Abstract
Store-operated Ca2+ entry (SOCE) occurs in diverse cell types in response to depletion of Ca2+ within the endoplasmic/sarcoplasmic reticulum and functions both to refill these stores and to shape cytoplasmic Ca2+ transients. Here we report that in addition to conventional SOCE, skeletal myotubes display a physiological mechanism that we term excitation-coupled Ca2+ entry (ECCE). ECCE is rapidly initiated by membrane depolarization. Like excitation-contraction coupling, ECCE is absent in both dyspedic myotubes that lack the skeletal muscle-type ryanodine receptor 1 and dysgenic myotubes that lack the dihydropyridine receptor (DHPR), and is independent of the DHPR l-type Ca2+ current. Unlike classic SOCE, ECCE does not depend on sarcoplasmic reticulum Ca2+ release. Indeed, ECCE produces a large Ca2+ entry in response to physiological stimuli that do not produce substantial store depletion and depends on interactions among three different Ca2+ channels: the DHPR, ryanodine receptor 1, and a Ca2+ entry channel with properties corresponding to those of store-operated Ca2+ channels. ECCE may provide a fundamental means to rapidly maintain Ca2+ stores and control important aspects of Ca2+ signaling in both muscle and nonmuscle cells.
Auniversal response to depletion of Ca2+ within the endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR) is to enhance cation entry through the plasma membrane through a mechanism known as store-operated Ca2+ entry (SOCE). A form of SOCE has recently been demonstrated to exist in skeletal muscle (1). The primary function of SOCE is to refill ER/SR stores and to shape cytoplasmic Ca2+ transients (2). Activation of SOCE by a mechanism involving conformational coupling has been previously inferred from broken-cell analyses (3-5).
In skeletal muscle cells, ryanodine receptor 1 (RyR1) regulates intracellular Ca2+ signaling by controlling the release of Ca2+ from intracellular stores. In particular, orthograde conformational coupling between the l-type Ca2+ channel [dihydropyridine receptor (DHPR)] of the plasma membrane and RyR1 underlies excitation-contraction (EC) coupling, whereas retrograde coupling between these two proteins regulates both the organization of the DHPR complex into tetrads and the magnitude of the l-type inward Ca2+ current carried by the DHPR (6, 7). Here we show in intact primary skeletal myotubes a form of conformational coupling that is to our knowledge previously uncharacterized. Physiological stimuli that do not produce substantial depletion of stores rapidly activate Ca2+ entry through channels having properties corresponding to those of store-operated Ca2+ channels (SOCCs). These findings represent evidence of a form of conformational coupling that depends on interactions among three different Ca2+ channels (the DHPR, RyR1, and a Ca2+ entry channel) through a mechanism we term excitation-coupled Ca2+ entry (ECCE).
Methods
Primary Cultures. Preparation of primary cultures of skeletal myotubes from primary WT, dyspedic (lacking RyR1), and dysgenic (lacking DHPR) mice has been described in refs. 8 and 9. Primary WT, dyspedic, and dysgenic myoblasts were cultured in 10-cm, cell-culture-treated Corning dishes coated with calf skin collagen (Calbiochem) in F-10 medium containing 20% (vol/vol) FBS, 2 mM l-glutamine, 4 ng/ml fibroblast growth factor (Promega), penicillin G, and streptomycin sulfate at 37°C in 10% CO2/5% O2. For fura-2 imaging, cells were plated onto 96-well microclear plates (Greiner, Nurtingen, Germany) coated with MATRIGEL (Becton Dickinson) or collagen. Upon reaching ≈80% confluence, the cells were differentiated into myotubes over a period of 3-5 days in DMEM containing 2% (vol/vol) heat-inactivated horse serum and 2 mM l-glutamine, at 37°C in 10% CO2/10% O2.
cDNA Transfection. A Glu-to-Lys mutation in the repeat III pore region of the skeletal muscle DHPR (SkEIIIKDHPR) was constructed by using PCR as described in ref. 10. Transfection of primary dysgenic myotubes with cDNA coding for SkEIIIKDHPR was performed with TransIT LT1 reagent (Mirus, Madison, WI) 3-5 days after withdrawal of serum in a 5:1 μl/μg cDNA ratio as described in ref. 11.
Ca2+ Imaging, Mn2+ Quench, and Electrical-Field Stimulation. Ca2+ transients and Mn2+ influx were measured as described in refs. 12 and 13. Briefly, differentiated primary WT, dyspedic, or dysgenic myotubes were loaded with fura-2; the cells were imaged with an intensified charge coupled device camera with a 40× objective, and data were collected from 3-10 individual cells at ≥6 frames per sec. Activators or inhibitors were dissolved in imaging buffer and rapidly puffed onto the cells by micropipette. When KCl was used to depolarize the cells (≥40 mM), the concentration of Na+ was lowered accordingly to maintain osmolarity. In the Mn2+ quench experiments, a final concentration of 500 μM MnCl2 was used and emission was monitored at 510 nm.
For electrical-field stimulation, two platinum electrodes were fixed to opposite sides of the well and connected to a stimulator. Myotubes were loaded with either fura-2 or fluo-4 and stimulated with 25-msec, 3-V pulses over a range of frequencies (2-20 Hz). In these experiments, data were acquired at 50-msec intervals by photometry. Caffeine was added to dyspedic myotubes loaded with Ca2+ indicator to test for possible influence on the properties of the Ca2+ dye. Caffeine had negligible effects on fura-2 responses.
Results and Discussion
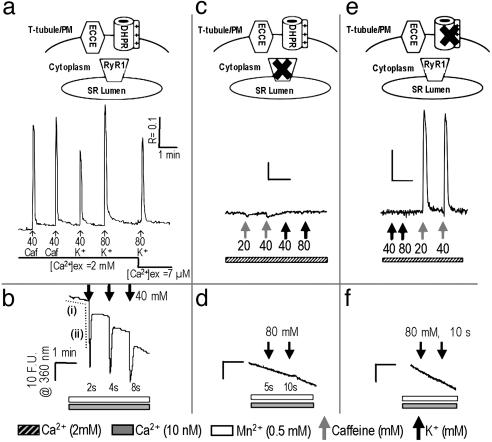
In the presence of extracellular Ca2+, membrane depolarization of primary skeletal myotubes by K+ (40 or 80 mM) induced Ca2+ transients that were of slightly higher magnitude (base to peak) compared with those produced by caffeine, a RyR1 agonist (Fig. 1a). Moreover, transients were elicited immediately after reducing the extracellular Ca2+ ([Ca2+]o) concentration from 2 mM to 7 μM, consistent with skeletal-type EC coupling and with SR stores being the principal source of the Ca2+ transients. However, the transients measured after reducing Ca2+ to 7 μM were on average 25% smaller in height and returned to baseline significantly faster than those elicited in the presence of 2 mM [Ca2+]o, indicating that these transients depend in part on external Ca2+. Fig. 1b demonstrates that, in addition to eliciting the efflux of Ca2+ from SR, depolarization also caused the influx of extracellular cations through plasmalemmal Ca2+ channels, which can be monitored by the quench of intracellular fura-2 fluorescence in the presence of external Mn2+ and the absence of [Ca2+]o (12, 14). In response to depolarization, Mn2+ entry displayed a very large rate of entry compared with that at rest [Fig. 1b, compare dashed traces i (horizontal) and ii (vertical)], and the duration of the influx was proportional to the length of the depolarizing stimulus (Fig. 1b). The initial rate of Mn2+ entry triggered by the depolarization of WT myotubes with 2 sec of 40 mM K+ was 59-fold that measured at rest (Table 1). The depolarization-induced Mn2+ influx was entirely blocked by the SOCC blocker SKF-96365 (20 μM) (15) and reduced by 80% with 100 μM 2-aminoethoxydiphenyl borate (2-APB) (16, 17), indicating involvement of a Ca2+ entry channel possessing SOCC-like pharmacology.
Fig. 1.
RyR1 and α1sdhpr are both necessary for engaging ECCE. (a) Response of WT myotubes (n = 25 cells) to 5-sec stimulation with caffeine (caf) or K+ in the presence of [Ca2+]o and to K+ immediately after reducing the [Ca2+]o concentration to 7 μM. PM, plasma membrane. (b) Depolarization (2, 4, and 8 sec) of primary myotubes (n = 25) resulted in an extremely rapid entry of Mn2+ as determined by monitoring the quench of fura-2 fluorescence. The response of fura-2 to depolarization exhibited an overshoot in quench, which is likely the consequence of slight deviations from excitation (ex) at 360 nm, indicating that Ca2+ was released during EC coupling. The average rates of baseline quench and that induced by depolarization are indicated by dashed traces i (horizontal) and ii (vertical), respectively, and are summarized in Table 1. (c) Response of dyspedic myotubes (n = 25) to 10 sec of stimulation with caffeine or K+ in the presence of [Ca2+]o. (d) Dyspedic myotubes (n = 56) did not produce detectable quench of fura-2 fluorescence upon K+ depolarization for 5 or 10 sec. (e) Response of dysgenic myotubes (n = 35) to 10 sec of stimulation with K+ or caffeine in the presence of [Ca2+]o. (f) Dysgenic myotubes (n = 45) did not produce detectable quench of fura-2 fluorescence upon K+ depolarization. The very slow basal rate of Mn2+ entry in quiescent cells was linear over several minutes and comparable across all cell types. (Scale: R represents a 340- to 380-nm excitation ratio for fluorescence emission at 510 nm; F.U. are at an excitation of 360 nm.)
Table 1. Initial rates of Mn2+ entry into primary myotubes in the presence and absence of RyR1, α1sdhpr, or the Ca2+-entry mutant SKEIIIKdhpr.
| WT RyR1
|
Dyspedic
|
Dysgenic
|
SkEIIIKDHPR
|
|||||
|---|---|---|---|---|---|---|---|---|
| Ryanodine | - | + | - | + | - | + | - | + |
| Rest | 0.195 ± 0.025 | 0.485 ± 0.061 | 0.056 ± 0.003 | 0.053 ± 0.004 | 0.111 ± 0.008 | 0.216 ± 0.022 | 0.163 ± 0.015 | 0.415 ± 0.030 |
| KCl | 11.42 ± 1.08 | 5.35 ± 0.75 | 0.055 ± 0.004 | 0.057 ± 0.005 | 0.110 ± 0.011 | 0.195 ± 0.022 | 9.99 ± 0.89 | 6.1 ± 1.4 |
Rates are shown for naïve myotubes (-) and myotubes pretreated with 500 μM ryanodine for 1 hr (+) to block RyR1 when present. For each condition, initial rates are shown for myotubes in resting solution (Rest) and in response to a 5-sec wash in a depolarizing solution containing 80 mM K+ (KCl). Data were obtained with high-speed imaging, are shown in arbitrary F.U., and represent the mean ± SE of 6-26 myotubes.
Primary dyspedic myotubes failed to produce a Ca2+ transient in response to either K+ or caffeine (Fig. 1c), consistent with previous reports (18, 19). In addition to having a very slow rate of Mn2+ entry at rest, dyspedic myotubes also failed to produce a detectable increase in the rate of quench of fura-2 fluorescence by Mn2+ in response to depolarization, indicating that expression of RyR1 is required to engage the depolarization-triggered cation influx (Fig. 1d and Table 1). Dysgenic myotubes (20) that lack α1SDHPR but express RyR1 also failed to show Ca2+ transients in response to membrane depolarization but responded vigorously to caffeine (Fig. 1e). As with dyspedic cells, depolarization of dysgenic myotubes was unable to trigger a measurable increase in the rate of Mn2+ influx (Fig. 1f and Table 1). However, after complete depletion of SR stores with thapsigargin (TG) in a Ca2+-free medium, replenishment of Ca2+ from the external medium of WT, dyspedic, or dysgenic cells caused a rise in cytosolic Ca2+ consistent with SOCE. Consistent with the results of Pan et al. (21), dyspedic myotubes exhibit attenuated SOCE in response to depletion of SR by TG. Collectively, these results indicate the existence of two forms of Ca2+ entry in myotubes. Classic SOCE depends on the depletion of ER/SR Ca2+ stores but does not require the presence of RyR1 and α1SDHPR. A previously uncharacterized form of depolarization-triggered Ca2+ entry, which we term ECCE, requires the presence of both RyR1 and α1SDHPR.
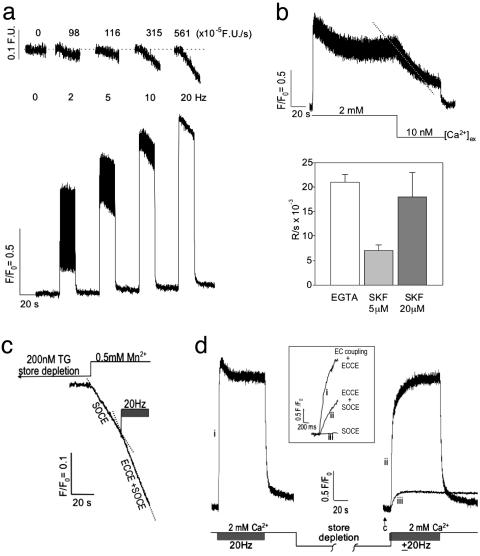
In the presence of [Ca2+]o, depolarization of fluo-4-loaded myotubes by a train of electrical pulses induced a frequency-dependent (2-20 Hz) summation of Ca2+ transients (Fig. 2a Lower). Measurements of fura-2 quench in myotubes revealed that electrical stimulation initiated a Mn2+ influx with rates that were proportional to the frequency of stimulation (Fig. 2a Upper). The contribution of cation entry triggered by depolarization to the sustained component of the Ca2+ transients was further examined (Fig. 2b). The amplitude of the Ca2+ transients produced by each pulse train exhibited an initial peak followed by decay to a “steady state” (S-S) that was attained within 20 sec and sustained for the length of the stimulus. Upon achieving S-S responses, the reduction of the [Ca2+]o concentration to 10 nM resulted in a rapid decay of Ca2+ transient amplitude. Although skeletal EC coupling is independent of [Ca2+]o, our results show that depolarization-induced Ca2+ influx is essential to sustain Ca2+ transients during pulse trains. The type of Ca2+ entry channel responsible for the sustained phase was probed with the SOCC blocker SKF-96365. Fig. 2b (Lower) shows that low-micromolar SKF-96365 rapidly caused a decay of the S-S phase of the Ca2+ transients produced by 20-Hz pulses. The blocking action of SKF-96365 was dose-dependent, with 20 μM giving the same rate of decay seen in the presence of 10 nM [Ca2+]o (Fig. 2b Lower), and these effects of SKF-96365 were completely reversible with a 5-min wash out (data not shown). The existence of ECCE was further examined under extreme conditions of chronic store depletion. Myotubes loaded with fura-2 were placed in a nominally Ca2+-free [Ca2+]o concentration and challenged with TG, an irreversible blocker of SR/ER Ca2+-ATPase (SERCA). An initial rise in intracellular Ca2+ concentration was monitored as Ca2+ leaked from stores, followed by a decline to the original baseline as Ca2+ was extruded from the cell. At this time, cells failed to respond to caffeine. After depletion, the external solution was changed to one containing Mn2+, excitation was shifted to 360 nm, and the rate of quench of fura-2 monitored (Fig. 2c). Under conditions of severe depletion, the quench rate ascribed to SOCE was 0.47 ± 0.05 fluorescence units (F.U.)·sec-1 (n = 10 cells). A 20-Hz pulse train enhanced the rate of Mn2+ entry ≈2-fold (ECCE plus SOCE = 0.81 ± 0.07 F.U.·sec-1), revealing the rate of Mn2+ entry to be ascribable to an ECCE of 0.34 ± 0.04 F.U.·sec-1 (n = 10). Experiments aimed at depleting SR stores were also under-taken in the absence of TG. After measuring responses to a pulse train (Fig. 2d), we exchanged the external medium for one lacking [Ca2+]o and challenged cells (30 sec each) repeatedly with 20 mM caffeine until they failed to respond to the last caffeine application (Fig. 2d, arrow). A subsequent pulse train initiated immediately before return of 2 mM [Ca2+]o (using a micropipette directly over the cells being monitored) showed that under these conditions Ca2+ entry was extremely rapid (2.15 ± 0.30 F.U.·sec-1; n = 5) but slower than the rate of skeletal EC coupling plus ECCE (7.13 ± 1.06 F.U.·sec-1; n = 5) (Fig. 2d Inset, compare traces i and ii). Thus, the initial rate attributable to ECCE, under conditions of chronic store depletion is ≈43% of that of EC coupling when elicited by a 20-Hz pulse train. Significantly, the initial rate of rise in cell Ca2+ in the presence of depolarizing pulses (ECCE plus SOCE) was monotonic and reached the S-S level originally observed before Ca2+ depletion, at which time individual transient summation ascribable to EC coupling was observed. Also significant was that in the presence of SERCA activity, the apparent rate of SOCE alone was much slower than ECCE (0.055 ± 0.006; n = 5) (Fig. 2d Inset, compare traces ii and iii).
Fig. 2.
Electrical-field stimulation induces ECCE. (a)Ca2+ transients produced in primary myotubes (n = 15) in response to trains of electrical stimuli at 2, 5, 10, and 20 Hz (25-msec duration, 3 V) in the presence of 2 mM [Ca2+]o.(Upper) The rate of Mn2+ entry is proportional to the stimulus frequency monitored by the quench in fura-2 fluorescence. (Lower) Ca2+ transients exhibit an initial peak that decrements to a S-S level that is sustained for the duration of the pulse train. (b) (Upper) Removal of external Ca2+ results in the loss of the S-S response (dashed trace). (Lower) Likewise, SKF-96365 produces a decay of the S-S phase of the response with a rate that depends on the concentration of this SOCC blocker. The decay of the S-S phase subsequent to buffering [Ca2+]o with EGTA or brief addition of SKF-96365 is summarized (mean and SE from n = 8, 3, and 3 myotubes, respectively). The decay rate of the S-S phase observed in the presence of 20 μM SKF-96365 (SKF) was not significantly different from that measured when [Ca2+]o was removed from primary myotubes (P > 0.05). Field stimuli are at 3 V and 25 ms in duration at 20 Hz, and Ca2+ transients were recorded with fluo-4. (c) Quench of fura-2 fluorescence in response to Mn2+ entry from the external medium subsequent to complete store depletion with 200 nM TG was ascribable to SOCE. A pulse train initiated after measurement of the SOCE component resulted in an ≈2-fold increase in the rate of quench (ECCE plus SOCE). The trace shown is an average from three myotubes. See the text for rates. (d) The responses of WT myotubes to a pulse train before and after store depletion (without the use of SERCA blocker TG) using fluo-4 gave a rapid rate of Ca2+ rise (time to peak, <100 msec). After an initial pulse train in the presence of 2 mM [Ca2+]o, aCa2+-free solution was applied and the cells were challenged four to six times (30 sec each) with 20 mM caffeine. The last caffeine challenge failed to produce measurable Ca2+ release from the SR (arrow). Rapid substitution of 2 mM [Ca2+]o in the presence (trace ii) or absence (trace iii) of a train of electrical pulses resulted in a significantly different initial rates of rise for cytoplasmic Ca2+. (Inset) An expanded time scale of the initial rates of Ca2+ entry for EC coupling plus ECCE (trace i), ECCE plus SOCE (trace ii), and SOCE (trace iii). Rates are given in the text. Data were acquired at 20 Hz by using photometry. Data shown is an average of three myotubes and is representative of data acquired from at least 10 cells in separate culture wells and on separate days.
Collectively, these data indicated that sustained Ca2+ transients induced in electrically stimulated myotubes required [Ca2+]o entry through an ECCE mechanism. Even under non-physiological conditions of complete store depletion (with or without blocking SERCA), two distinct mechanisms of Ca2+ entry exist, one that depends on the level of store depletion (SOCE) another triggered by membrane depolarization (ECCE).
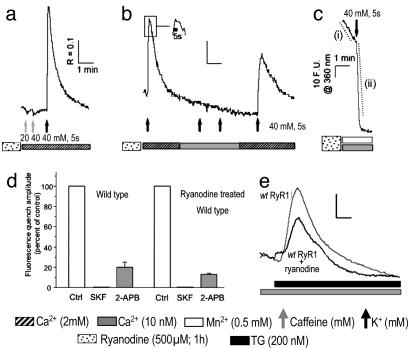
The lack of ECCE in dyspedic myotubes raises the possibility that intracellular Ca2+ release might be important for activation of the entry current. We therefore tested whether depolarization could trigger Mn2+ entry in WT myotubes pretreated with high-micromolar ryanodine, which have been shown to elicit sequential biphasic activation followed by persistent inactivation of RyR1 channels (22-24). We first demonstrated that pretreatment of WT primary myotubes with 500 μM ryanodine for 1 hr locked RyR1 into an inactive conformation by showing a complete loss of cellular responses to caffeine in treated cells (Fig. 3 a and b). Despite the lack of any response to caffeine, depolarization of ryanodine-treated myotubes with K+ triggered a large Ca2+ transient that persisted for the duration of the K+ application (Fig. 3b Inset) and slowly returned to baseline upon removal of K+ from the external medium. Virtually the entire Ca2+ transient triggered by depolarization of ryanodine-treated myotubes can be accounted for by Ca2+ entry because these transients were eliminated when the [Ca2+]o concentration was lowered to 10 nM (Fig. 3b). Mn2+ quench experiments revealed that the baseline rate of Mn2+ entry in quiescent myotubes pretreated with ryanodine was 2.5-fold greater than naïve WT myotubes (Fig. 3c, dashed trace i, and Table 1). Depolarization of ryanodine-treated myotubes with addition of K+ triggered a Mn2+ influx that lasted the length of the stimulus, with an initial rate that was 11-fold greater than the respective baseline entry in quiescent cells treated with ryanodine (Fig. 3c, dashed trace ii, and Table 1). As observed with depolarization-induced Mn2+ influx in untreated myotubes, a Mn2+ influx triggered by depolarization in ryanodine-treated myotubes was abolished by the SOCC blocker SKF-96356 (20 μM) or GdCl3 (data not shown) and was reduced by 80% and 87% when naïve and ryanodine-pretreated cells were exposed to 100 μM 2-APB (Fig. 3d). The depolarization-induced cation influx observed after ryanodine pretreatment of WT myotubes was neither a consequence of, nor dependent on, a significant chronic depletion of SR Ca2+ stores, because the addition of TG to ryanodine-treated myotubes resulted in a robust mobilization of SR Ca2+ stores (Fig. 3e). The levels of Ca2+ within TG-sensitive stores in ryanodine-treated myotubes were on average 76 ± 5% that of naïve cells. The enhanced basal entry seen in ryanodine-treated myotubes was likely the result of a partial depletion of the SR Ca2+ store and the result of a SOCE phenomenon previously shown to exist in skeletal myotubes (Fig. 2d) (1, 25). The lack of any measurable effect of ryanodine on basal- or K+-evoked Mn2+ quench in dyspedic cells supports this interpretation (Table 1). In contrast to the dynamic regulation of ECCE seen with naïve myotubes, ryanodine treatment induces a persistent conformational change of RyR1 that slows activation of ECCE 2-fold (Table 1), maintains its activity for the duration of a depolarization (Fig, 3b Inset), and significantly slows its inactivation kinetics upon membrane repolarization (Fig. 3b). These findings are evidenced by the prolonged Ca2+ transient observed in ryanodine-pretreated myotubes in response to a 5-sec depolarization compared with the response of naïve myotubes to a 10-sec depolarization (compare Figs. 1a and 3b). The persistent activation maintained by membrane depolarization is unlikely to have a component of SOCE because SOCE is inhibited by membrane depolarization (26). Because ryanodine pretreatment partially depletes SR stores, enhanced SOCE may contribute to slowing the recovery kinetics of the transient once the cell is repolarized. Because activation of cation influx depends on depolarization rather than chronic SR store depletion, ECCE is distinct from the classic depletion-activated Ca2+ influx mechanism, such as that elicited by administration of the SERCA blockers TG and cyclopiazonic acid or the depletion of stores by repetitive stimuli in the absence of [Ca2+]o (1, 25, 26). Interestingly concentrations of ryanodine that lock RyR1 into a blocked conformation have recently been shown to cause an ≈2-nm shift between DHPRs within tetrads, indicating that ryanodine induces large conformational changes in the α1SDHPR-RyR1 complex (27). The present finding that the ryanodine-modified junctional complex also alters the kinetics of ECCE suggests a tight functional coupling among three Ca2+ channels.
Fig. 3.
ECCE persists after inactivation of RyR1 by ryanodine pretreatment. (a) Response of WT (wt) myotubes pretreated with ryanodine (n = 33) to 5 sec of stimulation with caffeine or K+ in the presence of [Ca2+]o.(b) Response of myotubes pretreated with ryanodine (n = 16) to stimulation by K+ for 5 sec in the absence and presence of [Ca2+]o. (Inset) Illustrated is the sustained response of myotubes pretreated with ryanodine to K+ for 5 sec by showing an expanded time axis of the transient region indicated within the box. (c) Myotubes pretreated with ryanodine (n = 45) exhibited large Mn2+ entry in response to depolarization as monitored by the quench of fura-2 fluorescence. The average quench rates indicated by dashed traces i and ii are summarized in Table 1. (d) Inhibitory effect of SOCC blockers SKF-96365 (20μM, 1- to 2-min exposure) and 2-APB (100 μM, 5-min exposure) on a 5-sec, 40 mM, K+-induced Mn2+ quench in control (ctrl; n = 18) and ryanodine-pretreated (500 μM, 1 hr; n = 17) WT myotubes. The absolute peak amplitudes of Mn2+ quench were 18.2 ± 0.6 and 100 ± 5.7 (in arbitrary F.U.) for naïve and ryanodine-pretreated cells, respectively. (Scale: R represents a 340- to 380-nm excitation ratio; F.U. are at an excitation of 360 nm.) (e) Representative responses of control (n = 11) and ryanodine-pretreated (n = 27) WT primary myotubes challenged with 200 nM TG in the absence of [Ca2+]o.
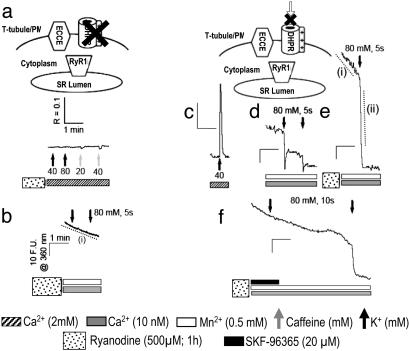
The α1SDHPR appears to serve as the voltage sensor for ECCE because depolarization of dysgenic myotubes, which lack α1SDHPR, fails to trigger ECCE whether or not the cells were pretreated with ryanodine (Fig. 4 a and b). SkEIIIKDHPR contains a Glu-to-Lys mutation within the pore region of repeat III that, when expressed in dysgenic myotubes, fails to support inward Ca2+ current at any potential but restores both evoked contractions and depolarization-dependent intracellular Ca2+ transients with properties similar to those for the WT DHPR (10). To test the hypotheses that the DHPR acts solely as the voltage sensor for ECCE and that ECCE is not dependent on l-type Ca2+ entry, we expressed SkEIIIKDHPR in dysgenic myotubes. Expression of SkEIIIKDHPR in primary dysgenic myotubes restored both skeletal EC coupling (Fig. 4c) and depolarization-activated Mn2+ entry (Fig. 4d) to levels comparable with those seen in WT myotubes (Fig. 1b). Thus, although the depolarization-triggered cation entry ascribed to ECCE is initiated by activation of the α1SDHPR voltage sensor, l-type Ca2+ entry is neither responsible nor necessary for engaging ECCE. As is seen in WT myotubes, Mn2+ entry in cells expressing SkEIIIKDHPR is completely blocked by the presence of 20 μM SKF-96365 (18 of 18 cells; data not shown). As seen in WT myotubes, large depolarization-induced Mn2+ entry is observed after ryanodine pretreatment (Fig. 4e and Table 1), and entry is completely inhibited by SKF-96365 in a reversible manner (Fig. 4f) in cells expressing SkEIIIKDHPR.
Fig. 4.
Role of DHPR in ECCE. (a) Response of primary dysgenic myotubes pretreated with ryanodine (n = 35) to 5 sec of stimulation with K+ or caffeine in the presence of [Ca2+]o.(b) Dysgenic myotubes pretreated with ryanodine (n = 47) did not produce additional quench of fura-2 fluorescence upon depolarization for 5 sec with 80 mM K+. The mean rate of quench indicated by dashed trace i is given in Table 1. (c) Response of dysgenic myotubes transfected with SkEIIIKdhpr (n = 30) to 5 sec of stimulation with K+ in the absence of [Ca2+]o. Cells pretreated with ryanodine (500 μM) failed to respond to caffeine but produced a large Ca2+ entry upon depolarization ascribable to ECCE (data not shown). (d) Dysgenic myotubes transfected with SkEIIIKdhpr (n = 42) produced transient entry of Mn2+ upon being depolarized for 5 sec, as determined by monitoring the quench in fura-2 fluorescence. (e) Dysgenic myotubes transfected with SkEIIIKdhpr and pretreated with ryanodine exhibited a basal quench in the absence of a K+ bolus (n = 40; rate measured from region indicated by dashed trace i) (Table 1). Depolarization by addition of K+ dramatically enhanced rate of Mn2+ entry (dashed trace ii; see Table 1). (f) Mn2+ entry was reversibly blocked by SKF-96365 in dysgenic myotubes expressing SkEIIIKdhpr and pretreated with ryanodine (n = 20). (Scale: R represents a 340- to 380-nm exitation ratio; F.U. are at an excitation of 360 nm.)
Further characterization of the ECCE pathway engaged by depolarization in ryanodine-treated primary dysgenic myotubes expressing either WT DHPR or SkEIIIKDHPR showed that this channel allowed permeation of Sr2+ in addition to Ca2+ and Mn2+. The magnitude of cation flux depended on the concentration of Ca2+ or Sr2+ in the external medium, and the concentrations that gave half-maximal fluxes were 100 and 250 μM, respectively (data not shown). Thus, with the exception that they require voltage gated conformational coupling for activation, based on both pharmacological blockade and cation selectivity, the channels involved in ECCE appear to have SOCC-like properties.
RyR1 and RyR3 have been shown to interact with and influence the gating of the putative SOCC, human transient receptor potential cation channel 3 (hTRPC3) (4). However, unlike RyR1, RyR3 is unable to engage α1SDHPR to support either EC coupling or tetradic organization of DHPRs when it is expressed in dyspedic myotubes (13, 28). The ability of RyR1 to functionally and/or physically interact with α1SDHPR appears essential for ECCE because depolarization of RyR3-expressing dyspedic myotubes before or after pretreatment with ryanodine yielded no voltage-triggered Ca2+ transient and no detectable Mn2+ entry (data not shown).
In this communication, we present evidence for a physiological mechanism of Ca2+ entry that we term ECCE. This mechanism is distinct from conventional SOCE, which is activated by substantial depletion of intracellular Ca2+ stores. Instead, ECCE is rapidly triggered by depolarization of the plasma membrane and requires an interaction among the α1SDHPR, RyR1, and a yet to be identified Ca2+ entry channel that possesses the pharmacological and permeation properties of a SOCC. The Ca2+ entry associated with ECCE responds rapidly and in a use-dependent manner to maintain the store of Ca2+ contained within the SR during physiological trains of excitation. ECCE may also provide an independent Ca2+ signal of yet unknown function. Importantly, the data clearly demonstrate that Ca2+ entry activated by ECCE shuts off rapidly upon repolarization of the myotubes and thus cannot be the consequence of substantial, chronic store depletion, as is necessary for conventional capacitative Ca2+ entry. The model we propose for ECCE is depicted in Fig. 5. The α1SDHPR initially senses plasma membrane depolarization triggering concurrent signals to RyR1 and a sarcolemmal Ca2+ entry channel. The signal from the DHPR to RyR1 induces a conformational change of RyR1 that allows Ca2+ efflux from the SR (EC coupling) as well as a (likely distinct) conformational change such that RyR1 engages ECCE and enables Ca2+ influx. Although the channel mediating ECCE is not identified, the extreme rapidity with which it is activated by membrane depolarization indicates that it may be part of the DHPR tetrad. Alternatively, ECCE channels may be located across alternate RyR1 feet where DHPR is absent. Although activation of SOCE by SR depletion can be influenced by conformational changes in RyR1 or RyR3 alone (24, 26), experiments with dysgenic myotubes and those expressing SkEIIIKDHPR clearly identify α1SDHPR as an essential element of ECCE. Functional coupling between DHPR and Ca2+ entry is supported by the inability to trigger ECCE in primary dysgenic cells whether or not they are pretreated with ryanodine and the observation that Ca2+ influx remains activated in WT myotubes pretreated with ryanodine until the depolarizing stimulus is removed. Furthermore, because the pretreatment with ryanodine causes RyR1 to assume a conformation that is refractory for EC coupling, it seems likely that at least some conformations of RyR1 that regulate ECCE may differ from those essential for EC coupling. The present results suggest that dynamic changes in RyR1 conformation known to be elicited during EC coupling provides retrograde signals not only to the DHPR but to a Ca2+ influx channel having SOCC-like properties, possibly by direct conformational coupling of three types of channels. Recent work by Eltit et al. (29) identified slow Ca2+ signals elicited by tetanic electrical stimulation of skeletal myotubes that are mediated by the diffusible second messenger inositol 1,4,5-trisphosphate (IP3). Several properties distinguish ECCE from IP3-mediated slow Ca2+ signals. Most significant is the difference in activation kinetics [t1/2 = 300 msec for ECCE elicited by a 20-Hz train versus t1/2 = 54 sec elicited by a 45-Hz train for a IP3-generated slow Ca2+ wave (29)]. Although we cannot discount the involvement of a diffusible second messenger in the activation of ECCE, rapid activation of ECCE by depolarization suggests a more direct mechanism.
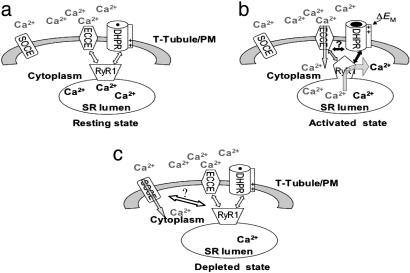
Fig. 5.
Proposed model for ECCE. (a) Resting state. (b) Activated state. α1Sdhpr initially senses plasma membrane depolarization (ΔEM), which triggers concurrent signals to RyR1 and possibly SOCC. The signal to RyR1 induces a conformational change (represented by changing from a trapezoid in the resting state to a pentagon in the activated state) such that RyR1 engages SOCC to activate [Ca2+]o influx (gray arrow) into the cytoplasm. The Ca2+ is then resequestered into the SR lumen to replenish the Ca2+ released from the SR by means of RyR1 (black arrow) during EC coupling. (c) Depleted state showing the activation of conventional SOCE.
The distinguishing feature of SOCE is that its activation is consequential to the emptying of intracellular Ca2+ stores, a process also commonly referred to as capacitative Ca2+ entry (CCE). The most studied form of CCE is produced by the intracellular signaling molecule IP3 (2, 30). However, there is compelling evidence that CCE is not the only Ca2+ entry pathway regulated by receptors that couple to the production of phospholipase C, IP3, and diacylglycerol. Such noncapacitative Ca2+ entry pathways can contribute significantly to the Ca2+ signals evoked by physiological stimuli (31, 32). Several Ca2+-permeable channels, many of which are assembled from TRPC proteins, are regulated by phosphatidylinositol 4,5-bisphosphate or elements of the DAG limb of the phosphoinositide pathway. TRPC proteins have been described as putative SOCCs and have been shown to share significant structural homology with the α1 subunit of voltage-gated Ca2+ channels (33). Kiselyov et al. (4) have presented data consistent with the hypothesis that RyR1 and RyR3 can conformationally couple with hTRPC3 expressed in HEK293 cells. More recently, TRPC 1, 2, 3, 4, and 6 have been shown to be present in adult skeletal myotubes (34). It is therefore attractive to speculate that the channel activated by ECCE resembles a member of the TRPC family and interacts with RyR1 in a manner analogous to that documented for the interaction between DHPR and RyR1. However, the cation channel that is responsible for ECCE remains to be elucidated. Also yet to be determined is whether the channel(s) underlying Ca2+ entry in response to store depletion and the channels involved in ECCE exist as a single population regulated by both processes or as two distinct populations.
We conclude that ECCE is a context-specific mechanism for producing Ca2+ entry during EC coupling. The absence of either the α1SDHPR or RyR1 or the substitution of RyR1 with RyR3 abolishes this physiological coupling with Ca2+ entry. The properties of ECCE identified here in skeletal myotubes suggests that although Ca2+ entry activated by ER/SR store depletion is a generalized phenomenon, a specialized, highly controlled refilling of stores can occur in response to normal physiological triggers as a result of functional and physical associations of Ca2+-release channels of the ER/SR with SOCC and voltage-gated channels of the plasma membrane. ECCE may also occur in nonmuscle cells, such as cerebellar granule neurons, where RyR1, l-type Ca2+ channels, and SOCC, such as TRPCs, all occur (35, 36). It would be of significant interest to determine whether ECCE, if given the correct cue(s), could also be activated in other nonmuscle cells as a means to refill and maintain Ca2+ stores and control important aspects of Ca2+ signaling.
Acknowledgments
This work was supported by National Institutes of Health Grants 2PO1 AR17605 and 1PO1 ES11269.
Author contributions: G.C. and I.N.P. designed research; G.C. and A.M.H. performed research; P.D.A. and K.G.B. contributed new reagents/analytical tools; G.C., A.M.H., E.H.L., and I.N.P. analyzed data; G.C., A.M.H., and I.N.P. wrote the paper; E.H.L. provided supporting data; E.H.L. and K.G.B. edited the paper; and J.D.F. made the original observation that ryanodine enhances depolarization-triggered Ca entry in myotubes.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PM, plasma membrane; ER, endoplasmic reticulum; SR, sarcoplasmic retic- ulum; SOCE, store-operated Ca2+ entry; EC, excitation-contraction; DHPR, dihydropyridine receptor; RyR1, ryanodine receptor 1; SOCC, store-operated Ca2+ channels; ECCE, excitation-coupled Ca2+ entry; [Ca2+]o, extracellular Ca2+; 2-APB, 2-aminoethoxydiphenyl borate; TG, thapsigargin; S-S, steady state; SERCA, ER/SR Ca2+-ATPase; IP3, inositol 1,4,5-trisphosphate; F.U., fluorescence unit.
References
- 1.Ma, J. & Pan, Z. (2003) Front. Biosci. 8, D242-D255. [DOI] [PubMed] [Google Scholar]
- 2.Putney, J. W., Broad, L. M., Braun, F.-J., Lievremont, J.-P. & Bird, G. S. J. (2001) J. Cell Sci. 114, 2223-2229. [DOI] [PubMed] [Google Scholar]
- 3.Launikonis, B. S., Barnes, M. & Stephenson, D. G. (2003) Proc. Natl. Acad. Sci. USA 100, 2941-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiselyov, K., Shin, D. M., Wang, Y., Pessah, I. N., Allen, P. D. & Muallem, S. (2000) Mol. Cell 6, 421-431. [DOI] [PubMed] [Google Scholar]
- 5.Kiselyov, K., Xu, X., Mozhayeva, G., Kuo, T., Pessah, I. N., Mignery, G., Zhu, Z., Birnbaumer, L. & Muallem, S. (1998) Nature 396, 478-482. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa, Y., Kurebayashi, N. & Murayama, T. (1999) Adv. Biophys. 36, 27-64. [DOI] [PubMed] [Google Scholar]
- 7.Protasi, F. (2002) Front. Biosci. 7, D650-D658. [DOI] [PubMed] [Google Scholar]
- 8.Rando, T. A. & Blau, H. M. (1994) J. Cell Biol. 125, 1275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beam, K. G. & Knudson, C. M. (1988) J. Gen. Physiol. 91, 781-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dirksen, R. T. & Beam, K. G. (1999) J. Gen. Physiol. 114, 393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahern, C. A., Sheridan, D. C., Cheng, W., Mortenson, L., Nataraj, P., Allen, P., De Waard, M. & Coronado, R. (2003) Biophys. J. 84, 942-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clementi, E., Scheer, H., Zacchetti, D., Fasolato, C., Pozzan, T. & Meldolesi, J. (1992) J. Biol. Chem. 267, 2164-2172. [PubMed] [Google Scholar]
- 13.Fessenden, J. D., Wang, Y., Moore, R. A., Chen, S. R., Allen, P. D. & Pessah, I. N. (2000) Biophys. J. 79, 2509-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merritt, J. E., Jacob, R. & Hallam, T. J. (1989) J. Biol. Chem. 264, 1522-1527. [PubMed] [Google Scholar]
- 15.Leung, Y. M. & Kwan, C. Y. (1999) Jpn. J. Pharmacol. 81, 253-258. [DOI] [PubMed] [Google Scholar]
- 16.Diver, J. M., Sage, S. O. & Rosado, J. A. (2001) Cell Calcium 30, 323-329. [DOI] [PubMed] [Google Scholar]
- 17.Dobryndeva, Y. & Blackmore, P. (2001) Mol. Pharmacol. 60, 541-552. [PubMed] [Google Scholar]
- 18.Nakai, J., Dirksen, R. T., Nguyen, H. T., Pessah, I. N., Beam, K. G. & Allen, P. D. (1996) Nature 380, 72-75. [DOI] [PubMed] [Google Scholar]
- 19.Dirksen, R. T. (2002) Front. Biosci. 7, D659-D670. [DOI] [PubMed] [Google Scholar]
- 20.Beam, K. G., Knudson, C. M. & Powell, J. A. (1986) Nature 320, 168-170. [DOI] [PubMed] [Google Scholar]
- 21.Pan, Z., Yang, D., Nagaraj, R. Y., Nosek, T. A., Nishi, M., Takeshima, H., Cheng, H. & Ma, J. (2002) Nat. Cell Biol. 4, 379-383. [DOI] [PubMed] [Google Scholar]
- 22.Pessah, I. N. & Zimanyi, I. (1991) Mol. Pharmacol. 39, 679-689. [PubMed] [Google Scholar]
- 23.Zimanyi, I., Buck, E., Abramson, J. J., Mack, M. M. & Pessah, I. N. (1992) Mol. Pharmacol. 42, 1049-1057. [PubMed] [Google Scholar]
- 24.Sutko, J. L., Airey, J. A., Welch, W. & Ruest, L. (1997) Pharmacol. Rev. 49, 53-98. [PubMed] [Google Scholar]
- 25.Ma, J. & Pan, Z. (2003) Cell Calcium 33, 375-384. [DOI] [PubMed] [Google Scholar]
- 26.Kurebayashi, N. & Ogawa, Y. (2001) J. Physiol. (London) 533, 185-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paolini, C., Fessenden, J. D., Pessah, I. N. & Franzini-Armstrong, C. (2004) Proc. Natl. Acad. Sci. USA 101, 12748-12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Protasi, F., Takekura, H., Wang, Y., Chen, S. R., Meissner, G., Allen, P. D. & Franzini-Armstrong, C. (2000) Biophys. J. 79, 2494-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eltit, J. M., Hidalgo, J., Liberona, J. L. & Jaimovich, E. (2004) Biophys. J. 86, 3042-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatachalam, K., van Rossum, D. B., Patterson, R. L., Ma, H.-T. & Gill, D. L. (2002) Cell Biol. 4, E263-E272. [DOI] [PubMed] [Google Scholar]
- 31.Montell, C., Birnbaumer, L. & Flockerzi, V. (2002) Cell 108, 595-598. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, C. W. (2002) Cell 111, 767-769. [DOI] [PubMed] [Google Scholar]
- 33.McFadzean, I. & Gibson, A. (2002) Br. J. Pharmacol. 135, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandebrouck, C., Martin, D., Colson-Van Schoor, M., Debaix, H. & Gailly, P. (2002) J. Cell Biol. 158, 1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavis, P., Fagni, L., Lansman, J. B. & Bockaert, J. (1996) Nature 382, 719-722. [DOI] [PubMed] [Google Scholar]
- 36.Riccio, A., Medhurst, A. D., Mattei, C., Kelsell, R. E., Calver, A. R., Randall, A. D., Benham, C. D. & Pangalos, M. N. (2002) Brain Res. Mol. Brain Res. 109, 95-104. [DOI] [PubMed] [Google Scholar]