Abstract
The interplay of environmental and genetic factors in the developmental organization of the hippocampus has not been fully elucidated. The neuropeptide corticotropin-releasing factor (CRF) is released from hippocampal interneurons by environmental signals, including stress, to increase synaptic efficacy. In the early postnatal hippocampus, we have previously characterized a transient population of CRF-expressing Cajal-Retzius-like cells. Here we queried whether this stress-activated neuromodulator influences connectivity in the developing hippocampal network. Using mice deficient in the principal hippocampal CRF receptor [CRF1(-/-)] and organotypic cultures grown in the presence of synthetic CRF, or CRF receptor antagonists, we found robust effects of CRF on dendritic differentiation in hippocampal neurons. In CRF1(-/-) mice, the dendritic trees of hippocampal principal cells were exuberant, an effect that was induced in normal hippocampi in vitro by the presence of CRF1 antagonists. In both cases, total dendritic length and dendritic branching were significantly increased. In contrast, exogenous synthetic CRF blunted the dendritic growth in hippocampal organotypic cultures. Taken together, these findings suggest that endogenous CRF, if released excessively by previous early postnatal stress, might influence neuronal connectivity and thus function of the immature hippocampus.
Keywords: corticotropin-releasing hormone, stress, corticotropin-releasing factor receptor, neuropeptide, Cajal-Retzius cells
The developmental organization of the hippocampal network is a complex process, requiring the interplay of genetic and environmental factors. Establishment of basic hippocampal connectivity is governed by genetically determined mechanisms, including the coordinated activation of transcription factors (1, 2, 3), and the expression of both local and more generally acting guidance molecules (4, 5, 6). Once the basic elements are in place, refinement of connectivity is achieved through environmental stimuli that influence neuronal activity (7, 8, 9). Among the elements that contribute to developmental organization of the hippocampus are the Cajal-Retzius (CR) cells (10, 11). CR cells, found in hippocampal marginal zones, release reelin, an extracellular matrix protein required for layer formation and positioning of cortical neurons (12, 13). Because lack of reelin causes perturbation of cortical lamination (14), control of neuronal positioning has been considered the major function of CR cells. However, recent results suggest additional roles for CR cells that are independent of reelin (15, 16, 17, 18).
We have previously characterized a subset of CR cells that do not express reelin but release the neuropeptide corticotropin-releasing factor (CRF).∥ CRF functions primarily as a regulator of the neuroendocrine stress response (19, 20) but is also widely expressed within the central nervous system, where it acts as a neuromodulator (21, 22, 23, 24). In mature hippocampus, although CRF is exclusively expressed in GABAergic interneurons (18, 25, 26), its physiological actions are excitatory (21, 27), enhancing synaptic efficacy (28, 29). Thus, in mature hippocampus, CRF activates and stabilizes synaptic transmission.
The role of CRF derived from CR cells and other CRF-expressing neurons in immature hippocampus has remained elusive (18). Here we investigated the function(s) of CRF in developing hippocampus by using CRF receptor-deficient [CRF1(-/-)] mice and in vitro hippocampal organotypic cultures exposed to exogenous CRF or to specific CRF1 antagonist NBI 30775 (30, 31, 32). Our results indicate that CRF influences dendritic differentiation, an essential component of neuronal communication. These findings suggest new roles for hippocampal CRF and for CRF-expressing CR cells.
Materials and Methods
Animals. Sprague-Dawley rats [postnatal days (P) 0-7] and CRF1(-/-) P6-P7 mice (C57 background) were used. Mice were genotyped by PCR (33), and CRF1(-/-) mice were compared to CRF1(+/+) littermates (WT). Animals were maintained in uncrowded, National Institutes of Health-approved facilities on a 12-h light cycle, with ad libitum access to lab chow and water. Experiments were approved by University of California and Federal Animal Care Committees.
Organotypic Slice Cultures and Manipulation of CRF Receptor Activation. Entorhinal cortex-hippocampal cultures (n = 338) were prepared from P0-P1 rat brains (34). Concentrations of CRF1 antagonist NBI 30775 {3-[6-(dimethylamino)-4-methyl-pyrid-3-yl]-2,5-dimethyl-N,N-dipropyl-pyrazolo[2,3-a]pyrimidin-7-amine, previously R121919} were 0.1 or 1 μM (for Golgi studies; n = 60 per group) and 0.01, 0.1, or 10 μM (for calbindin D-28K; n = 6-10); those of CRF (n = 56; Bachem) were 1 μM.
Tissue Processing and Immunocytochemistry. Animals were anesthetized and then perfused with a formaldehyde solution made from 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4) (18). Organotypic cultures were fixed in the perfusion solution for 30 min. Abs included rabbit anti-CRF (1:40,000, W. W. Vale, Salk Institute for Biological Studies, San Diego), goat anti-CRF1 (1:10,000, Santa Cruz Biotechnology), mouse anti-vimentin (1:100, Developmental Studies Hybridoma Bank, Iowa City, IA), mouse anti-SMI32 (1:2,000, Sternberger Monoclonals, Baltimore), and rabbit anti-calbindin D-28K (1:8,000, Chemicon). Immunocytochemistry was performed on free-floating sections (20 μm; ref. 18) by using standard avidin-biotin methods. Double labeling of CRF1 and vimentin (delineates radial glia; ref. 35) or SMI32 (a marker of subgroup of pyramidal neurons; ref. 36) were performed as described (18). Visualization used Alexa Fluor 488-anti-goat IgG and Alexa Fluor 568-anti-mouse IgG (Molecular Probes). Images were obtained by using a Zeiss LSM510 Meta confocal microscope.
Golgi Impregnation. P6-P7 CRF1(-/-) (n = 4) and WT (n = 5) mice were processed for Golgi staining (37), in parallel and “blindly.” After immersion in Golgi-Cox solution (9 d) and 30% sucrose (2 d), both in the dark, coronal sections (200 μm) were processed in 10% NH4OH (30 min) and then fixed (Kodak Fix, Kodak). For organotypic cultures, Golgi-gold toning was used (8).
Quantitative and Statistical Analyses. Individual Golgi-impregnated cells were reconstructed blindly by using camera lucida. Neurons for analysis were chosen by using unbiased systematic sampling (8-12 neurons per region per animal; ref. 18). For cultures, 9-18 neurons per region per group were analyzed. Sholl analysis (38), a method providing quantitative description of the dendritic tree by evaluating the number of dendrites that cross or branch within virtual concentric circles drawn at fixed distances from the cell body, was used. Numbers of primary dendrites, total dendritic length, and number of dendritic intersections at each concentric circle (20 μm, 40 μm, 60 μm, etc.) were measured. An independent analysis of average dendritic length used the calcium-binding protein calbindin D-28K, which serves as dendritic marker in developing hippocampal neurons. Dendritic length (distance from dendritic tip to cell body) was measured at three points (midpoint of the granule cell layer and two equidistant points 60 μm away) and averaged for comparison among experimental groups. Differences among groups were compared by using two-way ANOVA (for genotype/treatment and distance from soma), with post hoc analyses (prism, GraphPad, San Diego). Dendritic length and primary dendrites were compared by using Student's t test. Data are expressed as mean ± SEM.
Results
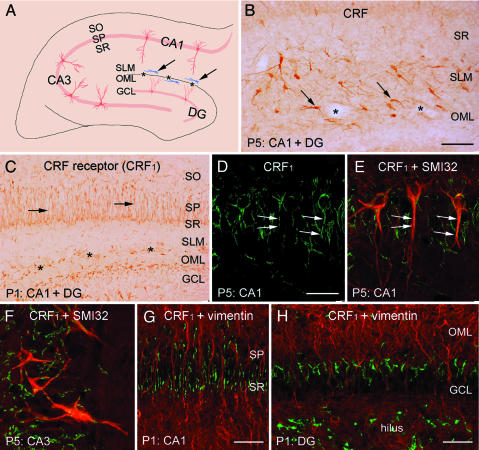
CRF and Its Receptor, CRF1, Are Expressed in the Early Postnatal Hippocampus. During the first postnatal week, a population of CRF-expressing neurons, many with characteristics of CR cells, occupied the area abutting the hippocampal fissure (Fig. 1 A and B). These cells comprise the majority of CRF-expressing cells in hippocampus at this age (18). The actions of CRF on hippocampal neurons are mediated mainly by the type 1 receptor, CRF1. Therefore, if CRF contributes to early postnatal hippocampal development, then CRF1 should be expressed during these periods, and its distribution and sub-cellular localization may provide clues for the nature of CRF influence on hippocampal organization. Indeed, CRF1 was detected in principal hippocampal neurons (Fig. 1 C-F) but not in radial glia (Fig. 1 G and H). Interestingly, in the P1 rat, CRF1 was localized primarily to dendrites (Fig. 1C), whereas at older ages, it was found also perisomatically (Fig. 1 D-F). This early dendritic localization of CRF1 suggested a role for its ligand, CRF, in dendritic development.
Fig. 1.
CRF and its receptor, CRF1, are present in early postnatal hippocampus. (A) Diagram illustrates the organization of the developing hippocampus. CR cells expressing CRF (in blue) are highlighted by arrows. (B) In P5 rat hippocampus, many CRF-immunoreactive neurons resemble CR cells (arrows). (C-H) Distribution of CRF1 in neonatal hippocampus supports a role for CRF in dendritic development. In P1 (C, G, and H) and P5 (D-F), CRF1 is expressed primarily in dendrites of pyramidal (arrows) and granule cells. Confocal images indicate that CRF1 (green) is colocalized with neurofilament SMI32 (red, E and F), a pyramidal cell marker, but not with vimentin (red, G and H), a glia marker. *, hippocampal fissure; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; SLM, stratum lacunosum-moleculare; OML, outer molecular layer; GCL, granule cell layer. (Scale bars: B and C, 75 μm; D-F, 25 μm; G, 100 μm; H, 50 μm.)
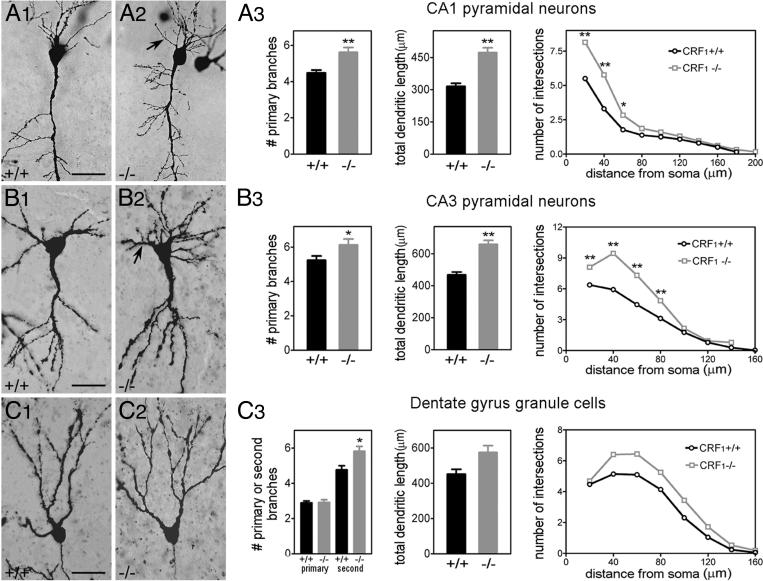
CRF1 Receptor Deletion Results in Increased Dendritic Length and Arborization. If CRF is involved in shaping dendritic development, then mice lacking CRF or CRF1 should have altered dendrites. At P6, whereas CRF1(-/-) hippocampi exhibited normal lamination and cell orientation, the size and organization of dendritic trees were abnormal (Fig. 2 A1-A3 and B1-B3). Numbers of primary dendrites and total dendritic length of CA1 pyramidal cells in CRF1(-/-) mice were higher than in WT littermates (P < 0.01, Fig. 2 A3). Using Sholl analysis (38), two-way ANOVA indicated that the null genotype significantly affected the number of dendritic intersections. The analysis further pinpointed this effect to increased proximal dendrites (Fig. 2 A3). Similarly, in CA3 pyramidal cells, numbers of dendritic intersections at 20-80 μm from the soma in CRF1(-/-) mice exceeded those of WT controls (Fig. 2B3). Granule cells were analyzed at comparable locations within the granule cell layer, and only in the earlier-maturing suprapyramidal blade to avoid maturational differences (Fig. 2 C1-C3). Lack of CRF1 led to significant increase of secondary dendrites. Two-way ANOVA of the number of dendritic intersections showed a strong effect of genotype (F[1, 48] = 15.88; P = 0.0002).
Fig. 2.
Numbers of proximal dendrites and dendritic length in hippocampal pyramidal cells are higher in CRF1(-/-) mice. Photographs (light microscopy) show Golgi-impregnated CA1 (A1 and A2) and CA3 (B1 and B2) pyramidal neurons and dentate gyrus granule cells (C1 and C2) from P6 CRF1(+/+) (A1, B1, and C1) and CRF1(-/-) (A2, B2, and C2) mice. Proximal dendrites and dendritic length are higher (*, P < 0.05; **, P < 0.01) in CA1 and CA3 pyramidal neurons from CRF1(-/-) mice (A3 and B3). CRF1 deletion has a significant effect on numbers of intersections in CA1 (F[1, 56] = 61.97; P < 0.0001) and CA3 (F[1, 49] = 62.71; P < 0.0001). These differences stem from increased proximal dendrites (*, P < 0.05; **, P < 0.01; Bonferroni's post hoc test). In dentate gyrus (C3), the number of secondary dendrites is significantly higher in CRF1(-/-) mice than in WT controls. Two-way ANOVA demonstrates an overall effect of CRF1 deletion on numbers of dendritic intersections (F[1, 48] = 15.88; P = 0.0002). n = 8-10 cells per region per animal [n = 5 WT and 4 CRF1(-/-)]. Arrows, basal dendrites in CRF1(-/-) mice. (Scale bars: A1 and A2, 40 μm; B1 and B2, 30 μm; C1 and C2, 25 μm.)
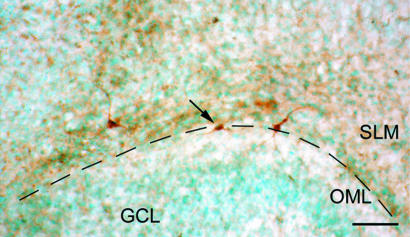
CRF1 Receptor Blockade Augments the Proximal Dendritic Trees of Pyramidal Neurons. The enlarged dendritic tree in CRF1(-/-) mice suggested that lack of actions of CRF via CRF1 may influence dendritic differentiation. However, genetically engineered mice may possess characteristics that are independent of the function of the deleted gene (39, 40). Therefore, we investigated the role of CRF1-mediated actions of CRF on dendritic development by using hippocampal organotypic culture. In cultures obtained on P1 and grown for 7 d, CRF was expressed in a pattern recapitulating in vivo hippocampus, including CRF-expressing CR cells near the hippocampal fissure (Fig. 3).
Fig. 3.
The in vivo expression pattern of CRF is recapitulated in organotypic cultures. The hippocampal slice was harvested at P1, cultured for 7 d, and then subjected to CRF immunocytochemistry (with methyl green counterstain). CRF-expressing neurons (arrow) with characteristics of CR cells are found close to the hippocampal fissure (broken line). SLM, stratum lacunosum-moleculare; OML, outer molecular layer; GCL, granule cell layer. (Scale bar: 60 μm.)
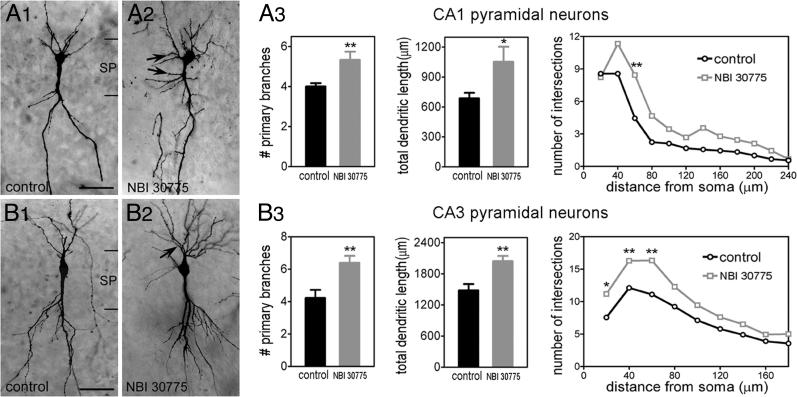
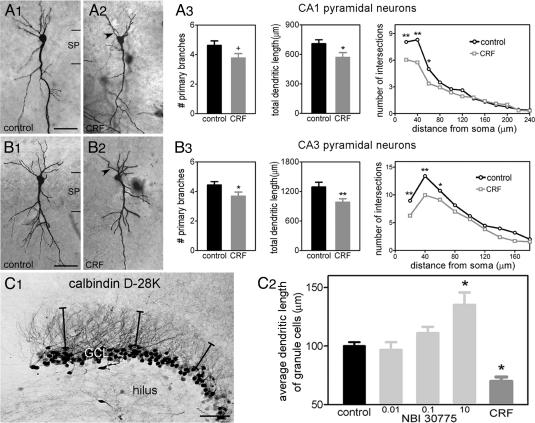
Exposure of cultures to CRF1 antagonist NBI 30775 (0.1 or 1 μM) for 6 d strongly modified the dendritic tree of principal cells, leading to hyperarborization (Fig. 4) and increased dendritic length of granule cells (Fig. 5 C1 and C2). CRF1 blockade increased the number of primary dendrites, total dendritic length, and number of dendritic intersections at 60 μm from the soma of CA1 pyramidal neurons (Fig. 4A3). The CRF1 antagonist also influenced CA3 pyramidal neurons (Fig. 4B3) and exerted a dose-dependent increase of dendritic length of granule cells as measured by using the dendritic marker calbindin D-28K (Fig. 5 C1 and C2).
Fig. 4.
CRF1 blockade increases the number of proximal dendrites (arrows) of CA1 and CA3 pyramidal cells. Slice cultures from P1 hippocampi were grown in the presence of 0.1 or 1 μM CRF1 antagonist NBI 30775 (A2 and B2) and controls (A1 and B1). Because of their indistinguishable effects, the two doses were combined for analysis. NBI 30775 treatment increased total dendritic length (*, P < 0.05) and proximal dendritic intersections (F[1, 192] = 24.77; P < 0.0001) in CA1 pyramidal cells (A3). Similarly increased were the number of dendritic intersections in CA3 (B3) (F[1, 275] = 45.81; P < 0.0001). n = 9-18 neurons per treatment per region. SP, stratum pyramidale. (Scale bars: A1 and A2, 45 μm; B1 and B2, 55 μm.)
Fig. 5.
Addition of synthetic CRF (A2 and B2) to hippocampal slice cultures reduces dendritic branching and length, evident from both Golgi impregnation and quantification of a dendritic marker. CRF exposure reduces number of primary dendrites (+, P = 0.056), total dendritic length (*, P < 0.05), and overall number of intersections (F[1, 360] = 13.50; P = 0.0003) (A3) in CA1. In CA3, number of primary dendrites (*,P < 0.05), dendritic length (**, P < 0.01), and number of intersections (F[1, 330] = 21.52; P < 0.0001) (B3) are reduced. Cultures were grown with CRF (1 μM, added on day 2) or CRF1 antagonist (0.01, 0.1, or 10 μM). n = 16 neurons per treatment per region. (C) Differential and opposing effects of CRF and CRF1 antagonist on dendritic growth also are evident when the dendritic marker calbindin D-28K is used (shown here for the granule cell dendrites in suprapyramidal blade of dentate gyrus). C1 illustrates calbindin-immunoreactive granule cells dendrites in a control culture. Lines from cell body to the tip of the dendrites were used to assess dendritic length. (C2) Exposure to CRF1 antagonist increases average dendritic length in a dose-dependent manner, whereas growing cultures in the presence of CRF reduces dendritic length (*, P < 0.05). SP, stratum pyramidale; GCL, granule cell layer. (Scale bars: A1 and A2, 45 μm; B1 and B2, 55 μm; C1, 25 μm.)
Altered Dendritic Development of Pyramidal Neurons in the Presence of CRF. If CRF (via CRF1) contributes to the selective process by which hippocampal dendrites develop or persist, then the presence of supranormal levels of the peptide should reduce the number and perhaps differentiation of dendrites. Indeed, 6 d in the presence of CRF (1 μM) reduced total dendritic length and primary branches in pyramidal cells (Fig. 5 A1-A3 and B1-B3), and using dendritic markers, reduced average dendritic length of granule cells (Fig. 5 C1 and C2). Again, the principal effect was on proximal dendrites: fewer intersections at 20, 40, and 60 μm in the CRF group (Bonferroni's post hoc test).
Discussion
The major findings of this study are as follows. (i) In early postnatal hippocampus, CRF is expressed by CR cells, and its principal receptor, CRF1, is found in neurons but not in radial glia. (ii) Absence (in vivo) or pharmacological blockade (in vitro) of CRF1 leads to exuberant dendritic arborization. (iii) High levels of CRF have the opposite effect, reducing dendritic differentiation. (iv) These effects are primarily on proximal dendritic segments and do not influence hippocampal lamination. Taken together, these findings suggest that endogenous CRF, which may be released excessively in developing hippocampus by stress, may influence hippocampal dendritic differentiation.
The distribution and function of CRF in mature hippocampus are being progressively elucidated. In juvenile and adult hippocampus, CRF is primarily expressed in GABAergic interneurons (18, 25, 26). When released from GABAergic terminals (41), CRF acts as an excitatory neuromodulator: Although not provoking action potentials, CRF amplifies excitatory signals that may then reach firing threshold (1, 24). These actions of CRF enhance normal synaptic function, promoting learning and memory processes (e.g., refs. 28 and 29). Under pathological conditions, these actions may also underlie the proconvulsant effects of the peptide (27). In general, the effects of CRF are mediated via binding to G-protein-coupled CRF receptors, CRF1 and CRF2 (42). CRF1 is predominant in rodent hippocampus (43, 44, 45), where it is selectively responsible for mediating the actions of the peptide (46, 47, 48). Recent studies show that CRF1 is preferentially located on dendritic spines, in close to excitatory synapses (41). These data indicate that on release from perisomatic GABAergic terminals, CRF needs to traverse a substantial distance to reach its receptor, assuring that only under conditions of extensive release, e.g., during stress, will sufficient peptide amounts reach CRF1 to enhance synaptic efficacy. Thus, in mature hippocampus, CRF and CRF1 may constitute an important element of the molecular machinery that mediates stress-related synaptic plasticity (29, 41).
Early in postnatal life, CRF expression differs from the mature pattern (18): During the first postnatal week, most CRF-expressing hippocampal neurons are CR cells, whose numbers decline drastically thereafter. This unique expression pattern of CRF raised the possibility of development-specific functions, and a dendritic-modulating role has been proposed in cerebellum (49, 50, 51). The exclusive location of CRF1 on neuronal dendrites excluded the possibility that CRF might act in concert on radial glia differentiation, suggesting instead that early postnatal CRF may influence the maturation of hippocampal neurons, specifically dendrites, directly.
The current studies provide evidence for a role of CRF in dendritic growth and pruning. In the absence of CRF-evoked CRF1-mediated neuromodulation, i.e., in CRF1(-/-) mice or when hippocampi are grown in vitro in the presence of CRF1 antagonists, dendritic branching and total dendritic length were increased. In contrast, increasing CRF levels artificially during the first postnatal week blunted dendritic growth. How do these findings, together with the known functions of CRF on synaptic efficacy, inform us about the role of the peptide in dendritic differentiation?
The developing dendritic arbor is a dynamic structure (52), governed by both intrinsic genetic programs and extrinsic signals (53, 54). Dendrites actively contribute to initial synaptogenic intercellular contacts via immature dendritic protrusions. Because the majority of contacts do not survive, selective stabilization of these immature synapses may be a key element in the generation of mature dendritic structures (55). The molecules and mechanisms for this selective stabilization are not fully understood and include neuronal activity (8, 56). Thus, genetic programs and guidance cues influence early phase of dendritic growth whereas activity-mediated increase of strength of select synapse may govern final neuronal connectivity (53).
The current studies suggest a role for CRF in this later phase of dendritic differentiation: The “hyperarborization” when CRF1 is absent or blocked suggests a lack of selective stabilization of synaptic contacts. This possibility is attractive because it is consonant with the established roles of the peptide in mature hippocampus, i.e., increasing synaptic efficacy (24, 28, 29). Thus, immature dendritic contacts expressing CRF1 are preferentially strengthened and stabilized by ambient CRF, compared to those lacking the receptor. Alternatively, the peptide might function to inhibit nonstabilized connections: In the absence of CRF or CRF1, excessive numbers of dendritic elements would persist as found here. In contrast, under conditions of pathological release of CRF, as occurs in immature hippocampus during stress (20, 41), excessive strengthening of few synapses and/or excessive pruning might contribute to stress-associated dendritic atrophy (57).
The downstream effects of CRF1 activation on developing dendrites are unknown. Increased cellular cAMP levels (58, 59), promoting phosphorylation of cAMP-response element-binding protein transcription factor (41, 60, 61) is an attractive possibility, because cAMP-response element-binding protein is selectively activated in developing hippocampal neurons (62) and is involved in dendritic differentiation (63). The selective effects of CRF on proximal dendrites may be dictated by the selective distribution of CRF1 at this age (Fig. 1). This intriguing laminar selectivity also suggests that CRF and CRF1 may influence the selective termination of associational/commissural connections on proximal dendrites (6, 17).
In summary, the hippocampal neuromodulator CRF acts via the CRF1 receptor to influence the structure of dendritic trees in developing hippocampal neurons. Because this peptide's release is regulated by environmental signals, it might contribute to neuroplasticity of hippocampal synaptic connectivity. In addition, under pathological conditions, excessive CRF release may contribute to abnormal dendritic patterns in certain human neurological disorders (64, 65).
Acknowledgments
We thank R. Gonzalez-Vega for excellent technical assistance. This work was supported by National Institutes of Health Grants NS28912 and NS39307 (to T.Z.B.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CRF, corticotropin-releasing factor; CR, Cajal-Retzius; Pn, postnatal day n.
Footnotes
The nomenclature of corticotropin-releasing hormone/corticotropin-releasing factor is in accordance with ref. 66.
References
- 1.Pellegrini, M., Mansouri, A., Simeone, A., Boncinelli, E. & Gruss, P. (1996) Development (Cambridge, U.K.) 122, 3893-3898. [DOI] [PubMed] [Google Scholar]
- 2.Pleasure, S. J., Anderson, S., Hevner, R., Bagri, A., Marin, O., Lowenstein, D. H. & Rubenstein, J. L. (2000) Neuron 28, 727-740. [DOI] [PubMed] [Google Scholar]
- 3.Schwab, M. H., Bartholomae, A., Heimrich, B., Feldmeyer, D., Druffel-Augustin, S., Goebbels, S., Naya, F. J., Zhao, S., Frotscher, M., Tsai, M. J., et al. (2000) J. Neurosci. 20, 3714-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chédotal, A., Del Río, J. A., Ruiz, M., He, Z., Borrell, V., de Castro, F., Ezan, F., Goodman, C. S., Tessier-Lavigne, M., Sotelo, C., et al. (1998) Development (Cambridge, U.K.) 125, 4313-4323. [DOI] [PubMed] [Google Scholar]
- 5.Skutella, T., Savaskan, N. E., Ninnemann, O. & Nitsch, R. (1999) Dev. Biol. 211, 277-292. [DOI] [PubMed] [Google Scholar]
- 6.Zhao, S., Förster, E., Chai, X. & Frotscher, M. (2003) J. Neurosci. 23, 7351-7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kossel, A. H., Williams. C. V., Schweizer, M. & Kater, S. B. (1997) J. Neurosci. 17, 6314-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drakew, A., Frotscher, M. & Heimrich, B. (1999) Neuroscience 94, 767-774. [DOI] [PubMed] [Google Scholar]
- 9.Galvan, C. D., Hrachovy, R. A., Smith, K. L. & Swann, J. W. (2000) J. Neurosci. 20, 2904-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derer, P. & Derer, M. (1990) Neuroscience 36, 839-856. [DOI] [PubMed] [Google Scholar]
- 11.Del Río, J. A., Martínez, A., Fonseca, M., Auladell, C. & Soriano, E. (1995) Cereb. Cortex 5, 13-21. [DOI] [PubMed] [Google Scholar]
- 12.D'Arcangelo, G., Miao, G. G., Chen, S. C., Soares, H. D., Morgan, J. I. & Curran, T. (1995) Nature 374, 719-723. [DOI] [PubMed] [Google Scholar]
- 13.Hirotsune, S., Takahara, T., Sasaki, N., Hirose, K., Yoshiki, A., Ohashi, T., Kusakabe, M., Murakami, Y., Muramatsu, M., Watanabe, S., et al. (1995) Nat. Genet. 10, 77-83. [DOI] [PubMed] [Google Scholar]
- 14.Caviness, V. S., Jr., Crandall, J. E. & Edwards, M. A. (1988) in Cerebral Cortex, eds. Jones, E. G. & Peters, A. (Plenum, New York), Vol. 7, pp. 59-89. [Google Scholar]
- 15.Del Río, J. A., Heimrich, B., Borrell, V., Förster, E., Drakew, A., Alcántara, S., Nakajima, K., Miyata, T., Ogawa, M., Mikoshiba, K., et al. (1997) Nature 385, 70-74. [DOI] [PubMed] [Google Scholar]
- 16.Ceranik, K., Deng, J., Heimrich, B., Lubke, J., Zhao, S., Forster, E. & Frotscher, M. (1999) Eur. J. Neurosci. 11, 4278-4290. [DOI] [PubMed] [Google Scholar]
- 17.Deller, T., Drakew, A. & Frotscher, M. (1999) Exp. Neurol. 156, 239-253. [DOI] [PubMed] [Google Scholar]
- 18.Chen, Y., Bender, R. A., Frotscher, M. & Baram, T. Z. (2001) J. Neurosci. 21, 7171-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vale, W., Spiess, J., Rivier, C. & Rivier, J. (1981) Science 213, 1394-1397. [DOI] [PubMed] [Google Scholar]
- 20.Avishai-Eliner, S., Brunson, K. L., Sandman, C. A. & Baram, T. Z. (2002) Trends Neurosci. 25, 518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldenhoff, J. B., Gruol, D. L., Rivier, J., Vale, W. & Siggins, G. R. (1983) Science 221, 875-877. [DOI] [PubMed] [Google Scholar]
- 22.Swanson, L. W., Sawchenko, P. E., Rivier, J. & Vale, W. W. (1983) Neuroendocrinology 36, 165-186. [DOI] [PubMed] [Google Scholar]
- 23.Curtis, A. L., Pavcovich, L. A., Grigoriadis, D. E. & Valentino, R. J. (1995) Neuroscience 65, 541-550. [DOI] [PubMed] [Google Scholar]
- 24.Hollrigel, G. S., Chen, K., Baram, T. Z. & Soltesz, I. (1998) Neuroscience 84, 71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merchenthaler, I. (1984) Peptides 5, Suppl. 1, 53-69. [DOI] [PubMed] [Google Scholar]
- 26.Yan, X. X., Toth, Z., Schultz, L., Ribak, C. E. & Baram, T. Z. (1998) Hippocampus 8, 231-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baram, T. Z. & Hatalski, C. G. (1998) Trends Neurosci. 21, 471-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, H. L., Wayner, M. J., Chai, C. Y. & Lee, E. H. (1998) Eur. J. Neurosci. 10, 3428-3437. [DOI] [PubMed] [Google Scholar]
- 29.Blank, T., Nijholt, I., Eckart, K. & Spiess, J. (2002) J. Neurosci. 22, 3788-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keck, M. E., Welt, T., Wigger, A., Renner, U., Engelmann, M., Holsboer, F. & Landgraf, R. (2001) Eur. J. Neurosci. 13, 373-380. [DOI] [PubMed] [Google Scholar]
- 31.Heinrichs, S. C., De Souza, E. B., Schulteis, G., Lapsansky, J. L. & Grigoriadis, D. E. (2002) Neuropsychopharmacology 27, 194-202. [DOI] [PubMed] [Google Scholar]
- 32.Gutman, D. A., Owens, M. J., Skelton, K. H., Thrivikraman, K. V. & Nemeroff, C. B. (2003) J. Pharmacol. Exp. Ther. 304, 874-880. [DOI] [PubMed] [Google Scholar]
- 33.Preil, J., Muller, M. B., Gesing, A., Reul, J. M., Sillaber, I., van Gaalen, M. M., Landgrebe, J., Holsboer, F., Stenzel-Poore, M. & Wurst, W. (2001) Endocrinology 142, 4946-4955. [DOI] [PubMed] [Google Scholar]
- 34.Bender, R., Heimrich, B., Meyer, M. & Frotscher, M. (1998) Exp. Brain Res. 120, 399-402. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez-Buylla, A., Buskirk, D. R. & Nottebohm, F. (1987) J. Comp. Neurol. 264, 159-170. [DOI] [PubMed] [Google Scholar]
- 36.Sternberger, L. A. & Sternberger, N. H. (1983) Proc. Natl. Acad. Sci. USA 80, 6126-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibb, R. & Kolb, B. (1998) J. Neurosci. Methods 79, 1-4. [DOI] [PubMed] [Google Scholar]
- 38.Sholl, D. A. (1953) J. Anat. 89, 33-46. [PMC free article] [PubMed] [Google Scholar]
- 39.Schauwecker, P. E. & Steward, O. (1997) Proc. Natl. Acad. Sci. USA 94, 4103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfer, D. P., Crusio, W. E. & Lipp, H. P. (2002) Trends Neurosci. 25, 336-340. [DOI] [PubMed] [Google Scholar]
- 41.Chen, Y., Brunson, K. L., Adelmann, G., Bender, R. A., Frotscher, M. & Baram, T. Z. (2004) Neuroscience 126, 533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grigoriadis, D. E., Lovenberg, T. W., Chalmers, D. T., Liaw, C. & De Souza, E. B. (1996) Ann. N.Y. Acad. Sci. 780, 60-80. [DOI] [PubMed] [Google Scholar]
- 43.Chalmers, D. T., Lovenberg, T. W. & De Souza, E. B. (1995) J. Neurosci. 15, 6340-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen, Y., Brunson, K. L., Müller, M. B., Cariaga, W. & Baram, T. Z. (2000) J. Comp. Neurol. 420, 305-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Pett, K., Viau, V., Bittencourt, J. C., Chan, R. K., Li, H. Y., Arias, C., Prins, G. S., Perrin, M., Vale, W. & Sawchenko, P. E. (2000) J. Comp. Neurol. 428, 191-212. [DOI] [PubMed] [Google Scholar]
- 46.Lovenberg, T. W., Liaw, C. W., Grigoriadis, D. E., Clevenger, W., Chalmers, D. T., De Souza, E. B. & Oltersdorf, T. (1995) Proc. Natl. Acad. Sci. USA 92, 836-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baram, T. Z., Chalmers, D. T., Chen, C., Koutsoukos, Y. & De Souza, E. B. (1997) Brain Res. 770, 89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunson, K. L., Grigoriadis, D. E., Lorang, M. T. & Baram, T. Z. (2002) Exp. Neurol. 176, 75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cummings, S. L., Young, W. S., III, & King, J. S. (1994) J. Comp. Neurol. 350, 534-549. [DOI] [PubMed] [Google Scholar]
- 50.Ha, B. K., Bishop, G. A., King, J. S. & Burry, R. W. (2000) J. Neurosci. Res. 62, 789-798. [DOI] [PubMed] [Google Scholar]
- 51.Swinny, J. D., Metzger, F., IJkema-Paassen, J., Gounko, N. V., Gramsbergen, A. & van der Want, J. J. (2004) Eur. J. Neurosci. 19, 1749-1758. [DOI] [PubMed] [Google Scholar]
- 52.Dailey, M. E. & Smith, S. J. (1996) J. Neurosci. 16, 2983-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cline, H. T. (2001) Curr. Opin. Neurobiol. 11, 118-126. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, L. I. & Poo, M. M. (2001) Nat. Neurosci. 4, Suppl., 1207-1214. [DOI] [PubMed] [Google Scholar]
- 55.Changeux, J. P. (1983) Prog. Brain Res. 58, 465-478. [DOI] [PubMed] [Google Scholar]
- 56.Goodman, C. S. & Shatz, C. J. (1993) Cell 72, Suppl., 77-98. [DOI] [PubMed] [Google Scholar]
- 57.Magarinos, A. M., Verdugo, J. M. & McEwen, B. S. (1997) Proc. Natl. Acad. Sci. USA 94, 14002-14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Battaglia, G., Webster, E. L. & De Souza, E. B. (1987) Synapse 1, 572-581. [DOI] [PubMed] [Google Scholar]
- 59.Haug, T. & Storm, J. F. (2000) J. Neurophysiol. 83, 2071-2079. [DOI] [PubMed] [Google Scholar]
- 60.Kovacs, K. J. & Sawchenko, P. E. (1996) J. Neurosci. 16, 262-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen, Y., Hatalski, C. G., Brunson, K. L. & Baram, T. Z. (2001) Mol. Brain Res. 96, 39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bender, R. A., Lauterborn, J. C., Gall, C. M., Cariaga, W. & Baram, T. Z. (2001) Eur. J. Neurosci. 13, 679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy, D. D. & Segal, M. (1997) Proc. Natl. Acad. Sci. USA 94, 1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raymond, G. V., Bauman, M. L. & Kemper, T. L. (1996) Acta Neuropathol. 91, 117-119. [DOI] [PubMed] [Google Scholar]
- 65.Lewis, D. A. & Levitt, P. (2002) Annu. Rev. Neurosci. 25, 409-432. [DOI] [PubMed] [Google Scholar]
- 66.Hauger, R. L., Grigoriadis, D. E., Dallman, M. F., Plotsky, P. M., Vale, W. W. & Dautzenberg, F. M. (2003) Pharmacol. Rev. 55, 21-26. [DOI] [PubMed] [Google Scholar]