Abstract
The pre-T cell receptor (TCR) functions as a critical checkpoint during αβ T cell development. Signaling through the pre-TCR controls the differentiation of immature CD4-CD8-CD25+CD44- [double-negative (DN)3] thymocytes into CD4+CD8+ double-positive (DP) cells through the CD4-CD8-CD25-CD44-(DN4) stage. In addition, pre-TCR activity triggers expansion and survival of thymocytes and inhibits TCRβ gene rearrangement through a process referred to as allelic exclusion. Whereas many proteins involved in the pre-TCR transduction cascade have been identified, little is known about the nuclear factors associated with receptor function. Here, we use gene targeting to inactivate the Ets-1 transcription factor in mice and analyze pre-TCR function in developing Ets-1-deficient (Ets-1-/-) thymocytes. We find that inactivation of Ets-1 impairs the development of DN3 into DP thymocytes and induces an elevated rate of cell death in the DN4 subset. This defect appears specific to the αβ lineage because γδ T cells maturate efficiently. Finally, the percentage of thymocytes coexpressing two different TCRβ chains is increased in the Ets-1-/- background and, in contrast with wild type, forced activation of pre-TCR signaling does not block endogenous TCRβ gene rearrangement. These data identify Ets-1 as a critical transcription factor for pre-TCR functioning and for allelic exclusion at the TCRβ locus.
Lymphocytes develop from multipotent stem cells through a regulated sequence of events that controls the production of functional T, B, and natural killer cells (1). The great majority of immunocompetent T cells are generated in the thymus where their maturation can be followed by expression of specific cell-surface markers (2). The most immature thymocyte population is found within the double-negative (DN) subset of cells lacking the CD4 and CD8 coreceptors. DN cells are further subdivided into four consecutive populations; DN1 (CD44+CD25-), DN2 (CD44+CD25+), DN3 (CD44-CD25+), and DN4 (CD44-CD25-). As immature DN1 cells differentiate to the DN2 and DN3 stages, they begin to commit into the T cell lineage and start rearranging their T cell receptor (TCR) loci (3). In-frame rearrangement of the TCRβ gene allows the production of a β-chain, which is expressed at the thymocyte cell surface within the pre-TCR (4). Expression of the pre-TCR activates a set of intracellular signaling pathways that allow a specific genetic program to be switched on (5-7). This program results in CD25 down-regulation, rescue of differentiating cells from apoptosis, and intense proliferation and progression to the double-positive (DP) stage (CD4+CD8+). In addition, the function of the pre-TCR is essential to inhibit further rearrangements at the TCRβ locus, insuring that each T cell expresses a unique β-chain through a process referred to as allelic exclusion. Thus, by modulating the transcription of specific genes, the pre-TCR signaling selects for DP thymocytes bearing a unique functional TCRβ chain through a process referred to as β-selection.
Ets-1 is the founding member of a family of winged helix-turn-helix transcription factors that share homologies with the v-ets sequence of the E26 avian leukemia virus (8, 9). In a variety of species, ETS proteins are involved in the regulation of developmental processes in response to extracellular signals (10). For instance, activation of the Ras pathway modulates the activities of Ets-1 through an extracellular signal-regulated kinase-mediated phosphorylation on a threonine residue (11). Further regulations are triggered by the calcium/calmodulindependent protein kinase II, through phosphorylations of serine residues located near the Ets-1 DNA-binding domain (12-14). Interestingly, both of these regulatory pathways have been shown to target Ets-1 after lymphocyte activation. Gene targeting experiments in mice have established that Ets-1 is essential for the development of both the natural killer and T cell lineages. Despite increased numbers of splenic IgM-secreting plasma cells, Ets-1 deficiency does not affect the numbers of IgM+B220+ splenic B cells. In contrast, the size of the peripheral T cell pool is reduced and displays functional defects in vitro. Consistently, thymi of Ets-1-deficient mice are markedly hypocellular and the percentage of DP thymocytes is decreased, whereas that of DN thymocytes is increased.
In this article, we analyzed the role of Ets-1 in the context of the four known pre-TCR functions: (i) transition from DN3 to DN4 and further differentiation to DP, (ii) inhibition of apoptosis of DN cells, (iii) cellular expansion, and (iv) allelic exclusion at the TCRβ locus. We demonstrate that development of Ets-1-/- DN3 thymocytes into DP cells is severely inhibited. Furthermore, Ets-1-/- DN4 thymocytes appear to undergo normal cell cycle after pre-TCR expression but are highly susceptible to cell death. Finally, we show that allelic exclusion at the TCRβ locus is inefficient in Ets-1-/- thymocytes. Our results indicate that the activity of Ets-1 is critical for the functional integrity of the pre-TCR. They suggest a model where Ets-1 participates in the genetic response to pre-TCR signaling.
Materials and Methods
Generation of Mutant Mice. Generation of mutated J1 embryonic stem cells has been described (15). Heterozygous ES cells (Ets-1+/-) were injected into C57BL/6 blastocysts, and chimera mice were bred to C57BL/6 mice to give germ-line transmission. The Ets-1 mutation was backcrossed into the C57BL/6 background for more than seven generations, and heterozygous mutant mice were then interbred to produce Ets-1-deficient animals. All mice used in this study, including wild-type C57BL/6 mice, were maintained in our specific pathogen-free breeding facility (Departement d'Experimentation Animale, Institut Universitaire d'Hématologie, Hôpital Saint Louis, Paris) and killed for analysis between 4 and 5 weeks of age.
In Vivo Cell Transfer. A total of 106 liver cells from day 17.5 postcoitus (p.c.) Ly5.2 embryos were injected in the tail vein of 600-rad-irradiated C57BL/6-Ly5.1-RAG2-/- mice. Injections into irradiated mice deficient for Rag2 and the common-chain γ (CD132) C57BL/6-Ly5.1-RAG2-/-/γc-/- (a gift from J. P. DiSanto, Institut Pasteur, Paris) were performed for analysis of early thymocytes. Five to 6 weeks after transfer, cells from thymuses of the recipient mice were collected and analyzed by flow cytometry.
Flow Cytometry. Single-cell thymus suspensions were stained with antibodies following standard procedures and analyzed on a FACSCalibur flow cytometer (Becton Dickinson). The following Abs were purchased from Becton Dickinson: anti-CD8α (53-6.7), anti-CD4 (L3T4), anti-CD25 (3C7), anti-CD3ε (clone 145-2C11), anti-CD44 (IM7), anti-TCRβ (H57-597), anti-Vβ8 (MR5-2), anti-Vβ9 (MR10-2), anti-Vβ7 (TR310), anti-Vβ5 (MR9-4), and anti-TCRγδ (GL3). mAbs were conjugated with FITC, phycoerythrin (PE), biotin (bi), or allophycocyanin (APC) and streptavidin CyChrome. The data were analyzed on a FACSCalibur f low cytometer by using the program cellquestpro (Becton Dickinson). Cell sorting was performed on a FACSVantage SE flow cytometry system (Becton Dickinson), and in all cases, the purity of the sorted cells was estimated at >99%.
In Vivo BrdUrd Labeling. Day 18.5 p.c. pregnant mice received two pulse injections of BrdUrd (Sigma) 1 mg in 0.2 ml at a 2-h interval. Embryos were collected 1 h after the second BrdUrd injection and thymuses were dissected. Single-cell suspensions of thymocytes were stained with anti-CD25-PE, anti-CD44-bi, and anti-CD4+8+3-APC antibodies following standard procedures, and subpopulations were sorted on a FACSVantage flow cytometry system. To produce nucleus, sorted thymocytes were fixed in 70% ethanol at -20°C for 30 min, washed in PBS, and resuspended in 0.1 M HCl/0.5% Nonidet P-40 for 45 min at room temperature. Cells were washed in PBS and resuspended in 0.1 M Na2B4O7 for 5 min at room temperature. Nuclei were labeled with antibody anti-BrdUrd-FITC (Becton Dickinson) for 30 min on ice, washed, and incubated for 30 min in PBS containing 10 μg/ml propidium iodide. The data were analyzed on FACSCalibur (Becton Dickinson) by using the doublet discrimination system.
Cell Death Analysis. Flow cytometric analysis of apoptotic cells was performed with the R & D Systems Apoptosis Detection kit according to the manufacturer's instructions. Briefly, cells were washed in PBS and resuspended in 1× binding buffer at 106 cells per ml. Annexin V-FITC was added to a final concentration of 0.05 μg/ml, incubated for 10 min at room temperature, and analyzed on a FACSCalibur with cellquestpro software (Becton Dickinson).
PCR Assays. PCRs were performed in a 50-μl reaction containing the indicated amounts of genomic DNA, 2 ng/μl each primer, 0.2 μM each dNTP, 2 mM MgCl2, 50 mM KCl, 10 mM Tris·HCl (pH 8.8), 0.1% Triton X-100, and 1 unit/50 μl of Taq polymerase. Reactions were 4 min at 95°C; 35 cycles of 1 min at 95°C, 1 min at 57°C, and 1.5 min at 72°C; and 5 min at 72°C. PCR primers used in this study have been described (16). PCR products were electrophoresed on a 1% agarose gel, transferred to Zeta-probe membranes, and probed with 32P-end-labeled specific oligonucleotides hprt#654, 5′-GGATATGCCCTTGACTATAATG-3′, or Jβ2.6, 5′-CCGTGAGCCTGGTGCCGGGACCGA-3′. Filters were hybridized overnight at 42°C in 6× SSC/1% SDS/3× Denhardt's solution, washed at 42°C in 2× SSC/0.1% SDS, and subjected to autoradiography.
Results
Inactivation of Ets-1 Severely Impaired the Development of αβ but Not γδ Thymocytes. To investigate the function of Ets-1 during T cell development, we generated Ets-1-deficient mice from previously described embryonic stem cells carrying a targeted inactivation of the ets-1 gene (15). Up to day 18.5 p.c., Ets-1-/- embryos were produced in Mendelian ratio. However, at 3 weeks of age, only 2% of the total offspring carried the homozygous Ets-1 mutation (data not shown). Ets-1-/--viable mice displayed marked growth retardation and 4-week-old animals weighed 50% less than their heterozygous or wild-type littermates (data not shown).
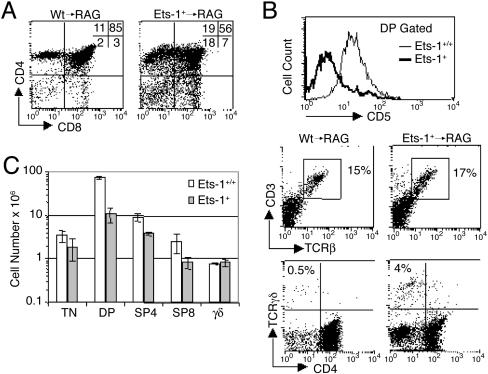
To analyze the role of Ets-1 in T cell development, we generated chimera mice by injecting Ly5.2 Ets-1-/- or wild-type fetal liver cells into irradiated Ly5.1-RAG-2-/- hosts. Five to 6 weeks after injection, the thymus cell populations were stained with specific antibodies and analyzed by flow cytometry. Thymuses were reconstituted at >99.5% by Ly5.2+ cells of donor origin (data not shown); however, fluorescence-activated cell sorter (FACS) analysis was performed by using the Ly5 system to focus exclusively on engrafted cells. In agreement with previous reports, the thymus of Ets-1-deficient chimeras displayed decreased percentages of DP thymocytes and increased percentages of DN cells (Fig. 1A). Although the CD3ε and TCRβ chains were expressed in wild-type and mutated thymocytes, expression of the CD5 molecule, which is up-regulated after pre-TCR signaling (17), was decreased in Ets-1-/- DP cells (Fig. 1B). Interestingly, the percentage of γδ T cells was markedly increased in Ets-1-/-/RAG-2-/- chimera and represented 4% of the total thymus cell population (Fig. 1B). However, among DN thymocytes, the percentage of γδ cells was not significantly increased (data not shown), indicating that Ets-1 mutation diminished the number of αβ thymocytes rather than increasing the number of γδ cells. Moreover, whereas the total number of DP and single-positive (SP) appeared severely reduced in Ets-1-deficient chimeras, the number of γδ cells matched that in wild-type controls (Fig. 1C). These results indicate that Ets-1 inactivation severely affects the differentiation of αβ thymocytes, which depends on the pre-TCR but not the development of γδ thymocytes, which is pre-TCR-independent.
Fig. 1.
Ets-1 is required for efficient differentiation of αβ thymocytes. (A and B) Flow cytometric analysis of thymocytes from wild-type/RAG-2-/- (Wt → RAG) and Ets-1-/-/RAG-2-/- (Ets-1-/- → RAG) chimera mice. Numbers represent the percentage of cells found in the indicated quadrant or region. Histograms show the level of CD5 expression on CD4+CD8+ DP-gated thymocytes. Wild-type thymocytes are indicated by fine lines, and Ets-1-/- thymocytes are indicated by thick lines. (C) Analysis of thymus cell numbers. Thymus cell suspensions were counted, stained with anti-CD3-APC, anti-CD8-FITC, anti-CD4-bi, and anti-γδ-PE, and the percentages of triple-negative (CD4-CD8-CD3-), DP (CD4+CD8+), SP4 (CD4+), SP8 (CD8+), and γδ+ cells determined by FACS. Cell numbers were calculated by multiplying the total number of thymocytes by the percentage of each subset. Wild-type and Ets-1-/- subsets are indicated by open and shaded bars, respectively. These results are representative of six independent chimeras generated from different fetal liver cell injections. For each staining, potential RAG2-/--derived cells were gated out by using the Ly5.2 cell-surface marker. Heterozygous chimeras were identical to wild-type chimeras and are not shown.
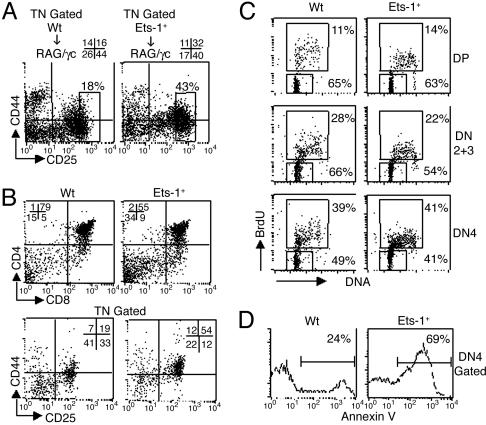
CD44-25- Ets-1-/- DN4 Thymocytes Undergo Normal Cell Cycle but Increased Cell Death. Pre-TCR signaling is known to induce the transition from DN3 to DN4 and to promote thymocyte survival. To investigate the effect of Ets-1 inactivation on immature thymocytes, we generated chimera mice by injecting wild-type or Ets-1-/- fetal liver cells into irradiated Ly5.1-RAG-2-/-/γc-/- hosts. RAG-2-/-/γc-/- mice display almost no thymocytes and therefore are suitable recipients for immature thymocytes analysis in chimera systems (18). FACS analysis of CD44 and CD25 expression on CD3-CD4-CD8-Ly5.1- and propidium iodide-negative Ets-1-/- thymocytes revealed that the percentage of DN4 cells was reduced, whereas that of the DN2 cells was increased 2-fold (Fig. 2A). Furthermore, the percentage of CD25 bright thymocytes, which is elevated in mice carrying a deficient pre-TCR signaling (5, 19), was increased ≈2.5 times in Ets-1-/- chimeras (Fig. 2 A). Similarly, day 18.5 p.c. Ets-1-/- embryonic thymocytes show a partial block of T cell development characterized by a decreased percentage of DP and DN4 cell subsets and an increased percentage of DN2 cells compared with controls (Fig. 2B). Although we noticed a reduction of the DN3 fraction, the development into DN4 thymocytes, which is triggered by the pre-TCR, appeared to occur in a fraction of Ets-1-deficient thymocytes (Fig. 2 A and B).
Fig. 2.
Impaired development of Ets-1-/--immature thymocytes. (A) Flow cytometric analysis of immature thymocytes from wild-type/RAG-2-/-/γc-/- (Wt → RAG/γc) and Ets-1-/-/RAG-2-/-/γc-/- (Ets-1-/- → RAG/γc) chimera mice. Thymus cell suspensions were stained with anti-CD8-bi, anti-CD4-bi, anti-CD3-bi, anti-Ly5.1-bi, anti-CD25-PE, anti-CD44-FITC, and propidium iodide. Dot plots represent CD25 and CD44 expression on live (propidium iodide-) CD3-CD4-CD8-Ly5.1- thymocytes. Numbers indicate the percentage of cells in each quadrant. (B) Flow cytometric analysis of day 18.5 p.c. embryonic thymocytes from wild-type (Wt) and Ets-1-/-. Thymus cell suspensions were stained with anti-CD8-FITC and anti-CD4-PE and analyzed by FACS. For analysis of early thymocytes (Lower), cells were stained with anti-CD8-APC, anti-CD4-APC, anti-CD3-APC, anti-CD25-PE, and anti-CD44-FITC. Dot plots represent the expression of CD25 and CD44 on CD3-CD4-CD8- triple-negative thymocytes. Percentages of cells are shown in the dot plots. (C) Cell-cycle analysis of sorted CD25+CD3-CD4-CD8- (DN2/3), CD25-CD44-CD3-CD4-CD8- (DN4), and CD4+CD8+ (DP) thymocytes of wild-type (Wt) and Ets-1-/- day 18.5 p.c. embryos. The percentages of BrdUrd+ cycling cells and of BrdUrd- quiescent cells are shown in each dot plot. (D) Cell death analysis. Thymocytes of adult wild-type (Wt) and Ets-1-/- mice were stained with CD25-, CD44-, CD3-, CD4-, and CD8-specific antibodies, further labeled with annexin V, and analyzed by FACS. The percentage of annexin V+ cells among CD25-CD44-CD3-CD4-CD8- (DN4) is shown in each histogram. These results are representative of three independent experiments.
We next investigated the ability of Ets-1-deficient embryonic thymocytes to undergo cell cycle after pre-TCR expression. Day 18.5 p.c. embryos were treated in utero with BrdUrd, thymi were dissected out, and sorted live DN2 plus DN3, DN4, and DP thymocytes were analyzed for BrdUrd incorporation and DNA content. As a result of efficient pre-TCR signaling, the percentage of BrdUrd+ cycling wild-type cells increased in the DN4 subset (39%) compared with DN2 plus DN3 or DP cells (28% and 11%, respectively; Fig. 2C). The percentages of BrdUrd+ cells in sorted live DN2 plus DN3, DN4, and DP Ets-1-deficient thymocytes appear similar to wild type (Fig. 2C). Thus, Ets-1 is not absolutely required for DN thymocytes to enter the cell cycle after pre-TCR expression.
To further analyze the function of the pre-TCR in Ets-1 deficient thymocytes, we used annexin V staining and flow cytometry to monitor the level of cells undergoing apoptosis among β-selected thymocytes. The majority of wild-type DN4 thymocytes that were rescued from cell death by the pre-TCR signal did not stain for annexin V (Fig. 2D). In contrast, almost 70% of Ets-1-deficient DN4 cells were stained by annexin V, indicating a high level of apoptosis among β-selected thymocytes (Fig. 2D). In summary, our data demonstrate that Ets-1-deficient DN4 thymocytes can develop and undergo cell cycle but are poorly rescued from apoptosis.
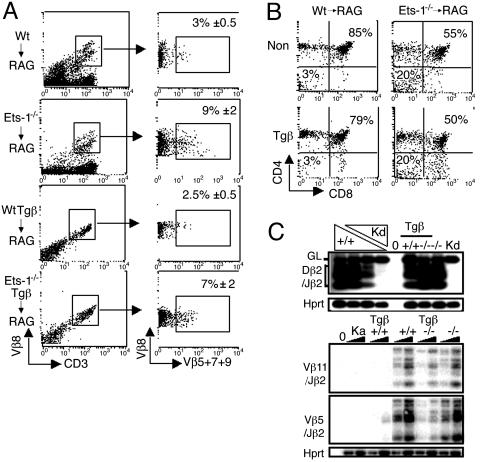
Allelic Exclusion Is Inefficient in Ets-1-/- Thymocytes. One function of the pre-TCR is to initiate an inhibitory feedback loop, referred to as allelic exclusion, that prevents rearrangements at the TCRβ locus. This process relies on pre-TCR signaling because it is deficient in pre-Tα, CD3ε-, or SLP-76-null mice (20). Inversely, allelic exclusion can be artificially triggered by transgenic expression of a TCRβ chain or an activated LckF505 (20). Because some pre-TCR functions may be impaired in the absence of Ets-1 activity, we assessed the status of allelic exclusion in Ets-1-deficient thymocytes. We used antibodies specific for several Vβ domains (Vβ5, Vβ7, Vβ8, and Vβ9), to test whether different endogenous TCRβ chains could be concomitantly expressed on thymocytes. As a result of efficient allelic exclusion, wild-type thymocytes expressing endogenous Vβ8 TCRβ chains infrequently express other TCRβ chains (Vβ5, Vβ7, and Vβ9; Fig. 3A). In contrast, a significant percentage (≈9%) of Ets-deficient thymocytes stained for Vβ8 as well as for Vβ5, Vβ7, or Vβ9 (Fig. 3A). Thus, whereas expression of endogenous Vβ8 chains on wild-type thymocytes can exclude expression of other TCRβ chains, this process is less efficient in Ets-1-/- thymocytes.
Fig. 3.
Allelic exclusion at the TCRβ locus is inefficient in Ets-1-/- thymocytes. (A) Thymocytes from wild-type/RAG-2-/- (Wt → RAG), Ets-1-/-/RAG-2-/- (Ets-1-/- → RAG), TCRβ-transgenic wild-type/RAG-2-/- (WtTgb → RAG), and TCRβ-transgenic Ets-1-/-/RAG-2-/- (Ets-1-/-Tgb → RAG) chimera mice were stained with anti-CD3-FITC, anti-Vβ8-PE, anti-Vβ5-bi, anti-Vβ7-bi, and anti-Vβ9-bi antibodies and analyzed by FACS. CD3+, Vβ8+ cells (Left) were analyzed for Vβ 5,7,9 expression (Right). The percentage of Vβ8+ cells that also express Vβ 5,7,9 is shown in the dot plot. (B) Thymocytes from wild type/RAG-2-/- (Wt → RAG), Ets-1-/-/RAG-2-/- (Ets-1-/- → RAG) chimera mice carrying a TCRβ transgene (Tgβ) or not transgenic (Non Tg), were stained with antibodies directed against CD4, CD8, and Ly5.2 and analyzed by FACS. Dot plots represent CD4 and CD8 expression on Ly5.2+ cells. The percentage of DN and DP cells is shown in the dot plots. (C) PCR analysis of TCRβ gene rearrangements. Genomic DNA isolated from thymocytes of nontransgenic or TCRβ-transgenic (Tβ), wild-type (+/+), or Ets-1-/- (-/-) chimera mice was amplified with primers that detect rearrangements among Dβ2 and Jβ2(Upper)or among Vβ11 or Vβ5 and Jβ2(Lower). PCR products were analyzed by Southern blot. Lanes Kd and 0 correspond to RAG2-/- kidney and to no DNA, respectively. For Dβ2 and each Vβ, there are six bands that correspond to rearrangements to the Jβ2.1 through Jβ2.6 gene segments. (Upper) Triangles represent serial dilutions at a 1:5 ratio of wild type (+/+) thymocyte DNA into RAG2-/- kidney (Kd) DNA. Products generated by the germ-line Dβ2-Jβ2 (GL) region are indicated. (Lower) Two quantities of DNA (100 and 20 ng) were used in the assay. Equivalent loading was confirmed by amplification of a fragment of the Hprt gene.
To further investigate allelic exclusion at the TCRβ locus, we introduced a Vβ8 TCRβ transgene (21) in Ets-1-deficient thymocytes. The TCRβ transgene was expressed at the same level on the surface of wild-type and Ets-1-deficient thymocytes (Fig. 3A). Moreover, in wild-type and Ets-1-/- TCRβ-transgenic chimeras, the percentage of the DN3 (CD44-CD25+) cells was decreased, indicating that the transgene was similarly expressed and functional in wild-type and Ets-1-/--immature thymocytes (data not shown). Despite expression of the TCRβ transgene, Ets-1-deficient DP thymocytes developed as poorly as nontransgenic Ets-1-/- DP cells (50% and 55%, respectively, versus 79% and 85% for controls; Fig. 3B). In addition, expression of the TCRβ transgene was unable to rescue the thymus cellularity of Ets-1-deficient chimera mice (data not shown).
We used FACS analysis to further investigate the process of allelic exclusion in the TCRβ-transgenic system. As expected, few wild-type thymocytes expressing the Vβ8-transgenic chain expressed other Vβ chains at their cell surface (Fig. 3A). In contrast, 7% of Ets-1-deficient TCRVβ8-transgenic thymocytes expressed endogenous Vβ5, Vβ7, or Vβ9 (Fig. 3A). We next used a semiquantitative PCR assay (16) to detect endogenous Dβ2 to Jβ2 and Vβ to DJβ2 rearrangements in transgenic thymocytes. In these experiments, TCRβ gene rearrangements involving representative Dβ2 and Vβ segments (Vβ5 and Vβ11) were amplified from genomic DNA samples isolated from thymocytes of TCRβ-transgenic or -nontransgenic Ets-1-/-/RAG-2-/- or Ets-1+/+/RAG-2-/- chimera mice. The amplified DNA fragments corresponding to rearrangements between TCRβ gene segments were analyzed by Southern blot with a Jβ2-specific probe. Recombination of Dβ2 to Jβ2 segments was observed in all samples, confirming that expression of a TCRβ chain is not sufficient to block this initial step of TCRβ gene rearrangements (Fig. 3C). Vβ to DJβ junctions were readily detectable in both Ets-1+/+ and Ets-1-/- cells and introduction of the TCRβ transgene into Ets-1+/+ thymocytes almost completely blocked Vβ to DJβ recombination of the endogenous TCRβ locus (Fig. 3C). In contrast, rearrangements of the endogenous TCRβ genes occurred at significant levels in TCRβ-transgenic Ets-1-deficient DP thymocytes. Together, these results demonstrate that the process of allelic exclusion at the TCRβ locus that is known to be triggered by the pre-TCR signaling is inefficient in Ets-1-deficient thymocytes.
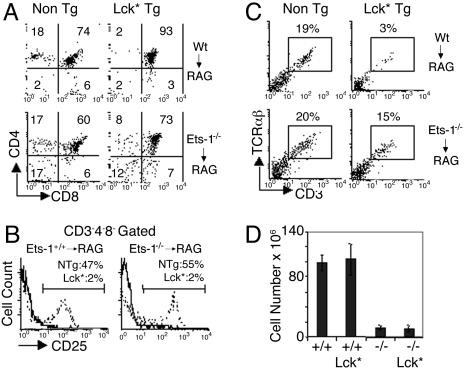
Expression of an Activated Lck Transgene (LckF505) Only Partially Mimics Pre-TCR Function in Ets-1-Deficient Thymocytes. To further investigate the role of Ets-1 in the pre-TCR function, we introduced an activated form of the Lck protein tyrosine kinase (LckF505) into the Ets-1+/+ or Ets-1-/- background and analyzed the development of thymocytes in the RAG2 chimera system. Expression of LckF505 has been shown to trigger proliferation and differentiation of pre-TCR-deficient DN thymocytes (22). In addition, by mimicking the process of allelic exclusion, overexpression of Lck inhibits TCRβ gene rearrangements, thereby impairing the development of αβ+ SP thymocytes (23). Expression of the LckF505 transgene allowed wild-type thymocytes to expand and differentiate to the DP stage, but, as a result of increased Lck activity, few SP cells developed (Fig. 4 A and D). Furthermore, FACS analysis revealed that CD25 was dramatically down-regulated in wild-type DN thymocytes expressing the LckF505 protein (Fig. 4B). Similarly, introduction of the LckF505 transgene in the Ets-1-/- background also triggered CD25 down-regulation in mutant DN thymocytes (Fig. 4B). Thus, the LckF505-transgenic protein appeared equally active in wild-type and Ets-1-deficient DN thymocytes. Despite expression of the LckF505 transgene, the total number of thymocytes and the percentage of DN cells remained similarly low in Ets-1-/-/RAG-2-/-/LckF505-transgenic and Ets-1-/-/RAG-2-/- chimera mice (Fig. 4 A and D). Furthermore, whereas expression of the LckF505 protein reduced the percentage of wild-type CD3+TCRαβ+ cells from 19% to 3%, the reduction was modest in the Ets-1-/- background and 15% of LckF505 mutant thymocytes could express a TCRβ chain and develop to the CD3+TCRαβ+ stage (Fig. 4C). Thus, forced activation of the pre-TCR transduction cascade induces CD25 down-regulation in Ets-1-/- DN thymocytes but is unable to trigger allelic exclusion at the TCRβ locus or to drive expansion of DP mutant cells.
Fig. 4.
Analysis of thymocyte development in Ets-1-/- chimeras mice expressing a LckF505 transgene. (A) Thymocytes from wild-type/RAG-2-/- (Wt → RAG), Ets-1-/-/RAG-2-/- (Ets-1-/- → RAG) chimera mice expressing an activated Lck transgene (Lck*Tg) or none (Non Tg) were analyzed for CD4 and CD8 expression. The percentage of cells falling in each quadrant is indicated. (B) Histogram showing CD25 expression on CD3-CD4-CD8-Ly5.2+ thymocytes from wild-type/RAG-2-/- (Wt → RAG), Ets-1-/-/RAG-2-/- (Ets-1-/- → RAG) chimera mice expressing LckF505 (thick lines) or nontransgenic (dotted lines). The percentages of cells falling in the gate are shown in the histograms. (C) Thymocytes from the indicated chimeras (referenced as in A) were analyzed for cell-surface expression of CD3 and TCRαβ. The percentages of CD3+ and TCRαβ+ cells are shown in the dot plots. For each staining, potential RAG2-/--derived cells were gated out by using the Ly5.2 cell-surface marker. (D) Histogram showing the total number of cells in the thymus of RAG chimera mice injected with wild-type (+/+), Ets-1 (-/-), wild-type/Lck* transgenic (+/+ Lck*), and Ets-1-/-/Lck* transgenic (-/- Lck*) fetal liver cells. These results are representative of three independent chimeras of each genotype.
Discussion
The results of our study indicate that the Ets-1 transcription factor is essential for the normal program of αβ T cell development in the thymus. In the absence of Ets-1, the numbers of DP thymocytes were reduced, and, although the transition from CD25+CD44- to CD25-CD44- could occur, DN4 cells were poorly rescued from apoptosis. This defect affected specifically the αβ lineage, because γδ thymocytes could be generated in normal numbers. Furthermore, the process of allelic exclusion, which is triggered by pre-TCR signaling, appeared less efficient in Ets-1-deficient thymocytes. Altogether, these data demonstrate that the Ets1 transcription factor is essential for complete pre-TCR functions.
Ets-1 and other Ets proteins participate into the transcriptional regulation of numerous genes whose products are critical for the development or the function of lymphoid cells (24-29). Thus, inactivation of Ets-1 could lower or extinguish expression of genes whose products are components of the pre-TCR or are involved in pre-TCR function. In mice that are compromised for TCRβ expression, introduction of a TCRβ transgene can completely rescue the development and expansion of DP thymocytes (30). Moreover, expression of an activated Lck protein is sufficient to induce cellular proliferation and development of DP thymocytes in the absence of the pre-TCR complex (22). If Ets-1 controlled the transcription of an essential component of the pre-TCR complex, one would expect the defect to be complemented by the LckF505 transgene. Our results show that expression of a β-chain or LckF505 did not rescue the normal differentiation of Ets-1-/- DP thymocytes (Figs. 3 and 4); therefore, the potential Ets-1 target gene might encode a more distal element of the pre-TCR transduction pathway.
Expression of the pre-TCR activates numerous intracellular signaling pathways, including the Ras/mitogen-activated protein kinase, the phospholipase Cγ1, and downstream signaling events such as Ca2+ mobilization and PKC (31). Interestingly, activation of the Ras pathways is known to modulate the activity of Ets-1 through a phosphorylation mechanism (32). The “pointed” domain of Ets-1 contains an extracellular signal-regulated kinase 2 docking site that allows efficient Ets-1 phosphorylation, thereby enhancing its transactivation function (11). Furthermore, calcium flux can inhibit Ets-1 DNA-binding activity through calcium/calmodulin-dependent protein kinase II-mediated phosphorylation of serine residues (12). Together, these data indicate that Ets-1 could modulate nuclear events such as gene expression or TCRβ chromatin remodeling in response to pre-TCR engagement.
Interestingly, our results show a decreased number of DP thymocytes and a normal percentage of cycling DN4 cells (Figs. 1C and 2C). These data could appear paradoxical, considering that the large number of DP thymocytes is thought to be a direct consequence of DN4 cellular expansion. Two mechanisms could leads to these results. First, upon pre-TCR signal, a significant fraction of DN thymocytes enter the cell cycle, but the number of divisions that the mutant cells can do is limited compared with their wild-type counterparts and they reach the DP stage prematurely. Second, only a small fraction of Ets-1-deficient DN thymocytes enter the cell cycle after pre-TCR expression, whereas the rest undergo apoptosis. In both cases, the level of live DN4 thymocytes undergoing cell-cycle analysis appears normal, but DP cells are produced in decreased numbers. Testing these issues in a dynamic system such as thymocyte differentiation could turn out to be difficult because developing cells are not synchronized. However, a functional assay design to evaluate how apoptosis contributes to the defect of Ets-1-/- DP thymocytes should shed light on the role of Ets-1 in the differentiation/expansion process.
Development of immature thymocytes appears to be a complex process that is regulated by several mechanisms. Although the pre-TCR acts as a critical checkpoint, additional receptors also control T cell lymphopoiesis. Notch signaling, which is initiated by the interaction of Notch receptors with Notch ligands (jagged and δ-like), also plays a critical role in T cell differentiation (33). In early T cells, interaction of Notch with its ligands induces the cleavage of intracellular Notch that then translocates to the nucleus and associates with DNA-binding protein CSL (CBF1/RBP) to relieve transcriptional repression of T cell-lineage-specific genes. Interestingly, the product of the pointed gene, which is the homologue for Ets-1 in Drosophila, competes with CSL (SuH) of the Notch pathway for the regulation of the yan gene (34). In this system that regulates the cell-fate specification during fly eye development, Pointed, which is phosphorylated by engagement of the sevenless receptor protein-tyrosine kinase inhibits yan expression, whereas Notch signaling enhances it. In T lymphocytes, both Notch and TCR/pre-TCR signals regulate cell decision to commit into αβ versus γδ or CD8 versus CD4 lineage (35, 36). Although the molecular mechanism that potentially connects these two pathways is not known, our results showing a more pronounced defect in αβ than γδ T cell differentiation could indicate that Notch signaling is altered in Ets-1-deficient thymocytes.
In conclusion, our results demonstrate that the Ets-1 transcription factor is essential for pre-TCR function. Pre-TCR and TCR use several common signaling pathways such as Ras, Ca2+, and phospholipase C. Furthermore, Ets-1-deficient mature T cells were shown to respond poorly to anti-CD3 crosslinking in vitro, suggesting that the TCR function may rely on Ets-1 activity (15, 37). Considering the similarities between TCR and pre-TCR signaling and the role of Ets-1 for both receptor functions, one can hypothesize that Ets-1 is involved in the TCR-mediated T cell maturation steps.
Acknowledgments
We thank J. P. Di Santo, M. Goodhardt, R. Mostoslavsky, and B. Malissen for critically reading the manuscript. This work was supported by l'Association pour la Recherche sur le Cancer Grant 1320.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DP, double-positive; DN, double-negative; SP, single-positive; TCR, T cell receptor; p.c., postcoitus; PE, phycoerythrin; bi, biotin; APC, allophycocyanin; FACS, fluorescence-activated cell sorter.
References
- 1.Osborne, B. A. (2000) Curr. Opin. Immunol. 12, 301-306. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. & Jenkinson, E. J. (2001) Nat. Rev. Immunol. 1, 31-40. [DOI] [PubMed] [Google Scholar]
- 3.Muljo, S. A. & Schlissel, M. S. (2000) Immunol. Rev. 175, 80-93. [PubMed] [Google Scholar]
- 4.von Boehmer, H. & Fehling, H. J. (1997) Annu. Rev. Immunol. 15, 433-452. [DOI] [PubMed] [Google Scholar]
- 5.Fehling, H. J., Krotkova, A., Saint-Ruf, C. & von Boehmer, H. (1995) Nature 375, 795-798. [DOI] [PubMed] [Google Scholar]
- 6.Cantrell, D. A. (2002) Nat. Rev. Immunol. 2, 20-27. [DOI] [PubMed] [Google Scholar]
- 7.Malissen, B., Ardouin, L., Lin, S. Y., Gillet, A. & Malissen, M. (1999) Adv. Immunol. 72, 103-148. [DOI] [PubMed] [Google Scholar]
- 8.Leprince, D., Gegonne, A., Coll, J., de Taisne, C., Schneeberger, A., Lagrou, C. & Stehelin, D. (1983) Nature 306, 395-397. [DOI] [PubMed] [Google Scholar]
- 9.Nunn, M. F., Seeburg, P. H., Moscovici, C. & Duesberg, P. H. (1983) Nature 306, 391-395. [DOI] [PubMed] [Google Scholar]
- 10.Oikawa, T. & Yamada, T. (2003) Gene 303, 11-34. [DOI] [PubMed] [Google Scholar]
- 11.Seidel, J. J. & Graves, B. J. (2002) Genes Dev. 16, 127-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabault, B. & Ghysdael, J. (1994) J. Biol. Chem. 269, 28143-28151. [PubMed] [Google Scholar]
- 13.Pognonec, P., Boulukos, K. E., Gesquiere, J. C., Stehelin, D. & Ghysdael, J. (1988) EMBO J. 7, 977-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowley, D. O. & Graves, B. J. (2000) Genes Dev. 14, 366-376. [PMC free article] [PubMed] [Google Scholar]
- 15.Bories, J. C., Willerford, D. M., Grevin, D., Davidson, L., Camus, A., Martin, P., Stehelin, D. & Alt, F. W. (1995) Nature 377, 635-638. [DOI] [PubMed] [Google Scholar]
- 16.Eyquem, S., Lagresle, C., Fasseu, M., Sigaux, F. & Bories, J. C. (2002) Eur. J. Immunol. 32, 3256-3266. [DOI] [PubMed] [Google Scholar]
- 17.Azzam, H. S., Grinberg, A., Lui, K., Shen, H., Shores, E. W. & Love, P. E. (1998) J. Exp. Med. 188, 2301-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiSanto, J. P., Muller, W., Guy-Grand, D., Fischer, A. & Rajewsky, K. (1995) Proc. Natl. Acad. Sci. USA 92, 377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malissen, M., Gillet, A., Ardouin, L., Bouvier, G., Trucy, J., Ferrier, P., Vivier, E. & Malissen, B. (1995) EMBO J. 14, 4641-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khor, B. & Sleckman, B. P. (2002) Curr. Opin. Immunol. 14, 230-234. [DOI] [PubMed] [Google Scholar]
- 21.Uematsu, Y., Ryser, S., Dembic, Z., Borgulya, P., Krimpenfort, P., Berns, A., von Boehmer, H. & Steinmetz, M. (1988) Cell 52, 831-841. [DOI] [PubMed] [Google Scholar]
- 22.Mombaerts, P., Anderson, S. J., Perlmutter, R. M., Mak, T. W. & Tonegawa, S. (1994) Immunity 1, 261-267. [DOI] [PubMed] [Google Scholar]
- 23.Anderson, S. J., Abraham, K. M., Nakayama, T., Singer, A. & Perlmutter, R. M. (1992) EMBO J. 11, 4877-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassuk, A. G. & Leiden, J. M. (1997) Adv. Immunol. 64, 65-104. [DOI] [PubMed] [Google Scholar]
- 25.Lin, J. X., Bhat, N. K., John, S., Queale, W. S. & Leonard, W. J. (1993) Mol. Cell. Biol. 13, 6201-6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wotton, D., Prosser, H. M. & Owen, M. J. (1993) Leukemia 7, Suppl. 2, S55-S60. [PubMed] [Google Scholar]
- 27.Ho, I. C., Bhat, N. K., Gottschalk, L. R., Lindsten, T., Thompson, C. B., Papas, T. S. & Leiden, J. M. (1990) Science 250, 814-818. [DOI] [PubMed] [Google Scholar]
- 28.Leung, S., McCracken, S., Ghysdael, J. & Miyamoto, N. G. (1993) Oncogene 8, 989-997. [PubMed] [Google Scholar]
- 29.McCracken, S., Leung, S., Bosselut, R., Ghysdael, J. & Miyamoto, N. G. (1994) Oncogene 9, 3609-3615. [PubMed] [Google Scholar]
- 30.Shinkai, Y., Koyasu, S., Nakayama, K., Murphy, K. M., Loh, D. Y., Reinherz, E. L. & Alt, F. W. (1993) Science 259, 822-825. [DOI] [PubMed] [Google Scholar]
- 31.Michie, A. M. & Zuniga-Pflucker, J. C. (2002) Semin. Immunol. 14, 311-323. [DOI] [PubMed] [Google Scholar]
- 32.Yang, B. S., Hauser, C. A., Henkel, G., Colman, M. S., Van Beveren, C., Stacey, K. J., Hume, D. A., Maki, R. A. & Ostrowski, M. C. (1996) Mol. Cell. Biol. 16, 538-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radtke, F., Wilson, A., Mancini, S. J. & MacDonald, H. R. (2004) Nat. Immunol. 5, 247-253. [DOI] [PubMed] [Google Scholar]
- 34.Rohrbaugh, M., Ramos, E., Nguyen, D., Price, M., Wen, Y. & Lai, Z. C. (2002) Curr. Biol. 12, 576-581. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Hoyos, G. & Alberola-Ila, J. (2003) Semin. Cell Dev. Biol. 14, 121-125. [DOI] [PubMed] [Google Scholar]
- 36.Bellavia, D., Campese, A. F., Vacca, A., Gulino, A. & Screpanti, I. (2003) Semin. Immunol. 15, 107-112. [DOI] [PubMed] [Google Scholar]
- 37.Muthusamy, N., Barton, K. & Leiden, J. M. (1995) Nature 377, 639-642. [DOI] [PubMed] [Google Scholar]