Abstract
Numerous beneficial effects have been attributed to probiotic lactic acid bacteria (LAB), such as the stimulation of the immune system, the prevention of enteric infections by enteropathogens, and the regression of immunodependent tumors. It has been shown that biologically active metabolites released during fermentation, in particular biopeptides, could act as immunomodulatory agents. However, no studies have been conducted to evaluate the implication of these bioactive peptides in the induction of a protective immune response against enteric infections. The present study aimed to evaluate the possible immunomodulatory and anti-infectious effects of a peptidic fraction released in milk fermented by Lactobacillus helveticus. The immune response in the mucosa-associated lymphoid tissue was monitored following an administration of the potentially bioactive peptidic fraction. The total immunoglobulin A (IgA) immune response was evaluated after an Escherichia coli O157:H7 infection in a BALB/c murine model. Immunohistochemical and enzyme-linked immunosorbent assays revealed an increase in the number of IgA-secreting B lymphocytes in the intestinal lamina propria and an enhanced total secretory and systemic IgA response. Cytokine profiling also revealed stimulation of a Th2 response in mice fed the peptidic fraction, whereas infected controls demonstrated a proinflammatory Th1 response. These results indicate that bioactive peptides released during fermentation by LAB could contribute to the known immunomodulatory effects of probiotic bacteria.
Since its first documented outbreak in 1982 (62), enterohemorrhagic Escherichia coli O157:H7 has been recognized as an emerging foodborne pathogen. Although various pathogenic serotypes exist (43), E. coli O157:H7 has been the most frequently isolated in North America (50). Pathogenesis of E. coli O157:H7 is linked to numerous virulent factors (24), leading to pathological conditions such as hemorrhagic colitis, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, acute renal failure, and even death (6). E. coli O157:H7 is considered a worldwide threat not only because of its increasing incidence and low infectious dose, but also due to the severity of clinical presentation and complications during treatment, particularly with the controversial role of antibiotics (12). Recent studies have explored alternative therapeutic strategies, such as the use of probiotic lactic acid bacteria (LAB).
In accordance with Metchnikoff's theory of the prolongation of life by lactobacilli in yogurt (39), probiotic LAB have shown numerous strain-dependant beneficial roles in the protection of host organisms against a wide variety of enteropathogens, including Salmonella enterica serovar Typhimurium (18), Clostridium difficile (2), Listeria monocytogenes (11), and even E. coli O157:H7 (20, 49, 65). Terms such as colonization resistance (68), competitive exclusion (29), and immunomodulation (53-58, 69) have been used to describe mechanisms in which live bacteria could prevent bacterial infections.
Milks fermented by LAB have previously been shown to enhance both specific and nonspecific immune responses. Though most related studies focus on the administration of live bacteria, there is a lack of recognition of the possible immunomodulatory role of the bioactive peptides or other compounds released in the culture medium during fermentation with LAB. Indeed, many beneficial effects have been attributed to bioactive peptides derived from milk, including opiate activity, antimicrobial activity, antihypertension, antithrombotic activity, and immunomodulation (8, 35, 37, 64).
Cell-free supernatants have been used to study the possible role of bioactive compounds released during milk fermentation. Laffineur et al. (22) reported that cell-free supernatants of Lactobacillus helveticus-fermented β-casein-enriched medium modulated lymphocyte proliferation in vitro. In parallel, Ng and Griffiths (47) used cultured macrophages to demonstrate that cell-free supernatants of L. helveticus-fermented milks exhibit higher interleukin-6 (IL-6) production than with lipopolysaccharide alone. More recently, peptidic fractions of cell-free supernatants of L. helveticus-fermented milks have been shown to significantly reduce fibrosarcoma in vivo (25). However, cell-free supernatants of L. helveticus-fermented milks have not yet been implicated in the prevention or attenuation of bacterial infections in vivo. In the present study, a BALB/c murine model was used to examine the role of an L. helveticus-fermented milk supernatant fraction on the total immunoglobulin A (IgA) response following an E. coli O157:H7 infection. Immunohistochemical and double antibody sandwich-enzyme-linked immunosorbent assay (DAS-ELISA) techniques have demonstrated that a cell-free peptidic fraction of L. helveticus-fermented milk could enhance both the total humoral and systemic IgA responses.
MATERIALS AND METHODS
Bacterial strains.
L. helveticus R389 (33) was maintained in BBL MRS broth medium for lactobacilli (Becton Dickinson, Cockeysville, Md.) and grown to stationary phase at 37°C for 17 h. Lactobacillus growth was determined by counting CFU after plating serial dilutions on MRS agar (Becton Dickinson) and incubation at 37°C for 48 h. Enterohemorrhagic E. coli O157:H7 (ATCC 35150) was grown with agitation (100 rpm) in Difco tryptic soy broth (Difco Laboratories, Detroit, Mich.) at 37°C for 7 h using 2% of an overnight culture and resuspended in sterile phosphate-buffered saline (PBS) to the desired concentration of 1010 CFU/ml.
Milk fermentation.
Milk fermentation was achieved by methods described by LeBlanc et al., (25). Nonfat, dried, low-heat-grade, non-vitamin A- and D-added milk (Dairytown Products Ltd., Sussex, New Brunswick, Canada) was rehydrated (12% [wt/vol]) and then autoclaved at 121°C for 15 min (Sanyo Vertical Labo autoclave; NB Scientific, Edison, N.J.). The milk was inoculated (2% [vol/vol]) with an overnight culture of L. helveticus R389 containing 108 to 109 CFU/ml and incubated at 37°C for 24 h. The inoculum was then added (2% [vol/vol]) to 2 liters of rehydrated milk (12% [wt/vol]) to start the milk fermentation. Fermentation was achieved using a Bioflow 3000 Biofermentor (NB Scientific) at 37°C with an agitation rate of 100 rpm and CO2 spurging (10 lb/in2; 0.2 liters/min); pH was buffered to 6.00 by automatic addition of 8 M NaOH as required. Samples were obtained under sterile conditions after 0, 6, 12, and 24 h to verify L. helveticus growth. The extent of milk protein proteolysis was evaluated using the o-phtaldialdehyde method developed by Church et al. (7) (data not shown).
Size-exclusion HPLC.
Protein-peptide fractions were obtained by size-exclusion high-performance liquid chromatography (HPLC) with an HP1100 HPLC system (Agilent Technologies). Milk samples were prepared prior to HPLC injection by centrifugation at 6,000 × g for 20 min at 4°C (Micromax RF; IEC). The supernatant was filtered using a 0.22-μm Millex-GP syringe filter (Millipore) and maintained at 4°C until injection. A 200-μl aliquot of the sample was loaded on an LKB TSK-G2000SW gel filtration column (600 by 7.5 mm; TosoHaas) using 5 mM ammonium acetate (pH 6.5) (Fisher Scientific) as the elution buffer prepared as described previously (26). Protein-peptides were eluted with a flow rate of 0.7 ml/min and monitored at 214 nm by using a HP1100 diode array detector. Fractions were selected based on elution time and were named fractions I, II, and III (see Fig. 2B, below) for large, medium, and small peptides, respectively (25). Fractions were collected with a Gilson FC104 fraction collector and then pooled and concentrated using an automatic environmental SpeedVac system (Savant) and stored at 4°C for later use. Quantification of protein-peptide fractions was evaluated with the Bradford assay (3) (Bio-Rad Laboratories, Hercules, Calif.).
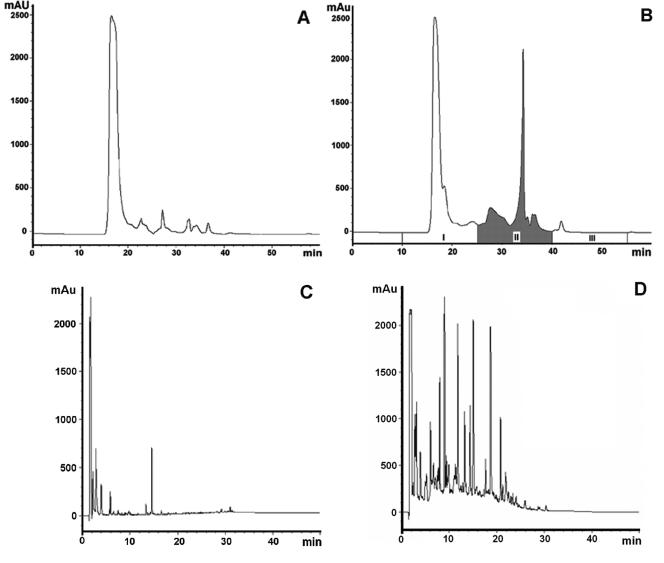
FIG. 2.
HPLC analysis of fermented and unfermented milk. (A and B) Size-exclusion HPLC profiles of unfermented milk (A) and milk fermented with L. helveticus R389 at 37°C for 24 h at pH 6.0 (B). In panel B, the peptidic fraction analyzed in this study (fraction II) is shaded. (C and D) RP-HPLC profiles of fraction II (D) and the respective control (C). Absorbance was monitored at 214 nm, representing the peptide bond.
RP-HPLC.
To confirm the liberation of numerous peptides in fraction II, reverse-phase HPLC (RP-HPLC) was performed as described previously (33) using a 3.5-μm Zorbaz SB-C18 column (4.6 by 150 mm; Agilent Technologies). Briefly, the column was equilibrated with solvent A (0.115% trifluoroacetic acid) at a flow rate of 1 ml/min, and peptides were eluted with solvent B (60% acetonitrile in 0.1% trifluoroacetic acid) as follows: 0 to 30 min, 0 to 60% B; 30 to 35 min, 60 to 100% B; 35 to 42 min, 100 to 0% B. As a control, nonfermented milk was analyzed under same conditions. Eluted peptides were monitored at 214 nm.
Immune system stimulation studies.
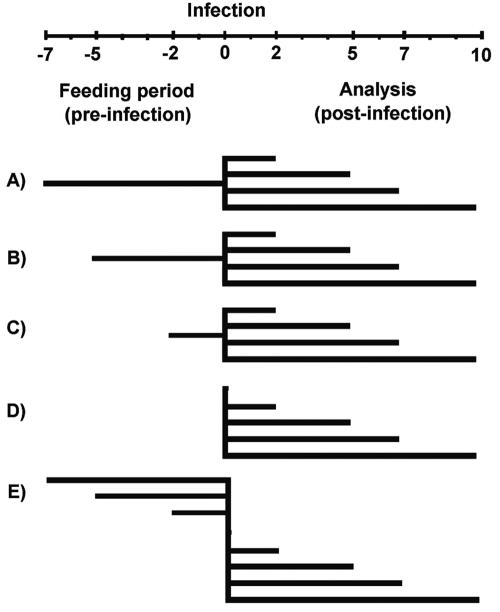
The immunomodulatory effect of the peptidic fraction was determined using the in vivo model developed by Perdigon et al. (54). All animal procedures were performed or supervised by qualified technicians following standard operation methods with respect to the Canadian Council on Animal Care and the Comité de protection des animaux de l'Université Laval (www.ssp.ulaval.ca/Da). Throughout the experiment, animals were fed ad libitum water and standard rodent chow (Charles River Laboratories, Wilmington, Mass.), and feed and litter were replaced daily. All experiments were performed under controlled conditions (temperature [21 ± 2°C], humidity, and a 12-h light-dark cycle). After an acclimatization period of 48 h, 100 female BALB/c mice (Charles River Laboratories) weighing 18 to 20 g (6 to 8 weeks old) were assigned randomly to groups corresponding to three different periods of feeding (7, 5, or 2 days preinfection [groups A, B, and C, respectively]). Each group was also divided in five subgroups corresponding to the day of sacrifice postinfection (days 2, 5, 7, and 10). Infected controls did not receive the peptidic fraction preinfection (group D). To assess the effect of the peptidic fraction on the day of the infection, the noninfected control group received the feedings for 7, 5, and 2 days preinfection and were sacrificed on day 0 (group E). Noninfected controls also consisted of mice that had not received the peptide fraction preinfection and that were sacrificed on days 2, 5, 7, and 10 postinfection. Figure 1 presents a summary of the animal model.
FIG. 1.
Schematic representation of the animal model. Mice were fed peptidic fraction II for 7 (A), 5 (B), and 2 (C) days preinfection, as described in the time chart. On day zero, groups A to D were challenged with 1010 CFU of E. coli O157:H7/ml. Groups D and E represent the infected and noninfected controls, respectively. Analysis was performed on days 0, 2, 5, 7, and 10 postinfection.
Mice were fed by gavage, using 20-gauge by 38-mm malleable animal feeding needles (Poper & Sons, Inc.), 50 μg of the peptidic fraction II/day (25) (or sterile water for the control groups) for the duration of the feeding period (2, 5, or 7 days). After feeding periods, mice were challenged intragastrically with 0.5 ml of E. coli O157:H7 suspension at 1010 CFU/ml or PBS for noninfected controls. This dose was needed to observe pathological symptoms, such as weight loss, in mice. Following challenge, total body weight was assessed daily. On days 2, 5, 7, and 10 postinfection, mice were anesthetized with IsoFlo isofluorane (Abbott Laboratories Ltd., St.-Laurent, Quebec, Canada). Approximately 0.5 ml of blood was withdrawn via cardiac puncture, and mice were subsequently sacrificed by cervical dislocation. Blood was left to coagulate overnight at 4°C and centrifuged (1,000 × g for 15 min at 4°C), and serum was stored at −20°C. The small intestine from each mouse was recovered (by truncation at the stomach-duodenum junction and the ileum-ascending colon junction), its contents were flushed with 5 ml of PBS, and particulate material was removed by centrifugation (10,000 × g for 10 min at 4°C). The remaining supernatant fluid was stored in triplicate at −20°C. Both serum and intestinal fluid were used to measure the mucosal IgA response following E. coli O157:H7 infection. Intestinal tissues were prepared in triplicate for histological evaluation using standard paraffin-embedding methods (63).
Immunofluorescence assay.
The number of cells secreting IgA was determined by direct immunofluorescence assay as described previously (63). For each intestinal tissue, 10 microscope slides were prepared with 4-μm serial paraffin sections. Slides were incubated with a 1/50 dilution of α-chain monospecific antibody conjugated with fluorescein isothiocyanate (Sigma, St. Louis, Mo.) for 30 min and observed by using a Hund H600 fluorescence light microscope. Results were expressed as the number of fluorescent cells counted in 10 fields of vision at 1,000× magnification.
DAS-ELISA.
Total IgA antibodies were detected by standard DAS-ELISA. Briefly, affinity-purified monoclonal goat anti-IgA (α-chain specific) was added at 1.25 μg/well in 0.05 M carbonate-bicarbonate buffer (pH 9.6) to Costar 96-well, U-bottomed, high-binding polystyrene microplates (Corning Inc.) and incubated at 37°C for 1 h. The plates were then washed three times using an ELX405 Auto plate washer (Bio-Tek Instruments, Inc., Winooski, Vt.) with PBS containing 0.05% Tween 20 (PBS-T) and blocked for 1 h at 25°C with 0.5% nonfat dry milk in PBS. Plates were washed five times with PBS-T and incubated for 2 h at 37°C with either 50 μl of standard kappa IgA or 50-μl samples of intestinal fluid or serum (diluted 1/4,000 and 1/20,000 in blocking solution, respectively), both of which were added in triplicate. Plates were washed seven times with PBS-T and incubated in the presence of horseradish peroxidase-conjugated anti-IgA-specific antibodies at 1.25 μg/well for 1 h at 37°C. Plates were again washed seven times, and 100 μl of trimethylbenzidine reagent containing peroxide (BD Biosciences, Mississauga, Ontario, Canada) was added to each well. Reactions were terminated with 100 μl of H2SO4 (2 N) with gentle shaking. The optical density was read at 450 nm by using a μQuant automatic microplate reader (Bio-Tek Instruments). For the IgA-specific DAS-ELISA, all antibodies, standards, and buffers were purchased from Sigma Chemical Co.
Cytokine production.
Serum IL-4 and gamma interferon (IFN-γ) levels were evaluated with mouse OptEIA ELISA sets (BD Biosciences, San Diego, Calif.) following the instructions provided by the manufacturer.
Statistical analysis.
Results were expressed as the mean ± standard deviation, and data were subjected to analysis of variance with SPSS software version 11.5 (SPSS Inc., Chicago, Ill.). Tukey's multiple-range test was used to compare the least square means of all treatments. A P value of <0.05 was considered significantly different.
RESULTS
Milk fermentation.
Size-exclusion and RP-HPLC elution profiles confirmed the proteolysis of milk proteins during fermentation by L. helveticus (Fig. 2).
As observed previously (25), Fig. 2B revealed the appearance of small oligopeptides and peptides ranging from 2 to 10 kDa derived from larger milk proteins (compare Fig. 2A and B). RP-HPLC analysis indicated that the peptidic fraction used in this study (Fig. 2B) was effectively composed of several peptides that were not observed in the control (Fig. 2C and D).
Postchallenge body weight.
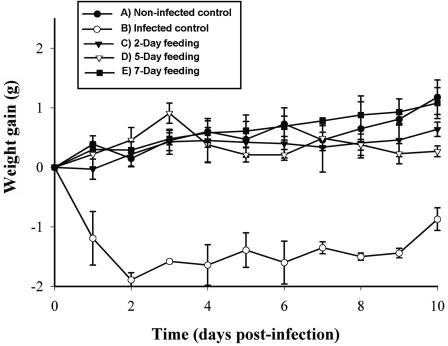
Initial body weights were subtracted from subsequent values. It is noteworthy that no differences in initial mean body weight were detected (17.70 ± 1.03, 19.13 ± 0.62, 19.38 ± 0.82, 19.22 ± 1.46, and 18.11 ± 0.18 g for the groups fed for 2, 5 and 7 days preinfection and the infected and noninfected controls, respectively). Following challenge with E. coli O157:H7, the infected control showed a significant loss in body weight, particularly on days 1 and 2 postinfection (Fig. 3).
FIG. 3.
Mean (± standard deviation) weight gain for mice challenged with E. coli O157:H7 (n = 4). Results are expressed as the cumulative weight change relative to day zero weight.
There were no significant differences observed between mice receiving any of the peptide regimens (2, 5, and 7 days preinfection) and the noninfected control; therefore, all regimens of peptide feeding prevented the weight loss caused by the E. coli O157:H7 challenge.
Effect of the peptidic fraction on the number of IgA+ B cells in the intestinal lamina propria.
As shown in Fig. 4, the peptidic fraction significantly altered the B-lymphocyte response to E. coli O157:H7 infection after all feeding periods (days 2, 5, and 7).
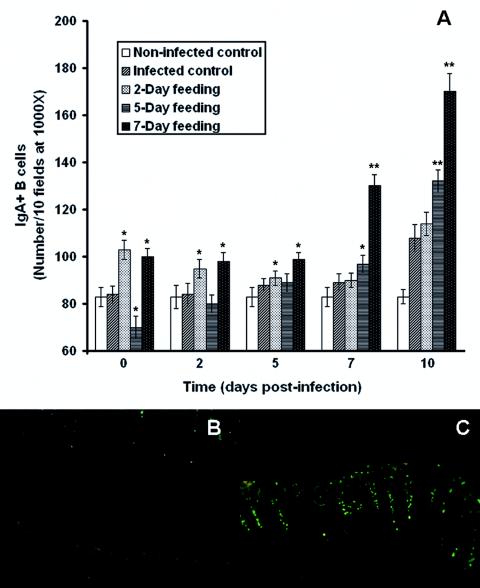
FIG. 4.
(A) Effect of consumption of a peptidic fraction derived from L. helveticus-fermented milk on the intestinal lamina propria IgA+ B cells of mice infected with E. coli O157:H7. Values are means for n = 4 (± standard deviations), and significance was determined by comparison to the infected control (*, P < 0.05; **, P < 0.01). (B and C) Images of IgA+ B cells in the small intestine on day 10 postinfection of the infected control (B) and mice that received the 7-day feeding (C). Magnification, ×100.
The infected control did not differ in the number of IgA-producing B lymphocytes compared to the noninfected control (83 ± 4 B cells/10 fields of vision at a magnification of ×1,000) on days 0 and 2 postinfection (84 ± 4 and 84 ± 5, respectively); nonetheless, on the 5th, 7th, and 10th days postinfection, numbers reached significant levels (88 ± 3, 89 ± 4, and 108 ± 6, respectively). In parallel with infected controls, a 2-day feeding period with the peptidic fraction (preinfection) demonstrated a significant increase in the number of IgA+ B cells in the intestinal lamina propria on days 0 (103 ± 4) and 2 (95 ± 4) postinfection; however, numbers dropped after 5, 7, and 10 days (91 ± 3, 90 ± 3, and 114 ± 5, respectively). A 5-day feeding preinfection showed an increase in the number of IgA+ B lymphocytes to significant levels on the 7th and 10th days postinfection (97 ± 4 and 132 ± 5, respectively); however, no significant changes were observed 2 or 5 days following infection (80 ± 4 and 89 ± 4, respectively). Moreover, on day 0, a significant decrease (70 ± 5) was also observed. For all days following E. coli O157:H7 challenge (0, 2, 5, 7, and 10), the 7-day feeding period induced a strong increase in the number of IgA+ B cells (100 ± 4, 98 ± 4, 99 ± 3, 130 ± 5, and 170 ± 8, respectively).
Effect of the peptidic fraction on total intestinal IgA secretion.
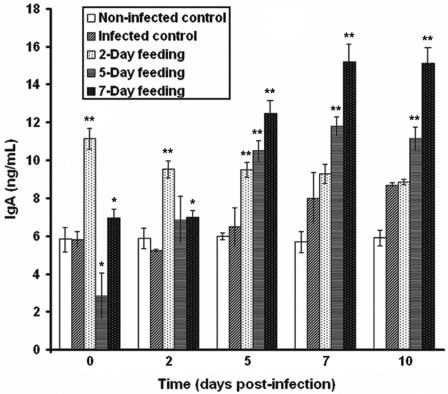
In parallel with the immunofluorescence assay, an IgA-specific DAS-ELISA confirmed that the altered B-lymphocyte response to E. coli O157:H7 infection was caused by the peptidic fraction derived from L. helveticus-fermented milk. In comparison to the number of IgA+ B cells in the intestinal lamina propria (Fig. 4), similar patterns were observed in IgA content in the intestinal fluid (Fig. 5).
FIG. 5.
Effect of consumption of a peptidic fraction derived from L. helveticus-fermented milk on total IgA in the intestinal secretions of mice infected with E. coli O157:H7. Values are means for n = 4 (± standard deviations), and significance compared to the values for the infected control is shown (*, P < 0.05; **, P < 0.01).
In comparison to the noninfected control (5.84 ± 0.68 μg/ml), the infected control did not differ in IgA secretion on days 0 and 2 postinfection (5.83 ± 0.46 and 5.24 ± 0.13 μg/ml, respectively); nonetheless, on the 5th, 7th, and 10th days postinfection, IgA production reached significant levels (6.51 ± 1.04, 8.01 ± 1.35, and 8.70 ± 0.14 μg/ml, respectively). In comparison to the infected controls, a 2-day feeding period with the peptidic fraction (preinfection) led to a significant increase in IgA production on days 0 (11.14 ± 0.58 μg/ml), 2 (9.52 ± 0.46 μg/ml), and 5 (9.49 ± 0.41 μg/ml) postinfection; however, the antibody response did not significantly differ from that in the infected control after 7 and 10 days (9.27 ± 0.55 and 8.85 ± 0.18 μg/ml, respectively). Although IgA levels showed a significant increase on days 5, 7, and 10 postinfection (10.50 ± 0.57, 11.80 ± 0.51, and 11.12 ± 0.68 μg/ml, respectively), a significant decrease in total IgA was observed on day 0 (2.85 ± 1.26 μg/ml) and values were not significantly different on day 2 (6.87 ± 1.26). On the other hand, for all days of analysis postinfection (0, 2, 5, 7, and 10), a strong IgA response was observed, with the results for the 7-day feeding period reaching concentrations of 6.97 ± 0.52, 6.99 ± 0.41, 12.46 ± 0.72, 15.19 ± 0.96, and 15.14 ± 0.85 μg/ml, respectively. The results seem to confirm those observed in the immunofluorescence study (compare Fig. 4 and 5).
Effect of the peptidic fraction on total serum IgA.
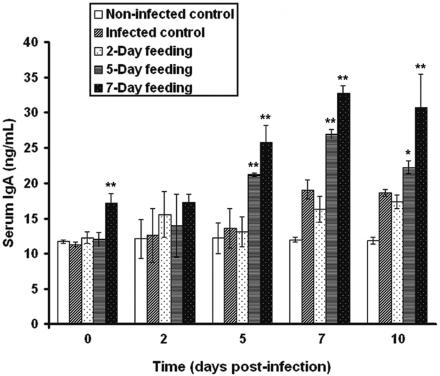
In order to determine if the peptidic fraction increased the systemic immune response to E. coli O157:H7, total serum IgA was evaluated by DAS-ELISA (Fig. 6).
FIG. 6.
Effect of consumption of a peptidic fraction derived from L. helveticus-fermented milk on total serum IgA response of mice infected with E. coli O157:H7. Values are means for n = 4 (± standard deviations), and significance compared to the values of the infected control is shown (*, P < 0.05; **, P < 0.01).
In comparison with the noninfected control (11.70 ± 0.22 μg/ml), the infected control did not differ in total serum IgA on days 0, 2, and 5 postinfection (11.27 ± 0.34, 12.61 ± 3.83, and 13.62 ± 2.80 μg/ml, respectively); however, IgA levels differed significantly on the 7th and 10th days postinfection (19.07 ± 1.30 and 18.67 ± 0.49 μg/ml, respectively). A 2-day feeding period with the peptidic fraction preinfection showed no apparent differences in total serum IgA (12.27 ± 0.86, 15.58 ± 3.22, 13.11 ± 2.12, 16.29 ± 1.82, and 17.38 ± 1.00 μg/ml, respectively) relative to the response in infected controls. A 5-day gavage preinfection did not significantly alter serum IgA on days 0 (12.02 ± 1.01 μg/ml) and 2 (13.98 ± 4.47 μg/ml) postinfection; however, this group demonstrated increases on days 5, 7, and 10 postinfection (21.12 ± 0.23, 26.90 ± 0.69, and 22.18 ± 0.88 μg/ml, respectively). For all days of sampling, the group previously fed the peptidic fraction for 7 days showed a significant increase in serum IgA (17.21 ± 1.31, 17.29 ± 1.16, 25.73 ± 2.47, 32.76 ± 1.06, and 30.72 ± 4.72 μg/ml, respectively).
Cytokine production.
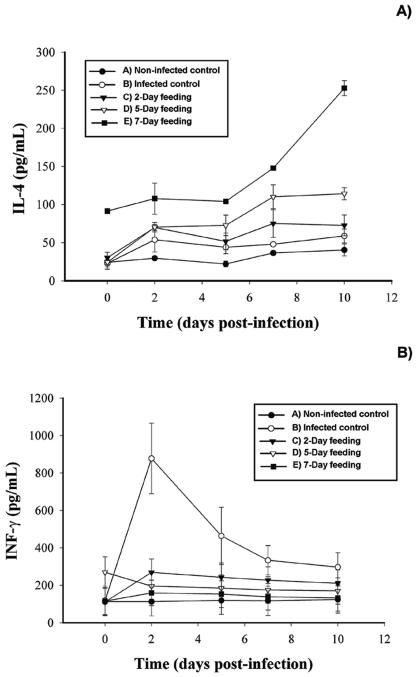
In order to determine possible mechanisms implicated in the enhanced IgA immune response to E. coli O157:H7, serum IL-4 and IFN-γ levels were determined by DAS-ELISA (Fig. 7).
FIG. 7.
Influence of consumption of a peptidic fraction derived from L. helveticus-fermented milk on serum IL-4 (A) and IFN-γ (B) following infection with E. coli O157:H7. Values are means for n = 4 (± standard deviations), and significance was determined for a P level of <0.01 compared to infected controls.
As illustrated in Fig. 7A, a remarkable increase in IL-4 was observed throughout the experiment for the group that had received a 7-day gavage of the peptidic fraction preinfection. Interestingly, the IL-4 production was also significantly elevated on the day of challenge compared to responses in the other groups. A 5-day feeding period resulted in significantly increased IL-4 production on days 2, 5, 7, and 10 postinfection; however, IL-4 levels did not differ from those in other groups on day 0. The group that received a 2-day feeding showed a slight increase in IL-4 production on day 2 postinfection, but IL-4 concentrations did not differ from those in the noninfected controls on days 5, 7, and 10 postinfection. No apparent differences were observed between the infected and noninfected controls.
As shown in Fig. 7B, increased IFN-γ levels were observed in the infected controls, particularly on day 2 postinfection. Although IFN-γ levels in this group decreased on days 5, 7, and 10 postinfection, they remained significantly higher than in other groups. The group fed the peptidic fraction for 2 days preinfection showed a slight increase in IFN-γ production during infection. However, IFN-γ levels were significantly lower than those of the infected control. A 5-day feeding period preinfection demonstrated high levels of IFN-γ on day 0 but dropped to statistically insignificant amounts for the remainder of the experiment. Remarkably, the 7-day feeding period showed no significant difference from the noninfected control on all days of analysis.
DISCUSSION
This study dealt with the effects of peptides derived from fermented milk on the enhancement of the immune response following an E. coli O157:H7 challenge. The immunopotentiating effect of these peptides was analyzed in vivo by oral administration to BALB/c mice during 2, 5, and 7 days prior to infection with E. coli O157:H7. The peptidic fraction (Fig. 2B) resulted from extensive proteolysis by L. helveticus and was been isolated by size-exclusion HPLC. LAB such as L. helveticus possess proteases and peptidases that permit milk protein degradation (33, 37), yet their specific activities differ markedly between bacterial species (16) and strains (14, 15, 23, 41, 72). Consequently, different peptide profiles may be released (34). A better hydrolysis of milk proteins by LAB can increase the yield of bioactive peptides (15, 33, 34, 36, 41). Immunologically active peptides have been obtained from both the casein and whey fractions of human and bovine milk (8, 10, 17, 30, 71).
Size-exclusion and RP-HPLC profiles illustrated that milk proteins were effectively proteolysed by L. helveticus (Fig. 2). Fermentation of milk by L. helveticus increased the yield of small protein-derived compounds, shown by the appearance of novel peaks after milk fermentation that were not present beforehand. This is consistent with the observations made by LeBlanc et al. (25) and Matar et al. (33). In comparison with other LAB, L. helveticus is known to be a highly proteolytic bacterium (41). Previous studies have shown that cell-free supernatants of L. helveticus-fermented casein hydrolysates could induce immunomodulatory responses that were not observed in media supernatants or by the bacterium itself (47). It should also be noted that previous results showed that a nonproteolytic mutant of L. helveticus did not demonstrate immunomodulatory responses, even when cultured in milk (36). Since the proteolysis increases during fermentation, it has been postulated that the immunomodulatory effect could be attributed to peptides released during fermentation (25, 33). It should also be noted that the peptidic fraction used in our study has previously been shown to be immunomodulatory (25). This fraction is highly enriched with peptides (from 2 to 10 kDa) resulting from protein degradation, and their concentration (0.8 to 3.0 mg/ml) exceeds that of any secondary metabolites or bacterial components whose concentrations in the final product are minute in comparison to that of the peptides. The peptide profiles for the fraction mentioned above and a nonfermented control are reported in the RP-HPLC chromatogram (Fig. 2C and D).
Recent theories suggest that bacterial cleavage of peptide bonds within milk proteins could promote the unfolding of these molecules, facilitating the release of bioactive peptides (34). Supporting this theory, Sütas et al. (66) demonstrated the liberation of immunomodulatory peptides in Lactobacillus casei GG-fermented milk that were not released with the digestive enzymes pepsin and trypsin. Other protein hydrolysates have been shown to have an activity towards immune cells. Migliore-Samour et al. (40) have isolated biologically active casein peptides implicated in immunomodulation. Sütas et al. (66) have demonstrated that the proteolysis of κ-caseins by Lactobacillus rhamnosus GG, combined with pepsin and trypsin hydrolysis, could stimulate lymphocyte responses in vitro. Recently, Low et al. (30) reported that when BALB/c mice were fed a whey protein concentrate, they produced elevated levels of specific intestinal tract and serum antibodies against orally and parenterally administered antigens. Peptides from L. helveticus-fermented milk have also been shown to increase the number of IgA-secreting B cells and decrease the growth of fibrosarcomas (25). In the present study, a peptidic fraction derived from L. helveticus-fermented milk supernatants was evaluated for its capacity to prime immune system parameters in BALB/c mice following an E. coli O157:H7 challenge.
First, total IgA levels in the intestinal fluid and serum were analyzed to better assess the effect of the peptidic fraction on the induction of an IgA antiadherence barrier against E. coli O157:H7, a concept referred to as immune exclusion. The immunofluorescence assay and IgA-specific DAS-ELISA revealed that various feeding durations with the peptidic fraction modulated not only the number of IgA-producing B lymphocytes in the intestinal lamina propria following E. coli O157:H7 challenge (Fig. 4), but also enhanced the secretory (Fig. 5) and the systemic (Fig. 6) immune responses. Indeed, mice fed the peptidic fraction for 2 days preinfection demonstrated a slight increase in the number of IgA+ B cells in the intestinal lamina propria on days 0 and 2 postinfection. However, the 2-day feeding period seemed insufficient to enhance the local and systemic IgA response after 5, 7, and 10 days. A 5-day or, to a higher extent, a 7-day feeding showed a strong increase in the number of IgA+ B lymphocytes and total intestinal and serum IgA, particularly on the 7th and 10th days postinfection. These results suggest that peptides derived from L. helveticus-fermented milk are capable of enhancing total secretory and systemic IgA responses following an E. coli O157:H7 challenge. Interestingly, a 5-day feeding period demonstrated a significant decrease in IgA+ B cells and total intestinal IgA on day zero postinfection. Although this observation could be attributed to oral tolerance (70), the low antibody production could also be associated with a transient Th1 cytokine profile (discussed below).
The concept of biologically active peptides has evoked much interest in the past years. Numerous authors have reported that immunomodulatory peptides can produce local effects on the gastrointestinal tract and stimulate immunocompetent cells through the gut-associated lymphoid tissue (4, 5, 30, 33-35, 38, 45, 54-56, 58). Unlike epithelial enterocytes that exclude peptides and macromolecules with antigenic potential, specialized mucosal epithelial cells (M cells), located in the follicle-associated epithelium of Peyer's patches, transport luminal antigen across the intestinal barrier (21, 46). The apical surface of M cells lacks the brush border microvilli and possesses a large endocytic domain which enables them to uptake and release luminal antigen on their basolateral surface, where there is a complex network of antigen-presenting cells and Th CD4+ lymphocytes capable of stimulating the differentiation of underlying B cells into Ig-producing plasma cells (44). The specific humoral (secretory) mucosal immune response is mediated through an increase of IgA-producing B cells and secretory IgA (sIgA) synthesis (48). Since sIgA is considered the major immunological barrier against enteropathogens, an increase in total sIgA could prevent adherence and the colonization by enteropathogens such as E. coli O157:H7 (33, 48, 56).
Protective effects of LAB against numerous enteropathogenic bacteria have been reported in several studies (2, 11, 18, 20, 49, 55, 65). Perdigon et al. (52) demonstrated that L. casei decreased the infection caused by S. enterica serovar Typhimurium by enhancing the secretion of specific IgA. Link-Amster et al. (28) demonstrated production of specific IgA against S. enterica serovar Typhimurium in the serum of volunteers who had previously consumed Lactobacillus acidophilus. LAB have also demonstrated beneficial effects against E. coli O157:H7 infections. For example, Ogawa et al. (49) demonstrated that L. casei strain Shirota enhances the local immune response to E. coli O157:H7 and decreases the production of its toxins. Paton et al. (51) have demonstrated that antibodies specific to Shiga toxin-producing E. coli could inhibit pathogen adherence in vitro. There is also evidence that vaccination against E. coli O157:H7 could be successful by increasing the humoral response to virulent factors, such as the translocated intimin receptor (Tir) (27). Since E. coli O157:H7 preferentially binds itself to M cells in Peyer's patches (13, 61), mechanisms that induce an increase of sIgA might be sufficient to prevent or attenuate the infection. Indeed, Conlan and Perry (9) correlated the presence and persistence of serum and fecal anti-O157 IgA to an enhanced resistance to E. coli O157:H7 in BALB/c mice.
Interestingly, the immune response to probiotics and probiotic-derived products is strain dependent. Indeed, enhancement of the immune system by orally administered LAB cannot be generalized for genera, or even species. Perdigon et al. (55) have reported that L. casei and Lactobacillus plantarum interact with Peyer's patches; L. rhamnosus, Streptococcus thermophilus, Lactobacillus delbruecki subsp. bulgaricus, and Lactococcus lactis interact with epithelial cells of the small intestine or in Peyer's patches; and L. acidophilus interacts mainly with epithelial cells of the large intestine. These results suggest that the different immunostimulatory action of LAB could result from strain-specific interactions in the intestinal tract (55, 58). Immune system modulation might also be strongly dependent on the lactobacillus species, or on the metabolites they produce. Thus, species differing in their metabolic activities, such as proteolysis, could release peptides that may (or may not) interact with immune cells to induce distinct immunomodulatory response.
LAB have been known to influence cellular and humoral responses through modulation of the Th1/Th2 balance (31, 56). The classic Th1 cytokine IFN-γ is a potent multifunctional proinflammatory cytokine that triggers innate immune responses, such as phagocytosis and antimicrobial activity, whereas the predominant Th2 cytokine IL-4 regulates a number of events, including antibody production (19). Both IL-4 and IFN-γ generally exist in an antagonistic relationship and have been considered the pivotal cytokines in the induction of Th1 and Th2 responses, respectively (19, 31, 42, 56). In contrast to the strong IFN-γ levels in the infected control (Fig. 7B), the 7-day and, to a lesser extent, the 5-day feeding of the peptidic fraction preinfection led to a remarkable IL-4 increase throughout the experiment (Fig. 7A). These results indicate that the peptidic fraction favors a Th2 response and can be correlated to a high antibody response. In contrast, the infected control displayed high IFN-γ levels with a maximum value on day 2 postinfection. Interestingly, this proinflammatory response could be correlated to the drastic weight loss observed in Fig. 3. Asahara et al. (1) illustrated that E. coli O157:H7 proliferated in the intestine of mice within 24 h of infection, and infected mice showed a dramatic decrease in body weight starting at 2 days postchallenge. In our study, weight loss was only observed in the infected control. Mice fed the peptidic regimen were shown to have an enhanced intestinal IgA response even before infection, suggesting that immune modulation by the peptidic fraction could involve homeostasis between Th1 and Th2 activity (31, 56). In mice, this phenomenon is reflected in specific cytokine patterns (31, 56). Since the Th2 response is linked to antibody production and the Th1 response reflects an inflammatory response (19, 56), it is possible that the enhance IgA production could influence the ability of E. coli O157:H7 to colonize the intestinal epithelium. However, bacterial clearance studies were not undertaken to confirm this hypothesis. It was also noted that the 2-day feeding demonstrated only an IgA increase in the intestine (Fig. 4 and 5), but it did not show an increase in serum IgA (Fig. 6) or serum IL-4 (Fig. 7). Nonetheless, this local IgA stimulation was sufficient to prevent the weight loss associated with the infection. Overall, these results suggest that the peptidic fraction derived from L. helveticus-fermented milk enhances the humoral immune response following an E. coli O157:H7 challenge by favoring a Th2 cytokine profile and reducing the proinflammatory Th1 response.
Although LAB have been shown to influence cytokine profiles, little attention has been paid to the modulation of cytokine production following protein hydrolysis by LAB. Sütas et al. (67) indicated that caseins could up-regulate IL-4 and IFN-γ production, whereas L. rhamnosus GG-degraded caseins down-regulated IL-4 production with no effect on IFN-γ. Pessi et al. (59) reported that L. rhamnosus GG-degraded caseins suppressed T-cell activation through down-regulation of IL-2 synthesis and protein kinase C activity. In contrast, this study demonstrated a predominant effect on Th2 cells after biopeptide ingestion. These opposing effects could be attributed to the liberation of distinct peptides released during proteolysis with LAB. It is generally accepted that orally administered LAB produce peptides in vivo (32, 60); therefore, immunomodulatory peptides released from dietary proteins could account for the strain-specific probiotic effects. It is clear that the effects of probiotics are complex and multifactorial and result from different modes of interaction with the immune cells. Immune cells, such as macrophages and lymphocytes, possess specific receptors that can interact with cell wall components of bacteria, such as the peptidoglycan and lipopolysaccharides. LAB-derived peptides might also interact with such receptors or selectively influence the immune system through different immunopotentiating mechanisms. In all, LAB-derived peptides could greatly contribute to the known probiotic effects of immunomodulation.
This study represents the first documented evidence that peptides derived from L. helveticus-fermented milk can enhance the total IgA humoral and systemic immune response following an E. coli O157:H7 challenge. An increase in total IgA can be beneficial to prevent the entry or colonization of enteropathogens, a concept referred to as immune exclusion. However, subsequent studies will need to address the specific quantification of antipeptide and anti-E. coli IgA responses in relation to bacterial clearance. Isolation and purification of the peptide(s) and dose-dependent relationships will undoubtedly reveal the role of immunomodulatory peptides derived from dietary proteins and their suitability for prevention or attenuation of lethal bacterial infections.
Acknowledgments
We give special thanks to Gabriella Perdigon for her invaluable expertise in all areas of this study. We thank Julie Brassard and all of the technicians at the Pavillon Agathe Lacerte (Université Laval, Sainte-Foy, Québec, Canada) for help during animal procedures. We are also grateful to Jean Guy LeBlanc and Luc Martin for a critical review of the manuscript and for helpful discussions and encouragement.
The research was funded by the Natural Sciences and Engineering Research Council of Canada and by the Canadian Foundation for Innovation.
REFERENCES
- 1.Asahara, T., K. Shimizu, K. Nomoto, T. Hamabata, A. Ozawa, and Y. Takeda. 2004. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 72:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biller, J. A., A. J. Katz, A. F. Flores, T. M. Buie, and S. L. Gorbach. 1995. Treatment of C. difficile colitis with Lactobacillus GG. J. Pediatr. Gastroenterol. Nutr. 21:224-226. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P. 2002. Current understanding of gastrointestinal immunoregulation and its relation to food allergy. Ann. N. Y. Acad. Sci. 964:13-45. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., I. N. Farstad, F. E. Johansen, H. C. Morton, I. N. Norderhaug, and T. Yamanaka. 1999. The B-cell system of the human mucosae and exocrine glands. Immunol. Rev. 171:45-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chart, H. 2000. VTEC enteropathogenicity. J. Appl. Microbiol. Symp. Suppl. 88:12S-23S. [DOI] [PubMed] [Google Scholar]
- 7.Church, F. C., H. E. Swaisgood, D. H. Porter, and G. L. Catignani. 1983. Spectrophotometric assay using o-phtaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 66:1219-1227. [Google Scholar]
- 8.Clare, D. A., and H. E. Swaisgood. 2000. Bioactive milk peptides: a prospectus. J. Dairy Sci. 83:1187-1195. [DOI] [PubMed] [Google Scholar]
- 9.Conlan, J. W., and M. B. Perry. 1998. Susceptibility of three strains of conventional adult mice to intestinal colonization by an isolate of Escherichia coli O157:H7. Can. J. Microbiol. 44:800-805. [DOI] [PubMed] [Google Scholar]
- 10.Cross, M. L., and H. S. Gill. 2000. Immunomodulatory properties of milk. Br. J. Nutr. 84(Suppl. 1):81-89. [DOI] [PubMed] [Google Scholar]
- 11.De Waard, R., J. Garssen, G. C. Bokken, and J. G. Vos. 2002. Antagonistic activity of Lactobacillus casei strain Shirota against gastrointestinal Listeria monocytogenes infection in rats. Int. J. Food Microbiol. 73:93-100. [DOI] [PubMed] [Google Scholar]
- 12.Dundas, S., and W. T. A. Todd. 2000. Clinical presentation, complications and treatment of infection with verotoxin-producing Escherichia coli. Challenges for the clinician. J. Appl. Microbiol. Symp. Suppl. 88:24S-30S. [DOI] [PubMed] [Google Scholar]
- 13.Fitzhenry, R. J., D. J. Pickard, E. L. Hartland, S. Reece, G. Dougan, A. D. Phillips, and G. Frankel. 2002. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 50:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortina, M. G., G. Nicastro, D. Carminati, E. Neviani, and P. L. Manachini. 1998. Lactobacillus heterogeneity in natural cheese starters: the diversity in phenotypic characteristics. J. Appl. Microbiol. 84:72-80. [DOI] [PubMed] [Google Scholar]
- 15.Foucaud, C., and V. Juillard. 2000. Accumulation of casein-derived peptides during growth of proteinase-positive strains of Lactococcus lactis in milk: their contribution to subsequent bacterial growth is impaired by their internal transport. J. Dairy Res. 67:233-240. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert, C., B. Blanc, J. Frot-Coutez, R. Portalier, and D. Atlan. 1997. Comparison of cell surface proteinase activities within the Lactobacillus genus. J. Dairy Res. 64:561-571. [Google Scholar]
- 17.Gill, H. S., F. Doull, K. J. Rutherford, and M. L. Cross. 2000. Immunoregulatory peptides in bovine milk. Br. J. Nutr. 84(Suppl. 1):S111-S117. [DOI] [PubMed] [Google Scholar]
- 18.Gill, H. S., Q. Shu, H. Lin, K. J. Rutherfurd, and M. L. Cross. 2001. Protection against translocating Salmonella typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med. Microbiol. Immunol. (Berlin) 190:97-104. [DOI] [PubMed] [Google Scholar]
- 19.Kidd, P. 2003. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 8:223-246. [PubMed] [Google Scholar]
- 20.Kim, S. H., S. J. Yang, H. C. Koo, W. K. Bae, J. Y. Kim, J. H. Park, Y. J. Baek, and Y. H. Park. 2001. Inhibitory activity of Bifidobacterium longum HY8001 against Vero cytotoxin of Escherichia coli O157:H7. J. Food Prot. 64:1667-1673. [DOI] [PubMed] [Google Scholar]
- 21.Kraehenbuhl, J. P., and M. R. Neutra. 2000. Epithelial M cells: differentiation and function. Annu. Rev. Cell. Dev. Biol. 16:301-332. [DOI] [PubMed] [Google Scholar]
- 22.Laffineur, E., N. Genetet, and J. Leonil. 1996. Immunomodulatory activity of β-casein permeate medium fermented by lactic acid bacteria. J. Dairy Sci. 79:2112-2120. [DOI] [PubMed] [Google Scholar]
- 23.Law, J., and A. Haandrikman. 1997. Proteolytic enzymes of lactic acid bacteria. Int. Dairy J. 7:1-11. [Google Scholar]
- 24.LeBlanc, J. 2003. Implication of virulence factors in Escherichia coli O157:H7 pathogenesis. Crit. Rev. Microbiol. 29:277-296. [DOI] [PubMed] [Google Scholar]
- 25.LeBlanc, J. G., C. Matar, J. C. Valdéz, J. LeBlanc, and G. Perdigon. 2002. Immunomodulating effects of peptidic fractions issued from milk fermented with Lactobacillus helveticus. J. Dairy Sci. 85:2733-2742. [DOI] [PubMed] [Google Scholar]
- 26.Lemieux, L., J. M. Piot, D. Guillochon, and J. Amiot. 1991. Study of the efficiency of a mobile phase used in size-exclusion HPLC for the separation of peptides from a casein hydrolysate according to their hydrodynamic volume. Chromatographia 31:499-504. [Google Scholar]
- 27.Li, Y., E. Frey, A. M. Mackenzie, and B. B. Finlay. 2000. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun. 68:5090-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Link-Amster, H., F. Rochat, K. Y. Saudan, O. Mignot, and J. M. Aeschlimann. 1994. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol. Med. Microbiol. 10:55-63. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd, A. B., R. B. Cumming, and R. D. Kent. 1977. Prevention of Salmonella typhimurium infection in poultry by pretreatment of chickens and poults with intestinal extract. Aust. Vet. J. 53:82-87. [DOI] [PubMed] [Google Scholar]
- 30.Low, P. P. L., K. J. Rutherford, H. S. Gill, and M. L. Cross. 2003. Effect of dietary whey protein concentrate on primary and secondary antibody responses in immunized BALB/c mice. Int. Immunopharmacol. 3:393-401. [DOI] [PubMed] [Google Scholar]
- 31.Maassen, C., C. van Holten-Neelen, F. Balk, M. J. Heijne den Bak-Glashouwer, R. J. Leer, J. D. Laman, W. J. Boersma, and E. Claassen. 2000. Strain-dependent induction of cytokine profiles in the gut by orally-administered Lactobacillus strains. Vaccine 18:2613-2623. [DOI] [PubMed] [Google Scholar]
- 32.MacFarlane, G. T., J. H. Cummings, and C. Allison. 1986. Protein degradation by human intestinal bacteria. J. Gen. Microbiol. 132:1647-1656. [DOI] [PubMed] [Google Scholar]
- 33.Matar, C., J. Amiot, L. Savoie, and J. Goulet. 1996. The effect of milk fermentation by Lactobacillus helveticus on the release of peptides during in vitro digestion. J. Dairy Sci. 79:971-979. [DOI] [PubMed] [Google Scholar]
- 34.Matar, C., J. Goulet, R. L. Bernier, and E. Brochu. 2000. Bioactive peptides from fermented foods: their role in the immune system, p. 193-212. In R. Fuller and G. Perdigon (ed.), Probiotics 3: immunomodulation by the gut microflora and probiotics. Kluwer Academic Publishers, Norwell, Mass.
- 35.Matar, C., J. G. LeBlanc, L. Martin, and G. Perdigon. 2003. Biologically active peptides released from fermented milk: role and functions, p. 177-201. In E. R. Farnworth (ed.), Handbook of fermented functional foods. CRC Press, Boca Raton, Fla.
- 36.Matar, C., J. C. Valdez, M. Medina, M., Rachid, and G. Perdigon. 2001. Immunomodulating effects of milks fermented by Lactobacillus helveticus and its non-proteolytic variant. J. Dairy Res. 68:601-609. [DOI] [PubMed] [Google Scholar]
- 37.Meisel, H., and W. Bockelmann. 1999. Bioactive peptides encrypted in milk proteins: proteolytic activation and tropho-functional properties. Antonie Leeuwenhoek 76:207-215. [PubMed] [Google Scholar]
- 38.Meisel, H., H. Frister, and E. Schlimme. 1989. Biologically active peptides in milk proteins. Z. Ernährungswiss. 28:267-278. [DOI] [PubMed] [Google Scholar]
- 39.Metchnikoff, E. 1908. Étude sur la flore intestinale. Annu. Inst. Pasteur Paris 22:929-955. [Google Scholar]
- 40.Migliore-Samour, D., F. Floc'h, and P. Jollès. 1989. Biologically active casein peptides implicated in immunomodulation. J. Dairy Res. 56:357-362. [DOI] [PubMed] [Google Scholar]
- 41.Moineau, S., and J. Goulet. 1991. Effect of feeding fermented milks on pulmonary macrophage activity in mice. Milchwissenschaft 46:551-554. [Google Scholar]
- 42.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 43.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neutra, M. 1999. M cell sampling in mucosal tissues. Curr. Top. Microbiol. Immunol. 236:17-33. [DOI] [PubMed] [Google Scholar]
- 45.Neutra, M. R., N. J. Mantis, and J.-P. Kraehenbuhl. 2001. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2:1004-1009. [DOI] [PubMed] [Google Scholar]
- 46.Neutra, M. R., H. E. Pringault, and J.-P. Kraehenbuhl. 1996. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 14:275-300. [DOI] [PubMed] [Google Scholar]
- 47.Ng, K.-Y., and M. W. Griffiths. 2002. Enhancement of macrophage cytokine release by cell-free fractions of fermented milk. Milchwissenschaft 57:66-70. [Google Scholar]
- 48.Norderhaug, I. N., F.-E. Johansen, H. Schjerven, and P. Brandtzaeg. 1999. Regulation of the formation and external transport of secretory immunoglobulins. Crit. Rev. Immunol. 19:481-508. [PubMed] [Google Scholar]
- 49.Ogawa, M., K. Shimizu, K. Nomoto, M. Takahashi, M. Watanuki, R. Tanaka, T. Tanaka, T. Hamabata, S. Yamasaki, and Y. Takeda. 2001. Protective effect of Lactobacillus casei strain Shirota on Shiga toxin-producing Escherichia coli O157:H7 infection in infant rabbits. Infect. Immun. 69:1101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parry, S. M., and S. R. Palmer. 2000. The public health significance of VTEC O157. Symp. Ser. Soc. Appl. Microbiol. 29:1S-9S. [DOI] [PubMed] [Google Scholar]
- 51.Paton, A. W., E. Voss, P. A. Manning, and J. C. Paton. 1998. Antibodies to lipopolysaccharide block adherence of Shiga toxin-producing Escherichia coli to human intestinal epithelial (Henle 407) cells. Microb. Pathog. 24:57-63. [DOI] [PubMed] [Google Scholar]
- 52.Perdigon, G., S. Alvarez, and A. Pesce de Ruiz Holgado. 1991. Immunoadjuvant activity of oral Lactobacillus casei: influence of dose on the secretory immune response and protective capacity in intestinal infections. J. Dairy Res. 58:485-496. [DOI] [PubMed] [Google Scholar]
- 53.Perdigon, G., S. Alvarez, M. E. Nader de Macias, M. E. Roux, and A. D. Pesce de Ruiz Holgado. 1990. The oral administration of lactic acid bacteria increases the mucosal intestinal immunity in response to enteropathogens. J. Food Prot. 53:404-410. [DOI] [PubMed] [Google Scholar]
- 54.Perdigon, G., S. Alvarez, M. Rachid, G. Aguero, and N. Gobbato. 1995. Immune system stimulation by probiotics. J. Dairy Sci. 78:1597-1606. [DOI] [PubMed] [Google Scholar]
- 55.Perdigon, G., R. Fuller, and R. Raya. 2001. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2:27-42. [PubMed] [Google Scholar]
- 56.Perdigon, G., C. Maldonado Galdeano, J. C. Valdez, and M. Medici. 2002. Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 56(Suppl. 4):S21-S26. [DOI] [PubMed] [Google Scholar]
- 57.Perdigon, G., M. E. Nader de Macias, S. Alvarez, G. Oliver, and A. Pesce de Ruiz Holgado. 1990. Prevention of gastrointestinal infection using immunobiological methods with milk fermented with Lactobacillus casei and Lactobacillus acidophilus. J. Dairy Res. 57:255-264. [DOI] [PubMed] [Google Scholar]
- 58.Perdigon, G., E. Vintini, S. Alvarez, M. Medina, and M. Medici. 1999. Study of the possible mechanisms involved in the mucosal immune system activation by lactic acid bacteria. J. Dairy Sci. 82:1108-1114. [DOI] [PubMed] [Google Scholar]
- 59.Pessi, T., E. Isolauri, Y. Sütas, H. Kankaanranta, E. Moilanen, and M. Hurme. 2001. Suppression of T-cell activation by Lactobacillus rhamnosus GG-degraded bovine casein. Int. Immunopharmacol. 1:211-218. [DOI] [PubMed] [Google Scholar]
- 60.Pessi, T., Y. Sütas, A. Marttinen, and E. Isolauri. 1998. Probiotics reinforce mucosal degradation of antigens in rats: implications for therapeutic use of probiotics. J. Nutr. 128:2313-2318. [DOI] [PubMed] [Google Scholar]
- 61.Phillips, A. D., S. Navabpour, S. Hicks, G. Dougan, T. Wallis, and G. Frankel. 2000. Enterohaemorrhagic Escherichia coli O157:H7 target Peyer's patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut 47:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 63.Sainte-Marie, G. 1962. A paraffin embedding technique for studies employing immunofluorescence. J. Histochem. Cytochem. 10:150-156. [Google Scholar]
- 64.Shah, N. P. 2000. Effects of milk-derived bioactives: an overview. Br. J. Nutr. 84(Suppl. 1):S3-S10. [DOI] [PubMed] [Google Scholar]
- 65.Shu, Q., and H. S. Gill. 2002. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 34:59-64. [DOI] [PubMed] [Google Scholar]
- 66.Sütas, Y., E. Soppi, H. Korhonen, E. L. Syvaoja, M. Saxelin, T. Rokka, and E. Isolauri. 1996. Suppression of lymphocyte proliferation in vitro by bovine caseins hydrolyzed with Lactobacillus casei GG-derived enzymes. J. Allergy Clin. Immunol. 98:216-224. [DOI] [PubMed] [Google Scholar]
- 67.Sütas, Y., M. Hurme, and E. Isolauri. 1996. Downregulation of anti-CD3 antibody-induced IL-4 production by bovine caseins hydrolysed with Lactobacillus GG-derived enzymes. Scand. J. Immunol. 43:687-689. [DOI] [PubMed] [Google Scholar]
- 68.Van der Waaij, D., J. M. B.-D. Vries, and J. E. C. van der Lekkerkerk-Wees. 1971. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. 69:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vintini, E., S. Alvarez, M. Medina, M. Medici, M. V. de Budeguer, and G. Perdigon. 2000. Gut mucosal immunostimulation by lactic acid bacteria. Biocell 24:223-232. [PubMed] [Google Scholar]
- 70.Weiner, H. L. 1997. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol. Today 18:335-343. [DOI] [PubMed] [Google Scholar]
- 71.Wong, C. W., and D. L. Watson. 1995. Immunomodulatory effects of dietary whey proteins in mice. J. Dairy Res. 72:219-227. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto, N., A. Akino, and T. Takano. 1994. Antihypertensive effects of different kinds of fermented milk in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 58:776-778. [Google Scholar]