Malaria parasites are major human pathogens annually associated with 300 million to 500 million clinical cases worldwide and 0.5 million to 3 million deaths, mostly among children under the age of 5 years living in sub-Saharan Africa (50). Human malaria is caused by four species of parasitic protozoa of the genus Plasmodium: Plasmodium falciparum, P. vivax, P. malariae, and P. ovale. The natural history of infection with P. falciparum, which causes the most severe infections and nearly all malaria-related deaths, has been well characterized in areas of high endemicity in Africa (27). Children have a primary malaria attack during their first year of life, while most toddlers and juveniles have already developed resistance against severe disease but still experience a few clinical episodes. African adolescents and adults, in contrast, are often clinically immune; they remain free of malaria symptoms, despite continuous exposure to the parasite, but maintain low-grade infections throughout the transmission season. Clinical immunity is usually lost during pregnancy, especially among primigravid women, or after migration to areas where the disease is not endemic. Life-long exposure to malaria parasites rarely leads to sterile immunity; blood-stage infections remain detectable by sensitive methods, such as PCR, in all age groups (71).
Much less is known at present about the acquisition of clinical immunity to P. vivax, which accounts for 70 million to 80 million malaria infections each year in Asia, Oceania, and South America but which is rare in most of Africa (73). Asymptomatic P. vivax infections, however, occur after continuous exposure to this species (3, 43, 95). Interestingly, cocirculation of more than one species of human malaria parasites is a common feature of most areas where malaria is endemic, creating complex patterns of interspecies interactions (15, 68).
The extensive diversity of malarial surface antigens is one of the main reasons why clinical immunity develops only after repeated infections with the same species over several years (27). Antigenic diversity has a dual origin. One is the classical genetic mechanism of nucleotide replacement and recombination that creates allelic polymorphism, the existence of genetically stable alternative forms of antigen-coding genes. The second mechanism is antigenic variation, whereby a clonal lineage of parasites expresses successively alternate forms of an antigen without changes in genotype. This minireview focuses on the origins and patterns of allelic polymorphism and antigenic variation in natural parasite populations and their possible implications for naturally acquired immunity and malaria vaccine development.
The complex parasite biology.
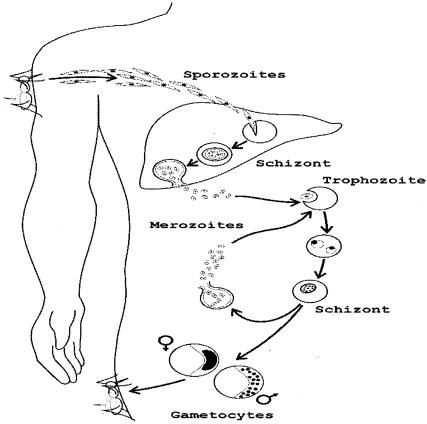
The life cycle of malaria parasites comprises morphologically and antigenically distinct stages that are targeted by stage-specific immunity (Fig. 1). A few (n = 5 to 20) sporozoites are inoculated by blood-feeding female Anopheles mosquitoes. After 30 to 60 min in the bloodstream, these uninucleate extracellular stages penetrate hepatocytes and start intense mitotic activity and nuclear division. The resulting mature, multinucleate liver-stage schizont bursts within 9 to 16 days and releases thousands of free merozoites into the bloodstream. Two of the human malaria parasite species, P. vivax and P. ovale, produce dormant liver stages, known as hypnozoites, that are the sources of relapses weeks or months after the primary infection. Within 1 to 2 min of release, merozoites invade red blood cells (RBCs), in which, over the next 48 or 72 h, they develop from early to late trophozoites and undergo a further phase of mitotic division, which generates erythrocytic-stage schizonts. When infected RBCs rupture, each mature schizont releases 8 to 32 merozoites, each of which invades new erythrocytes. Fever paroxysms, the hallmark of malaria, occur when infected RBCs rupture and release parasite-derived molecules that stimulate the production of proinflammatory cytokines by the host. After a few cycles, some merozoites develop into sexual stages known as gametocytes. When gametocytes are taken up by feeding Anopheles mosquitoes, they mature into male and female gametes and unite to form a zygote in the midgut of the vector. The zygote is the sole diploid stage of malaria parasites; the only meiosis event during this life cycle occurs within a few hours of zygote formation, eventually generating infective sporozoites, which migrate to the salivary glands.
FIG. 1.
Simplified life cycle of malaria parasites in human hosts. The parasite development within mosquito vectors is not represented.
The precise targets of protective immunity against malaria remain unknown, although present evidence implicates about 20 candidate antigens, most of them polymorphic surface proteins. Because structurally different antigens are expressed during each part of the parasite's life cycle, naturally acquired immunity is mostly stage specific (12). Both antibodies and T cells are required for naturally acquired immunity; CD4+ and CD8+ T-cell responses are particularly effective against intracellular liver-stage parasites, while antibodies may block host cell invasion by sporozoites and merozoites. In addition, antibodies to variant antigens expressed on the surface of RBCs may facilitate phagocytosis of infected cells and inhibit or revert their adhesion to endothelial receptors. T-cell help is essential for an effective antibody response, but relatively little is known about the additional roles of cell-mediated immunity in the clearance of blood-stage malaria parasites.
REPETITIVE ANTIGENS AND ALLELIC POLYMORPHISM
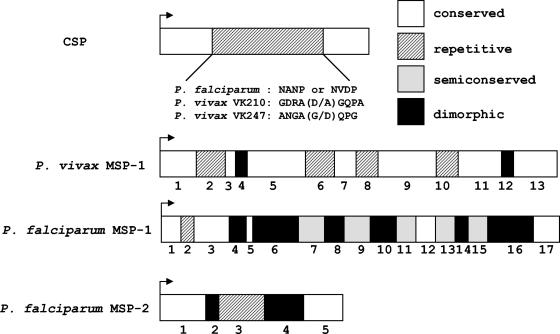
A notable feature of most malaria surface antigens is the presence of tandem arrays of relatively short amino acid motifs (12). The circumsporozoite protein (CSP) (Fig. 2), an abundant sporozoite surface antigen extensively investigated as a target in vaccine development (79), was the first well-studied example of a repetitive malarial antigen. The CSPs of all malaria parasites studied so far comprise central tandem repeats that form immunodominant B-cell epitopes (Fig. 2). The P. falciparum CSP, for example, contains 37 to 50 copies of 4-mer motifs (32, 91), but relatively short repetitive synthetic peptides are thought to express the full antigenicity of this molecule (121). Interestingly, different nucleotide motifs, termed repeat allotypes, code for the same NANP and NVDP repeats in natural P. falciparum isolates, indicating that conservation is maintained at the amino acid level (putatively due to functional constraints) but not at the nucleotide level (91). Different nonapeptide repeats are found in P. vivax CSP variants VK210 and VK247 (94) (Fig. 2); although these variants are found in sympatric parasite populations, no example of a hybrid csp allele with both types of repeats is known. Monoclonal antibodies to CSP repeats inhibit the infectivity of P. falciparum and P. vivax sporozoites in chimpanzees (78), but no clear association has been found between the levels of naturally acquired antibodies to CSP and human protection from malaria in Africa (53) and Asia (119).
FIG. 2.
Schematic representation of three major malarial surface antigens. Amino acid sequences of CSP repeat motifs are shown for P. falciparum and the two variants of P. vivax. MSP-1 of P. falciparum was divided into 17 blocks according to the levels of interallele sequence divergence (111). MSP-1 of P. vivax was divided into 13 blocks (87). MSP-2 of P. falciparum was divided into five blocks (107); the central repeats differ both between and within dimorphic families.
Merozoite surface protein type 1 (MSP-1) and MSP-2 are additional examples of blood-stage vaccine-candidate antigens with repetitive arrays (Fig. 2). MSP-2 has been characterized so far only in P. falciparum and the closely related chimpanzee parasite, P. reichenowi (29). The centrally located immunodominant repeats of P. falciparum MSP-2 consist of variable numbers of either relatively conserved 32-mer and 12-mer motifs, which characterize the allelic family FC27, or polymorphic 2- to 10-mer GSA-rich motifs, which are typical of the allelic family IC1 (54). These repeats are targeted by parasite-inhibitory antibodies (31, 34, 88) and contain T-cell epitopes (98). Naturally acquired human antibodies to MSP-2 predominantly recognize its variable domains (5, 30, 113) and are putatively associated with clinical immunity from malaria (4, 5, 74, 112).
Direct repeats in P. falciparum MSP-1 are thought to be restricted to the short domain known as block 2 (Fig. 2), and some alleles display nonrepetitive sequences in this region (20). However, cryptic repeats were recently described in other variable blocks of P. falciparum MSP-1 (90); repetitive elements are also widespread in the MSP-1 homologue of P. vivax (87) and the rodent parasites P. chabaudi and P. yoelii (58). Most naturally acquired antibodies to MSP-1 recognize its variable domains (55). Significantly, block 2 repeats are targeted by P. falciparum-inhibitory monoclonal antibodies (66) and naturally acquired antibodies associated with clinical immunity in humans (23, 85).
Repeat sequences and immune evasion.
Polymorphisms in repeats may enable parasites to evade immune responses elicited by past exposure to diverse forms of the same antigen. In addition, tandem repeats may stimulate T-cell-independent B-cell responses (104) that fail to generate memory B cells or somatic hypermutation, leading to antibody affinity maturation (76). Moreover, cross-reactive epitopes on otherwise different repetitive antigens may prevent the affinity maturation of antibodies by causing an abnormally high proportion of mutated B cells to be preserved during clonal expansion (6). A well-known example of such a cross-reactive epitope is the pentapeptide VTEEI, present in repeat arrays of unrelated blood-stage (RESA and Pf332) and gametocyte (Pf11.1) antigens of P. falciparum (2, 70).
Mutations and genetic recombination.
Minor amino acid diversity is created in malarial antigens by single-nucleotide replacements. These point mutations are relatively rare events (10−9 replacements/nucleotide/replication) that occur at any site, but they are often clustered in sequences coding for B- or T-cell epitopes of malarial antigens. At the csp locus of P. falciparum, for example, nearly all nonsynonymous nucleotide replacements (i.e., those leading to amino acid replacements) map to the T-cell epitopes Th2R and Th3R, located downstream of the central repeats (56), putatively due to the positive selection of novel polymorphisms associated with immune evasion (24). In fact, amino acid replacements in the CSP T-cell epitopes Th2R and Th3R abolish their recognition by naturally exposed people (14, 49). Amino acid replacements are found in other T-cell epitopes of malarial antigens, but their impact on T-cell recognition and immune evasion remains to be confirmed. Genetic recombination, however, accounts for most variation seen in malarial antigens, since it occurs several orders of magnitude more frequently than mutations. In addition, a single event changes several nucleotides at once. Recombination events comprise both exchanges of blocks of homologous sequences during meiosis and rearrangements in repeat arrays during both mitosis and meiosis. The extensive variation in CSP, MSP-2, and many other antigens results primarily from insertions and deletions of repeat motifs (12, 33, 54, 90). In contrast, both exchanges of nonrepetitive sequences and rearrangements within repeat arrays seem to generate new msp-1 alleles in natural populations of P. falciparum (35) and P. vivax (87).
Origin of allelic dimorphism.
Allelic dimorphism, the occurrence of only two alternate forms at a given locus, characterizes several malarial antigens. The dimorphic allelic families are not necessarily homogeneous (i.e., there is some within-family heterogeneity); members of different families, however, differ at more than 30% of the amino acid residues in variable sequences (i.e., there is extensive between-family divergence). Allelic dimorphism was originally described for the msp-1 locus (111) and was later found in other blood-stage antigens of P. falciparum, such as the merozoite surface proteins MSP-2 and MSP-3, the serine repeat antigen (SERA), and EBA-175, a parasite molecule that binds to sialic acid and glycophorin A during RBC invasion (22, 54, 99). The msp-1 locus of P. vivax is also essentially dimorphic (48, 86, 87). How did the dimorphic regions develop in these antigens? The repetitive sequences in block 2 of the P. falciparum msp-1 gene may be taken as an example: the proliferation of trinucleotide motifs and fusion with nearby triplets, followed by several nucleotide replacements, may explain the origin of the whole repertoire of 9-bp repeats of the dimorphic allelic families (42). Similar processes have been postulated to operate at the msp-2 locus of P. falciparum (33, 34, 90). Alternatively, dimorphic alleles could arise by gene duplication followed by sequence divergence between paralogous genes as a result of gene conversion events (52). This process may readily explain the origin of allelic dimorphism in EBA-175 and SERA, which are encoded by members of multigene families. Nevertheless, it remains unclear why only two of the several possible allelic families of dimorphic blood-stage antigens are detected in natural isolates.
Allelic polymorphism and immune recognition.
Cross-reactivity is a major factor driving the emergence and persistence of novel antigenic variants of malaria parasite antigens in human populations; new variants are theoretically selected if mutant parasites evade the host's immunity (72). Thus, the high recombination rate typically found in repeats, coupled with the positive selection of non-cross-reacting variants, might account for most of the antigenic diversity seen in natural malaria parasite populations (90).
Whether rearrangements in the NANP and the NVDP repeat arrays affect the recognition of B-cell epitopes on P. falciparum CSP remains unknown. This topic has elicited limited interest among vaccine-minded researchers, despite the conformational nature of these epitopes (77, 100) and the structural consequences putatively associated with changes in the number of NANP repeats (32). On the other hand, there is little antibody cross-reactivity between the P. vivax CSP variants VK210 and VK247 (94).
Naturally acquired immune responses discriminate between antigenic variants belonging to different dimorphic families of P. falciparum MSP-1 and MSP-2 (18, 19, 30, 113) and P. vivax MSP-1 (69). Significantly, experimental immunization with a multivalent vaccine prototype containing the 3D7 variant of MSP-2 partially protected humans from infection with homologous (IC1-type) but not heterologous (FC27-type) parasites (47). However, do insertions and deletions of repeat units within dimorphic allelic families affect immune recognition and favor immune evasion? The most plausible answer is yes: (i) narrowly specific antibodies to repetitive block 2 variants of MSP-1 are found in subjects exposed to malaria (26, 59, 85), and (ii) murine (66) and human (108) monoclonal antibodies are able to discriminate between repeat variants within the same dimorphic allelic family. The monoclonal antibody described by Sowa and colleagues (108), for example, discriminates between the MAD20 and HB3 variants of block 2, which differ only in the number of copies of the hexapeptide SGGSVA.
Similar data are available for MSP-2: (i) deletions of 12-mer repeats in FC27-type MSP-2 variants decrease their ability to be recognized by naturally acquired antibodies (89); (ii) a murine monoclonal antibody discriminates between IC1-type antigens that differ in the number of copies of the tetrapeptide GGSA (34); and (iii) antibodies that discriminate between MSP-2 variants within the same allelic family are found during acute P. falciparum infections (60, 118) and in experimentally immunized mice (115), although examples of extensive cross-reactivity to structurally different MSP-2 variants have been described in African children (39). Interestingly, several instances of complete mismatch between the specificities of detectable antibodies during acute infections and the MSP-2 type in infecting parasites have been found in areas of endemicity (30, 39, 60, 118). These results may be explained by a selective unresponsiveness to MSP-2 variants expressed by infecting parasites, by the presence of MSP-2 variants in infecting parasites that were missed during the typing procedure, or by clonal imprinting. Recent data from a longitudinal study with African children are consistent with the clonal imprinting hypothesis, since infection with novel msp-2 variants frequently boosted antibodies that cross-recognized relatively conserved domains within local variants instead of eliciting antibodies with new specificities (39).
ANTIGENIC VARIATION
The expression of variant antigens on the surface of infected RBCs is an additional strategy of immune evasion used by all Plasmodium species so far studied. At a first glimpse, the expression of foreign antigens on RBCs, which are devoid of major histocompatibility complex molecules and which thus represent the ideal hideout for intracellular organisms, seems a disadvantage for the parasite's survival in the presence of a competent immune system. However, this may be an intrahost mechanism used to control parasite populations, since rapid parasite multiplication would kill the host in a very short time, decreasing the chances of transmission to mosquitoes (83, 101).
At least two P. falciparum variant antigens are expressed on the surface of infected RBCs: P. falciparum erytrocyte membrane protein 1 (PfEMP-1), encoded by var genes, and repetitive interspersed family proteins (RIFINs), encoded by rif genes. Members of a third family of variant antigens, encoded by stevor (subtelomeric variant open reading frame) genes, were recently found to be associated with Maurer's clefts, a parasite-derived tubular network that is present in the cytoplasm of infected RBCs. These clefts are thus not directly exposed on the RBC surface (61), although they may become visible at the late schizont stage due to augmented access of antibodies to the cytoplasmic structures of infected RBCs (57). These three proteins are encoded by multigene families, and most members of each family are located in the subtelomeric regions of chromosomes (45).
Cytoadherence, var gene transcription, and antigenic switching.
RBCs infected with very young P. falciparum trophozoites (known as ring stages) circulate in the peripheral blood, but nearly all RBCs containing mature trophozoites and schizonts are sequestered in capillary vessels of different organs (67), thus avoiding their clearance by the spleen. Large parasite-encoded surface variant antigens were found in 1984 to be responsible for the binding of RBCs infected with mature parasites to endothelial receptors; these proteins were originally named PfEMP-1 (64). Over the following years, PfEMP-1 proteins were found to bind to a wide range of endothelial receptors, such as CD36 (82), intercellular adhesion molecule 1 (ICAM-1) (11), chrondroitin sulfate A (CSA) (93), platelet endothelial cell adhesion molecule (PECAM) (80), vascular cell adhesion molecule (VCAM) (80), hyaluronic acid (10), heparan sulfate (116), and other molecules, such as complement receptor 1 (97), immunoglobulins G (37), and ABO blood group antigens (8). The adherence of infected RBCs to endothelial receptors is believed to be causally associated with severe disease, although striking evidence for a direct connection between PfEMP-1 expression and pathology was shown only for CSA-binding PfEMP-1 variants (41; for a review, see reference 75). The var gene family was described in 1995 (9, 105, 109); each P. falciparum genome contains 40 to 60 different var genes (38, 45, 105), mostly located in subtelomeric regions of chromosomes (36, 114). The var gene alleles found in different strains are so variable (38, 110) that the whole repertoire of different var genes seems unlimited.
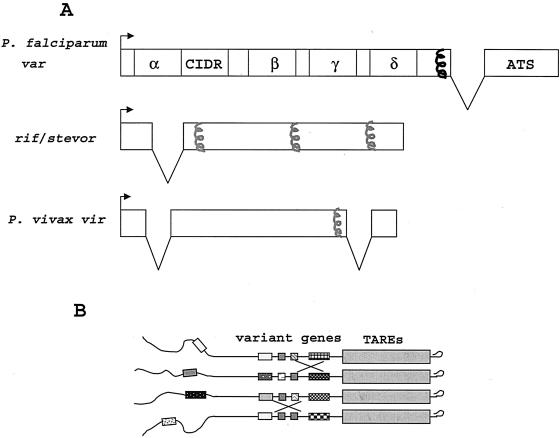
Most functional var genes share a two-exon structure (Fig. 3): exon 1 codes for highly variant domains exposed on the surface of infected RBCs that bind to endothelial receptors, while exon 2 codes for the more conserved acidic terminal segment of PfEMP-1. This domain is attached to electron-dense complexes of parasite-derived proteins known as “knobs,” located in the inner RBC membrane, that confer the mechanical stability required for adherence to the capillary endothelium under flow conditions (25). A single PfEMP-1 gene seems to be expressed by parasites within each infected RBC; var gene transcription is tightly controlled by a mutually exclusive transcription mechanism that silences all but one or a few var genes per genome (21, 103). This process, the details of which have yet to be uncovered, is probably steered by epigenetic processes. However, switching from one PfEMP-1 variant to another occurs at a certain rate (92; G. Wunderlich, unpublished data), which may depend on which var gene is initially transcribed (84). The frequent switch guarantees the parasite's survival under immune pressure and the constant removal of RBCs expressing known PfEMP-1 variants.
FIG. 3.
(A) Structures of the different variant multigene families of P. falciparum and P. vivax. Var genes have two exons and code for a various number of domains with different adhesive properties, termed Duffy binding-like domains (α, β, γ, δ, etc.) and cysteine-rich interdomain regions (CIDR) (106). Vir genes have three exons. Probable (gray) or confirmed (black) regions coding for transmembrane domains are indicated with helices. ATS, acidic terminal segment. (B) Proposed model of ectopic exchange of telomeres. Chromosome telomeres cluster in bouquet-like structures at perinuclear regions. The similarity among the repetitive telomere elements (TAREs) and the var, stevor, and rif genes (represented by boxes with different patterns) are believed to facilitate the recombination between chromosomes. Modified from reference 102 with permission of the publisher.
The 200- to 400-kDa PfEMP-1 protein is a major target of naturally acquired antibodies (16); recognition of a few commonly expressed antigenic determinants may protect against severe disease (51). Human T-cell responses to PfEMP-1 have not been investigated so far. Infection with parasites expressing common PfEMP-1 variants is associated with severe disease in childhood, but the acquisition of variant-specific immunity decreases the probability of severe outcomes with increasing age (16). In fact, the number of different PfEMP-1 subdomains recognized by antibodies from African adults correlates inversely with the probability that these subjects will develop symptomatic malaria episodes during the next transmission season (81). PfEMP-1 domains might be regarded as ideal candidates for subunit vaccine development, since most complications that characterize severe malaria are likely to be prevented by antibodies that inhibit the PfEMP-1-mediated binding of infected RBCs to specific endothelial receptors. Although the extensive diversity in PfEMP-1 represents a major obstacle for immunization, its motifs that bind to CD36 and CSA are structurally conserved (44, 96), pointing to these domains as attractive targets for vaccines aimed at the inhibition or reversal of cytoadherence (65, 120).
The second group of parasite-encoded antigens expressed on the surface of P. falciparum-infected RBCs, RIFINs (63), are encoded by the family of rif genes (Fig. 3), most of which are located in subtelomeric regions of chromosomes, often in tight proximity to var genes (45). Few data regarding their transcription modes are available; but rif genes, in contrast to var genes, appear to be transcribed only by immature trophozoites for a very short period (62). It remains unknown whether switching occurs and how many different RIFINs are coexpressed on the surface of a single infected RBC. RIFINs are targets of naturally acquired antibodies, and the number of different RIFIN variants recognized by antibodies correlates with the strength of clinical immunity in Africans (1). More than 200 rif genes are found per haploid P. falciparum genome, indicating that the repertoire of rif genes is even larger than that of var genes; recent sequence data from field samples support this notion (G. Wunderlich, unpublished). RIFINs do not mediate cytoadherence, and their role in parasite virulence remains unknown.
The third family of variant proteins comprises STEVOR antigens (117). Although stevor genes are located in tandem with rif and var genes, they seem to be much more conserved among strains than the rif and var genes (G. Wunderlich, unpublished). The stevor genes (30 to 40 copies per haploid genome) have, similarly to the rif genes, a two-exon structure (Fig. 3) and code for 30- to 40-kDa proteins with a rather short intracellular domain that are expressed over a brief period by mature trophozoites and, possibly, by sporozoites and gametocytes as well (13). Their biological function remains unknown.
Variant antigens of P. vivax.
Sequence analysis of a P. vivax chromosome telomere revealed the presence of a large family of variant genes named vir genes (Fig. 3) (28). The VIR proteins are exposed on the surface of infected RBCs but do not mediate cytoadherence. VIR proteins are targets of naturally acquired antibodies (28), but the repertoire of VIR proteins is extraordinarily vast: at least 600 copies of vir genes are estimated to exist per haploid genome. The genome of the rodent species P. yoelii, which has a telomere structure similar to that of P. vivax, also contains variant gene families, termed yir (17).
Generation of new alleles by ectopic recombination.
New insights into the origins of sequence diversity in subtelomeric variant genes were recently provided by Freitas-Junior and colleagues (40), who detected nonparental var gene alleles in the progeny of a genetic cross between P. falciparum strains HB3 and Dd2 much more frequently than expected from the available estimates of meiotic recombination rates (Fig. 3). In addition, the chromosome location of some parental var alleles had changed in the progeny. Fluorescent in situ hybridization experiments revealed that, in both trophozoites and gametocytes, four to five heterologous telomeres cluster tightly together during mitosis, forming “bouquet”-like structures that favor ectopic recombination events (Fig. 3B). Some questions, however, remain to be addressed: (i) How frequent are ectopic exchanges in natural parasite populations? (ii) Is chromosome proximity in bouquet-like structures sufficient for the occurrence of ectopic exchanges of telomeres, or is a gametocyte-specific recombinase required? Interestingly, if parasites are capable of ectopic recombination during their asexual replication, transpositions of var genes from one chromosome to another could putatively occur during long-term in vitro parasite culture and chronic human infections. These findings imply that the huge antigenic diversity seen in PfEMP-1 (and perhaps in other P. falciparum variant antigens) results from both antigenic variation that involves no change in the parasite's genome and the generation of new alleles by frequent ectopic recombination events during the asexual propagation of the parasites.
CONCLUDING REMARKS
The notion that polymorphic antigens elicit variant-specific clinical immunity is supported by the finding that most PfEMP-1 variants expressed by parasites obtained during clinical malaria episodes in African children are not recognized by the child's own preexisting antibodies (16). Clinical disease is mostly caused by parasite variants corresponding to gaps in the developing repertoire of variant-specific antibodies to PfEMP-1, and the occurrence of placental malaria (accumulation of infected RBCs in the intervillous space) has been associated with the lack of antibodies to PfEMP-1 variants that are able to bind to CSA, the locally abundant receptor for infected RBCs (16). We are testing whether gaps in the repertoire of antibodies to merozoite surface antigens may explain the susceptibility to disease due to both P. falciparum and P. vivax in areas of endemicity in Brazil.
Most malaria vaccine-candidate antigens are highly polymorphic surface proteins that elicit variant-specific immunity. How do investigators deal with this fact in malaria vaccine development? The conventional approach, which has been highly successful for common viral and bacterial infections, consists of the tailoring of vaccines to match all or most local variants (7) or those associated with the most severe disease (51). As an alternative, evolutionary relationships might be explored for the design of vaccines based on ancestral sequences, with the potential for inducing cross-protection against a wide range of antigenic variants (46). Thus, understanding the mechanisms and patterns of genetic recombination and sequence variation may help in designing vaccines that represent the worldwide repertoire of polymorphic malarial surface antigens.
Acknowledgments
Research in our laboratories has been supported by grants from the UNDP/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases, the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). M.U.F. and G.W. are recipients of CNPq research scholarships, and M.D.S.N. is a recipient of a FAPESP Ph.D. scholarship.
REFERENCES
- 1.Abdel-Latif, M. S., K. Dietz, S. Issifou, P. G. Kremsner, and M. Q. Klinkert. 2003. Antibodies to Plasmodium falciparum rifin proteins are associated with rapid parasite clearance and asymptomatic infections. Infect. Immun. 71:6229-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahlborg, N., K. Berzins, and P. Perlmann. 1991. Definition of the epitope recognized by the Plasmodium falciparum-reactive human monoclonal antibody 33G2. Mol. Biochem. Parasitol. 46:89-95. [DOI] [PubMed] [Google Scholar]
- 3.Alves, F. P., R. R. Durlacher, M. J. Menezes, H. Krieger, L. H. Silva, and E. P. Camargo. 2002. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am. J. Trop. Med. Hyg. 66:641-648. [DOI] [PubMed] [Google Scholar]
- 4.al-Yaman, F., B. Genton, R. Anders, J. Taraika, M. Ginny, S. Mellor, and M. P. Alpers. 1995. Assessment of the role of the humoral response to Plasmodium falciparum MSP2 compared to RESA and SPf66 in protecting Papua New Guinean children from clinical malaria. Parasite Immunol. 17:493-501. [DOI] [PubMed] [Google Scholar]
- 5.al-Yaman, F., B. Genton, R. F. Anders, M. Falk, T. Triglia, D. Lewis, J. Hii, H. P. Beck, and M. P. Alpers. 1994. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am. J. Trop. Med. Hyg. 51:593-602. [DOI] [PubMed] [Google Scholar]
- 6.Anders, R. F. 1986. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 8:529-539. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, R. M., C. A. Donnelly, and S. Gupta. 1997. Vaccine design, evaluation, and community-based use for antigenically variable infectious agents. Lancet 350:1466-1470. [DOI] [PubMed] [Google Scholar]
- 8.Barragan, A., P. G. Kremsner, M. Wahlgren, and J. Carlson. 2000. Blood group A antigen is a coreceptor in Plasmodium falciparum rosetting. Infect. Immun. 68:2971-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 10.Beeson, J. G., S. J. Rogerson, B. M. Cooke, J. C. Reeder, W. Chai, A. M. Lawson, M. E. Molyneux, and G. V. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 6:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berendt, A. R., D. L. Simmons, J. Tansey, C. I. Newbold, and K. Marsh. 1989. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 341:57-59. [DOI] [PubMed] [Google Scholar]
- 12.Berzins, K., and R. F. Anders. 1999. The malaria antigens, p. 181-216. In M. Wahlgren and P. Perlmann (ed.), Malaria—molecular and clinical aspects. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 13.Blythe, J., T. Surentheran, and P. R. Preiser. 2004. STEVOR—a multifunctional protein? Mol. Biochem. Parasitol. 134:11-14. [DOI] [PubMed] [Google Scholar]
- 14.Bonelo, A., D. Valmori, F. Triponez, J. M. Tiercy, G. Mentha, J. Oberholzer, P. Champagne, J. F. Romero, F. Esposito, I. Nebie, C. Barbey, P. Romero, S. Herrera, G. Corradin, and J. A. Lopez. 2000. Generation and characterization of malaria-specific human CD8+ lymphocyte clones: effect of natural polymorphism on T cell recognition and endogenous cognate antigen presentation by liver cells. Eur. J. Immunol. 30:3079-3088. [DOI] [PubMed] [Google Scholar]
- 15.Bruce, M. C., C. A. Donnelly, M. P. Alpers, M. R. Galinski, J. W. Barnwell, D. Walliker, and K. P. Day. 2000. Cross-species interactions between malaria parasites in humans. Science 287:845-848. [DOI] [PubMed] [Google Scholar]
- 16.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlton, J. M., S. V. Angiuoli, B. B. Suh, T. W. Kooij, M. Pertea, J. C. Silva, M. D. Ermolaeva, J. E. Allen, J. D. Selengut, H. L. Koo, J. D. Peterson, M. Pop, D. S. Kosack, M. F. Shumway, S. L. Bidwell, S. J. Shallom, S. E. van Aken, S. B. Riedmuller, T. V. Feldblyum, J. K. Cho, J. Quackenbush, M. Sedegah, A. Shoaibi, L. M. Cummings, L. Florens, J. R. Yates, J. D. Raine, R. E. Sinden, M. A. Harris, D. A. Cunningham, P. R. Preiser, L. W. Bergman, A. B. Vaidya, L. H. van Lin, C. J. Janse, A. P. Waters, H. O. Smith, O. R. White, S. L. Salzberg, J. C. Venter, C. M. Fraser, S. L. Hoffman, M. J. Gardner, and D. J. Carucci. 2002. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature 419:512-519. [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh, D. R., I. M. Elhassan, C. Roper, V. J. Robinson, H. Giha, A. A. Holder, L. Hviid, T. G. Theander, D. E. Arnot, and J. S. McBride. 1998. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J. Immunol. 161:347-359. [PubMed] [Google Scholar]
- 19.Cavanagh, D. R., and J. S. McBride. 1997. Antigenicity of recombinant proteins derived from Plasmodium falciparum merozoite surface protein 1. Mol. Biochem. Parasitol. 85:197-211. [DOI] [PubMed] [Google Scholar]
- 20.Certa, U., D. Rotmann, H. Matile, and R. Reber-Liske. 1987. A naturally occurring gene encoding the major surface antigen precursor p190 of Plasmodium falciparum lacks tripeptide repeats. EMBO J. 6:4137-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen, Q., V. Fernandez, A. Sundstrom, M. Schlichtherle, S. Datta, P. Hagblom, and M. Wahlgren. 1998. Developmental selection of var gene expression in Plasmodium falciparum. Nature 394:392-395. [DOI] [PubMed] [Google Scholar]
- 22.Conway, D. J., and J. Baum. 2002. In the blood—the remarkable ancestry of Plasmodium falciparum. Trends Parasitol. 18:351-355. [DOI] [PubMed] [Google Scholar]
- 23.Conway, D. J., D. R. Cavanagh, K. Tanabe, C. Roper, Z. S. Mikes, N. Sakihama, K. A. Bojang, A. M. Oduola, P. G. Kremsner, D. E. Arnot, B. M. Greenwood, and J. S. McBride. 2000. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 6:689-692. [DOI] [PubMed] [Google Scholar]
- 24.Conway, D. J., and S. D. Polley. 2002. Measuring immune selection. Parasitology 125(Suppl.):S3-S16. [DOI] [PubMed] [Google Scholar]
- 25.Crabb, B. S., B. M. Cooke, J. C. Reeder, R. F. Waller, S. R. Caruana, K. M. Davern, M. E. Wickham, G. V. Brown, R. L. Coppel, and A. F. Cowman. 1997. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 89:287-296. [DOI] [PubMed] [Google Scholar]
- 26.Da Silveira, L. A., M. L. Dorta, E. A. Kimura, A. M. Katzin, F. Kawamoto, K. Tanabe, and M. U. Ferreira. 1999. Allelic diversity and antibody recognition of Plasmodium falciparum merozoite surface protein 1 during hypoendemic malaria transmission in the Brazilian amazon region. Infect. Immun. 67:5906-5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day, K. P., and K. Marsh. 1991. Naturally acquired immunity to Plasmodium falciparum. Immunol. Today 12:A68-A71. [DOI] [PubMed] [Google Scholar]
- 28.del Portillo, H. A., C. Fernandez-Becerra, S. Bowman, K. Oliver, M. Preuss, C. P. Sanchez, N. K. Schneider, J. M. Villalobos, M. A. Rajandream, D. Harris, L. H. Pereira da Silva, B. Barrell, and M. Lanzer. 2001. A superfamily of variant genes encoded in the subtelomeric region of Plasmodium vivax. Nature 410:839-842. [DOI] [PubMed] [Google Scholar]
- 29.Dubbeld, M. A., C. H. Kocken, and A. W. Thomas. 1998. Merozoite surface protein 2 of Plasmodium reichenowi is a unique mosaic of Plasmodium falciparum allelic forms and species-specific elements. Mol. Biochem. Parasitol. 92:187-192. [DOI] [PubMed] [Google Scholar]
- 30.Ekala, M. T., H. Jouin, F. Lekoulou, O. Mercereau-Puijalon, and F. Ntoumi. 2002. Allelic family-specific humoral responses to merozoite surface protein 2 (MSP2) in Gabonese residents with Plasmodium falciparum infections. Clin. Exp. Immunol. 129:326-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epping, R. J., S. D. Goldstone, L. T. Ingram, J. A. Upcroft, R. Ramasamy, J. A. Cooper, G. R. Bushell, and H. M. Geysen. 1988. An epitope recognised by inhibitory monoclonal antibodies that react with a 51 kilodalton merozoite surface antigen in Plasmodium falciparum. Mol. Biochem. Parasitol. 28:1-10. [DOI] [PubMed] [Google Scholar]
- 32.Escalante, A. A., H. M. Grebert, R. Isea, I. F. Goldman, L. Basco, M. Magris, S. Biswas, S. Kariuki, and A. A. Lal. 2002. A study of genetic diversity in the gene encoding the circumsporozoite protein (CSP) of Plasmodium falciparum from different transmission areas. XVI. Asembo Bay Cohort Project. Mol. Biochem. Parasitol. 125:83-90. [DOI] [PubMed] [Google Scholar]
- 33.Felger, I., V. M. Marshal, J. C. Reeder, J. A. Hunt, C. S. Mgone, and H. P. Beck. 1997. Sequence diversity and molecular evolution of the merozoite surface antigen 2 of Plasmodium falciparum. J. Mol. Evol. 45:154-160. [DOI] [PubMed] [Google Scholar]
- 34.Fenton, B., J. T. Clark, C. M. Khan, J. V. Robinson, D. Walliker, R. Ridley, J. G. Scaife, and J. S. McBride. 1991. Structural and antigenic polymorphism of the 35-to 48-kilodalton merozoite surface antigen (MSA-2) of the malaria parasite Plasmodium falciparum. Mol. Cell. Biol. 11:963-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira, M. U., W. L. Ribeiro, A. P. Tonon, F. Kawamoto, and S. M. Rich. 2003. Sequence diversity and evolution of the malaria vaccine candidate merozoite surface protein-1 (MSP-1) of Plasmodium falciparum. Gene 304:65-75. [DOI] [PubMed] [Google Scholar]
- 36.Fischer, K., P. Horrocks, M. Preuss, J. Wiesner, S. Wunsch, A. A. Camargo, and M. Lanzer. 1997. Expression of var genes located within polymorphic subtelomeric domains of Plasmodium falciparum chromosomes. Mol. Cell. Biol. 17:3679-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flick, K., C. Scholander, Q. Chen, V. Fernandez, B. Pouvelle, J. Gysin, and M. Wahlgren. 2001. Role of nonimmune IgG bound to PfEMP1 in placental malaria. Science 293:2098-2100. [DOI] [PubMed] [Google Scholar]
- 38.Fowler, E. V., J. M. Peters, M. L. Gatton, N. Chen, and Q. Cheng. 2002. Genetic diversity of the DBLα region in Plasmodium falciparum var genes among Asia-Pacific isolates. Mol. Biochem. Parasitol. 120:117-126. [DOI] [PubMed] [Google Scholar]
- 39.Franks, S., L. Baton, K. Tetteh, E. Tongren, D. Dewin, B. D. Akanmori, K. A. Koram, L. Ranford-Cartwright, and E. M. Riley. 2003. Genetic diversity and antigenic polymorphism in Plasmodium falciparum: extensive serological cross-reactivity between allelic variants of merozoite surface protein 2. Infect. Immun. 71:3485-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freitas-Junior, L. H., E. Bottius, L. A. Pirrit, K. W. Deitsch, C. Scheidig, F. Guinet, U. Nehrbass, T. E. Wellems, and A. Scherf. 2000. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407:1018-1022. [DOI] [PubMed] [Google Scholar]
- 41.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 42.Frontali, C. 1994. Genome plasticity in Plasmodium. Genetica 94:91-100. [DOI] [PubMed] [Google Scholar]
- 43.Gamage-Mendis, A. C., J. Rajakaruna, R. Carter, and K. N. Mendis. 1991. Infectious reservoir of Plasmodium vivax and Plasmodium falciparum malaria in an endemic region of Sri Lanka. Am. J. Trop. Med. Hyg. 45:479-487. [DOI] [PubMed] [Google Scholar]
- 44.Gamain, B., L. H. Miller, and D. I. Baruch. 2001. The surface variant antigens of Plasmodium falciparum contain cross-reactive epitopes. Proc. Natl. Acad. Sci. USA 98:2664-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaschen, B., J. Taylor, K. Yusim, B. Foley, F. Gao, D. Lang, V. Novitsky, B. Haynes, B. H. Hahn, T. Bhattacharya, and B. Korber. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354-2360. [DOI] [PubMed] [Google Scholar]
- 47.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. F. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. V. Brown, D. Pye, D. O. Irving, T. A. Smith, H. P. Beck, and M. P. Alpers. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185:820-827. [DOI] [PubMed] [Google Scholar]
- 48.Gibson, H. L., J. E. Tucker, D. C. Kaslow, A. U. Krettli, W. E. Collins, M. C. Kiefer, I. C. Bathurst, and P. J. Barr. 1992. Structure and expression of the gene for Pv200, a major blood-stage surface antigen of Plasmodium vivax. Mol. Biochem. Parasitol. 50:325-333. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert, S. C., M. Plebanski, S. Gupta, J. Morris, M. Cox, M. Aidoo, D. Kwiatkowski, B. M. Greenwood, H. C. Whittle, and A. V. Hill. 1998. Association of malaria parasite population structure, HLA, and immunological antagonism. Science 279:1173-1177. [DOI] [PubMed] [Google Scholar]
- 50.Guerin, P. J., P. Olliaro, F. Nosten, P. Druilhe, R. Laxminarayan, F. Binka, W. L. Kilama, N. Ford, and N. J. White. 2002. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect. Dis. 2:564-573. [DOI] [PubMed] [Google Scholar]
- 51.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 52.Hartl, D. L., S. K. Volkman, K. M. Nielsen, A. E. Barry, K. P. Day, D. F. Wirth, and E. A. Winzeler. 2002. The paradoxical population genetics of Plasmodium falciparum. Trends Parasitol. 18:266-272. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman, S. L., C. N. Oster, C. V. Plowe, G. R. Woollett, J. C. Beier, J. D. Chulay, R. A. Wirtz, M. R. Hollingdale, and M. Mugambi. 1987. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science 237:639-642. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann, E. H., L. A. da Silveira, R. Tonhosolo, F. J. Pereira, W. L. Ribeiro, A. P. Tonon, F. Kawamoto, and M. U. Ferreira. 2001. Geographical patterns of allelic diversity in the Plasmodium falciparum malaria-vaccine candidate, merozoite surface protein-2. Ann. Trop. Med. Parasitol. 95:117-132. [DOI] [PubMed] [Google Scholar]
- 55.Holder, A. A., and E. M. Riley. 1996. Human immune response to MSP-1. Parasitol. Today 12:173-174. [DOI] [PubMed] [Google Scholar]
- 56.Hughes, A. L. 1991. Circumsporozoite protein genes of malaria parasites (Plasmodium spp.): evidence for positive selection on immunogenic regions. Genetics 127:345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hui, G. S., and W. A. Siddiqui. 1988. Characterization of a Plasmodium falciparum polypeptide associated with membrane vesicles in the infected erythrocytes. Mol. Biochem. Parasitol. 29:283-293. [DOI] [PubMed] [Google Scholar]
- 58.Jennings, V. M., A. A. Lal, and R. L. Hunter. 1998. Evidence for multiple pathologic and protective mechanisms of murine cerebral malaria. Infect. Immun. 66:5972-5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jouin, H., C. Rogier, J. F. Trape, and O. Mercereau-Puijalon. 2001. Fixed, epitope-specific, cytophilic antibody response to the polymorphic block 2 domain of the Plasmodium falciparum merozoite surface antigen MSP-1 in humans living in a malaria-endemic area. Eur. J. Immunol. 31:539-550. [DOI] [PubMed] [Google Scholar]
- 60.Kanunfre, K. A., F. M. Leoratti, E. H. Hoffmann, R. R. Durlacher, A. W. Ferreira, S. L. Moraes-Avila, and M. U. Ferreira. 2003. Differential recognition of Plasmodium falciparum merozoite surface protein 2 variants by antibodies from malaria patients in Brazil. Clin. Diagn. Lab. Immunol. 10:973-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaviratne, M., S. M. Khan, W. Jarra, and P. R. Preiser. 2002. Small variant STEVOR antigen is uniquely located within Maurer's clefts in Plasmodium falciparum-infected red blood cells. Eukaryot. Cell 1:926-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kyes, S., R. Pinches, and C. Newbold. 2000. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol. Biochem. Parasitol. 105:311-315. [DOI] [PubMed] [Google Scholar]
- 63.Kyes, S. A., J. A. Rowe, N. Kriek, and C. I. Newbold. 1999. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 96:9333-9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leech, J. H., J. W. Barnwell, L. H. Miller, and R. J. Howard. 1984. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 159:1567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lekana Douki, J. B., B. Traore, F. T. Costa, T. Fusai, B. Pouvelle, Y. Sterkers, A. Scherf, and J. Gysin. 2002. Sequestration of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A, a receptor for maternal malaria: monoclonal antibodies against the native parasite ligand reveal pan-reactive epitopes in placental isolates. Blood 100:1478-1483. [DOI] [PubMed] [Google Scholar]
- 66.Locher, C. P., L. Q. Tam, S. P. Chang, J. S. McBride, and W. A. Siddiqui. 1996. Plasmodium falciparum: gp195 tripeptide repeat-specific monoclonal antibody inhibits parasite growth in vitro. Exp. Parasitol. 84:74-83. [DOI] [PubMed] [Google Scholar]
- 67.Luse, S. A., and L. H. Miller. 1971. Plasmodium falciparum malaria. Ultrastructure of parasitized erythrocytes in cardiac vessels. Am. J. Trop. Med. Hyg. 20:655-660. [PubMed] [Google Scholar]
- 68.Maitland, K. A., T. N. Williams, and C. I. Newbold. 1997. Plasmodium vivax and Plasmodium falciparum: biological interactions and the possibility of cross-species immunity. Parasitol. Today 13:227-231. [DOI] [PubMed] [Google Scholar]
- 69.Mancilla, L. I., G. Levitus, K. Kirchgatter, F. Mertens, S. Herrera, and H. A. del Portillo. 1994. Plasmodium vivax: dimorphic DNA sequences from the MSP-1 gene code for regions that are immunogenic in natural infections. Exp. Parasitol. 79:148-158. [DOI] [PubMed] [Google Scholar]
- 70.Mattei, D., K. Berzins, M. Wahlgren, R. Udomsangpetch, P. Perlmann, H. W. Griesser, A. Scherf, B. Muller-Hill, S. Bonnefoy, M. Guillotte, et al. 1989. Cross-reactive antigenic determinants present on different Plasmodium falciparum blood-stage antigens. Parasite Immunol. 11:15-29. [DOI] [PubMed] [Google Scholar]
- 71.May, J., F. P. Mockenhaupt, O. G. Ademowo, A. G. Falusi, P. E. Olumese, U. Bienzle, and C. G. Meyer. 1999. High rate of mixed and subpatent malarial infections in southwest Nigeria. Am. J. Trop. Med. Hyg. 61:339-343. [DOI] [PubMed] [Google Scholar]
- 72.McKenzie, F. E., M. U. Ferreira, J. K. Baird, G. Snounou, and W. H. Bossert. 2001. Meiotic recombination, cross-reactivity, and persistence in Plasmodium falciparum. Evolution 55:1299-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendis, K., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97-106. [DOI] [PubMed] [Google Scholar]
- 74.Metzger, W. G., D. M. Okenu, D. R. Cavanagh, J. V. Robinson, K. A. Bojang, H. A. Weiss, J. S. McBride, B. M. Greenwood, and D. J. Conway. 2003. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol. 25:307-312. [DOI] [PubMed] [Google Scholar]
- 75.Miller, L. H., D. I. Baruch, K. Marsh, and O. K. Doumbo. 2002. The pathogenic basis of malaria. Nature 415:673-679. [DOI] [PubMed] [Google Scholar]
- 76.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655-692. [DOI] [PubMed] [Google Scholar]
- 77.Monette, M., S. J. Opella, J. Greenwood, A. E. Willis, and R. N. Perham. 2001. Structure of a malaria parasite antigenic determinant displayed on filamentous bacteriophage determined by NMR spectroscopy: implications for the structure of continuous peptide epitopes of proteins. Protein Sci. 10:1150-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nardin, E. H., V. Nussenzweig, R. S. Nussenzweig, W. E. Collins, K. T. Harinasuta, P. Tapchaisri, and Y. Chomcharn. 1982. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J. Exp. Med. 156:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nardin, E. H., and F. Zavala. 1998. Acquired immunity to sporozoites, p. 495-511. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 80.Ockenhouse, C. F., T. Tegoshi, Y. Maeno, C. Benjamin, M. Ho, K. E. Kan, Y. Thway, K. Win, M. Aikawa, and R. R. Lobb. 1992. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J. Exp. Med. 176:1183-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oguariri, R. M., S. Borrmann, M. Q. Klinkert, P. G. Kremsner, and J. F. Kun. 2001. High prevalence of human antibodies to recombinant Duffy binding-like alpha domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect. Immun. 69:7603-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oquendo, P., E. Hundt, J. Lawler, and B. Seed. 1989. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell 58:95-101. [DOI] [PubMed] [Google Scholar]
- 83.Paget-McNicol, S., M. Gatton, I. Hastings, and A. Saul. 2002. The Plasmodium falciparum var gene switching rate, switching mechanism and patterns of parasite recrudescence described by mathematical modelling. Parasitology 124:225-235. [DOI] [PubMed] [Google Scholar]
- 84.Peters, J., E. Fowler, M. Gatton, N. Chen, A. Saul, and Q. Cheng. 2002. High diversity and rapid changeover of expressed var genes during the acute phase of Plasmodium falciparum infections in human volunteers. Proc. Natl. Acad. Sci. USA 99:10689-10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Polley, S. D., K. K. Tetteh, D. R. Cavanagh, R. J. Pearce, J. M. Lloyd, K. A. Bojang, D. M. Okenu, B. M. Greenwood, J. S. McBride, and D. J. Conway. 2003. Repeat sequences in block 2 of Plasmodium falciparum merozoite surface protein 1 are targets of antibodies associated with protection from malaria. Infect. Immun. 71:1833-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porto, M., M. U. Ferreira, L. M. Camargo, S. Premawansa, and H. A. del Portillo. 1992. Second form in a segment of the merozoite surface protein 1 gene of Plasmodium vivax among isolates from Rondonia (Brazil). Mol. Biochem. Parasitol. 54:121-124. [DOI] [PubMed] [Google Scholar]
- 87.Putaporntip, C., S. Jongwutiwes, N. Sakihama, M. U. Ferreira, W. G. Kho, A. Kaneko, H. Kanbara, T. Hattori, and K. Tanabe. 2002. Mosaic organization and heterogeneity in frequency of allelic recombination of the Plasmodium vivax merozoite surface protein-1 locus. Proc. Natl. Acad. Sci. USA 99:16348-16353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramasamy, R., G. Jones, and R. Lord. 1990. Characterisation of an inhibitory monoclonal antibody-defined epitope on a malaria vaccine candidate antigen. Immunol. Lett. 23:305-309. [DOI] [PubMed] [Google Scholar]
- 89.Ranford-Cartwright, L. C., R. R. Taylor, N. Asgari-Jirhandeh, D. B. Smith, P. E. Roberts, V. I. Robinson, H. A. Babiker, E. M. Riley, D. Walliker, and J. S. McBride. 1996. Differential antibody recognition of FC27-like Plasmodium falciparum merozoite surface protein MSP2 antigens which lack 12 amino acid repeats. Parasite Immunol. 18:411-420. [DOI] [PubMed] [Google Scholar]
- 90.Rich, S. M., M. U. Ferreira, and F. J. Ayala. 2000. The origin of antigenic diversity in Plasmodium falciparum. Parasitol. Today 16:390-396. [DOI] [PubMed] [Google Scholar]
- 91.Rich, S. M., R. R. Hudson, and F. J. Ayala. 1997. Plasmodium falciparum antigenic diversity: evidence of clonal population structure. Proc. Natl. Acad. Sci. USA 94:13040-13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogerson, S. J., S. C. Chaiyaroj, K. Ng, J. C. Reeder, and G. V. Brown. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 182:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenberg, R., R. A. Wirtz, D. E. Lanar, J. Sattabongkot, T. Hall, A. P. Waters, and C. Prasittisuk. 1989. Circumsporozoite protein heterogeneity in the human malaria parasite Plasmodium vivax. Science 245:973-976. [DOI] [PubMed] [Google Scholar]
- 95.Roshanravan, B., E. Kari, R. H. Gilman, L. Cabrera, E. Lee, J. Metcalfe, M. Calderon, A. G. Lescano, S. H. Montenegro, C. Calampa, and J. M. Vinetz. 2003. Endemic malaria in the Peruvian Amazon region of Iquitos. Am. J. Trop. Med. Hyg. 69:45-52. [PubMed] [Google Scholar]
- 96.Rowe, J. A., S. A. Kyes, S. J. Rogerson, H. A. Babiker, and A. Raza. 2002. Identification of a conserved Plasmodium falciparum var gene implicated in malaria in pregnancy. J. Infect. Dis. 185:1207-1211. [DOI] [PubMed] [Google Scholar]
- 97.Rowe, J. A., J. M. Moulds, C. I. Newbold, and L. H. Miller. 1997. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388:292-295. [DOI] [PubMed] [Google Scholar]
- 98.Rzepczyk, C. M., R. Ramasamy, D. A. Mutch, P. C. Ho, D. Battistutta, K. L. Anderson, D. Parkinson, T. J. Doran, and M. Honeyman. 1989. Analysis of human T cell response to two Plasmodium falciparum merozoite surface antigens. Eur. J. Immunol. 19:1797-1802. [DOI] [PubMed] [Google Scholar]
- 99.Safitri, I., A. Jalloh, I. S. Tantular, S. Pusarawati, T. T. Win, Q. Liu, M. U. Ferreira, Y. P. Dachlan, T. Horii, and F. Kawamoto. 2003. Sequence diversity in the amino-terminal region of the malaria-vaccine candidate serine repeat antigen in natural Plasmodium falciparum populations. Parasitol. Int. 52:117-131. [DOI] [PubMed] [Google Scholar]
- 100.Satterthwait, A. C., T. Arrhenius, R. A. Hagopian, F. Zavala, V. Nussenzweig, and R. A. Lerner. 1989. The conformational restriction of synthetic peptides, including a malaria peptide, for use as immunogens. Philos. Trans. R. Soc. London B Biol. Sci. 323:565-572. [DOI] [PubMed] [Google Scholar]
- 101.Saul, A. 1999. The role of variant surface antigens on malaria-infected red blood cells. Parasitol. Today 15:455-457. [DOI] [PubMed] [Google Scholar]
- 102.Scherf, A., L. M. Figueiredo, and L. H. Freitas-Junior. 2001. Plasmodium telomeres: a pathogen's perspective. Curr. Opin. Microbiol. 4:409-414. [DOI] [PubMed] [Google Scholar]
- 103.Scherf, A., R. Hernandez-Rivas, P. Buffet, E. Bottius, C. Benatar, B. Pouvelle, J. Gysin, and M. Lanzer. 1998. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 17:5418-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schofield, L. 1991. On the function of repetitive domains in protein antigens of Plasmodium and other eukaryotic parasites. Parasitol. Today 7:269-275. [DOI] [PubMed] [Google Scholar]
- 105.Smith, J. D., C. E. Chitnis, A. G. Craig, D. J. Roberts, D. E. Hudson-Taylor, D. S. Peterson, R. Pinches, C. I. Newbold, and L. H. Miller. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith, J. D., G. Subramanian, B. Gamain, D. I. Baruch, and L. H. Miller. 2000. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 110:293-310. [DOI] [PubMed] [Google Scholar]
- 107.Snewin, V. A., M. Herrera, G. Sanchez, A. Scherf, G. Langsley, and S. Herrera. 1991. Polymorphism of the alleles of the merozoite surface antigens MSA1 and MSA2 in Plasmodium falciparum wild isolates from Colombia. Mol. Biochem. Parasitol. 49:265-275. [DOI] [PubMed] [Google Scholar]
- 108.Sowa, K. M., D. R. Cavanagh, A. M. Creasey, J. Raats, J. McBride, R. Sauerwein, W. F. Roeffen, and D. E. Arnot. 2001. Isolation of a monoclonal antibody from a malaria patient-derived phage display library recognising the block 2 region of Plasmodium falciparum merozoite surface protein-1. Mol. Biochem. Parasitol. 112:143-147. [DOI] [PubMed] [Google Scholar]
- 109.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 110.Tami, A., R. Ord, G. A. Targett, and C. J. Sutherland. 2003. Sympatric Plasmodium falciparum isolates from Venezuela have structured var gene repertoires. Malaria J. 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tanabe, K., M. Mackay, M. Goman, and J. G. Scaife. 1987. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 195:273-287. [DOI] [PubMed] [Google Scholar]
- 112.Taylor, R. R., S. J. Allen, B. M. Greenwood, and E. M. Riley. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58:406-413. [DOI] [PubMed] [Google Scholar]
- 113.Taylor, R. R., D. B. Smith, V. J. Robinson, J. S. McBride, and E. M. Riley. 1995. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect. Immun. 63:4382-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thompson, J. K., J. P. Rubio, S. Caruana, A. Brockman, M. E. Wickham, and A. F. Cowman. 1997. The chromosomal organization of the Plasmodium falciparum var gene family is conserved. Mol. Biochem. Parasitol. 87:49-60. [DOI] [PubMed] [Google Scholar]
- 115.Tonhosolo, R., G. Wunderlich, and M. U. Ferreira. 2001. Differential antibody recognition of four allelic variants of the merozoite surface protein-2 (MSP-2) of Plasmodium falciparum. J. Eukaryot. Microbiol. 48:556-564. [DOI] [PubMed] [Google Scholar]
- 116.Vogt, A. M., A. Barragan, Q. Chen, F. Kironde, D. Spillmann, and M. Wahlgren. 2003. Heparan sulfate on endothelial cells mediates the binding of Plasmodium falciparum-infected erythrocytes via the DBL1alpha domain of PfEMP1. Blood 101:2405-2411. [DOI] [PubMed] [Google Scholar]
- 117.Weber, J. L. 1988. Interspersed repetitive DNA from Plasmodium falciparum. Mol. Biochem. Parasitol. 29:117-124. [DOI] [PubMed] [Google Scholar]
- 118.Weisman, S., L. Wang, H. Billman-Jacobe, D. H. Nhan, T. L. Richie, and R. L. Coppel. 2001. Antibody responses to infections with strains of Plasmodium falciparum expressing diverse forms of merozoite surface protein 2. Infect. Immun. 69:959-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wongsrichanalai, C., H. K. Webster, B. Permpanich, N. Chuanak, and S. Ketrangsri. 1991. Naturally acquired circumsporozoite antibodies and their role in protection in endemic falciparum and vivax malaria. Am. J. Trop. Med. Hyg. 44:201-204. [DOI] [PubMed] [Google Scholar]
- 120.Yipp, B. G., D. I. Baruch, C. Brady, A. G. Murray, S. Looareesuwan, P. Kubes, and M. Ho. 2003. Recombinant PfEMP1 peptide inhibits and reverses cytoadherence of clinical Plasmodium falciparum isolates in vivo. Blood 101:331-337. [DOI] [PubMed] [Google Scholar]
- 121.Zavala, F., J. P. Tam, M. R. Hollingdale, A. H. Cochrane, I. Quakyi, R. S. Nussenzweig, and V. Nussenzweig. 1985. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 228:1436-1440. [DOI] [PubMed] [Google Scholar]