Abstract
In eukaryotic and prokaryotic cells, activity transcribed genes and, in some instances, the template strand of these genes have been found to be repaired 2-10 times more rapidly than nontranscribed genes or the coding strand of transcribed genes. We demonstrate here gene- and template strand-specific repair synthesis in vitro by using an Escherichia coli cell-free extract and a plasmid carrying a gene with the strong tac promoter. Strand-specific repair of UV, 4'-hydroxymethyl1-4,5',8-trimethylpsoralen, and cis-dicholorodiammine platinum(II) damage was dependent upon transcription and a functional nucleotide excision repair system and was stimulated by 6% (wt/vol) polyethylene glycol. A defined system consisting of the transcription and repair proteins in highly purified form did not perform strand-specific repair; however, active fractions of extract conferred strand specificity to the defined system. Transcription-repair coupling activity was partially purified from extract by successive DEAE-agarose and gel filtration chromatography. The coupling factor is heat-labile, with an estimated Mr of 100,000.
Full text
PDF

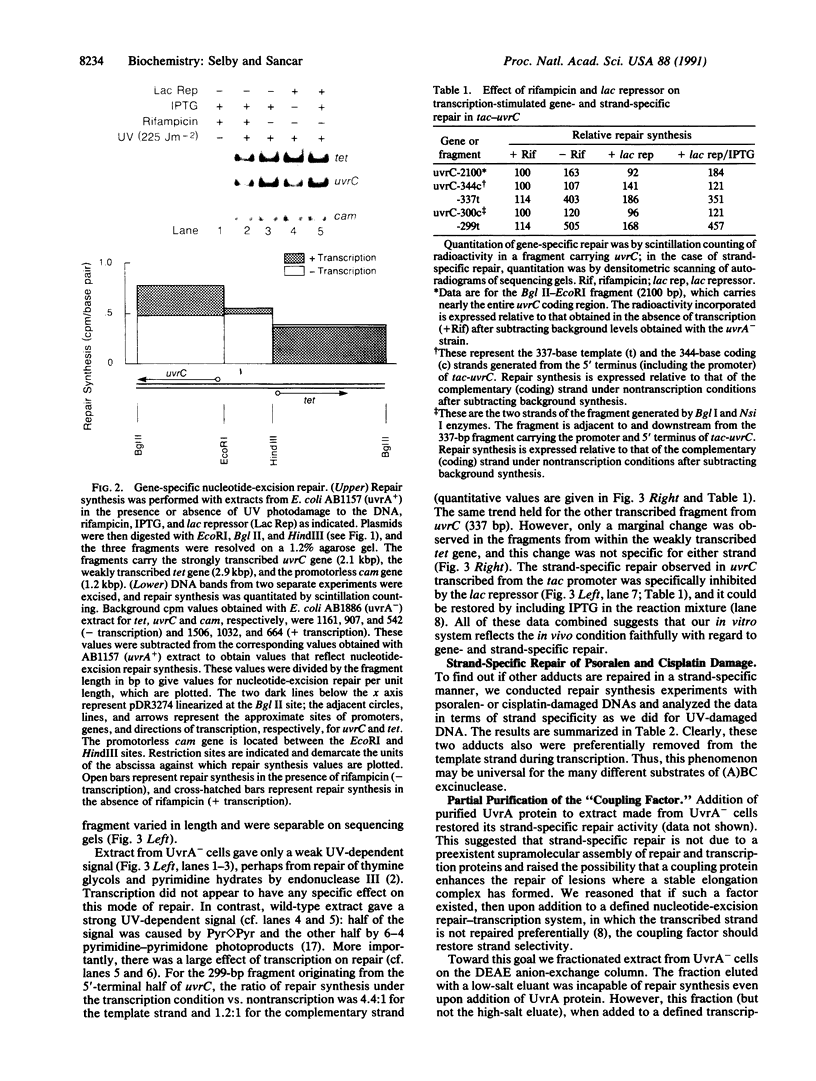
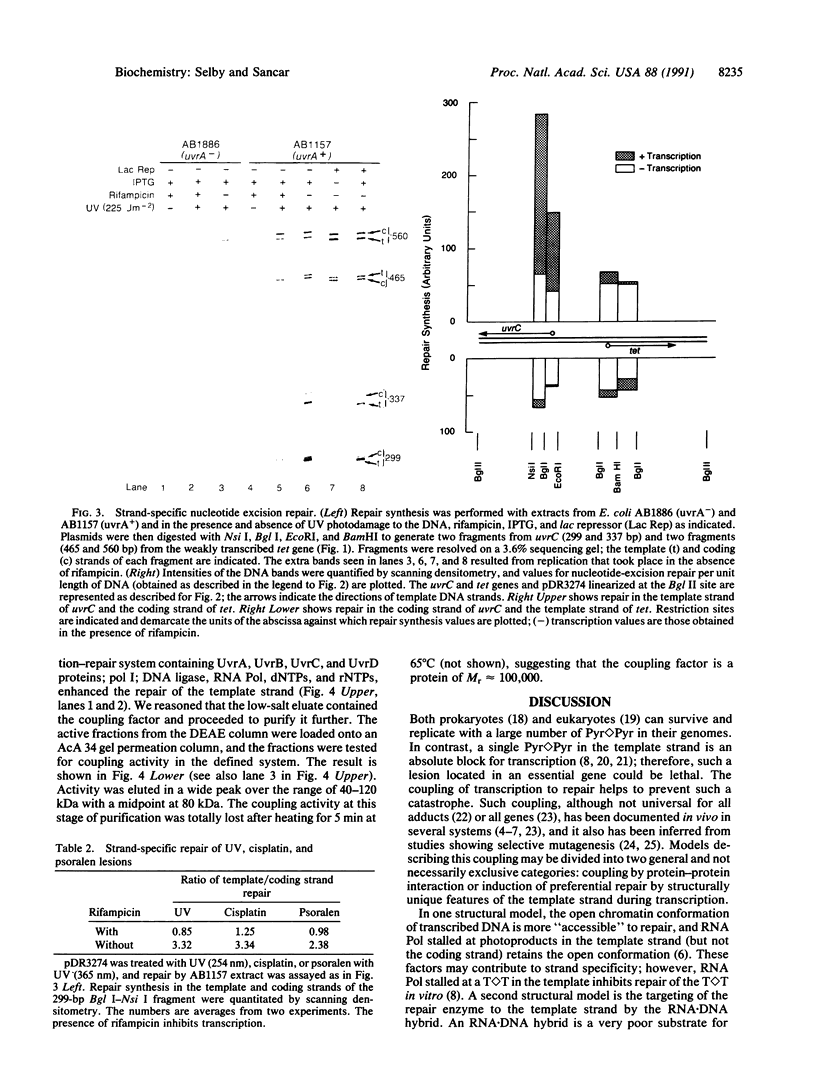
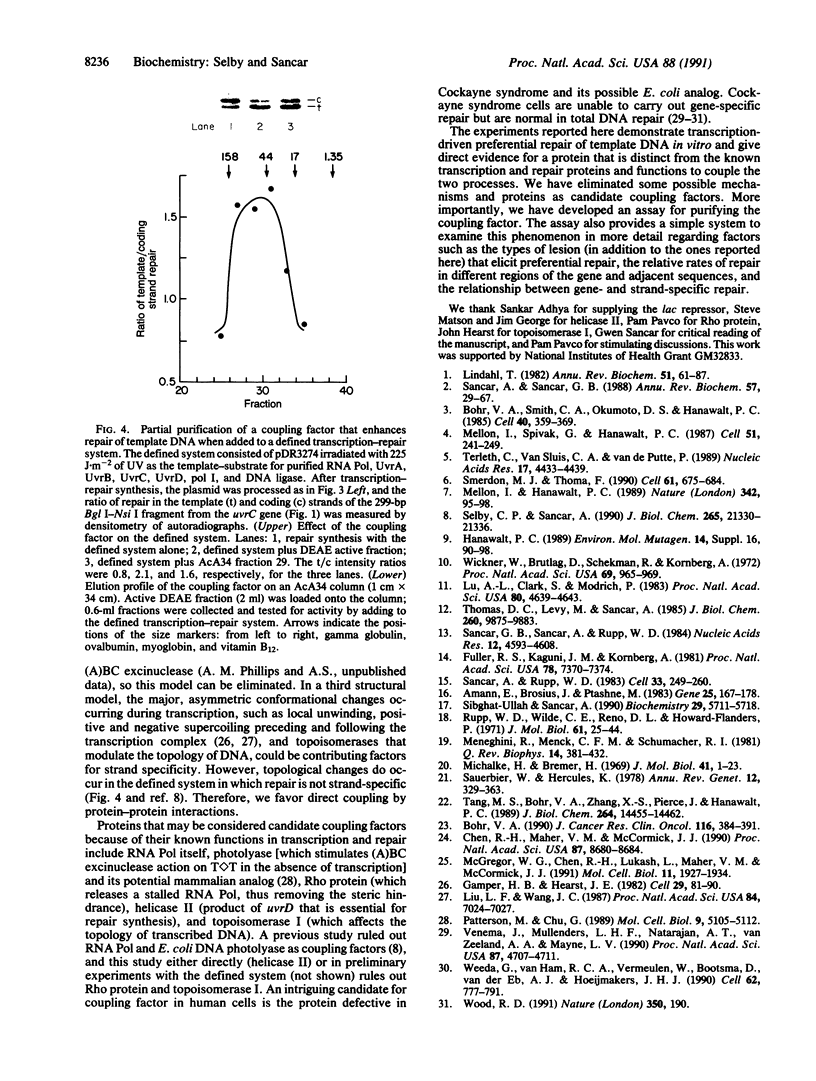
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Bohr V. A. DNA repair at the level of the gene: molecular and clinical considerations. J Cancer Res Clin Oncol. 1990;116(4):384–391. doi: 10.1007/BF01612922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Maher V. M., McCormick J. J. Effect of excision repair by diploid human fibroblasts on the kinds and locations of mutations induced by (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene in the coding region of the HPRT gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8680–8684. doi: 10.1073/pnas.87.21.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper H. B., Hearst J. E. A topological model for transcription based on unwinding angle analysis of E. coli RNA polymerase binary, initiation and ternary complexes. Cell. 1982 May;29(1):81–90. doi: 10.1016/0092-8674(82)90092-7. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. Concepts and models for DNA repair: from Escherichia coli to mammalian cells. Environ Mol Mutagen. 1989;14 (Suppl 16):90–98. doi: 10.1002/em.2850140617. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. L., Clark S., Modrich P. Methyl-directed repair of DNA base-pair mismatches in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4639–4643. doi: 10.1073/pnas.80.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor W. G., Chen R. H., Lukash L., Maher V. M., McCormick J. J. Cell cycle-dependent strand bias for UV-induced mutations in the transcribed strand of excision repair-proficient human fibroblasts but not in repair-deficient cells. Mol Cell Biol. 1991 Apr;11(4):1927–1934. doi: 10.1128/mcb.11.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Meneghini R., Menck C. F., Schumacher R. I. Mechanisms of tolerance to DNA lesions in mammalian cells. Q Rev Biophys. 1981 Aug;14(3):381–432. doi: 10.1017/s0033583500002353. [DOI] [PubMed] [Google Scholar]
- Michalke H., Bremer H. RNA synthesis in Escherichia coli after irradiation with ultraviolet light. J Mol Biol. 1969 Apr 14;41(1):1–23. doi: 10.1016/0022-2836(69)90122-3. [DOI] [PubMed] [Google Scholar]
- Patterson M., Chu G. Evidence that xeroderma pigmentosum cells from complementation group E are deficient in a homolog of yeast photolyase. Mol Cell Biol. 1989 Nov;9(11):5105–5112. doi: 10.1128/mcb.9.11.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sancar G. B., Sancar A., Rupp W. D. Sequences of the E. coli uvrC gene and protein. Nucleic Acids Res. 1984 Jun 11;12(11):4593–4608. doi: 10.1093/nar/12.11.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Gene and transcription unit mapping by radiation effects. Annu Rev Genet. 1978;12:329–363. doi: 10.1146/annurev.ge.12.120178.001553. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Transcription preferentially inhibits nucleotide excision repair of the template DNA strand in vitro. J Biol Chem. 1990 Dec 5;265(34):21330–21336. [PubMed] [Google Scholar]
- Sibghat-Ullah, Sancar A. Substrate overlap and functional competition between human nucleotide excision repair and Escherichia coli photolyase and (a)BC excision nuclease. Biochemistry. 1990 Jun 19;29(24):5711–5718. doi: 10.1021/bi00476a011. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Thoma F. Site-specific DNA repair at the nucleosome level in a yeast minichromosome. Cell. 1990 May 18;61(4):675–684. doi: 10.1016/0092-8674(90)90479-x. [DOI] [PubMed] [Google Scholar]
- Tang M. S., Bohr V. A., Zhang X. S., Pierce J., Hanawalt P. C. Quantification of aminofluorene adduct formation and repair in defined DNA sequences in mammalian cells using the UVRABC nuclease. J Biol Chem. 1989 Aug 25;264(24):14455–14462. [PubMed] [Google Scholar]
- Terleth C., van Sluis C. A., van de Putte P. Differential repair of UV damage in Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Jun 26;17(12):4433–4439. doi: 10.1093/nar/17.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Venema J., Mullenders L. H., Natarajan A. T., van Zeeland A. A., Mayne L. V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D. DNA repair. Seven genes for three diseases. Nature. 1991 Mar 21;350(6315):190–190. doi: 10.1038/350190a0. [DOI] [PubMed] [Google Scholar]






