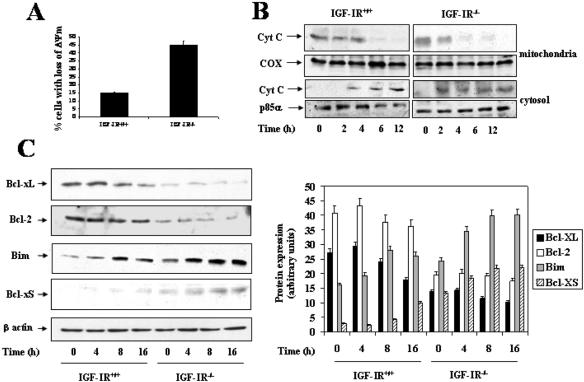
Mitochondria are deeply involved in the regulation of apoptosis (Green and Reed, 1998). The disruption of the Δψm has been defined as an early irreversible stage of apoptosis, initiating the intrinsic caspase activation pathway (Kluck et al., 1999). When wild-type and IGF-IR-/- brown adipocytes were cultured for 4 h in the absence of serum and growth factors, substantial differences were observed regarding DNA laddering and caspase-3 activation (Figure 1). Accordingly, we chose this time point to determine whether there were alterations in the Δψm due to the absence of IGF-I signals. After 4 h in serum-free medium, we noted a loss of Δψm in 14.92 ± 4.54% of wild-type cells per dish (Figure 2A), whereas 44.87 ± 6.22% of brown adipocytes lacking IGF-IR displayed defects in Δψm. Based on these results, we evaluated whether the loss of Δψm was consistent with a release of cytochrome c from mitochondria to cytosol. To analyze this, cells were cultured in serum-free medium for 2-12 h and then mitochondria were separated from cytosol. Cytochrome c content was analyzed in both fractions by direct Western blot analysis. As shown in Figure 2B, cytochrome c content decreased considerably in the mitochondria, occurring in the cytosol after 4-6 h of serum withdrawal in wild-type cells. However, IGF-IR-/- brown adipocytes showed a rapid decrease in mitochondrial cytochrome c content with a concurrent increase in the cytosol after only 2 h of serum withdrawal. Both mitochondrial and cytosolic fractions were pure because we did not detect cytochrome c oxidase (COX) in cytosolic extracts, and no p85α-PI 3-kinase (p85α) was found in mitochondrial extracts (our unpublished data).
Figure 2.
Serum withdrawal induced differential reduction of Δψm, release of cytochrome c, and expression of pro- and antiapoptotic proteins of the Bcl-2 family in wild-type and IGF-IR-/- brown adipocytes. (A) Wild-type and IGF-IR-/- brown adipocytes were cultured under growing conditions (10% FS) or serum deprived for 4 h. Then, cells were collected and incubated for 30 min with 0.5 μM TMRM. The intracellular fluorescence intensity was measured in a FACScan flow cytometer. Results are means ± SE from four independent experiments with duplicate dishes. (B) After incubation of cells in serum-free medium for 2-12 h, mitochondria were separated from cytosol, and cytochrome c content was analyzed in both fractions by Western blot. Protein loading was visualized with the anti-COX and anti-p85α antibodies for mitochondrial and cytosolic fractions, respectively. A representative experiment is shown. (C) Immortalized wild-type and IGF-IR-/- brown adipocytes cultured in 10% FS were serum deprived for 4, 8, and 16 h. Then, cells were collected and lysed. Total protein (50 μg) was submitted to Western blot analysis with the corresponding antibodies against Bcl-xL, Bcl-xS, Bim, and Bcl-2. Loading was normalized by blotting the membranes with the anti-β-actin antibody. The autoradiograms corresponding to a representative experiment out of four were quantitated by scanning densitometry and results are expressed as arbitrary units of Bcl-xL, Bcl-xS, Bim, and Bcl-2 protein content.
In an attempt to elucidate the molecular pathways that regulate the susceptibility of IGF-IR-/- brown adipocytes to apoptosis upon growth factor deprivation, the expression of several pro- and antiapoptotic members of the Bcl-2 family, which has been related to the mitochondrial changes during apoptosis, were analyzed. For this goal, both cell types were cultured either with or without serum and growth factors for various periods of time. Then, cells were collected and protein expression was analyzed by Western blot. The expression of the antiapoptotic protein Bcl-xL (29 kDa) was high in wild-type cells under growing conditions (time 0), but it gradually decreased with time after serum deprivation (Figure 2C). In sharp contrast, Bcl-xL was barely detected in IGF-IR-/- cells regardless of culture conditions. Bcl-2 expression, which elicits antiapoptotic effects in rat brown adipocytes (Navarro et al., 1998), was significantly higher in wild-type than in IGF-IR-deficient brown adipocytes under growing conditions, and its expression remained unchanged upon serum withdrawal in both cell types. Regarding proapoptotic genes, Bcl-xS (21-kDa) expression was almost undetectable in wild-type cells, with only slight expression being observed after 16 h of serum withdrawal. Conversely, in IGF-IR-/- brown adipocytes Bcl-xS protein content was detectable under growing conditions and gradually increased during 16 h of serum withdrawal. Under growing conditions, the expression of Bim was slightly higher in IGF-IR-deficient than in wild-type cells. Its expression gradually increases up to 16 h in both cell types after serum deprivation. Interestingly, Bim expression in IGF-IR-/- cells was always higher than that found in wild-type cells. Thus, the imbalanced expression of anti- and proapoptotic members of the Bcl-2 family provides one explanation for the rapid massive apoptosis in IGF-IR-/- brown adipocytes provoked by serum withdrawal.
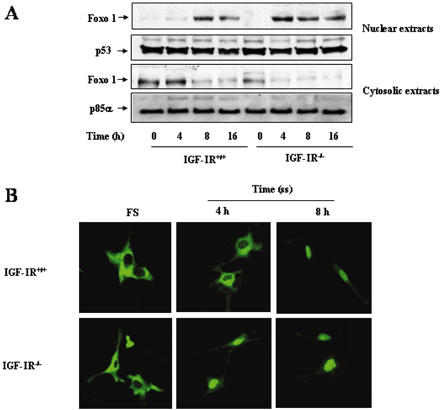
Serum Withdrawal Induced a Rapid Accumulation of Foxo1 into the Nucleus in IGF-IR-/- Brown Adipocytes
Transcription factors of the Foxo family (previously known as FKHR) have been recently implicated in the regulation of several proapoptotic genes, including mitochondrial-associated proteins such as Bim and members of the death receptor pathway such as FasL (Dijkers et al., 2000; Suhara et al., 2002). Growth factor stimulation induces the phosphorylation of Foxo1 by PKB/Akt that leads to its nuclear exclusion and subsequently, inactivation (Nakae et al., 2000). To assess whether the lack of IGF-IR modifies Foxo1 subcellular distribution in brown adipocytes, cells were cultured as described above, and nuclear and cytosolic proteins were purified. Under growing conditions, Foxo1 content in nuclear extracts was almost undetectable in either line of fetal brown adipocytes (Figure 3A). However, substantial differences were noted between the cell lines after serum withdrawal. Whereas nuclear Foxo1 was barely detected in nuclear extracts after 4 h of serum deprivation in wild-type cells, a significant amount of Foxo1 was localized in the nucleus in IGF-IR-/- brown adipocytes at this time point. Conversely, Foxo1 protein content decreased in the cytosolic fraction in parallel with its accumulation in the nucleus in both cell types. Both cytosolic and nuclear fractions were pure because we did not detect p53 in the cytosol, and no p85α was found in the nuclear extracts (our unpublished data). Interestingly, we have observed similar results by transiently transfecting brown adipocytes with the Foxo1-GFP construct. As shown in Figure 3B, Foxo1-GFP localized to the cytosol when both cell types were grown in serum. Consistent with cell fractionation results, serum withdrawal induced the entry of Foxo1-GFP into the nucleus after 4 h in IGF-IR-/- cells and after 8 h in wild-type brown adipocytes.
Figure 3.
Time course of Foxo1 entry into the nucleus in wild-type and IGF-IR-/- brown adipocytes. (A) Cells were cultured as described in Figures 1 and 2. At the end of the culture time, cells were collected and cytosolic and nuclear extracts were prepared. Nuclear and cytosolic protein (50 μg) was submitted to Western blot analysis with the anti-Foxo1 antibody. Protein loading was visualized with the anti-p53 and anti-p85α antibodies for nuclear and cytosolic fractions, respectively. A representative experiment out of three is shown. (B) Cells were grown to 80% confluence and then transiently transfected with a plasmid encoding Foxo1-GFP fusion protein. Twenty-four hours after transfection, cells were serum starved for 4 and 8 h. Then, cells were washed twice with PBS, fixed in methanol (-20°C) for 2 min, and processed to confocal immunofluorescence. A representative experiment is shown.
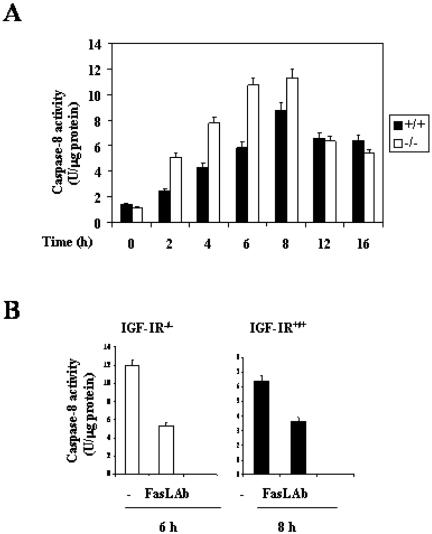
Fas-Ligand-neutralizing Antibody Impaired Caspase-8 Activity in Wild-Type and IGF-IR-/- Brown Adipocytes
Recently, it has been proposed that nuclear Foxo proteins induce the death receptor pathway by activating the FasL gene promoter (Suhara et al., 2002). Then, the clustering of Fas after the binding of FasL leads to caspase-8-dependent cell death (Juo et al., 1998). To assess whether nuclear Foxo1 accumulation is able to induce caspase-8 activity, brown adipocytes from both genotypes were cultured as described above, and caspase-8 activity was measured during a time course of serum deprivation. Wild-type cells showed a gradual increase in caspase-8 activity upon serum withdrawal, maximal activity being elicited at 8 h. In IGF-IR-/- brown adipocytes, caspase-8 activity was higher than that of control cells after 2-8 h of serum deprivation. Thus, caspase-8 was activated earlier in IGF-IR-/- cells, reaching a maximum after 6 h without serum. Furthermore, maximal caspase-8 enzymatic activity in both cell types was inhibited by 50% when a neutralizing antibody against FasL was added to the culture medium (Figure 4B).
Figure 4.
Time course of caspase-8 activity in wild-type and IGF-IR-/- brown adipocytes. Effect of FasL neutralizing antibody. (A) Cells were cultured as described in Figure 1A. At the end of the culture time, cells were collected and lysed. Caspase-8 activity was measured as described under Materials and Methods. Results are expressed as arbitrary units of caspase-8 activity per microgram of protein and are means ± SE from three independent experiments with duplicate dishes. (B) IGF-IR-/- and IGF-IR+/+ brown adipocytes were cultured for 6 and 8 h, respectively, in serum-free medium in the absence or presence of 1 μg/ml FasL neutralizing antibody. At the end of the culture time, cells were collected and lysed for determination of caspase-8 activity. Results are expressed as arbitrary units of caspase-8 activity per microgram of protein and are means ± SE from three independent experiments with duplicate dishes.
Constitutively Active Foxo1 Induces Apoptosis in Immortalized Fetal Brown Adipocytes
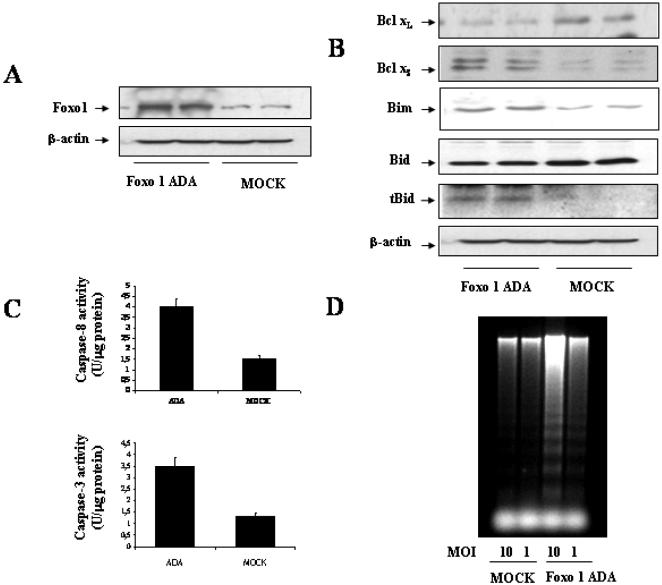
To directly demonstrate that nuclear Foxo1 induces apoptosis in brown adipocytes, we infected wild-type cells cultured under growing conditions with an adenovirus encoding a constitutively active Foxo1 mutant (Foxo1 ADA), in which the Akt phosphorylation sites have been mutated (Nakae et al., 2001). As a control, parallel dishes were infected with an adenovirus encoding β-gal. Figure 5A demonstrates Foxo1 ADA overexpression in wild-type brown adipocytes compared with β-gal-infected cells. Next, we analyzed the expression of pro- and antiapoptotic genes in transduced cells. As shown in Figure 5B, constitutively active Foxo1 increased Bim expression as well as decreased Bid expression with the concomitant presence of the 15-kDa Bid truncated fragment (tBid). Moreover, ADA overexpression resulted in a significant increase in Bcl-xS expression and a marked decrease in the antiapoptotic protein Bcl-xL, compared with the controls. Regarding caspases activation, overexpression of constitutively active Foxo1 increased both caspase-8 and caspase-3 enzymatic activities in wild-type brown adipocytes (Figure 5C). Finally, DNA fragmentation was visualized in Foxo1 ADA-transduced cells, but not in the controls (Figure 5D).
Figure 5.
Constitutively active Foxo1 induces apoptosis in immortalized fetal brown adipocytes. (A) Wild-type cells were cultured to 80% confluence. Then, cells were infected with adenoviral constructs encoding constitutively active Foxo1 (ADA) and mock (β-gal). Twenty-four hours after infection, cells were collected and lysed. Expression of total Foxo1 was analyzed by Western blot. (B) Wild-type brown adipocytes were cultured and infected with adenoviruses as described in A. Twenty-four hours after infection, cells were collected and lysed. Total protein (50 μg) was submitted to Western blot analysis with the anti-Bcl-xL, anti-Bcl-xs, anti-Bim, anti-Bid, and anti-β-actin antibodies. Results are representative of two independent experiments with duplicate dishes. (C) Twenty-four hours after infection, cells were collected and lysed. Caspase-3 and caspase-8 enzymatic activities were analyzed. Results are expressed as arbitrary units per microgram of total protein and are means ± SE from three independent experiments with duplicate dishes. (D) Twenty-four hours after infection, cells were scraped and subjected to extranuclear DNA extraction. Purified DNA was electrophoresed and visualized by UV fluorescence after staining with ethidium bromide. A representative experiment out of three is shown.
Overexpression of PGC-1α or UCP-1 Retards Serum Withdrawal-induced Apoptosis in IGF-IR-deficient Brown Adipocytes
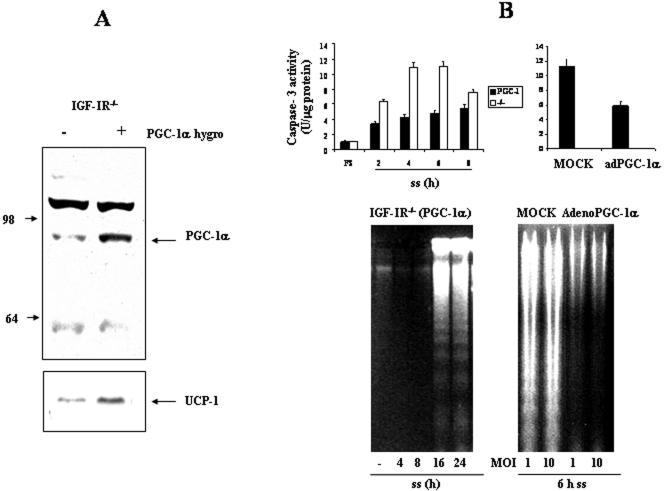
Efficient execution of apoptotic cell death machinery requires adequate supply of ATP (Dey and Moraes, 2000; Harris et al., 2000). In BAT, mitochondrial UCP-1 uncouples fatty acid oxidation from ATP synthesis allowing dissipation of energy from substrate oxidation as heat (Trayhurn and Milner, 1989). Thus, we studied the possibility to reduce the susceptibility to apoptosis of IGF-IR-/- brown adipocytes by exogenous expression of PGC-1α, which is a strong coactivator of several nuclear receptors that bind to the UCP-1 enhancer increasing its expression (Puigserver et al., 1998). In addition, PGC-1α stimulates mitochondrial biogenesis in skeletal muscle by binding NRF-1 and increasing the expression of its target genes, including mtTFA (Wu et al., 1999). Accordingly, we performed retrovirus-mediated PGC-1α gene transfer into IGF-IR-deficient brown adipocytes. After retroviral infection, PGC-1α was overexpressed threefold in IGF-IR-/- cell line. Moreover, PGC-1α-overexpressing IGFIR-/- cells [hereafter referred to as IGF-IR-/-(PGC-1α)] up-regulated UCP-1 expression compared with IGF-IR-/- brown adipocytes (Figure 6A). Next, we examined caspase-3 enzymatic activity in IGF-IR-/- and IGF-IR-/-(PGC-1α) brown adipocytes after serum withdrawal. As shown in Figure 6B (top), exogenous expression of PGC-1α in IGF-IR-deficient brown adipocytes significantly decreased the caspase-3 activity values at each time point (2-8 h). Alternatively, IGF-IR-/- brown adipocytes were infected with either mock (β-gal) or PGC-1α adenoviruses. Twenty-four hours after infection, cells were serum deprived for 6 h, and caspase-3 activity was determined. Adenoviral infection with PGC-1α significantly decreased caspase-3 activity in IGF-IR-deficient brown adipocytes compared with β-gal-infected cells, reaching values similar to those observed in wild-type cells (Figure 1B). Next, we evaluated whether the increased UCP-1 expression in IGF-IR-/-(PGC-1α) brown adipocytes might alter the pattern of DNA fragmentation observed in apoptotic cells. Figure 6B (bottom) shows a representative agarose gel of extranuclear DNA obtained from IGF-IR-/-(PGC-1α) brown adipocytes after serum withdrawal. Exogenous expression of PGC-1α in IGF-IR-/- adipocytes leads to a significant retardation of apoptosis as visualized by the DNA laddering profile (Figure 1). Furthermore, adenoviral expression of PGC-1α in IGF-IR-/- brown adipocytes completely abolished DNA laddering observed after 6 h of serum deprivation.
Figure 6.
Expression of PGC-1α in IGF-IR-/- brown adipocytes induces UCP-1 expression and retards the activation of caspase-3 and DNA fragmentation after serum withdrawal. (A) IGF-IR-/- fetal brown adipocyte cell line overexpressing PGC-1α [IGF-IR-/-(PGC-1α)] was generated as described under Materials and Methods. Nuclear protein extracts were prepared from growing cells (10% FS) and analyzed by Western blot with the anti-PGC-1α antibody. To analyze UCP-1 expression, mitochondrial protein extracts were isolated and analyzed by Western blot with the corresponding anti-UCP-1 antibody. (B) Top, after incubation of IGF-IR-/- and IGF-IR-/-(PGC-1α) brown adipocytes in serum-free medium for several time periods, cells were collected and lysed. Twenty-four hours after infection of IGF-IR-/- brown adipocytes with either mock (β-gal) or PGC-1α adenoviruses, cells were serum deprived for 6 h. Total protein content was determined and caspase-3 activity was measured. Results are expressed as arbitrary units per microgram of total protein and are means ± SE from three independent experiments with duplicate dishes. Bottom, after incubation of IGF-IR-/-(PGC-1α) brown adipocytes in serum-free medium for several time periods, cells were scraped and subjected to extranuclear DNA extraction. Twenty-four hours after infection of IGF-IR-/- brown adipocytes with either mock (β-gal) or PGC-1α adenoviruses, cells were serum deprived for 6 h and subjected to extranuclear DNA extraction. Purified DNA was electrophoresed and visualized by UV fluorescence after staining with ethidium bromide. A representative experiment out of three is shown.
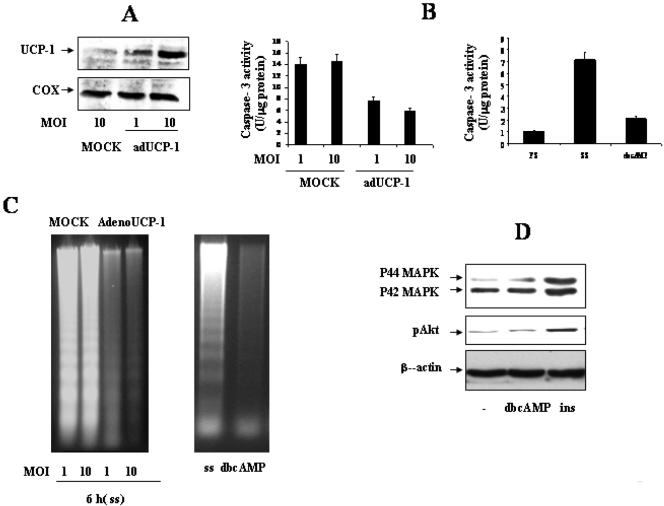
To test directly the protective effect of UCP-1 on brown adipocytes cell death, we infected IGF-IR-/- brown adipocytes with either mock or UCP-1 adenoviruses. UCP-1 overexpression was analyzed by Western blot (Figure 7A). Transduction with UCP-1 markedly decreased caspase-3 activity (Figure 7B), which correlated with the suppression of DNA laddering (Figure 7C). As s positive control, we cultured IGF-IR-/- brown adipocytes for 4 h with 0.5 mM dbcAMP, which strongly activates UCP-1. Under these conditions, activation of caspase-3 was reduced to levels observed in growing cells (10% FS) (Figure 7B), and DNA fragmentation was totally suppressed (Figure 7C). Finally, to rule out the possibility that dbcAMP could be acting as a survival factor by inducing either Akt or MAPK signaling pathways, quiescent IGF-IR-/- cells were stimulated for 10 min with 0.5 mM dbcAMP. Cells also were stimulated with 100 nM insulin, as a positive control. As shown in Figure 7D, dbcAMP failed to induce Akt or MAPK phosphorylation in IGF-IR-/- cells. These results indicate that the effects observed in dbcAMP-treated IGF-IR-deficient cells are the consequence of a direct PKA activation, and subsequently, increased UCP-1 expression rather than by the activation of survival signaling cascades.
Figure 7.
Transduction with UCP-1 or dbcAMP treatment protects IGF-IR-/- brown adipocytes from serum withdrawal-induced apoptosis. (A) Forty-eight hours after infection of IGF-IR-/- brown adipocytes with either mock or UCP-1 adenoviruses, mitochondrial protein was analyzed by Western blot with the anti-UCP-1 antibody. (B) Forty-eight hours after infection of IGF-IR-/- brown adipocytes with either mock or UCP-1 adenoviruses, cells were serum deprived for 6 h. Total protein content was determined and caspase-3 activity was measured. IGF-IR-/- brown adipocytes were cultured under growing conditions (10% FS) or for 4 h in serum-free medium either in the absence or in the presence of 0.5 mM dbcAMP. At the end of the culture time, caspase-3 activity was determined. Results are expressed as arbitrary units per microgram of total protein and are means ± SE from three independent experiments with duplicate dishes. (C) Left, 48 h after infection of IGF-IR-/- brown adipocytes with either mock or UCP-1 adenoviruses, cells were serum deprived for 6 h and subjected to extranuclear DNA extraction. Right, IGF-IR-/- brown adipocytes were cultured for 16 h in serum-free medium either in the absence or in the presence of 0.5 mM dbcAMP. At the end of the culture time, purified extranuclear DNA was electrophoresed and visualized by UV fluorescence after staining with ethidium bromide. A representative experiment of three is shown. (D) Quiescent (20-h serum-starved) IGF-IR-/- brown adipocytes were stimulated with 100 mM insulin or 0.5 mM dbcAMP for 10 min or maintained in the absence of the hormone. Cells were lysed and total protein (50 μg) was analyzed by Western blot with the corresponding antibodies against phospho-Akt, phospho-MAPK and β-actin. A representative experiment is shown.
Insulin Rescues IGF-IR-/- Brown Adipocytes from Apoptosis in a PI 3-Kinase-independent Manner
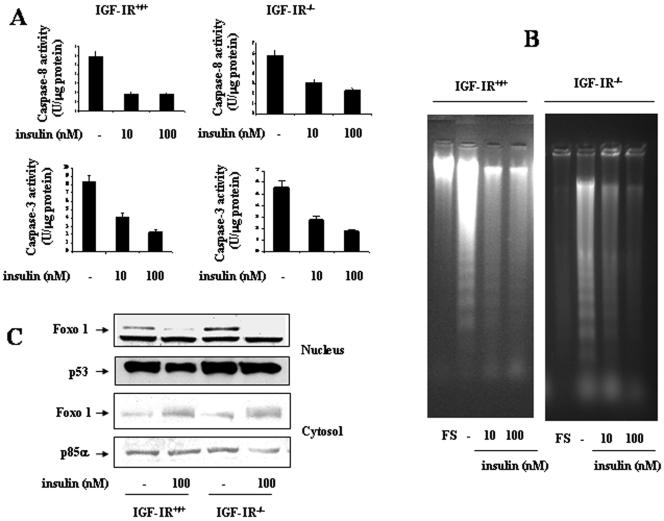
Insulin and IGF-I are survival factors that rescue brown adipocytes from apoptosis (Navarro et al., 1998). Accordingly, we studied whether insulin was able to suppress serum withdrawal-induced apoptosis in wild-type and IGFIR-deficient brown adipocytes. Cells were incubated for 4 and 8 h in serum-free medium either in the absence or presence of various doses of insulin and analyzed for caspase-3 and caspase-8 activation, respectively. As shown in Figure 8A, both caspase-8 and caspase-3 activities were significantly inhibited by the presence of insulin in the medium. Furthermore, insulin was able to rescue IGF-IR-/- brown adipocytes from DNA laddering. In control cells, insulin suppressed DNA laddering observed after 24 h of serum withdrawal, maximal effect being elicited at 10 nM insulin concentration (Figure 8B). In IGF-IR-/- brown adipocytes, DNA laddering was partially suppressed by 10 nM insulin concentration and totally at 100 nM. In addition, insulin was able to increase cytosolic and to decrease nuclear Foxo1 in both cell types (Figure 8C).
Figure 8.
Insulin rescues serum withdrawal-induced apoptosis in wild-type and IGF-IR-/- brown adipocytes. (A) Wild-type and IGF-IR-/- brown adipocytes were cultured for 8 and 4 h, respectively, in serum-free medium either in the absence or presence of insulin (10-100 nM). Then, cells were collected, lysed and caspase-8 and caspase-3 activities were measured. Results are expressed as arbitrary units per microgram of total protein and are means ± SE from three independent experiments with duplicate dishes. B. Wild-type and IGF-IR-/- brown adipocytes were cultured under growing conditions (FS) or in serum-free medium for 24 h either in the absence or in the presence of insulin (10-100 nM). At the end of the culture time, cells were scraped and subjected to extranuclear DNA extraction. Purified DNA was electrophoresed and visualized by UV fluorescence after staining with ethidium bromide. A representative experiment out of three is shown. C. Cells were cultured as described in B. At the end of the culture time, cells were collected and nuclear and cytosolic extracts were prepared. Nuclear or cytosolic protein (50 μg) was submitted to Western blot analysis with the anti-Foxo1 antibody. Protein loading was visualized with the anti-p53 and anti-p85α antibodies for nuclear and cytosolic fractions, respectively. A representative experiment of out three is shown.
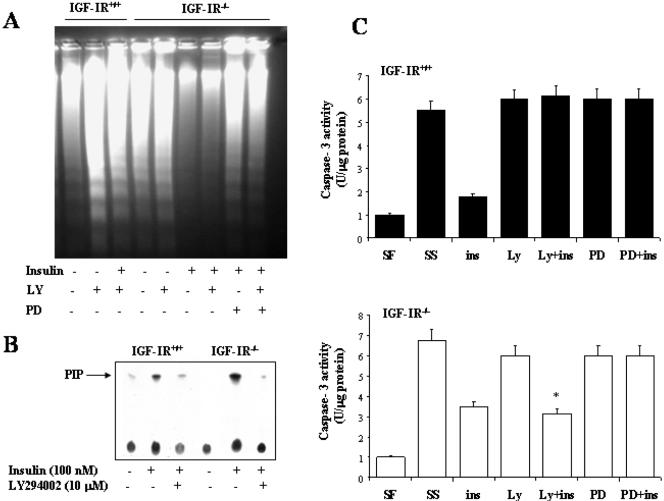
Next, we wanted to investigate the molecular mechanisms by which insulin elicits the survival effect in IGF-IR-/- brown adipocytes. In a previous article, we reported that both PI 3-kinase and MAPK signaling pathways are necessary for insulin rescue from apoptosis in rat brown adipocytes (Navarro et al., 1998). Furthermore, two very recent articles demonstrated that IGF-IR-/--immortalized brown adipocytes displayed an increased insulin sensitivity regarding IRS-1/Grb-2/MAPK and IRS-1/PI 3-kinase/Akt signaling pathways compared with wild-type cells (Mur et al., 2002, 2003). Accordingly, we cultured IGFIR-/- brown adipocytes for 16 h in serum-free medium without or with insulin (100 nM) either in the absence or in the presence of 10 μM LY294002 or 20 μM PD98059. Then, DNA fragmentation was analyzed. As a control, wild-type cells were incubated for 16 h with LY294002 alone or plus insulin. Although wild-type brown adipocytes are resistant to apoptosis after 16 h of serum deprivation (Figures 1A and 9A), a substantial DNA fragmentation was noted when LY294002 was present for 16 h. Thus, insulin was not able to protect wild-type brown adipocytes from DNA fragmentation when PI 3-kinase was inhibited with LY 294002. In contrast, IGF-IR-deficient cells undergo apoptosis after 16 h of serum withdrawal in the absence or presence of LY294002 (Figures 1A and 9A). Therefore, in these cells, insulin prevented apoptosis regardless PI 3-kinase activation. It is noteworthy that 10 μM LY294002 completely inhibited insulin-induced PI 3-kinase activity in both cell types (Figure 9B). However, inhibition of MAPK by PD098059 prevented insulin rescue from apoptosis (Figure 9A). To assess whether DNA fragmentation correlated with caspase-3 activation under the different experimental conditions, brown adipocytes were cultured for 6 h as described above. Then, cells were collected and caspase-3 activity was assayed. As shown in Figure 9C, insulin was not able to suppress caspase-3 activation in the presence of the mitogen-activated protein kinase kinase (MEK)-1 inhibitor PD098059 in both cell types. However, insulin prevented caspase-3 activation in IGF-IR-/- cells, but not in the wild type, when PI 3-kinase/Akt signaling was inhibited with LY294002.
Figure 9.
Insulin rescues from apoptosis IGF-IR-/- brown adipocytes in a PI 3-kinase independent-manner. (A) Wild-type and IGF-IR-/- brown adipocytes were cultured for 16 h in serum-free medium in the absence or presence of insulin, PD098059 (20 μM), and LY294002 (10 μM). Then, cells were scraped and subjected to extranuclear DNA extraction. Purified DNA was electrophoresed and visualized by UV fluorescence after staining with ethidium bromide. A representative experiment of three is shown. (B) Quiescent cells were stimulated with 100 nM insulin for 5 min or pretreated with 10 μM LY294002 for 20 min followed by 5-min insulin stimulation. PI 3-kinase activity was measured in antiphosphotyrosine immunoprecipitates as described under Materials and Methods. A representative experiment is shown. (C) Cells were cultured for 6 h as described in A. Total protein content was determined and caspase-3 activity was measured as described under Materials and Methods. Results are expressed as arbitrary units per microgram of total protein and are means ± SE from three independent experiments with duplicate dishes. Statistical significance was tested with a one-way analysis of variance, followed by the protected least significant difference test. p < 0.01 was considered significant.
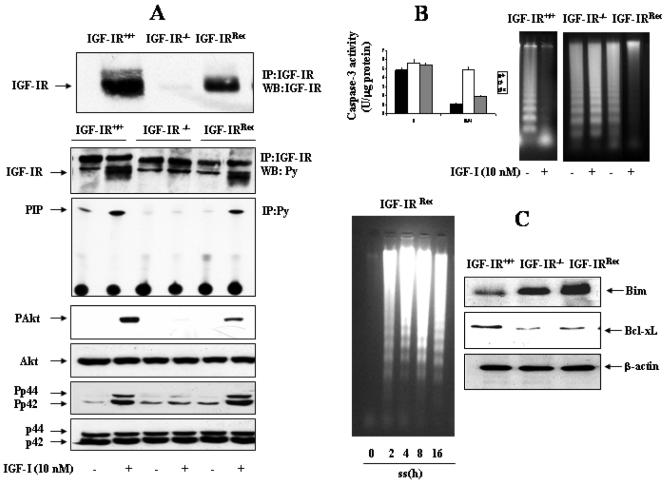
Reconstitution of IGF-IR-/- Brown Adipocytes with Wild-Type IGF-IR Restores IGF-I Signaling, but Not the Increased Susceptibility to Undergo Apoptosis
To ensure the role of IGF-IR in the increased susceptibility to undergo apoptosis in IGF-IR-deficient cells, we generated IGF-IR-/- brown adipocytes that reexpressed wild-type IGF-IR (IGF-IRRec). These cells were transfected with the pcDNA3.1-IGF-IRzeo construct, and stable cell lines were selected. Figure 10A (top) shows that IGF-IR reexpression in the reconstituted cell lines represents ∼70-80% of that seen in wild-type cells. Tyrosine phosphorylation of IGF-IR β-chain in response to IGF-I stimulation was recovered in reconstituted brown adipocytes in parallel to the level of IGF-IR protein reexpressed (Figure 10A, bottom). Likewise, IGF-I-induced anti-Tyr(P)-associated PI 3-kinase activity and the phosphorylation of Akt and MAPK, which was not detected in IGF-IR-/-cells, were recovered after IGF-IR reconstitution. It is noteworthy, that recovery of 70-80% of IGF-IR expression in deficient cells induced a level of phosphorylation of MAPK in response to IGF-I as in the wild type. However, phosphorylation of Akt parallels the level of IGF-IR reconstitution. Next, we evaluated whether the IGF-IR signaling, which was reconstituted in IGF-IR-deficient brown adipocytes, was able to restore the capacity of IGF-I to protect these cells from apoptosis. For that goal, cells were serum deprived for 8 h either in the absence or in the presence of 10 nM IGF-I and caspase-3 activity was determined. As shown in Figure 10B, reconstitution with IGF-IR down-regulated caspase-3 activity in the presence of IGF-I, as in wild-type cells. Furthermore, IGF-I suppressed DNA laddering in both wild-type and IGF-IRRec brown adipocytes, but not in IGF-IR-/- cells. Finally, the apoptotic sensitivity and the endogenous expression of pro- and antiapoptotic gene expression were analyzed in IGF-IRRec brown adipocytes. Although a substantial amount of IGF-IR was recovered in IGF-IRRec brown adipocytes (Figure 10A), these cells showed the same pattern of DNA laddering that IGF-IR-/- brown adipocytes upon serum withdrawal in a time-dependent manner (Figure 10C). Indeed, IGF-IRRec brown adipocytes maintained similar imbalance of Bim and Bcl-xL expression than IGF-IR-/- cells, compared with the wild type.
Figure 10.
Reconstitution of IGF-IR-deficient brown adipocytes does not rescue the susceptibility of apoptosis under conditions of serum withdrawal. (A) Top, IGF-IR-/- brown adipocytes were reconstituted with pcDNA3.1-IGF-IRzeo construct by generating stable cell lines (IGF-IRRec) as described under Materials and Methods. The expression of IGF-IR was assessed by immunoprecipitation of 600 μg of total protein with the anti-IGF-IR antibody followed by Western blot. Bottom, IGF-IR+/+, IGF-IR-/-, and IGF-IRRec brown adipocytes were serum starved for 16 h, stimulated with IGF-I (10 nM) for 5 min, and then lysed. Total protein (600 μg) was immunoprecipitated with anti-IGF-IR antibody, and the resulting immune complexes were analyzed by Western blot with the anti-Tyr(P) antibody. The band corresponding to the phosphorylated IGF-IR was indicated by an arrow. PI 3-kinase activity was measured in antiphosphotyrosine immunoprecipitates. Quiescent (20-h serum-starved) cells were stimulated with 10 nM IGF-I for 5 min or maintained in the absence of the hormone. Cells were lysed, and total protein (50 μg) was analyzed by Western blot with the corresponding antibodies against phospho-Akt, anti-Akt, antiphospho-MAPK, and anti-MAPK. A representative experiment is shown. (B) Left, cells were cultured for 8 h in serum-free medium either in the absence or presence of 10 nM IGF-I. Total protein content was determined and caspase-3 activity was measured. Results are expressed as arbitrary units per microgram of total protein and are means ± SE from three independent experiments with duplicate dishes. Right, IGF-IR+/+, IGF-IR-/-, and IGF-IRRec brown adipocytes were cultured in serum-free medium for 24 h either in the absence or in the presence of IGF-I (10 nM). At the end of the culture time, cells were scraped and subjected to extranuclear DNA extraction. Purified DNA was electrophoresed and visualized by UV fluorescence after staining with ethidium bromide. A representative experiment out of three is shown. (C) Left, IGF-IRRec brown adipocytes cultured in 10% FS were serum deprived for 2, 4, 8, 16, and 24 h. Then, cells were scraped and subjected to extranuclear DNA extraction. Purified DNA was electrophoresed and visualized by UV fluorescence after staining with ethidium bromide. A representative experiment out of three is shown. Right, cells were lysed and total protein (50 μg) was analyzed by Western blot with the anti-Bim, anti-Bcl-xL, and anti-β-actin antibodies. A representative experiment is shown.
DISCUSSION
BAT is specialized for adaptive thermogenesis and regulated energy expenditure. Embryonic development of BAT requires a balance between proliferation, necessary for organ growth, and apoptosis, essential for the orderly removal of certain cells. IGF-I is a potent mitogen in fetal rat brown adipocytes in primary culture (Lorenzo et al., 1993; Valverde et al., 1995) and also is a survival factor for immortalized rat (Navarro et al., 1998) and mouse (Tseng et al., 2002) brown adipocytes. Moreover, IGF-I induces adipogenic- and thermogenic-related gene expression in BAT at the end of the fetal life (Lorenzo et al., 1993; Valverde et al., 1997). It is well known that the biological effects of IGF-I in its target tissues are mediated by a complex network of intracellular signaling pathways initiated by the activation of the IGF-IR at the cell surface (Clemmons and Maile, 2003). In this regard, immortalized brown adipocytes derived from the IGF-IR-deficient mice are unique tools to study the role of IGF-I signaling in the biology of the brown adipose cell. Interestingly, these cells maintain the phenotypical features of wild-type brown adipocytes (Mur et al., 2002). In this study, we demonstrate that IGF-IR deficiency in brown adipocytes is associated with a rapid activation of the cellular apoptotic machinery in response to growth factor withdrawal. In previous works, we have reported the presence of functional insulin receptors in fetal brown adipocytes (Teruel et al., 1996), and more importantly, we have noted that IGF-IR-/- brown adipocytes display increased insulin receptor autophosphorylation upon insulin stimulation (Mur et al., 2002). However, the present results indicate that deficiency for the IGF-IR, which accelerates serum withdrawal-induced apoptosis, cannot be compensated by the insulin receptor and its signaling, thereby excluding functional redundancy of these receptors in BAT during fetal development.
The regulatory role of mitochondria in the apoptotic process has been well documented (Green and Reed, 1998; Kroemer, 1998). Caspase-3 may be activated by caspase-9, which is cleaved and activated by the release of cytochrome c from mitochondria to the cytosol (Li et al., 1997). In the current study, we report that the lack of IGF-IR accelerates the loss of Δψm transmembrane potential and cytochrome c release in brown adipocytes. These results, together with those discussed below, could explain the differences observed in the time course of caspase-3 activity and DNA laddering after serum withdrawal between IGF-IR-/- and control brown adipocytes.
The family of Bcl-2-related proteins constitutes one of the most biologically important classes of apoptosis-regulatory gene products. These proteins function like checkpoints through which survival and death signals must pass before they determine the cell fate. Indeed, a major site of activity of the Bcl-2 proteins is the mitochondrial membrane, promoting or preventing cytochrome c release. In this model, either up-regulation of proapoptotic members or down-regulation of antiapoptotic members triggers mitochondrial apoptosis. Immortalized mouse brown adipocytes growing in 10% FS express the antiapoptotic members Bcl-xL (29 kDa) and Bcl-2 (26 kDa), which prevent cytochrome c release from mitochondria to the cytosol. However, the proapoptotic member Bcl-xS (21 kDa), involved in cytochrome c release, was barely detected. Serum withdrawal stimulated a time-dependent decrease in Bcl-xL protein content that paralleled an increase in Bcl-xS expression at 16 h. This scenario was completely different in brown adipocytes lacking IGF-IR; when cultured with serum endogenous levels of Bcl-xL and Bcl-2 were much lower than that in wild-type cells. Moreover, in these cells Bcl-xS was constitutively expressed and its expression was further augmented by serum withdrawal. These results clearly indicate that the lack of IGF-IR in fetal brown adipocytes give rise to an imbalance between proand antiapoptotic genes, which may accelerate the sequential cellular events leading to DNA fragmentation and apoptosis. This imbalance cannot be rescued by reexpression of IGF-IR in deficient cells, although IGF-IRRec brown adipocytes restored IGF-IR signaling and rescue from serum withdrawal-induced apoptosis in response to IGF-I. Consequently, IGF-IRRec brown adipocytes show an increased susceptibility to undergo apoptosis under serum withdrawal as the IGF-IR-/- cells. These data suggest that the presence of IGF-IR signaling at very early stages of development is a requirement to maintain a balance between proand antiapoptotic genes in brown adipocytes. However, due to the absence of in vivo evidence that apoptosis is misregulated in IGF-IR-/- brown fat, we cannot exclude an indirect effect on brown adipocytes by altered signaling in other IGF-IR-dependent cells in a paracrine manner.
In contrast to the family of Bcl-related proteins, much less is known about the role of Foxo proteins in the mechanism of apoptosis. In the absence of Akt signaling, these unphosphorylated proteins predominantly localize in the nucleus where they bind to promoters of various target genes that induce cell death. These include FasL (Brunet et al., 1999), the BH3 domain-only member of the Bcl-2 family Bim (Dijkers et al., 2000; Stahl et al., 2002), TRAIL (Modur et al., 2002), TRADD (Rokudai et al., 2002), and BCL-6, a transcriptional repressor of Bcl-xL (Tzu-Ling Tang et al., 2002). Indeed, overexpression of Foxo1 or Foxo3a induces apoptosis in various cell types (Brunett et al., 1999; Tang et al., 1999). Interestingly, the lack of IGF-IR triggers substantial changes in Foxo1 cellular localization in brown adipocytes. After 4 h of serum deprivation, Foxo1 localization is entirely nuclear, whereas in wild-type cells nuclear localization of this molecule is detected only after 8 h. Bim is a well known target of Foxo transcription factors and exerts its proapoptotic activity through heterodimerization with antiapoptotic Bcl-2 members (O'Connor et al., 1998). Its constitutive expression is low in growing wild-type brown adipocytes and is up-regulated after serum starvation in parallel to Foxo1 entry into the nucleus. However, IGF-IR deficiency in brown adipocytes not only increases constitutive expression of Bim but also accelerates its expression upon serum withdrawal. Ectopic expression of Bim is sufficient to induce apoptosis in Ba/F3 cells (Dijkers et al., 2002). Moreover, Bim-/- lymphocytes and neurons have an increased resistance to cell death induced by cytokine withdrawal (Bouillet et al., 1999). Bim also has been shown to be an important mediator of the apoptosis seen in response to Foxo3 activation (Stahl et al., 2002). These data together with the results here presented suggest that in brown adipocytes Bim is an important transducer of death signals provoked by serum withdrawal.
Activation of caspase-8 was consistent with Foxo1 entry into the nucleus in brown adipocytes. Maximal activity was detected after 8 h of serum withdrawal in control cultures, whereas in IGF-IR-/- cells maximal activity was elicited at 6 h. The fact that caspase-8 activity was significantly inhibited by the neutralizing antibody against FasL in both cell types indicates that in immortalized brown adipocytes apoptosis induced by serum withdrawal is partly due to increased Foxo1-mediated FasL signaling, as proposed by Brunet et al. (1999). However, we cannot exclude the possibility that other targets of Foxo factors may play a role in this process. To further demonstrate the role of Foxo1 in controlling brown adipocyte survival, we performed adenoviral infections of wild-type brown adipocytes with an adenovirus encoding a form of Foxo1, which cannot be phosphorylated (ADA), and therefore is localized exclusively to the nucleus. Increased expression of proapoptotic proteins such as Bim and Bcl-xS was observed in ADA-infected cells. Likewise, the expression of the tBid indicated caspase-8 activation in these cells. Conversely, the antiapoptotic protein Bcl-xL was significantly decreased. As a result, caspase-3 activation and DNA fragmentation occurred in ADA-infected cells, but not in mock-infected cells. These data support the major role played by nuclear Foxo1 and its proapoptotic target genes in triggering death in brown adipocytes after serum withdrawal. More importantly, our data suggest that IGF-IR signaling is required to regulate the overall cell death process. In fact, recent data from Longo et al. (2002) demonstrate that protection against apoptosis elicited by Wnt signaling is due to the up-regulation of antiapoptotic genes such as IGF-I.
Most adaptive thermogenesis in small mammals takes place in BAT. Brown adipocytes contain multiple small lipid droplets and a high number of mitochondria, with fatty acids representing the main fuel to maintain the thermogenic capacity of the tissue (for review, see Ricquier and Bouillaud, 2000). The unique thermogenic capacity of BAT results from the expression of UCP-1 in the mitochondrial inner membrane, which is required to address the physiological hypothermia in newborn mammals (Nichols and Locke, 1984). UCP-1 increases proton leakage across the inner membrane of brown adipocyte mitochondria and thereby dissipates proton motive force as heat instead of synthesize ATP (Trayhurn and Milner, 1989). UCP-1 gene expression is highly cold inducible through the activation of the sympathetic nervous system, this effect being mediated by β-adenoreceptors and cAMP (Cassard-Doulcier et al., 1993; Kozak et al., 1994). PGC-1α is a strong coactivator of several nuclear receptors [i.e., poly(ADP-ribose) polymerase (PPAR)γ, PPARα, retinoic acid receptor, and thyroid hormone receptor that also binds to the UCP-1 enhancer (Puigserver et al., 1998). PGC-1α is induced in BAT when mice are exposed to cold or in vitro by the β-agonist isoproterenol (Puigserver et al., 1998; Boss et al., 1999). In fact, our data indicate that exogenous expression of PGC-1α in IGF-IR-deficient brown adipocytes increased UCP-1 protein content, which might implicate a decrease in ATP synthesis (Klingenspor, 2003). As a consequence, these cells were less susceptible to DNA fragmentation and caspases activation triggered by serum withdrawal compared with IGF-IR-deficient cells. Efficient execution of apoptosis requires an adequate supply of ATP (Dey and Moraes, 2000). This is particularly important in kidney and colon carcinomas because the selective repression of β-F1-ATPase expression hampered the apoptotic potential of the cancer cells, resulting in chemo- and radiotherapy resistance (Cuezva et al., 2002). Consistent with these findings, PGC-1α decreased the susceptibility of IGF-IR-deficient brown adipocytes to undergo apoptosis through increased UCP-1 expression. These results were reinforced by similar data obtained by transduction of UCP-1 directly in IGF-IR-/- cells. Although we cannot exclude that PGC-1α can retard apoptosis by UCP-1-independent mechanisms, our results indicate that UCP-1 might have an essential role probably by limiting the generation of ATP necessary for the apoptotic machinery. Recently, it has been proposed that one of the functions of proteins that uncouple respiration is the limitation of radical oxygen species (ROS) production (Nègre-Salvayre et al., 1997, Vicent et al., 2004). However, we have not found differences in the generation of ROS after 2-6 h of serum deprivation (our unpublished data), a critical time-point for induction of the apotoctic machinery in the cell lines used for the study.
Although most cells in culture undergo apoptosis after serum/growth factor deprivation, the supply of specific trophic factors prevents the apoptotic process in a number of cell types. Both wild-type and IGF-IR-/- brown adipocytes are insulin target cells with a fully functional insulin signaling cascade (Mur et al., 2002, 2003). In both cell types, insulin prevents serum withdrawal-induced cell death by increasing the amount of cytosolic Foxo1 with a concomitant decrease of caspase-8 enzymatic activity. Again, these results support the essential role of Foxo1 in maintaining the molecular balance of cell death/survival in brown adipocytes. Moreover, Bcl-xS expression is severely decreased by insulin in rat brown adipocytes (Navarro et al., 1998). An important effect of insulin is the inhibition of caspase-3 activity in wild-type and IGF-IR-/- cells. Because caspase activation is required for complete apoptotic phenotype, and caspase-3 is one of the executioners in this process (Shi, 2002), prevention of caspase-3 cleavage is certainly an essential mechanism by which insulin protects brown adipocytes from apoptosis. Together, these data indicate that insulin elicits its survival effect in brown adipocytes through its own receptor by preventing the nuclear accumulation of Foxo1 and the activation of its nuclear targets and by decreasing the levels of proapoptotic proteins of the Bcl family. Moreover, these protective effects of insulin are independent of the expression of the IGF-IR.
Studies on the signaling pathways involved in the antiapoptotic effect induced by insulin indicate that both PI 3-kinase/Akt and Ras/MAPK cascades participate in the control of this process. IGF-IR-deficient brown adipocytes are highly sensitive to insulin, displaying enhanced tyrosine phosphorylation of IR and IRS-1 compared with wild-type cells. Consequently, IRS-1/Grb-2/MAPK signaling, which is responsible of the mitogenic effect of insulin in brown adipocytes (Valverde et al., 2001), is augmented in IGF-IR-/- cells concomitantly with increased DNA synthesis, cell number, and proliferating cell nuclear antigen expression (Mur et al., 2002). Previous studies from our laboratory (Navarro et al., 1998) or others (Parrizas et al., 1997) indicated that the activation of both PI 3-kinase and MAPK signaling pathways is necessary for the full survival effect of insulin in brown adipocytes or PC12 cells, respectively. However, in brown adipocytes lacking IGF-IR, insulin maintains its survival effect even when PI 3-kinase is inhibited with LY294002. This effect is unique for this cell type, because insulin is unable to rescue apoptosis in wild-type brown adipocytes in the presence of the PI 3-kinase inhibitor. However, apoptosis does occur in IGF-IR-deficient cells in the presence of MEK-1 inhibitor, suggesting that activation of this pathway is not compensated by insulin-activated PI 3-kinase.
In conclusion, the lack of IGF-IR in brown adipocytes confers increased susceptibility to apoptosis upon serum withdrawal. This effect is the result of multiple defects reflecting altered mitochondrial integrity, including modified expression of Bcl-2 family genes, differential activation of caspases, and nuclear translocation of Foxo1, which activates the death receptor pathway through Fas ligand. Reexpression of IGF-IR cannot reverse this imbalance, although reconstituted brown adipocytes restored IGF-I signaling and rescue from serum withdrawal-induced apoptosis in response to IGF-I. Exogenous expression of the nuclear coactivator PGC-1α or UCP-1 prevents apoptosis in IGF-IR-deficient brown adipocytes, suggesting that cellular ATP content in these cells may be rate-limiting step in programmed cell death. Finally, the survival effect of insulin on IGF-IR-/- brown adipocytes was elicited even in the absence of PI 3-kinase/Akt signaling rescue pathway. Thus, our results demonstrate for the first time the unique role of IGF-IR in maintaining the balance cell death/survival in fetal brown adipocytes.
Acknowledgments
This work was supported by grant CAM 08.6/0015/2001.1 from the Comunidad deMadrid, Spain, and by Red deGrupos en Diabetes Mellitus (G03/212), Ministerio de Sanidad, Spain. We thankfully acknowledge Bruce S. Spiegelman and Pere Puiserverg (Dana-Farber Cancer Institute) for supplying the pBabe hygro PGC-1α construct and the adenovirus encoding PGC-1α, Domenico Accili (Columbia University, New York, NY) for supplying the adenovirus encoding Foxo1 ADA, R.W. Furlanetto (National Institutes of Health) for the pcDNA3.1-IGF-IRzeo construct, and T. Unterman (University of Chicago, Chicago, IL) for the Foxo1-GFP construct. We also acknowledge Isabel Fabregat, Margarita Fernandez (Universidad Complutense, Madrid, Spain), and Deborah J. Burks (Universidad de Salamanca, Spain) for suggestions and critical reading of the manuscript. We recognize the technical skill of M. López.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-11-0853. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-11-0853.
Abbreviations used: BAT, brown adipose tissue; FS, fetal calf serum; IGF-I, insulin-like growth factor-I; IGF-IR, insulin-like growth factor-I receptor; UCP-1, uncoupling protein-1; PBS, phosphate-buffered saline; PI, phosphatidylinositol.
References
- Baserga, R. (1999). The IGF receptor in cancer research. Exp. Cell Res. 253, 1-6. [DOI] [PubMed] [Google Scholar]
- Benito, M., Valverde, A.M., and Lorenzo, M. (1996). IGF-I: a mitogen also involved in differentiation processes in mammalian cells. Int. J. Biochem. Cell Biol. 28, 499-510. [DOI] [PubMed] [Google Scholar]
- Blakesley, V.A., Stannard, B.S., Kalebic, T., Helman, L.H., and Le Roith, D. (1997). Role of the IGF-I receptor in mutagenesis and tumor promotion. J. Endocrinol. 152, 339-344. [DOI] [PubMed] [Google Scholar]
- Bouillet, P., Metcalf, D., Huang, D.C., Tarlinton, D.M., Kay, T.W., Kontgen, F., Adams, J.M., and Strasser, A. (1999). Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735-17388. [DOI] [PubMed] [Google Scholar]
- Boss, O., Bachman, E., Vidal-Puig, A., Zhang, C.Y., Peroni, O., and Lowell, B.B. (1999). Role of the beta(3)-adrenergic receptor and/or a putative beta(4)-adrenergic receptor on the expression of uncoupling proteins and peroxisome proliferator-activated receptor-gamma coactivator-1. Biochem. Biophys. Res. Commun. 26, 870-876. [DOI] [PubMed] [Google Scholar]
- Brunet, A., Bonni, A., Zigmond, M.J., Lin, M.Z., Juo, P., Hu, L.S., Anderson, M.J., Arden, K.C., Blenis, J., and Greenberg, M.E. (1999). Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857-868. [DOI] [PubMed] [Google Scholar]
- Cassard-Doulcier, A-M., Gelly, C., Fox, N., Schrementi, J., Raimbault, S., Klaus, S., Forest, C., Bouillaud, F., and Ricquier, D. (1993). Tissue-specific and β-adrenergic regulation of the mitochondrial uncoupling protein gene: control by cis-acting elements in the 5′-flanking region. Mol. Endocrinol. 7, 497-506. [DOI] [PubMed] [Google Scholar]
- Clemmons, D.R., and Maile, L.A. (2003). Integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endocrinology 144, 1664-1670. [DOI] [PubMed] [Google Scholar]
- Cuezva, J.M., Krajewska, M., de Heredia, M.L., Krajewski, S., Santamaria, G., Kim, H., Zapata, J.M., Marusawa, H., Chamorro, M., and Reed, J.C. (2002). The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 62, 6674-6681. [PubMed] [Google Scholar]
- Dey, R., and Moraes, C.T. (2000). Lack of oxidative phosphorylation and low mitochondrial membrane potential decrease susceptibility to apoptosis and do not modulate the protective effect of Bcl-x(L) in osteosarcoma cells. J. Biol. Chem. 275, 7087-7094. [DOI] [PubMed] [Google Scholar]
- Dijkers, P.F., Birkenkamp, K.U., Lam, E.W., Thomas, N.S., Lammers, J.W., Koenderman, L., and Coffer, P.J. (2002). FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. 156, 531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers, P.F., Medema, R.H., Lammers, J.W., Koenderman, L., and Coffer, P.J. (2000). Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10, 1201-1204. [DOI] [PubMed] [Google Scholar]
- Dupont, J., and LeRoith, D. (2001). Insulin and insulin-like growth factor I receptors: similarities and differences in signal transduction. Horm. Res. 55 Suppl 2, 22-26. [DOI] [PubMed] [Google Scholar]
- Green, D.R., and Reed, J.C. (1998). Mitochondria and apoptosis. Science 281, 1309-1312. [DOI] [PubMed] [Google Scholar]
- Hazzis, M.H., Thompson, C.B. (2000). The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 7, 1182-1191. [DOI] [PubMed] [Google Scholar]
- Juo, P., Kuo, C.J., Yuan, J., and Blenis, J. (1998). Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8, 1001-1008. [DOI] [PubMed] [Google Scholar]
- Klingenspor, M. (2003). Cold-induced recruitment of brown adipose tissue thermogenesis. Exp. Physiol. 88, 141-148. [DOI] [PubMed] [Google Scholar]
- Kluck, R.M., et al. (1999). The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell Biol. 147, 809-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak, U.C., Kopecky, J., Teisinger, J., Enerback, S., Boyer, B., and Kozak, L.P. (1994). An upstream enhancer regulating brown-fat specific expression of the mitochondrial uncoupling protein gene. Mol. Cell. Biol. 14, 59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer, G. (1998). The mitochondrion as an integrator/coordinator of cell death pathways. Cell Death Differ. 5, 547. [DOI] [PubMed] [Google Scholar]
- Kulkarni, R.N., Holzenberger, M., Shih, D.Q., Ozcan, U., Stoffel, M., Magnuson, M.A., and Kahn, C.R. (2002). β-Cell-specific deletion of the IGF-1 receptor leads to hiperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat. Genet. 31, 111-115. [DOI] [PubMed] [Google Scholar]
- LeRoith, D., Werner, H., Beitner-Johnson, D., and Roberts, C.T. (1995). Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr. Rev. 16, 143-146. [DOI] [PubMed] [Google Scholar]
- Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S.M., Ahmad, M., Alnemri, E.S., and Wamg, X. (1997). Cytochrome c and ATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479-489. [DOI] [PubMed] [Google Scholar]
- Liu, J.P., Backer, J., Perkins, A.S., Robertson, E.J., and Efstradiatis, A. (1993). Mice carrying null mutations of the genes encoding insulin-like growth factor I (IGF-I) and type 1 IGF-I receptor. Cell 75, 59-72. [PubMed] [Google Scholar]
- Longo, K.A., Kennell, J.A., Ochocinska, M.J., Ross, S.E., Wright, W.S., and MacDougald, O.A. (2002). Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J. Biol. Chem. 277, 38239-38244. [DOI] [PubMed] [Google Scholar]
- Lorenzo, M., Valverde, A.M., Teruel, T., and Benito, M. (1993). IGF-I is a mitogen also involved in differentiation-related gene expression in fetal brown adipocytes. J. Cell Biol. 123, 1567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews, L.S., Hammer, R.E., Behringer, R.R., D′Ercole, A.J., Bell, G.I., Brinster, R.L., and Palmiter, R.D. (1988). Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology 123, 2827-2833. [DOI] [PubMed] [Google Scholar]
- Modur, V., Nagarajan, R., Evers, B.M., and Milbrandt, J. (2002). FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. J. Biol. Chem. 277, 47928-47937. [DOI] [PubMed] [Google Scholar]
- Moschos, S.J., and Mantzoros, C.S. (2002). The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology 63, 317-332. [DOI] [PubMed] [Google Scholar]
- Mur, C., Arribas, M., Benito, M., and Valverde, A.M. (2003). Essential role of insulin-like growth factor receptor in insulin-induced fetal brown adipocyte differentiation. Endocrinology 144, 581-593. [DOI] [PubMed] [Google Scholar]
- Mur, C., Valverde, A.M., Kahn, C.R., and Benito, M. (2002). Increased insulin sensitivity in IGF-I receptor deficient brown adipocytes. Diabetes 51, 743-754. [DOI] [PubMed] [Google Scholar]
- Nakae, J., Barr, V., and Accili, D. (2000). Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J. 19, 989-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae, J., Kitamura, T., Silver, D.L., and Accili, D. (2001). The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Investig. 108, 1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, Y., Mulshine, J.I., Kasprzyk, P.G., Natale, R.B., Maneckjee, R., Avis, A.M., and Teston, A.F. (1988). Insulin-like growth factor I can mediate autocrine proliferation of human small cell lung cancer cell lines. J. Clin. Investig. 82, 354-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, P., Valverde, A.M., Benito, M., and Lorenzo, M. (1998). Insulin/IGF-I rescues immortalized brown adipocytes from apoptosis down-regulating Bcl-xS expression, in a PI 3-kinase and MAP kinase-dependent manner. Exp. Cell Res. 243, 213-221. [DOI] [PubMed] [Google Scholar]
- Nègre-Salvayre, A., Hirtz, C., Carrera, G., Cazenave, R., Troly, M., Salvayre, R., Penicaud, L., and Casteilla, L. (1997). A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 11, 809-815. [PubMed] [Google Scholar]
- Nichols, D.G., and Locke, R.M. (1984). Thermogenic mechanisms in brown fat. Physiol. Rev. 64, 1-64. [DOI] [PubMed] [Google Scholar]
- O'Connor, L., Strasser, A., O′Reilly, A., Hausmann, G., Adams, J.M., Cory, S., and Huang, D.C.S. (1998). Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17, 384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrizas, M., Saltiel, A.R., and LeRoith, D. (1997). Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem. 272, 154-161. [DOI] [PubMed] [Google Scholar]
- Peruzzi, F., Frisco, M., Dews, M., Salomoni, P., Grassili, E., Romano, G., Calabretta, B., and Baserga, R. (1999). Multiple pathways of the insulin-like growth factor receptor in protection from apoptosis. Mol. Cell Biol. 19, 7203-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras, A., Alvarez, A.M., Valladares, A., and Benito, M. (1997). TNF-alpha induces apoptosis in rat fetal brown adipocytes in primary culture. FEBS Lett. 16, 324-328. [DOI] [PubMed] [Google Scholar]
- Powell-Braxton, L., Hollingshead, P., Warburton, C., Dowd, M., Pittis-Meek, S., Dalton, D., Gillet, N., and Stewar, T.A. (1993). IGF-I is required for normal embryonic growth in mice. Genes Dev. 7, 2609-2617. [DOI] [PubMed] [Google Scholar]
- Puigserver, P., Wu, Z., Park, C.W., Graves, R., Wright, M., and Spiegelman, B.M. (1998). A cold-inducible coactivator of nuclear receptors linked to adaptative thermogenesis. Cell 92, 829-839. [DOI] [PubMed] [Google Scholar]
- Resnicoff, M. (1998). Antitumor effects elicited by antisense-mediated down-regulation of the insulin-like growth factor I receptor. Int. J. Mol. Med. 1, 883-888. [DOI] [PubMed] [Google Scholar]
- Ricquier, D., and Bouillaud, F. (2000). The uncoupling protein homologues: UCP1, UCP2, UCP3 and AtUCP. Biochem. J. 345, 161-179. [PMC free article] [PubMed] [Google Scholar]
- Rininsland, F., Johnson, T.R., Chernicky, C.L., Schulze, E., Burfeind, P., and Ilan, J. (1997). Suppression of insulin-like growth factor type I receptor by a triple-helix strategy inhibits IGF-I transcription and tumorigenic potential of rat C6 glioblastoma cells. Proc. Natl. Acad. Sci. USA 94, 5854-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokudai, S., Fujita, N., Kitahara, O., Nakamura, Tsuruo, T. (2002). Involvement of FKHR-dependent TRADD expression in chemotherapeutic drug-induced apoptosis. Mol. Cell. Biol. 22, 8695-8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, D.N., Jones, B.G., Shapiro, L.H., Dias, P., and Houghton, P.J. (1994). Antisense-mediated reduction in insulin-like growth factor-I receptor expression suppresses the malignant phenotype of a human alveolar rhabdomyosarcoma. J. Clin. Investig. 94, 1235-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. (2002). Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9, 459-470. [DOI] [PubMed] [Google Scholar]
- Stahl, M., Dijkers, P.F., Koops, G.J., Lens, S.M., Coffer, P.J., Burgering, B.M.T., and Medema, R.H. (2002). The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 168, 5024-5031. [DOI] [PubMed] [Google Scholar]
- Suhara, T., Kim, H-S., Kirshenbaum, L.A., and Walsh, K. (2002). Suppression of Akt signaling induces Fas ligand expression: involvement of caspase and Jun kinase activation in Akt-mediated Fas ligand regulation. Mol. Cell. Biol. 22, 680-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, E.D., Nunez, G., Barr, F.G., and Guan, K.L. (1999). Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 27, 16741-16746. [DOI] [PubMed] [Google Scholar]
- Teruel, T., Valverde, A.M., Benito, M., and Lorenzo, M. (1995). Differentiation of rat brown adipocytes during late fetal development: role of insulin-like growth factor I. Biochem. J. 310, 771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruel, T., Valverde, A.M., Benito, M., and Lorenzo, M. (1996). Insulin-like growth factor and insulin induce adipogenic-related gene expression in fetal brown adipocyte primary cultures. Biochem. J. 319, 627-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruel, T., Valverde, A.M., Navarro, P., Benito, M., and Lorenzo, M. (1998). Inhibition of PI 3-kinase and Ras blocks IGF-I and insulin-induced uncoupling protein gene expression in brown adipocytes. J. Cell. Physiol. 176, 99-109. [DOI] [PubMed] [Google Scholar]
- Trayhurn, P., and Milner, R.E. (1989). A commentary on the interpretation of in vitro biochemical measures of brown adipose tissue thermogenesis. Can. J. Physiol. Pharmacol. 67, 811-819. [DOI] [PubMed] [Google Scholar]
- Tseng, Y.H., Ueki, K., Kriauciunas, K.M., and Kahn, C.R. (2002). Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J. Biol. Chem. 277, 31601-31611. [DOI] [PubMed] [Google Scholar]
- Tzu-Ling Tang, T., Dowbenko, D., Jackson, A., Toney, L., Lewin, D.A., Dent, A.L., and Lasky, L.A. (2002). The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J. Biol. Chem. 277, 14255-14265. [DOI] [PubMed] [Google Scholar]
- Ullrich, A., et al. (1986). Insulin-like growth factor receptor primary structure: comparision with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 5, 2503-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde,A.M.,Lorenzo,M.,Navarro,P.,andBenito,M.(1997).Phosphatidylinositol 3-kinase is a requirement for insulin-like growth factor-I-induced differentiation, but not for mitogenesis, in fetal brown adipocytes. Mol. Endocrinol. 11, 595-607. [DOI] [PubMed] [Google Scholar]
- Valverde, A.M., Lorenzo, M., Teruel, T., and Benito, M. (1995). cAMP inhibits IGF-I-induced proliferation in fetal rat brown adipocytes. Exp. Cell Res. 218, 305-309. [DOI] [PubMed] [Google Scholar]
- Valverde, A.M., Mur, C., Pons, S., Alvarez, A., White, M.F., Kahn, C.R., and Benito, M. (2001). Y895IRS-1/GRB-2 association mediates the insulin signaling involved in IRS-1-deficient brown adipocytes mitogenesis. Mol. Cell. Biol. 21, 2269-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent, A.M., Olzmann, J.A., Brownlee, M., Sivitz, W.I., and Russell, J.W. (2004). Uncoupling proteins prevent glucose-induced neuronal oxidative stress and programmed cell death. Diabetes 53, 726-734. [DOI] [PubMed] [Google Scholar]
- White, M.F. (2002). IRS proteins and the common path to diabetes. Am. J. Physiol. 283, E413-E422. [DOI] [PubMed] [Google Scholar]
- White, M.F., and Kahn, C.R. (1994). The insulin signaling system. J. Biol. Chem. 269, 1-4. [PubMed] [Google Scholar]
- Wu, Z., et al. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115-124. [DOI] [PubMed] [Google Scholar]
- Yee, D.S., Paik, G.S., Lebovic, R.R., Marcus, R.E., Favoni, K.J., Cullen, M.E., Lippman, M.E., and Rosen, N. (1989). Analysis of insulin-like growth factor I gene expression in malignancy: evidence for a paracrine role in human breast cancer. Mol. Endocrinol. 3, 509-514. [DOI] [PubMed] [Google Scholar]