Abstract
The human T-cell lymphotropic virus type 1 (HTLV-1) is the causative agent of HTLV-1-associated myelopathy/tropical spastic paraparesis (HT). Although it is widely believed that virus infection and host immune response are involved in the pathogenic mechanisms, the role of the immune system in the development and/or maintenance of HT remains unknown. We performed an analysis of the peripheral blood leukocyte phenotype for two different subcohorts of HTLV-1-infected individuals to verify the existence of similar immunological alterations, possible laboratory markers for HT. The leukocyte population balance, the activation status of the T lymphocytes, and the cellular migratory potential of T lymphocytes, monocytes, and neutrophils were evaluated in the peripheral blood of HTLV-1-infected individuals classified as asymptomatic individuals, oligosymptomatic individuals, and individuals with HT. Data analysis demonstrated that a decreased percentage of B cells, resulting in an increased T cell/B cell ratio and an increase in the CD8+ HLA-DR+ T lymphocytes, exclusively in the HT group could be identified in both subcohorts, suggesting its possible use as a potential immunological marker for HT for use in the laboratory. Moreover, analysis of likelihood ratios showed that if an HTLV-1-infected individual demonstrated B-cell percentages lower than 7.0%, a T cell/B cell ratio higher than 11, or a percentage of CD8+ HLA-DR+ T lymphocytes higher than 70.0%, this individual would have, respectively, a 12-, 13-, or 22-times-greater chance of belonging to the HT group. Based on these data, we propose that the T cell/B cell ratios and percentages of circulating B cells and activated CD8+ T lymphocytes in HTLV-1-infected patients are important immunological indicators which could help clinicians monitor HTLV-1 infection and differentiate the HT group from the asymptomatic and oligosymptomatic groups.
The human T-cell lymphotropic virus type 1 (HTLV-1) was the first retrovirus isolated from humans (20). Although this discovery was verified in the early 1980s, recent studies have demonstrated the presence of HTLV-1 proviral DNA in mummies over 1,500 years old (23). HTLV-1 is endemic in the southern region of Japan, the Caribbean, the equatorial region of Africa, and South America. In Brazil, the prevalence of HTLV-1 infection observed in blood banks between 1989 and 1996 ranged from 0.32% in Belo Horizonte, State of Minas Gerais, to 1.8% in Salvador, State of Bahia (6). HTLV-1 was shown to be the causative agent of adult T-cell leukemia/lymphoma (20, 21) as well as HTLV-1-associated myelopathy, which is also called tropical spastic paraparesis (HT), the most severe degenerative inflammatory syndrome observed in infected patients (9). This neurological disorder is a chronic, progressive, demyelinating disease which affects the spinal cord and white matter of the central nervous system (CNS) (1). HT is characterized by gait disturbance, weakness and stiffness in the lower limbs, spasticity, and frequently bladder and bowel dysfunction (21). Although it is widely believed that viral infection and host immune responses are involved in the pathogenesis of HT, the exact mechanism and role of the immune system in the development and/or maintenance of HT remain unknown. It seems that the immune response can play a dual role during this process, being associated with both pathology and protective events that involve antiviral activity as well as cellular damage induced by cytotoxic T cells. Recent studies have shown that infected CD4+ and CD8+ T lymphocytes may take part in a number of events leading to viral infections of cell populations in the CNS, activation of astrocytes and microglial cells, induction of proinflammatory cytokine, chemokine synthesis, recruitment of inflammatory infiltrate to the CNS, blood-brain barrier disruption, deregulation of oligodendrocyte homeostasis, demyelination, and axonal degradation (10). Despite the progression of HT, longitudinal studies of asymptomatic (AS) carriers have demonstrated that clinical symptoms can be observed in some of these individuals depending on the time of the infection. However, the factors that lead to this differentiated progression are still targeted for investigation (B. Catalan-Soares, personal communication). Therefore, the follow-up study of AS carriers represents an important tool to obtain a more complete understanding of the pathogenesis of HT. A number of altered immunological features, such as hypergammaglobulinemia (with high antibody titers to HTLV-1) and elevated levels of interleukin 6, tumor necrosis factor alpha, and interleukin 2, have been demonstrated for patients with HT in comparison to the immunological features of AS individuals (15, 25). Moreover, the presence of activated virus-specific CD8+ T lymphocytes in the peripheral blood and spontaneous proliferation of mononuclear cells in vitro have also been reported (17, 18, 19). Recently, our group has performed an ex vivo cytometric analysis of whole-blood leukocytes to evaluate their activation status, migratory potential, and cellular alterations in well-defined-HT patients in comparison to those of non-HT-infected individuals as well as noninfected (NI) blood donors. Our data have revealed that only the HT group showed a decreased percentage of B cells, leading to lower T lymphocyte/B lymphocyte ratios and higher percentages of activated circulating CD8+ HLA-DR+ cells than in the AS, oligosymptomatic (OL), and NI individuals (4). In addition, HT individuals showed a high expression of the adhesion molecule CD18 on the surfaces of both CD4+ and CD8+ T lymphocytes as well as on monocytes and neutrophils, resulting in an increase in cellular migratory potential in comparison to that in the AS group. In order to validate these immunological indexes as useful laboratory indicators for follow-up studies of HTLV-1-infected individuals, we performed a validation study in which these indexes were evaluated in a new subcohort, “B,” and the data obtained were compared with those obtained in our previous investigation where a different subcohort, “A,” of HTLV-1-infected individuals was examined. We have also performed a further analysis of important surface markers, CD38 and CD62L, involved in T-lymphocyte activation. The ex vivo condition was chosen since the phenotypic analysis may reflect the dynamic events of immune response that take place in vivo, particularly in the absence of exogenous stimuli.
MATERIALS AND METHODS
Subjects.
For this study, blood samples, with EDTA as an anticoagulant, were obtained from 121 HTLV-1-infected individuals distributed between two subcohorts and evaluated at different times. The attending physician defined their clinical statuses, and the HTLV-1 infection was determined by positive serology for anti-HTLV-1 antibodies through enzyme-linked immunosorbent assay and Western blot methods. All patients included in the present study demonstrated negative serology for other relevant blood-borne pathogens, including human immunodeficiency virus, hepatitis C virus, hepatitis B virus, Treponema pallidum, and Trypanosoma cruzi.
Subcohort A, evaluated in December of 1999, consisted of 74 HTLV-1-infected individuals distributed in three groups, including AS individuals (8 men and 10 women), OL individuals (8 men and 6 women), and individuals with well-defined HT (13 men and 29 women). Subcohort B, evaluated in December of 2002, was composed of 47 HTLV-1-infected individuals also classified as AS (10 men and 12 women), OL (5 men and 8 women), and having HT (5 men and 7 women).
The AS and OL groups were under medical care from two members of our research group (B.C.S. and J.G.R.) at the HEMOMINAS Foundation. The AS group had no clinical complaints and presented normal motor and sensory functions. Furthermore, they did not show any clinical signs as described by the standard neurological classification of spinal cord injury, namely, the American Spinal Injury Association (ASIA) impairment scale. HTLV-1-infected individuals were included in the OL group, based on their clinical status with respect to the ASIA impairment scale, being considered symptomatic but not having HT. OL patients presented impairment of the tendon reflexes (hyper or hypo reflexes); vesical impairment, including urinary dysfunctions; paresthesia; lumbar pain; and sexual dysfunction. However, the OL group did not present sufficient clinical signs, according to ASIA, to be classified as having HT.
The HT group attended the Sarah Kubitschek Hospital and presented a severe form of HTLV-1 chronic infection. This group included individuals receiving medical care from two members of our group (J.G.R and B.C.S.). HT patients were identified according to the ASIA impairment scale. These patients were evaluated at Sarah Kubitschek Hospital, where they underwent clinical and laboratory examinations. None of these patients received corticosteroids or other immunosuppressive chemotherapy before giving blood for immunophenotyping assays.
Healthy, NI volunteers, considered apt for blood donation with negative serology for HTLV-1 as well as the above-mentioned blood-borne pathogens, were included in the control group of NI individuals.
All subjects included in the present study gave their informed consent, and the study was approved by the Ethical Committee from the HEMOMINAS Foundation and Sarah Kubitschek Hospital.
Blood samples.
The biological samples consisted of 5 ml of venous peripheral blood, with EDTA as an anticoagulant. The samples were collected by trained professionals at the HEMOMINAS Foundation (from NI, AS, and OL individuals) and at Sarah Kubitschek Hospital (from HT patients). After collection, the whole peripheral blood was used for immunophenotypic analysis involving flow cytometry within 24 h.
Flow cytometric analysis of peripheral blood leukocytes.
White blood cell phenotypes were analyzed with an immunofluorescence procedure recommended by Becton Dickinson, modified as follows. In 12- by 75-mm tubes, 50 μl of EDTA-coagulated blood samples were incubated in the dark with 5 μl of undiluted monoclonal antibodies specific for several cell surface markers (anti-CD45 clone J33, anti-CD62L clone DREG56, anti-CD4 clone 13B8.2, and anti-CD8 clone B9.11 labeled with fluorescein isothiocyanate; and anti-CD3 clone UCTH-1, anti-CD16 clone 3G8, anti-CD19 clone J4.119, anti-CD18 clone 7E4, anti-CD38 clone LS198-4-3, and anti-HLA-DR clone TU36 labeled with phycoerythrin) for 20 to 30 min at room temperature. Following the incubation, erythrocytes were lysed by using 100 μl of lysing solution (Optlyse-B; Immunotech) for 5 min, followed by the addition of 900 μl of distilled water and reincubation for 10 min. White blood cells were then washed twice with 1 ml of phosphate-buffered saline containing 0.01% of sodium azide. Cell preparations were fixed in 500 μl of fluorescence-activated cell sorter fix solution (10 g of paraformaldehyde/liter, 10.2 g of sodium cacodylate/liter, 6.65 g of sodium chloride/liter). Cytofluorimetric data acquisition was performed with a Becton Dickinson FACSCalibur instrument. Cell phenotype analysis within gated lymphocyte populations and subpopulations was performed by using Cell-Quest software. Lymphocytes were first identified based on their forward and side laser scatter properties. After a lymphocyte gating strategy was used, cells were analyzed for fluorescence properties, and data were expressed as percentages of cells positive for a given cell marker or as fluorescence intensity for a given cell marker by using dual-color dot plot graphics or single histograms, respectively.
Statistical analysis.
Data analysis was performed by analysis of variance, followed by Student's t test. Additionally, we have used likelihood ratio analysis to evaluate the performance of a given phenotypic feature to discriminate HT patients from other HTLV-1-infected (AS and OL) individuals.
RESULTS
Analysis of circulating T and B lymphocytes.
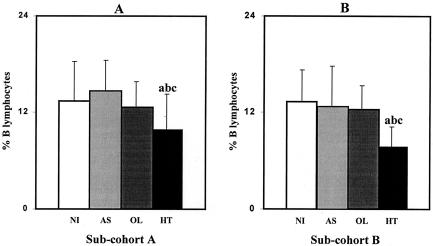
The analysis of B lymphocytes is presented in Fig. 1. Figure 1A represents the results of B-lymphocyte analysis performed for subcohort A in 1999. Figure 1B results confirm that the mean percentage ± standard deviation of circulating B lymphocytes (CD3− CD19+), when subcohort B was analyzed, was significantly lower (P < 0.01) in the HT group than in other groups (NI, 13.3% ± 3.9%; AS, 12.7% ± 5.1%; OL, 12.3% ± 2.9%; and HT, 7.7% ± 2.5%). The similarity of data obtained from the two subcohorts strongly suggests the usefulness of the quantification of circulating B cells as an immunophenotypic feature to discriminate HT patients from the other HTLV-1-infected individuals.
FIG. 1.
Mean percentage of B cells in the peripheral blood of different populations of HTLV-1-infected individuals (AS, OL, and HT individuals) and NI controls. (A) Analysis of subcohort A; (B) analysis of subcohort B. Significant differences (P < 0.05) are identified by the letters a, b, and c in comparison to results for NI, AS, and OL groups, respectively.
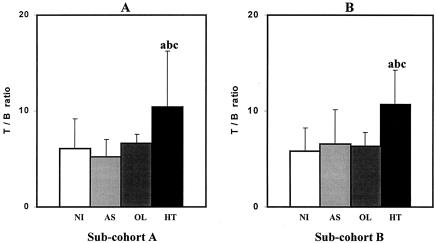
Additionally, as a consequence of B-lymphocyte decrease, which is another typical hallmark of HT patients observed in the present study, we also observed a distinctly low T lymphocyte/B lymphocyte ratio (CD3+/CD19+) (Fig. 2B). These data reiterate our previous observations when analyzing subcohort A (Fig. 2A). Moreover, these findings match observations reported by Furukawa et al. (8) of increased expression of phosphatidylserine, an early marker for apoptosis, on the surfaces of B cells from HT patients, which may explain the low levels of circulating B cells observed in HT patients. The possible involvement of this proapoptotic event in the B-lymphocyte populations might contribute to the decrease of this lymphocyte population in the peripheral blood as well as a down-regulation of the CD19 marker on differentiated B lymphocytes due to massive cell activation. The high levels of HTLV-1-specific antibodies observed in the peripheral blood and cerebrospinal fluid of HT patients compared to those in AS carriers reinforce this hypothesis of changes in the B-cell phenotypes, following antigen stimulation and their differentiation into antibody-producing plasm cells. However, additional studies using a range of B-cell markers must be performed to confirm this hypothesis.
FIG. 2.
T lymphocyte/B lymphocyte ratios (CD3+/CD19+) observed in different populations of HTLV-1-infected individuals (AS, OL, and HT individuals) and NI controls. (A) Analysis of subcohort A; (B) analysis of subcohort B. Significant differences (P < 0.05) are identified by the letters a, b, and c in comparison to results for NI, AS, and OL groups, respectively.
The differences observed in the percentages of circulating NK cells (CD3− CD16+) in subcohorts A and B do not suggest that such percentages are a useful immunological indicator for use in follow-up studies of HTLV-1-infected individuals (Table 1).
TABLE 1.
Phenotypic analysis of peripheral blood leukocytes in two subcohorts of HTLV-1-infected individuals
| Subcohort and subgroup (no. of subjects) | Total no. of leukocytes | % of indicated cell typea
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes | NK cells | T lymphocytes | CD4+ T lymphocytes | CD8+ T lymphocytes | CD4+ HLA- DR+ T cells | CD4+ CD38+ T cells | CD8+ CD38+ T cells | ||
| A | |||||||||
| NI (n = 32) | 6,175 ± 1,584 | 37.9 ± 7.1 | 13.0 ± 7.5 | 69.8 ± 8.5 | 43.6 ± 7.3 | 22.7 ± 6.0 | 12.8 ± 5.8 | ND | ND |
| AS (n = 18) | 7,483 ± 1,879 | 35.9 ± 8.2 | 12.5 ± 8.8 | 70 ± 8.5 | 42.6 ± 6.9a | 24.2 ± 5.2 | 20.6 ± 8.0a | ND | ND |
| OL (n = 14) | 7,029 ± 2,535 | 36.6 ± 5.3 | 12.8 ± 9.7 | 74.0 ± 9.0 | 48.9 ± 9.2a | 23.6 ± 4.9 | 33.8 ± 11.8a | ND | ND |
| HT (n = 42) | 6,617 ± 1,865 | 39.1 ± 7.1 | 7.9 ± 4.9a | 78.5 ± 7.2ab | 48.3 ± 10.3ab | 28.6 ± 9.2abc | 40.4 ± 16.8a | ND | ND |
| B | |||||||||
| NI (n = 16) | 6,480 ± 1,759 | 40.8 ± 5.9 | 9.2 ± 6.4 | 69.0 ± 8.4 | 43.7 ± 7.5 | 24.0 ± 5.3 | 12.1 ± 4.9 | 24.4 ± 9.5 | 63.7 ± 18.0 |
| AS (n = 22) | 6,800 ± 1,511 | 36.4 ± 6.6 | 12.2 ± 4.9cd | 69.7 ± 5.9 | 41.2 ± 7.6 | 26.9 ± 5.4 | 13.2 ± 3.3 | 22.6 ± 6.8 | 53.3 ± 10.7 |
| OL (n = 13) | 6,538 ± 1,752 | 40.7 ± 6.9 | 7.1 ± 3.7 | 74.2 ± 6.3 | 48.0 ± 5.9 | 27.4 ± 8.2 | 17.8 ± 10.4 | 22.6 ± 6.4 | 54.5 ± 14.3 |
| HT (n = 12) | 6,954 ± 1,712 | 37.5 ± 8.0 | 8.0 ± 5.7 | 77.2 ± 6.8ab | 52.3 ± 8.5abc | 23.5 ± 7.9 | 35.3 ± 16.2abc | 15.3 ± 3.9 | 54.9 ± 7.3 |
a, b, c, and d, significantly different from values for NI, AS, OL, and HT, respectively, at a P of <0.05. ND, not determined. Results are expressed as mean percentages±standard deviations.
Linkage between the activation status of CD8+ T lymphocytes and the HT clinical form of HTLV-1 infection.
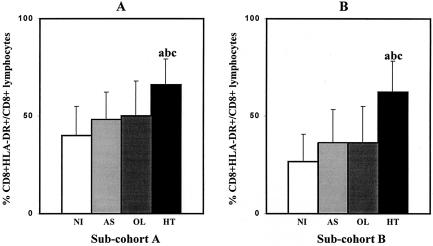
The importance of the CD8+-T-lymphocyte subset in the development and/or maintenance of HT has been documented in several reports (3, 7, 11, 13). It has been shown that activated CD8+ T lymphocytes accumulate in both peripheral blood and cerebrospinal fluid. In the present study, we have performed an analysis of the activation state of circulating CD8+ T lymphocytes in blood samples of HTLV-1-infected individuals (AS, OL, and HT individuals) and NI controls in order to establish a link between the activation state of this relevant lymphocyte subset and disease stage (Fig. 3). A two-color flow cytometry platform was used to identify CD8+ T lymphocytes coexpressing major histocompatibility complex class II molecules (HLA-DR), and the results obtained in subcohort B were compared to those in subcohort A. Interestingly, our data demonstrated that the HT group (P < 0.01) showed the highest level of activated CD8+ T lymphocytes among the four groups evaluated in both subcohorts (Fig. 3). It is relevant to note that the dimension of the differences in this immunological parameter observed between the groups was the same for the two distinct subcohort analyses. Despite the higher levels of CD4+ HLA-DR+ cells observed for HTLV-1-infected individuals in subcohort A than in the NI group, this phenotypic feature was not reproducible in subcohort B, in which the level of CD4+ HLA-DR+ cells enabled us to discriminate the HT group from the other HTLV-1-infected individuals. Therefore, we considered the levels of CD4+ HLA-DR+ cells useless for prognosis purposes (Table 1). In an additional approach, our group has also investigated, by flow cytometry, the intracytoplasmatic cytokine profile in the circulating CD4+ and CD8+ T lymphocytes, monocytes, and neutrophils in the same groups of HTLV-1-infected individuals after short-term stimulation in vitro. In the more recent data analysis, increased percentages of CD8+ gamma interferon-positive T lymphocytes and CD8+ tumor necrosis factor alpha-positive T lymphocytes were observed exclusively in the HT group (data not shown). These results reiterate the importance of the activated CD8+ T lymphocyte as a source of proinflammatory cytokines in the maintenance and/or development of HT (14).
FIG. 3.
Mean percentages of activated CD8+ T lymphocytes in the peripheral blood of different populations of HTLV-1-infected individuals (AS, OL, and HT individuals) and NI controls. (A) Analysis of subcohort A; (B) analysis of subcohort B. Significant differences (P < 0.05) are identified by the letters a, b, and c in comparison to results for NI, AS, and OL groups, respectively.
Patterns of CD18, CD38, and CD62L expression by T-lymphocyte subsets during chronic HTLV-1 infection.
The role of T-cell activation in the pathogenesis of clinical neurological manifestations of HT has been discussed in many reports (4, 19). However, a detailed mechanistic understanding of precise events in the immunopathological progression of the disease remains to be clarified. To obtain additional information regarding the activation status of circulating T lymphocytes observed by the analysis of the activation marker HLA-DR, we have expanded our ex vivo immunophenotyping analysis to other surface molecules involved in T-cell activation, including CD18, CD38, and CD62L.
No differences were observed in the percentages of CD4+ CD38+ and CD8+ CD38+ T cells between the groups evaluated (Table 1). Despite the up-regulation of CD18 on lymphocytes observed for the AS and HT groups in subcohort A, these differences were not observed in subcohort B. Although data from subcohort A suggested that CD18 expression by monocytes and neutrophils should be a good marker for the HT group, the analysis of subcohort B demonstrated that only neutrophils showed a level of expression of CD18 high enough to discriminate the HT group from the other HTLV-1-infected individuals (Table 1). The analysis of the CD62L molecule showed that all the infected individuals showed a down-regulation of this marker on the surfaces of both CD4+ and CD8+ T lymphocytes in comparison to response levels in the NI group (Table 2). As CD38 and CD62L were not investigated in subcohort A, further investigations using a new subcohort should be performed to validate these data. During T-cell activation, several phenotypic changes take place, including the substitution of L-selectin molecules for type 1 and 2 integrins, which has been observed on the surfaces of these activated cells (5, 12, 16, 22). This process is important for the migration of T lymphocytes from the secondary lymphoid organs or peripheral blood to the inflammatory focus. Wu (24), in an investigation of neurological disorders, including multiple sclerosis, HT, and hyperIgEaemic myelitis, demonstrated that HT individuals showed an increase in CD4+ CD62L− T lymphocyte level compared to that in a control group. Moreover, al-Fahim et al. (2) observed a down-regulation of CD62L expression on the surfaces of CD4+ and CD8+ T lymphocytes in individuals with HT. It has been shown that CD45+ RA+, or naïve, T lymphocytes express high levels of CD62L on their surfaces compared to CD45+ RO+ T lymphocytes, known as primed cells. These observations are in agreement with the hypothesis that infection by HTLV-1 produces a chronic activation of the circulating lymphocytes and an increase in the migratory potential of these cells, which might be sufficient to initiate the necessary events to cause inflammation in the CNS, contributing to the development and/or maintenance of the HT clinical form.
TABLE 2.
Analysis of the migration potential of the peripheral blood leukocytes in two subcohorts of HTLV-1-infected individuals
| Subcohort (no. of subjects) | Mean fluorescence channel of indicated cell typea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4+ CD18+ T lymphocytes | CD8+ CD18+ T lymphocytes | CD18+ monocytes | CD18+ neutrophils | CD4+ CD62L+ T lymphocytes | CD8+ CD62L+ T lymphocytes | CD62L+ monocytes | CD62L+ neutrophils | |
| A | ||||||||
| NI (n = 32) | 30.8 ± 10.6 | 49.0 ± 18.9 | 234.9 ± 111.8 | 148.5 ± 67.5 | ND | ND | ND | ND |
| AS (n = 18) | 40.9 ± 17.3a | 66.5 ± 34.7a | 255.7 ± 107.1 | 175.9 ± 90.5 | ND | ND | ND | ND |
| OL (n = 14) | 43.4 ± 13.5a | 62.4 ± 27.4 | 199.4 ± 73.08b | 111.9 ± 64.7b | ND | ND | ND | ND |
| HT (n = 42) | 49.0 ± 48.7a | 79.3 ± 20.8a | 455.2 ± 147.7abc | 338.6 ± 188.6abc | ND | ND | ND | ND |
| B | ||||||||
| NI (n = 16) | 34.7 ± 9.8 | 57.7 ± 21.1 | 217.4 ± 74.4 | 258.9 ± 158.6 | 360.6 ± 54.7 | 296.0 ± 73.4 | 393.7 ± 74.7 | 435.1 ± 99.0 |
| AS (n = 22) | 32.1 ± 18.4 | 61.6 ± 42.1 | 181.7 ± 114.8 | 120.7 ± 87.9a | 275.0 ± 103.7a | 210.9 ± 82.4a | 345.2 ± 84.1 | 368.5 ± 147.3 |
| OL (n = 13) | 33.6 ± 11.8 | 43.5 ± 24.9 | 162.8 ± 84.5 | 108.7 ± 63.4a | 229.3 ± 99.5a | 199.5 ± 83.4a | 326.8 ± 90.8 | 340.3 ± 171.3 |
| HT (n = 12) | 48.4 ± 27.1 | 65.6 ± 32.7 | 307.0 ± 155.9 | 256.7 ± 186.2bc | 260.0 ± 103.2a | 162.3 ± 65.1a | 319.0 ± 88.7 | 330.3 ± 15.4 |
a, b, c, and d, significantly different from values for NI, AS, OL, and HT, respectively, at a P value of <0.05. ND, not determined. Results are expressed as mean fluorescence channels±standard deviations.
Searching for leukocyte phenotypic features applicable as an indicator of HT syndrome in clinical laboratory immunology.
The immunophenotyping data from subcohort B have confirmed that a decreased percentage of B cells, resulting in a greater T cell/B cell ratio, and an increased percentage of CD8+ HLA-DR+ T lymphocytes are important immunological indexes of the HT group in comparison to the immunophenotyping data for all other HTLV-1-infected individuals. Aiming to evaluate this approach as a promising tool for clinical laboratory immunology, we have assessed these phenotypic features to identify cases of HT within HTLV-1-seropositive individuals by using analyses that evaluate chances, such as a likelihood ratio. For this purpose, considering the similarity between the data obtained from subcohorts A and B, the ability of the selected immunological features to discriminate HT patients within HTLV-1-infected individuals was investigated, taking into account the data obtained from both subcohorts. Analysis of likelihood ratios for different ranges of circulating B cells and circulating activated CD8+ HLA-DR+ cells, as well as T cell/B cell ratios, allowed the identification of specific values as the best measures for discriminating HT patients from AS and OL patients (Table 3). A likelihood ratio higher than 10 indicates that there is an outstanding probability of HTLV-1-infected individuals belonging to the HT group if they exhibit <7% B cells, a T cell/B cell ratio of >11, or >70% CD8+ HLA-DR+. Specifically, they will have a 12-, 13-, or 22-times-greater probability of belonging to the HT group than those HTLV-1-infected individuals with these phenotypic features at levels outside of their respective ranges. Longitudinal follow-up studies are in progress to assess the applicability of these immunological indexes as prognostic markers for monitoring HT onset.
TABLE 3.
Ability of the phenotypic features to identify patients with HT within HTLV-1-seropositive individuals
| Parameter | Likelihood ratioa |
|---|---|
| % of B cells < 7% | 13.2 |
| 7% < % of B cells < 13% | 0.8 |
| % of B cells > 13% | 0.4 |
| T cell/B cell ratio < 8 | 0.5 |
| 8 < T cell/B cell ratio < 11 | 1.7 |
| T cell/B cell ratio > 11 | 12.0 |
| % of CD8+ HLA-DR+ T lymphocytes < 30% | 0.0 |
| 30% < % of CD8+ HLA-DR+ T lymphocytes < 70% | 1.1 |
| % of CD8 T lymphocytes > 70% | 22.0 |
A likelihood ratio higher than 10 indicates a high probability of HTLV-1 infected individuals belonging to the HT group, with consideration of the given immunological parameters analyzed. Boldface values indicate significant likelihood ratios (>10).
Conclusions.
Despite a large number of reports regarding the involvement of the immune system in the pathogenesis of HTLV-1-associated disease, which led to a number of hypothetical models of HT pathogenesis, the available biological indexes to evaluate the clinical progression of this disease are still limited. We have been able to identify three promising immunological indexes to discriminate HT patients from other HTLV-1-infected individuals: the percentage of circulating B lymphocytes, the T cell/B cell ratio, and the percentage of circulating activated T lymphocytes within the CD8+ T cells. We are currently considering the application of these phenotypic features of peripheral blood leukocytes in a larger context in association with other clinical and laboratory parameters to further validate the applications of these markers at a population level. Furthermore, we reemphasize that our proposal does not support the use of these immunological indexes as definitive markers of HT but as alternative and additional laboratorial parameters to improve clinical diagnosis of the neurological disorder associated with HTLV-1 infection, just as the enumeration of CD4+ T cells in the peripheral blood of human immunodeficiency virus-infected individuals should not be used itself as a conclusive marker or a given clinical status. It is important to consider that other human pathological conditions may also lead to altered levels of circulating leukocytes. Moreover, further analysis of the performance of these three major immunophenotypic features of peripheral blood leukocytes observed in HT patients allows the identification of HT patients from other HTLV-1-infected individuals. A likelihood ratio analysis indicates that there is an outstanding probability for HTLV-1-infected individuals to belong to the HT group if they exhibit <7% B cells, T cell/B cell ratios of >11, or >70% CD8+ HLA-DR+ cells. Specifically, they will have a 12-, 13-, or 22-times-greater probability, respectively, of belonging to the HT group than those HTLV-1-infected individuals with these phenotypic features at levels outside of their respective ranges. These data confirmed the high association between specific ranges of each immunological parameter and the presence of HT. Therefore, our findings suggest that the monitoring of these immunological parameters, by use of a common flow cytometric technique, would yield the conclusion that there is a short-term prognosis of disease manifestation in currently AS individuals.
Acknowledgments
We thank Jamie Andrew Jacques Pennington and Mark Anthony Beinner for their critical reading of the manuscript.
The Grupo Interdisciplinar de Pesquisas em HTLV is composed of the following researchers: E. F. Barbosa-Stancioli, C. Bonjardim, G. E. A. Brito-Melo, A. B. F. Carneiro-Proietti, B. C. C. Catalan-Soares, D. U. Gonçalves, A. C. Guedes, E. G. Kroon, J. R. Lambertucci, M. L. Martins, O. A. Martins-Filho, J. G. A. C. Reis, V. Nobre, O. M. C. Pfeilstiker, S. R. Pinheiro, F. A. Proietti, J. G. R. Ribas, and G. W. Thorun.
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, the Oswaldo Cruz Foundation, FIOCRUZ, and Fundação HEMOMINAS, Minas Gerais, Brazil.
REFERENCES
- 1.Akizuki, S., O. Nakazato, Y. Higuchi, K. Tanabe, M. Setoguchi, S. Yoshida, S. Yamamoto, S. Sudou, K. Sannomiya, and T. Okajima. 1987. Necropsy findings in HTLV-I associated myelopathy. Lancet i:156-157. [DOI] [PubMed] [Google Scholar]
- 2.al-Fahim, A., P. Cabre, L. Kastrukoff, K. Dorovini-Sis, and J. Oger. 1999. Blood mononuclear cells in patients with HTLV-I-associated myelopathy: lymphocytes are highly activated and adhesion to endothelial cells is increased. Cell. Immunol. 198:1-10. [DOI] [PubMed] [Google Scholar]
- 3.Bieganowska, K., P. Hollsberg, G. J. Buckle, D. G. Lim, T. F. Greten, J. Schneck, J. D. Altman, S. Jacobson, S. L. Ledis, B. Hanchard, J. Chin, O. Morgan, P. A. Roth, and D. A. Hafler. 1999. Direct analysis of viral-specific CD8+ T cells with soluble HLA-A2/Tax11-19 tetramer complexes in patients with human T cell lymphotropic virus-associated myelopathy. J. Immunol. 2:1765-1771. [PubMed] [Google Scholar]
- 4.Brito-Melo, G. E. A., O. A. Martins-Filho, A. B. F. Carneiro-Proietti, B. Catalan-Soares, J. G. Ribas, G. W. Thorum, E. F. Barbosa-Stancioli, and Grupo Interdisciplinar de Pesquisas em HTLV. 2002. Phenotypic study of peripheral blood leucocytes in HTLV-I-infected individuals from Minas Gerais, Brazil. Scand. J. Immunol. 55:621-628. [DOI] [PubMed] [Google Scholar]
- 5.Cabre, P., A. al-Fahim, and J. Oger. 1999. Enhanced adherence of endothelial cells to blood mononuclear cells in HAM/TSP. Rev. Neurol. 155:273-279. [PubMed] [Google Scholar]
- 6.Carneiro-Proietti, A. B. F., J. G. Ribas, B. Catalan-Soares, M. L. Martins, G. E. A. Brito-Melo, O. A. Martins-Filho, S. R. Pinheiro, A. Q. C. Araújo, B. Galvão-Castro, M. S. Pombo de Oliveira, A. C. Guedes, and F. A. Proietti. 2002. Infection and disease caused by human T cell lymphotropic viruses type I and II in Brazil. Rev. Soc. Bras. Med. Trop. 35:499-508. [DOI] [PubMed] [Google Scholar]
- 7.Fujihara, K. 1999. Pathogenetic significance of HTLV-I infection and immune surveillance in HAM. Rinsho Shinkeigaku 39:21-23. [PubMed] [Google Scholar]
- 8.Furukawa, Y., C. R. Bangham, G. P. Taylor, J. N. Weber, and M. Osame. 2000. Frequent reversible membrane damage in peripheral blood B cells in human T cell lymphotropic virus type I (HTLV-I)-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Clin. Exp. Immunol. 120:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. De Thé. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407. [DOI] [PubMed] [Google Scholar]
- 10.Grant, C., K. Barmak, T. Alefantis, J. Yao, S. Jacobson, and B. Wigdahl. 2002. Human T cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J. Cell. Physiol. 190:133-159. [DOI] [PubMed] [Google Scholar]
- 11.Greten, T. F., J. E. Slansky, R. Kubota, S. S. Soldan, E. M. Jaffee, T. P. Leist, D. M. Pardoll, S. Jacobson, and J. P. Schneck. 1998. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8+ T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc. Natl. Acad. Sci. USA 95:7568-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichinose, K., T. Nakamura, Y. Nishiura, K. Nagasato, K. Ohishi, H. Watanabe, A. Fujita, K. Kurouji, M. Tsujihata, and S. Nagataki. 1994. Characterization of adherent T cells to human endothelial cells in patients with HTLV-I-associated myelopathy. J. Neurol. Sci. 122:204-209. [DOI] [PubMed] [Google Scholar]
- 13.Kubota, R., T. Kawanishi, H. Matsubara, A. Manns, and S. Jacobson. 1998. Demonstration of human T lymphotropic virus type I (HTLV-I) tax-specific CD8+ lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by intracellular cytokine detection. J. Immunol. 161:482-488. [PubMed] [Google Scholar]
- 14.Kubota, R., T. Kawanishi, H. Matsubara, A. Manns, and S. Jacobson. 2000. HTLV-I specific IFN-gamma+ CD8+ lymphocytes correlate with the proviral load in peripheral blood of infected individuals. J. Neuroimmunol. 102:208-215. [DOI] [PubMed] [Google Scholar]
- 15.Levin, M. C., and S. Jacobson. 1997. HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP): a chronic progressive neurologic disease associated with immunologically mediated damage to the central nervous system. J. Neurovirol. 3:126-140. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman, J., N. Manjunath, and P. Shankar. 2002. Avoiding the kiss of death: how HIV and other chronic viruses survive. Curr. Opin. Immunol. 14:478-486. [DOI] [PubMed] [Google Scholar]
- 17.Nagai, M., and S. Jacobson. 2001. Immunopathogenesis of human T cell lymphotropic virus type I-associated myelopathy. Curr. Opin. Neurol. 14:381-386. [DOI] [PubMed] [Google Scholar]
- 18.Nagai, M., K. Usuku, W. Matsumoto, D. Kodama, N. Takenouchi, T. Moritoyo, S. Hashiguchi, M. Ichinose, C. R. Bangham, S. Izumo, and M. Osame. 1998. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 4:586-593.10065900 [Google Scholar]
- 19.Osame, M. 2002. Pathological mechanisms of human T-cell lymphotropic virus type-I-associated myelopathy (HAM/TSP). J. Neurovirol. 8:359-364. [DOI] [PubMed] [Google Scholar]
- 20.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popovic, M., M. S. Reitz, Jr., M. G. Sarngadharan, M. Robert-Guroff, V. S. Kalyanaraman, Y. Nakao, I. Miyoshi, J. Minowada, M. Yoshida, Y. Ito, and R. C. Gallo. 1982. The virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus group. Nature 300:63-66. [DOI] [PubMed] [Google Scholar]
- 22.Romero, I. A., M. C. Prevost, E. Perret, P. Adamson, J. Greenwood, P. O. Couraud, and S. Ozden. 2000. Interactions between brain endothelial cells and human T-cell leukemia virus type 1-infected lymphocytes: mechanisms of viral entry into the central nervous system. J. Virol. 74:6021-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonoda, S., H. C. Li, L. Cartier, L. Nunez, and K. Tajima. 2000. Ancient HTLV type 1 provirus DNA of Andean mummy. AIDS Res. Hum. Retrovir. 16:1753-1756. [DOI] [PubMed] [Google Scholar]
- 24.Wu, X. M., M. Osoegawa, K. Yamasaki, Y. Kawano, H. Ochi, I. Horiuchi, M. Minohara, Y. Ohyagi, T. Yamada, and J. I. Kira. 2000. Flow cytometric differentiation of Asian and Western types of multiple sclerosis, HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and hyperIgEaemic myelitis by analyses of memory CD4 positive T cell subsets and NK cells subsets. J. Neurol. Sci. 177:24-31. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida, S., M. Osame, H. Kawai, M. Toita, N. Kuwasaki, Y. Nishida, Y. Hiraki, K. Takahashi, K. Nomura, S. Sonoda, N. Eiraku, and K. Usuku. 1989. Increased replication of HTLV-I in HTLV-I-associated myelopathy. Ann. Neurol. 26:331-335. [DOI] [PubMed] [Google Scholar]