Abstract
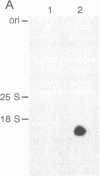
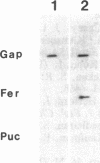
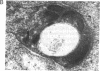
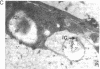
Iron-regulated ferritin synthesis in animals is dominated by translational control of stored mRNA; iron-induced transcription of ferritin genes, when it occurs, changes the subunit composition of ferritin mRNA and protein and is coupled to translational control. Ferritins in plants and animals have evolved from a common progenitor, based on the similarity of protein sequence; however, sequence divergence occurs in the C termini; structure prediction suggests that plant ferritin has the E-helix, which, in horse ferritin, forms a large channel at the tetrameric interface. In contemporary plants, a transit peptide is encoded by ferritin mRNA to target the protein to plastids. Iron-regulated synthesis of ferritin in plants and animals appears to be very different since the 50- to 60-fold increases of ferritin protein, previously observed to be induced by iron in cultured soybean cells, is accompanied by an equivalent accumulation of hybridizable ferritin mRNA and by increased transcription of ferritin genes. Ferritin mRNA from iron-induced cells and the constitutive ferritin mRNA from soybean hypocotyls are identical. The iron-induced protein is translocated normally to plastids. Differences in animal ferritin structure coincide with the various iron storage functions (reserve for iron proteins and detoxification). In contrast, the constancy of structure of soybean ferritin, iron-induced and constitutive, coupled with the potential for vacuolar storage of excess iron in plants suggest that rapid synthesis of ferritin from a stored ferritin mRNA may not be needed in plants for detoxification of iron.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Boyd D., Vecoli C., Belcher D. M., Jain S. K., Drysdale J. W. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem. 1985 Sep 25;260(21):11755–11761. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Daniels-McQueen S., Walden W. E., Patino M. M., Gaffield L., Bielser D., Thach R. E. Requirements for the translational repression of ferritin transcripts in wheat germ extracts by a 90-kDa protein from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13383–13386. [PubMed] [Google Scholar]
- Cairo G., Bardella L., Schiaffonati L., Arosio P., Levi S., Bernelli-Zazzera A. Multiple mechanisms of iron-induced ferritin synthesis in HeLa cells. Biochem Biophys Res Commun. 1985 Nov 27;133(1):314–321. doi: 10.1016/0006-291x(85)91877-7. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Ponce-Ortiz Y., Koch M. H., Parfait R., Stuhrmann H. B. Isolation and characterization of phytoferritin from pea (Pisum sativum) and Lentil (Lens esculenta). Biochem J. 1978 May 1;171(2):349–356. doi: 10.1042/bj1710349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R. Proteins of iron storage and transport. Adv Protein Chem. 1990;40:281–363. doi: 10.1016/s0065-3233(08)60288-0. [DOI] [PubMed] [Google Scholar]
- Dickey L. F., Sreedharan S., Theil E. C., Didsbury J. R., Wang Y. H., Kaufman R. E. Differences in the regulation of messenger RNA for housekeeping and specialized-cell ferritin. A comparison of three distinct ferritin complementary DNAs, the corresponding subunits, and identification of the first processed in amphibia. J Biol Chem. 1987 Jun 5;262(16):7901–7907. [PubMed] [Google Scholar]
- Dickey L. F., Wang Y. H., Shull G. E., Wortman I. A., 3rd, Theil E. C. The importance of the 3'-untranslated region in the translational control of ferritin mRNA. J Biol Chem. 1988 Mar 5;263(7):3071–3074. [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Hagen G., Guilfoyle T. J. Rapid induction of selective transcription by auxins. Mol Cell Biol. 1985 Jun;5(6):1197–1203. doi: 10.1128/mcb.5.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusterspreute M., Crichton R. R. Amino acid sequence of horse spleen apoferritin. FEBS Lett. 1981 Jul 6;129(2):322–327. doi: 10.1016/0014-5793(81)80193-7. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Koeller D. M., Casey J. L., Hentze M. W., Gerhardt E. M., Chan L. N., Klausner R. D., Harford J. B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulhere J. P., Laboure A. M., Briat J. F. Mechanism of the transition from plant ferritin to phytosiderin. J Biol Chem. 1989 Feb 25;264(6):3629–3635. [PubMed] [Google Scholar]
- Laulhere J. P., Lescure A. M., Briat J. F. Purification and characterization of ferritins from maize, pea, and soya bean seeds. Distribution in various pea organs. J Biol Chem. 1988 Jul 25;263(21):10289–10294. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure A. M., Massenet O., Briat J. F. Purification and characterization of an iron-induced ferritin from soybean (Glycine max) cell suspensions. Biochem J. 1990 Nov 15;272(1):147–150. doi: 10.1042/bj2720147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobreaux S., Briat J. F. Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem J. 1991 Mar 1;274(Pt 2):601–606. doi: 10.1042/bj2740601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Martin W., Lagrange T., Li Y. F., Bisanz-Seyer C., Mache R. Hypothesis for the evolutionary origin of the chloroplast ribosomal protein L21 of spinach. Curr Genet. 1990 Dec;18(6):553–556. doi: 10.1007/BF00327027. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Proudhon D., Briat J. F., Lescure A. M. Iron induction of ferritin synthesis in soybean cell suspensions. Plant Physiol. 1989 Jun;90(2):586–590. doi: 10.1104/pp.90.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland M., Briat J. F., Gagnon J., Laulhere J. P., Massenet O., Theil E. C. Evidence for conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J Biol Chem. 1990 Oct 25;265(30):18339–18344. [PubMed] [Google Scholar]
- Raguzzi F., Lesuisse E., Crichton R. R. Iron storage in Saccharomyces cerevisiae. FEBS Lett. 1988 Apr 11;231(1):253–258. doi: 10.1016/0014-5793(88)80742-7. [DOI] [PubMed] [Google Scholar]
- Rothenberger S., Müllner E. W., Kühn L. C. The mRNA-binding protein which controls ferritin and transferrin receptor expression is conserved during evolution. Nucleic Acids Res. 1990 Mar 11;18(5):1175–1179. doi: 10.1093/nar/18.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Haile D. J., Harford J. B., Klausner R. D. The iron-responsive element binding protein: a method for the affinity purification of a regulatory RNA-binding protein. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5768–5772. doi: 10.1073/pnas.86.15.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., Klausner R. D. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7958–7962. doi: 10.1073/pnas.87.20.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczekan S. R., Joshi J. G. Isolation and characterization of ferritin from soyabeans (Glycine max). J Biol Chem. 1987 Oct 5;262(28):13780–13788. [PubMed] [Google Scholar]
- Seckbach J. Remarks on ferritin from iron loaded plants. Planta Med. 1972 May;21(3):267–273. doi: 10.1055/s-0028-1099551. [DOI] [PubMed] [Google Scholar]
- Seckbach J. Studies on the deposition of plant ferritin as influenced by iron supply to iron-deficient beans. J Ultrastruct Res. 1968 Mar;22(5):413–423. doi: 10.1016/s0022-5320(68)90031-2. [DOI] [PubMed] [Google Scholar]
- Shih M. C., Goodman H. M. Differential light regulated expression of nuclear genes encoding chloroplast and cytosolic glyceraldehyde-3-phosphate dehydrogenase in Nicotiana tabacum. EMBO J. 1988 Apr;7(4):893–898. doi: 10.1002/j.1460-2075.1988.tb02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullfrog. J Biol Chem. 1982 Dec 10;257(23):14187–14191. [PubMed] [Google Scholar]
- Stevens P. W., Dodgson J. B., Engel J. D. Structure and expression of the chicken ferritin H-subunit gene. Mol Cell Biol. 1987 May;7(5):1751–1758. doi: 10.1128/mcb.7.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Theil E. C. The ferritin family of iron storage proteins. Adv Enzymol Relat Areas Mol Biol. 1990;63:421–449. doi: 10.1002/9780470123096.ch7. [DOI] [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Walden W. E., Thach R. E. Translational control of gene expression in a normal fibroblast. Characterization of a subclass of mRNAs with unusual kinetic properties. Biochemistry. 1986 Apr 22;25(8):2033–2041. doi: 10.1021/bi00356a030. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Sczekan S. R., Theil E. C. Structure of the 5' untranslated regulatory region of ferritin mRNA studied in solution. Nucleic Acids Res. 1990 Aug 11;18(15):4463–4468. doi: 10.1093/nar/18.15.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K., Munro H. N. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988 Jun 25;263(18):8938–8942. [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark F., Bienfait F., van den Ende H. Variable amounts of translatable ferritin mRNA in bean leaves with various iron contents. Biochem Biophys Res Commun. 1983 Sep 15;115(2):463–469. doi: 10.1016/s0006-291x(83)80167-3. [DOI] [PubMed] [Google Scholar]
- van der Mark F., van den Briel W., Huisman H. G. Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Studies on the synthesis and the uptake in vitro of the precursors of ferritin and ferredoxin by intact chloroplasts. Biochem J. 1983 Sep 15;214(3):943–950. doi: 10.1042/bj2140943. [DOI] [PMC free article] [PubMed] [Google Scholar]