Abstract
Introduction:
Craving is recognized as a formidable barrier in the management of patients with alcohol dependence. Among pharmacological agents that have been used in experimental studies for reduction in craving, baclofen appears to have a significant advantage over other agents.
Methodology:
The study is retrospective chart review of patients (n = 113) who have been treated with baclofen for alcohol dependence in a tertiary hospital of North India. Baseline assessments included sociodemography, motivation, quantity-frequency of alcohol use, and other alcohol-related clinical parameters. Weekly assessments, for a period of 4 weeks, were extracted from records which included dose of baclofen, craving intensity, and alcohol consumption.
Results:
The study sample was predominantly male, mean age of 41.49 (±9.75) years, most having a family history of substance use (70.97%), and many reporting binge use pattern in last year (49.46%). Baseline assessment revealed 48.7% of the sample was in precontemplation phase for alcohol use and 70% reported severe and persistent craving. This persistent craving was reported by only 15% of the sample by the end of 4 weeks treatment with baclofen (20–40 mg/day). Thirty-four percent of patients reported continued problematic use of alcohol by the end of 4 weeks.
Conclusion:
Our clinical experience suggests that baclofen reduces craving and alcohol consumption including in those with poor motivation. The drug causes few side effects and does not add to the intoxication effect of alcohol. Considering that baclofen is safe in those with liver cirrhosis and reduces withdrawal symptoms due to alcohol, a controlled trial comparing it with standard treatment is required.
Keywords: Alcohol abstinence, alcohol craving, alcohol dependence, baclofen, GABAB
Alcohol use and misuse are identified as a leading cause of prevention for disability and premature death in both developed and developing world.[1] Alcohol use disorders (AUD) comprise spectrum of alcohol use behaviors ranging from the heavy drinking, alcohol abuse, and the more severe pattern of alcohol dependence. A nation-wide epidemiological study in India has reported prevalence 21.4% for “current use” of alcohol and of these at least 17% may be dependent users.[2] Management of AUD provides best results when pharmacological and psychosocial interventions are combined.[3,4] Other than disulfiram, which is an aversive treatment, pharmacological treatment is needed during alcohol withdrawal state (including delirium and seizures) and to reduce craving after cessation of alcohol use.
The WHO defines craving as a strong desire or sense of compulsion to take the substance (either drugs or alcohol).[5] Craving is recognized as a formidable barrier in the management of patients with alcohol dependence.[6] The neurobiological underpinnings of craving are being discovered, and numerous possibilities for pharmacological management of craving are being postulated.[7,8,9,10]
Apart for the two widely used, the US-Food and Drug Administration approved, drugs acamprosate and naltrexone, many other agents have been used specifically for the management of craving. These include ondansetron,[11,12] buspirone,[13] fluoxetine, and other selective serotonin reuptake inhibitors,[14,15] topiramate,[16] and baclofen. Baclofen is primarily used to reduce spasticity due to variety of neurological disorders such as multiple sclerosis and cerebral palsy, in dose of 15–80 mg/day.[17] Its agonistic action on metabotropic G-protein (GABAB) receptors makes it a useful agent in the management of craving.
Preclinical studies have shown baclofen to suppress alcohol-stimulated dopamine release and in turn, dopamine-mediated, alcohol-reinforced, and motivated behaviors.[18] Clinical studies have demonstrated a reduction in alcohol withdrawal symptoms when baclofen is given either as prophylaxis or treatment.[19,20,21] Furthermore, since baclofen is primarily eliminated unchanged by the kidneys (85%), it can be prescribed in the presence of alcohol liver diseases.[17,22] Anticraving efficacy studies of baclofen in humans have been conducted in few countries. Apart for one study,[23] we are not aware of any systemic study for the baclofen use in India. In view of this, we used the drug with caution in our patients.
METHODOLOGY
The study is a retrospective chart review of patients who received treatment for alcohol dependence syndrome between June 2009 and June 2010 (13 months) from the de-addiction clinic of a tertiary healthcare center in North India. As a standard protocol in the department since 2009, the patients of alcohol dependence are detoxified either with benzodiazepine alone or with baclofen. Benzodiazepine-based detoxification was preferred in those with a history of withdrawal seizures, in those with no prior history of benzodiazepine dependence, and those who had a good response to naltrexone, acamprosate, and topiramate as anticraving agents. After detoxification, patients detoxified with benzodiazepines are started on either of the craving reduction drugs, namely., naltrexone, acamprosate, or topiramate, whereas patients detoxified with baclofen are continued on the same drug as baclofen itself has shown anticraving efficacy. Patients are followed twice a week for 1st 2 weeks and then weekly thereafter on outpatient basis. Patients admitted in the ward are detoxified and are generally discharged within 2 weeks. Pharmacotherapy for both outdoor and the indoor patients is largely same. In addition to pharmacotherapy, all the patients attended four group therapy sessions which are held once a week. The medical records of all the patients are stringently maintained and include personal details, drug use pattern, severity of dependence, level of motivation, severity of withdrawal, and degree of craving.
The inclusion criteria for the current study were: (1) patients with alcohol dependence syndrome diagnosed as per ICD-10, (2) age 18–65 years, and (3) patients with at least 1 week of baclofen use as per records. We included patients with additional diagnosis of substance use or other psychiatric and medical disorders, irrespective of their detoxification status, and treatment setup at the time of initiation of baclofen (inpatient/outpatient). We excluded those patients who were not accompanied by reliable informant at the time of initiation of treatment or doubtful compliance to therapy (as recorded in file).
For the purpose of the study, baseline assessment for socioepidemiological profile, substance use pattern, and treatment records was extracted from the records. As mentioned above, it is a practice in the department to record craving in terms of (a) no craving or minimal craving that can be easy to manage, noted “0” or “+” in records, (b) significant cue-associated craving that is often difficult to manage, noted “++” in records (henceforth called moderate craving), and (c) severe pervasive craving that cannot be managed, noted “+++” in records (henceforth called pervasive craving).
The drug use pattern during the follow-up is recorded as (a) abstinence, (b) intermittent or occasional drinking, and (c) problem drinking or dependent use (i.e., continued use). For the purpose of this study, we defined abstinent user as those who did not consume any amount of alcohol in the past 1 week; intermittent drinking as those who consumed <20% of their usual intake per day, never on three successive days, and not having any socio-occupational dysfunction due to alcohol use in the past 1 week; problem drinking as use beyond controlled use including any amount of alcohol use in patients with alcohol-related medical and psychiatric problem.
The follow-up data were extracted from the follow-up notes available in the personal case files of the patients. Data were analyzed for weekly visits till week 4. Cumulative abstinent duration (in days) was calculated for each week. Since many users consumed locally brewed alcohol which can have varied strength of alcohol content, changes in alcohol consumption are recorded in terms of percentage change from pretreatment use.
RESULTS
A total of 315 patients of alcohol dependence syndrome were registered during the study period. Subsequently, 223 patients (70.79%) turned up for follow-up by the end of 1 week in the de-addiction clinic. Out of 223 patients, 113 patients of alcohol dependence syndrome who were on baclofen were recruited for the study after applying the inclusion and exclusion criteria for the same. The sociodemographic features are as shown in Table 1. The age range of participants was 22–65 years (mean age 41.5 ± 9.8 years), age at dependence was 33.5 (±5.9) years. Approximately, 70% (n = 79) of the patients had abstained for a period of at least 1 month since the onset of dependence pattern while 47.8% (n = 54) of the patients had taken treatment for alcohol dependence in past. In terms of looking at the motivation of patients, the records showed that 55 patients (48.7%) of the sample were in precontemplation phase of the motivation cycle at baseline. In addition to alcohol dependence as a primary substance of abuse, eight patients (7.1%) had opioid dependence and one each had cannabis and sedative dependence. Family history of substance dependence was reported by 84 patients (73.3%).
Table 1.
Clinical profile of patients (n=113)
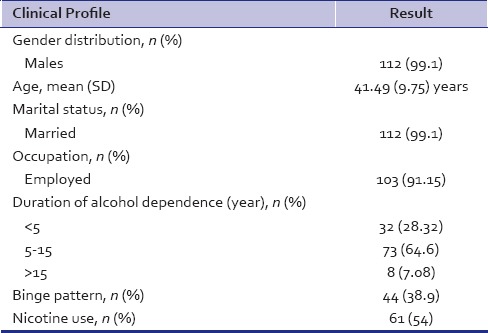
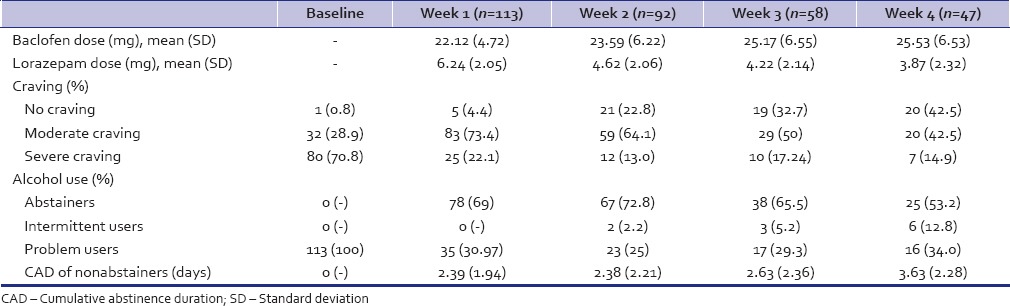
One hundred and thirteen patients who received baclofen, 57 (50.44%) of them were treated at outpatient service and rest were admitted in the ward. The change in dose of baclofen, craving intensity, and alcohol use at each assessment is noted in Table 2. The number of patients with pervasive craving at baseline (70.8%) steady decreased to 14.9% by week 4. About 65% of the patients were either abstinent or controlled users by 4 weeks.
Table 2.
Substance use profile of patients undergoing baclofen course (n=113)
DISCUSSION
Baclofen has shown evidence for decrease in withdrawal symptoms as well as reduction in craving.[17,19,20,21,22] In the present study, we attempted to study changes in craving and alcohol use pattern after prescription of baclofen. The study sample was predominantly male, married, and employed. Although most trials which have used immediate release formulation of baclofen (30 mg/day given in three divided doses),[21,22,24] we used the sustain release preparation (either 20 mg or 30 mg capsules) which reportedly is an efficacious, convenient, and better-tolerated alternatives to immediate release baclofen.[25]
All the patients reported craving for alcohol use with 70% reporting pervasive craving at baseline. Within 1 week of baclofen treatment, there was a reduction in intensity of craving from 70.8% (at baseline) to just 22.1% within a week and <15% at week 4. Many earlier research studies have shown that baclofen treatment reduces craving score as measured by the obsessive–compulsive drinking scale (OCDS; total score, obsessive subscale score, and compulsive subscale score) and the Penn Alcohol Craving Scale.[21,26]
Unlike acamprosate whose anticraving efficacy takes some time to be evident,[27] baclofen reduces craving within a week.[21,28] Although we did escalate the dose of baclofen in some patients who did not have any reduction in craving, about 50% of patients did not need dose escalation beyond 20 mg/day of baclofen. The maximum dose of 40 mg/day was prescribed to <8.6% of patients. None of the patients in this study reported side effects that warranted discontinuation or dose reduction. Baclofen can be prescribed in larger doses, and many experienced neurologists have used baclofen doses of 300 mg/day for control of spasticity. Two case reports have also described how high-dose of baclofen (140 mg and 270 mg/day) for craving reduction in patients of alcohol dependence.[29,30] Indeed, recent studies have used doses of 60 mg and 80 mg/day of baclofen for reducing craving among cocaine[31] and nicotine users.[32] Currently, no evidence exists on reduced efficacy/tolerability of baclofen in alcohol dependence patients at doses beyond those we prescribed.
The reduction in craving was also accompanied by decrease in alcohol use, with 69% becoming abstinent or intermittent user by week 1 and at least 65% maintaining this pattern by week 4. This finding is remarkable and is very close to two double-blind placebo-controlled baclofen trials[22,28] in patients of alcohol dependence patients where abstinence was reported as high as 70% and 71.4% in the baclofen arm of the trial. Both these trials were carried in outpatient setting; the former was a 4 week trial while the latter was 12 week trial conducted in those with additional diagnosis of liver cirrhosis. Although 25–35% of patients did not shift from problem drinking to abstinence or intermittent drinking, the cumulative abstinence duration did show a gradual increase over the 4 week period. It must be noted here that those who continue to use alcohol on prescribed doses of baclofen report a minimal increase in sedative effect of alcohol with no increase in positive subjective effects.[33]
Lorazepam was prescribed to patients, primarily as hypnotic during the 1st week at night time only and continued if the patient continued to have problems related to initiation and maintenance of sleep. Nearly, all patients needed lorazepam for sleep disturbances despite our best efforts to minimize it use. Baclofen, thus, does not appear to influence alcohol withdrawal insomnia even when given as night time dose. Since we did not exclude the patients of other substance dependence (multiple substance users) and patients with comorbid psychiatric illness, a higher dose of lorazepam was needed to induce or maintain sleep in them. To check for unprescribed use of lorazepam by patient, their informants were regularly asked for the same.
Although some patients were already on another anticraving medication (n = 17, one on disulfiram [250 mg/day], two on naltrexone [50 mg/day], and 14 on topiramate [mean dose 111 mg/day]), baclofen was added to their regimen as patients had not shown significant decrease in alcohol use or were having persistent craving. These patients did not appear to benefit from baclofen possibly because both topiramate and baclofen are involved in modulating the same (GABAnergic) transmitter system. The possible benefit of baclofen to those with additional diagnosis of opioid dependence, nicotine dependence, or cannabis dependence cannot be made with the available information in our records. No increase in levels of alanine transaminase or aspartate aminotransferase levels was evident although serum biochemistry was assessed only in those with clinical suspicion of hepatic dysfunction.
In India, it is a common clinical scenario for a person with AUD to report to treatment services against his/her will, i.e., on insistence of family members. Such patients tend to minimize the impact of alcohol use in their social, vocational, or interpersonal functioning. While motivation enhancement therapy is recommended in such situations, experience in India suggests that such individuals rarely come back for psychological consultation. We prescribed baclofen in such individuals and interestingly, precontemplators (noted as 'poor motivation' in records) at initial assessment (n = 55) when followed up at week 2 (n = 44), reported a reduction in alcohol craving. Pervasive craving reported by 95.5% of this sample at baseline was reported only by 11.4% of patients at week 2. Similarly, alcohol consumption reduced to abstinent or intermittent use in 77.3% of sample at the end of week 2. As previously mentioned, baclofen reduces withdrawal symptoms and craving at initiation consequently making it easier for precontemplators to reduce or stop alcohol use. Thus, baclofen may have a significant impact on treatment of alcoholic population in this country. Its safety and efficacy, even in those with cirrhosis, will encourage management of alcoholism by nonpsychiatric physician.[17]
The study has few limitations. Assessment of craving and alcohol use is recorded in nonstandard terms. A significant number of patients dropped out at various intervals within the 4 week period although dropout of about 65% by the end of 1 month has been reported by other de-addiction centers in India.[34] In case of baclofen use in alcoholism, dropouts have cited “lack of medication effect” as the main reason.[26] In our study, of those who did not follow-up, medical records of at least 68.18% (n = 66) of patients reported abstinence/intermittent drinking during the preceding week and 71.11% (n = 45) reported abstinence/intermittent drinking in the previous 2 weeks. Although we could not ascertain the reasons of their inability to follow-up for treatment, it is likely to be attributed to problems such as prolonged wait period in hospital, travel hassles, loss of daily wages, and belief that no further treatment is necessary[35] and not necessarily to “relapse to drinking.” Studies related to outcomes in alcohol dependence from India are few and have not included assessment of craving and baseline motivation as factors influencing outcome.[36,37,38,39]
In summary, our clinical experience with baclofen suggests that it definitively reduces alcohol craving and alcohol consumption in those with alcohol dependence at least in the early period of treatment. It benefits even those who are still in precontemplation stage of motivation. Research has shown that baclofen reduces withdrawal symptoms of alcohol and is safe in those with liver impairment. These findings make baclofen an essential drug for the management of AUDs. Further studies that compare long-term alcohol-related outcome of baclofen with established drugs such as naltrexone and disulfiram are needed.
Financial support and sponsorship
Baclofen 20 mg and 30 mg sustained release capsules were provide free of cost by Sun Pharmaceuticals to many patients.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Global Health Risk: Mortality and Burden of Disease Attributable to Certain Major Risk. Geneva: World Health Organization Press; 2009. [Google Scholar]
- 2.Ray R. National survey on extent, pattern and trends of drug abuse in India. New Delhi: Ministry of Social Justice and Empowerment & United Nations Office of Drugs and Crime-Regional Office of South Asia; 2004. [Google Scholar]
- 3.Szigethy E, Frieman E. In: Combined psychotherapy and pharmacology. Kaplan & Sadock's Comprehensive Textbook of Psychiatry. 9th ed. Sadock BJ, Sadock VA, Ruiz P, editors. Philadelphia: Wolters Kluwer, Lippincott Williams & Wilkins; 2009. pp. 2923–31. [Google Scholar]
- 4.Rounsville B, Carroll K, Back S. In: Individual psychotherapy. Substance Abuse: A Comprehensive Textbook. 4th ed. Lowinson J, Ruiz P, Millman R, editors. Philadelphia: Wolters Kluwer, Lippincott Williams & Wilkins; 2005. pp. 653–70. [Google Scholar]
- 5.World Health Organization. International Classification of Diseases and Death. 10th Revision. Geneva: World Health Organization Press; 1992. [Google Scholar]
- 6.Miller WR, Westerberg VS, Harris RJ, Tonigan JS. What predicts relapse. Prospective testing of antecedent models? Addiction. 1996;91(Suppl):S155–72. [PubMed] [Google Scholar]
- 7.McBride WJ, Li TK. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–69. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 8.Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: Recent advances and challenges. J Neurosci. 2002;22:3332–7. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addolorato G, Leggio L, Abenavoli L, Gasbarrini G Alcoholism Treatment Study Group. Neurobiochemical and clinical aspects of craving in alcohol addiction: A review. Addict Behav. 2005;30:1209–24. doi: 10.1016/j.addbeh.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Swift R. Emerging approaches to managing alcohol dependence. Am J Health Syst Pharm. 2007;64(5 Suppl 3):S12–22. doi: 10.2146/ajhp060644. [DOI] [PubMed] [Google Scholar]
- 11.Johnson BA, Roache JD, Ait-Daoud N, Zanca NA, Velazquez M. Ondansetron reduces the craving of biologically predisposed alcoholics. Psychopharmacology (Berl) 2002;160:408–13. doi: 10.1007/s00213-002-1002-9. [DOI] [PubMed] [Google Scholar]
- 12.Dawes MA, Johnson BA, Ait-Daoud N, Ma JZ, Cornelius JR. A prospective, open-label trial of ondansetron in adolescents with alcohol dependence. Addict Behav. 2005;30:1077–85. doi: 10.1016/j.addbeh.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Malec TS, Malec EA, Dongier M. Efficacy of buspirone in alcohol dependence: A review. Alcohol Clin Exp Res. 1996;20:853–8. doi: 10.1111/j.1530-0277.1996.tb05263.x. [DOI] [PubMed] [Google Scholar]
- 14.Kranzler HR, Burleson JA, Korner P, Del Boca FK, Bohn MJ, Brown J, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152:391–7. doi: 10.1176/ajp.152.3.391. [DOI] [PubMed] [Google Scholar]
- 15.Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001;21:143–53. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Johnson BA, Ait-Daoud N. Topiramate in the new generation of drugs: Efficacy in the treatment of alcoholic patients. Curr Pharm Des. 2010;16:2103–12. doi: 10.2174/138161210791516404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leggio L, Garbutt JC, Addolorato G. Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets. 2010;9:33–44. doi: 10.2174/187152710790966614. [DOI] [PubMed] [Google Scholar]
- 18.Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri S, Serra S, et al. Role of GABAB receptor in alcohol dependence: Reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res. 2004;6:403–14. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- 19.Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, et al. Baclofen in the treatment of alcohol withdrawal syndrome: A comparative study vs diazepam. Am J Med. 2006;119:276–e13-8. doi: 10.1016/j.amjmed.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Stallings W, Schrader S. Baclofen as prophylaxis and treatment for alcohol withdrawal: A retrospective chart review. J Okla State Med Assoc. 2007;100:354–60. [PubMed] [Google Scholar]
- 21.Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, et al. Rapid suppression of alcohol withdrawal syndrome by baclofen. Am J Med. 2002;112:226–9. doi: 10.1016/s0002-9343(01)01088-9. [DOI] [PubMed] [Google Scholar]
- 22.Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: Randomised, double-blind controlled study. Lancet. 2007;370:1915–22. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- 23.Mishra SN, Swain SP, Shukla RK, Sarkar P. A study of aomparative efficacy of baclofen vs acomprosate in reducing alcohol craving and abuse. Orissa J Psychiatry. 2010:48–53. [Google Scholar]
- 24.Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: A randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2010;34:1849–57. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampat NG, Kulkarni RV, Sase N, Joshi NH, Vora PB, Bhattacharya AK, et al. Once daily baclofen sustained release or gastro-retentive system are acceptable alternatives to thrice daily baclofen immediate release at same daily dosage in patients. Neurol India. 2009;57:295–9. doi: 10.4103/0028-3886.53284. [DOI] [PubMed] [Google Scholar]
- 26.Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, et al. Baclofen for alcohol dependence: A preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517–23. doi: 10.1097/01.alc.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- 27.SAMHSA/CSAT Treatment Improvement Protocols. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 1993. [Google Scholar]
- 28.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: A preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–8. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 29.Ameisen O. Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: A self-case report of a physician. Alcohol Alcohol. 2005;40:147–50. doi: 10.1093/alcalc/agh130. [DOI] [PubMed] [Google Scholar]
- 30.Bucknam W. Suppression of symptoms of alcohol dependence and craving using high-dose baclofen. Alcohol Alcohol. 2007;42:158–60. doi: 10.1093/alcalc/agl091. [DOI] [PubMed] [Google Scholar]
- 31.Kahn R, Biswas K, Childress AR, Shoptaw S, Fudala PJ, Gorgon L, et al. Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug Alcohol Depend. 2009;103:59–64. doi: 10.1016/j.drugalcdep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, et al. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend. 2009;103:30–6. doi: 10.1016/j.drugalcdep.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans SM, Bisaga A. Acute interaction of baclofen in combination with alcohol in heavy social drinkers. Alcohol Clin Exp Res. 2009;33:19–30. doi: 10.1111/j.1530-0277.2008.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandrasekaran R, Sivaprakash B, Chitraleka V. Five years of alcohol de-addiction services in a tertiary care general hospital. Indian J Psychiatry. 2001;43:58–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Chavan BS, Priti A. Treatment of alcohol and drug abuse in cAMP setting. Indian J Psychiatry. 1999;41:140–4. [PMC free article] [PubMed] [Google Scholar]
- 36.De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34:460–3. doi: 10.1016/j.jsat.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Basu D, Jhirwal OP, Mattoo SK. Clinical characterization of use of acamprosate and naltrexone: Data from an addiction center in India. Am J Addict. 2005;14:381–95. doi: 10.1080/10550490591006933. [DOI] [PubMed] [Google Scholar]
- 38.Sidana A, Rai S, Chavan BS. Alcohol dependence syndrome: One year follow up study. Delhi J Psychiatry. 2007;1:61–5. [Google Scholar]
- 39.Kuruvilla PK, Vijayakumar N, Jacob KS. A cohort study of male subjects attending an alcoholics anonymous program in India: One-year follow-up for sobriety. J Stud Alcohol. 2004;65:546–9. doi: 10.15288/jsa.2004.65.546. [DOI] [PubMed] [Google Scholar]


