Abstract
Hyperglycemia has been implicated in the development of endothelial dysfunction through heightened ROS production. Since nitrones reverse eNOS dysfunction, increase antioxidant enzyme activity, and suppress pro-apoptotic signaling pathway and mitochondrial dysfunction from ROS-induced toxicity, the objective of this study was to determine whether nitrone spin traps DMPO, PBN and PBN-LA were effective at duplicating these effects and improving glucose uptake in an in vitro model of hyperglycemia-induced dysfunction using bovine aortic endothelial cells (BAEC). BAEC were cultured in DMEM medium with low (5.5 mM glucose, LG) or high glucose (50 mM, HG) for 14 days to model in vivo hyperglycemia as experienced in humans with metabolic disease. Improvements in cell viability, intracellular oxidative stress, NO and tetrahydrobiopterin levels, mitochondrial membrane potential, glucose transport, and activity of antioxidant enzymes were measured from single treatment of BAEC cells with nitrones for 24 h after hyperglycemia. Chronic hyperglycemia significantly increased intracellular ROS by 50%, decreased cell viability by 25%, reduced NO bioavailability by 50%, and decreased BH4 levels by 15% thereby decreasing NO production. Intracellular glucose transport and SOD activity were also decreased by 50% and 25% respectively. Nitrone (PBN and DMPO, 50 μM) treatment of BAEC cells grown in hyperglycemic conditions resulted in in the normalization of outcome measures except for SOD and catalase activities. Our findings demonstrate that the nitrones reverse the deleterious effects of hyperglycemia in BAEC cells. We believe that in vivo testing of these nitrone compounds in models of cardiometabolic disease is warranted.
Keywords: endothelial dysfunction, ROS, NO, hyperglycemia
1. Introduction
Endothelium-derived nitric oxide (NO) is critical for vascular homeostasis [1]. Endothelial dysfunction (ED) results from a decrease in available NO [2] and is often present concomitantly in vivo with reduced vasodilation, increased platelet adhesion, abnormal cell proliferation and nutrient transport, and a general shift towards pro-inflammatory and pro-thrombic states. ED has been reported in cardiometabolic diseases (CMD), including atherosclerosis, coronary heart disease, diabetes and obesity [3]. Chronic hyperglycemia type 2 diabetes mellitus (T2DM) promotes endothelial dysfunction [4]. Impaired glucose uptake is due to endothelial cell insulin resistance, metabolite accumulation and increased reactive oxygen species (ROS) formation [5]. Chronic hyperglycemia promotes the formation of advanced glycation end products (AGEs), protein kinase C activation (PKC), and abnormal mitochondrial metabolism, all of which perturb the redox state of endothelial cells to favor the formation of pro-oxidant species. Abnormally high levels of ROS, along with insufficient antioxidant defenses (e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), or glutathione reductase (GR)) increase cellular oxidative stress [6, 7].
The proposed mechanism of hyperglycemia-induced uncoupling of eNOS resulting in low NO bioavailability, implicates ROS as the primary cause of ED [6]. Moreover, uncoupled eNOS produces superoxide radical anion (O2•−), thereby exacerbating ROS production. Superoxide radical also reacts with NO to form peroxynitrite (ONOO−) [x] which causes cytotoxicity via lipid peroxidation, protein nitrosylation, as well as eNOS inactivation through oxidation of tetrahydrobiopterin (BH4), an essential cofactor of eNOS [8]. Therefore, therapeutic antioxidant-based strategies have focused on the reduction of intracellular pro-oxidant species [9].
T2DM is a prime risk factor for cardiovascular diseases [10]. Interventions that exhibit synergistic effects in improving insulin sensitivity and vascular functions could be therapeutically effective. Alpha-lipoic acid (LA) is a known antioxidant with multiple therapeutic effects on CMD. LA has been shown to improve insulin signaling and glucose uptake, mitochondrial function, and decreases organ damage [11] due to its ability to accumulate in tissues and reduce inflammation and oxidative stress [12]. Nevertheless, studies on the effects of co-supplementation of LA with other antioxidants have been limited and do not address improvements [13].
Nitrones are structurally simple molecules that possess chemical and biological properties that make them important pharmacological agents [14, 15]. Previous studies show that nitrones can upregulate eNOS phosphorylation thereby reversing SIN-1-induced eNOS dysfunction, and have cytoprotective properties against SIN-1 induced peroxynitrite toxicity [16, 17]. Some nitrones can also induce endogenous antioxidant enzymes [18], inhibit NF-κB activation [19], as well as donate NO, thereby increasing NO bioavailability [20, 21]. Furthermore, c-Jun N-terminal kinase (JNK) signaling plays a central role in obesity and insulin resistance [22] and nitrone (PBN in particular) was found to inhibit activation of stress-regulated” mitogen-activated protein kinases and MAPK-activated protein kinase 2 by ischemia/reperfusion [23]. These chemical and biochemical properties support the use of nitrones in the management of hyperglycemia-induced endothelial dysfunction. Therefore, we explored the effect of nitrone treatment on mitochondrial function, intracellular ONOO and ROS levels, eNOS function, NO bioavailability, and antioxidant enzyme activity in endothelial cells cultured in normal and high glucose conditions.
2. Materials and Methods
2.1. Chemicals
PBN, LA, tris- HCl, DTT, EDTA, H3PO4, Iodine, KI, D-Glucose, Gel Mount Aqueous Mounting Medium, metaphosphoric acid, reagents used to determine SOD and CAT enzymatic activity, as well as reduced and oxidized GSH were all obtained from Sigma-Aldrich (St. Louis, MO, USA). DMEM medium, NEAA, PBS, pen-strep-neomycin, and ascorbic acid were purchased from Gibco (Rockville, MD, USA). CaI and the EPR probe RSSR were purchased from Enzo Biosciences (New York, NY, USA). BCA assay and NaOH were purchased from Thermo-Fisher (Rockford, IL, USA). DMPO was purchased from Dojindo Molecular Technologies (Rockville, MD, USA). DHE was purchased from Molecular Probes (Eugene, OR, USA). MTT and Griess assays were purchased from Promega Corporation (Madison, WI, USA). Primary BAEC (BW-6002) were obtained from Lonza Sciences(Walkersville, MD, USA). JC-1 dye was purchased from Invitrogen (CA, USA). Glucose uptake assay was purchased from Cayman Chemical (Ann Arbor, MI, USA). EGCS and chemiluminescent HRP substrate were obtained from Millipore Corporation (Billerica, MA, USA). FBS was obtained from HyClone Laboratories (South Logan, UT, USA). 4% paraformaldehyde was purchased from OSU Lab Stores (Columbus, OH, USA). Antibodies for ant- p-eNOS and and anti t-eNOS were purchased from Cell Signaling Technology (Boston, MA, USA) and Santa Cruz Biotechnology (Dallas, TX, USA), respectively. PBN-LA was provided by Prof. Durand (Avignon, France).
2.2. Cell Culture
BAEC (BW-6002) were obtained from Lonza (Walkersville, MD, USA) and cultured at 37°C, 20.7% O2, 5.0 % CO2 using DMEM containing 5.5 mM D-glucose (low glucose, LG) and 4 mM L-glutamine supplemented with 10% FBS, 2.5 mg/L ECGS, 1% MEM non-essential amino acids, and pen-strep-neomycin. Oxidative stress was induced by culturing BAEC (1.0× 105 cells/25 cm2) in 50 mM glucose medium (high glucose, HG) for at least 14 days. Confluent HG cultures were sub-passaged as necessary for all experiments, and similar passage numbers were used for both LG and HG conditions. BAEC cultures were washed with PBS, (1 mM pH = 7.4) and medium (LG or HG) was changed every 2–3 days. The effects of nitrones on hyperglycemia-induced oxidative stress were assessed by comparing nitrone-treated BAEC to nitrone-untreated BAEC cultured in LG or HG for at least 14 days. From here onwards, BAEC cultured in LG will be referred to as LG-BAEC, while BAEC cultured in HG for at least 14 days will be referred to as HG-BAEC..
2.3. Nitrone Cytotoxicity and Cytoprotection
Cell viability was assessed using MTT assay according to the procedure described elsewhere [18]. Intracellular dye intensity was spectrophotometrically measured at 570 nm. Cytotoxicity and cytoprotective studies were carried out on LG-BAEC and HG-BAEC incubated in the absence or presence of PBN, DMPO, LA or PBN-LA [24] at concentrations of 25 μM and 50 μM for 24 h.
2.4. Superoxide Radical Detection
Intracellular O2•− levels were measured using confocal microscopy. HG-BAEC were seeded on sterile cover slips in six-well plates (5.0 × 105 cells/well) and treated with 50 μM nitrones for 24 h in HG. The cover slips were washed twice with PBS and stained with 10 μM of DHE; for 30 min. Nuclei were counterstained with 1 μM of DAPI for 1 h. Cells were then fixed with 4% paraformaldehyde for 10 min and mounted onto glass slides using Gel-Mount mounting medium. Images were captured on an Olympus Fluoview-1000 confocal microscope at 40x magnification, using excitation lasers 543 nm and 405 nm for DHE and DAPI, respectively. Fluorescence was quantified using imageJ software and data was normalized to the mean fluorescence of untreated LG-BAEC (fluorescence of treatment ÷ fluorescence of LG-BAEC).
2.5. Nitric Oxide Detection
After cells were stimulated with CaI, NO levels was assessed by: 1) nitrite content using the Griess assay and; 2) EPR spin trapping of NO using the spin trap, iron (II)-N-methyl-D-glucamine dithiocarbamate (Fe(MGD)2 [21]. For nitrite content measurement, HG-BAEC were seeded onto sterile six-well plates (6.0 × 105 cells/well) and treated with PBN, DMPO, PBN-LA or LA for 24 h in HG medium. The cultures were then washed twice with PBS, and stimulated with 20.8 μM of CaI for 8 hr at 37°C in plain DMEM. The medium was then filtered using 0.2 micron syringe filters and the resulting nitrite was quantified using the Griess assay.
EPR spin trapping of NO was done following a published protocol [25]. Briefly, HG-BAEC were seeded onto sterile six-well plates (3.0 × 106 cells/well) and were treated with 50 μM of PBN for 24 h. Cells were then washed twice with PBS and incubated for 36 min at 37°C in a freshly prepared solution containing 9.2 μL of 1.9 mM CaI, 210 μL of 1.9 mM FeSO4.7H2O and 210 μL of 17.3 mM MGD from which 300 μL of the resulting supernatant was transferred to a quartz flat cell for analysis. Spectra were acquired on a EPR X-Band (Bruker EMX) spectrometer using the following parameters: microwave frequency, 9.8 GHz; center field, 3432.56 G; modulation amplitude, 6 G; microwave power, 12 mW; conversion time: 10 ms; time constant 20 ms; sweep width, 70 G; receiver gain 1× 105; total number of scans, 121, using incremental sweep. The 2-D spectra were integrated and baseline corrected using Bruker WinEPR Data Processing Software.
2.6. BH4 Analysis
Briefly, HG-BAEC were treated with PBN for 24 h and then scraped into cold lysis buffer (50 mM tris-HCl, pH 7.4, with 1 mM DTT and 1 mM EDTA and sonicated. Protein content was quantified using the BCA assay. Total BH4 and biopterin were measured by differential oxidation with iodine and quantified using HPLC [26]. Actual BH4 level was determined from the difference between biopterin level formed from acid oxidation and those formed from alkali oxidation. For acid oxidation, proteins were removed by adding 10 μL of a 1:1 mixture of 1.5 M HClO4 and 2 M H3PO4 to 90 μL of cell lysate and centrifuged at 13,000 rpm for 10 min. Iodine (10 μL, 1% in 2% KI) was then added to 90 μL of protein free extract and incubated for 1 h in the dark. After incubation, the excess iodine was reduced by adding 5 μL of ascorbic acid (20 mg/mL)
For alkali oxidation, 80 μL of cell lysate was treated with 10 μL of 1 M NaOH, followed by the addition 10 μL of iodine/KI solution for 1 h in the dark. The alkali samples were then acidified by adding 20 μL of 1 M H3PO4. HPLC was performed using an Apollo C18 5 μm 150 × 4.6 mm column with a 10/90 % methanol/water mobile phase. Fluorescent detection was done using an ESA FL detector 530, with excitation and emission set at 350 nm and 450 nm, respectively. Results were normalized to control mean, and represented as folds of pM BH4/mg of protein.
2.7. eNOS Protein
Western blot analysis was performed to determine the expression levels of p-eNOS, t-eNOS proteins in untreated LG-BAEC and HG-BAEC, and PBN treated LG-BAEC and HG-BAEC. 50 μg of protein from each set was used for western blotting. In a separate experimental set, the membrane was incubated with mouse monoclonal anti-p-eNOS antibody (1:1000 dilution), or mouse monoclonal anti-t-eNOS antibody (1:500 dilution) overnight at 4 °C. The protein bands were visualized using a chemiluminiscence kit and the bands were quantified densitometrically using imageJ software.
2.8. Mitochondrial Membrane Potential
Flow cytometry was used to assess changes in mitochondrial membrane potential (ΔPsi;m). HG-BAEC were treated with nitrones for 24 h and then stained with 0.5 μM of JC-1for 30 min at 37 °C in the dark [27]. JC-1 dye intensity was quantified using BD LSR II flow cytometer (BD Biosciences, San Jose, CA, USA) by measuring emission at 520 nm and 590 nm. Data was normalized to the mean fluorescence intensity of the control and plotted using FlowJo software (BD Biosciences, San Jose, CA, USA).
2.9. Glucose Uptake
Glucose uptake assay was performed using a commercially available kit. BAEC were cultured as previously described in LG or HG media and seeded onto a 96-well plate (2.0 × 104 cells/well) (n = 10) and grown for 24 h. Old cell culture medium was removed and washed with PBS to remove residual glucose. Supplied 2-NBDG was diluted to 100 μg/mL in serum and glucose free DMEM medium containing 50 μM PBN, DMPO, LA, PBN-LA, or PBS alone. A 100 μL of 2-NBDG glucose free medium with or without nitrones was added to the plated cells. After incubating at 37°C for 20 minutes, cells were washed gently twice with 200 μL of PBS. To each well, a volume of 200 μL PBS was added followed by fluorescence detection at λex/λem = 485/535 nm.
2.10. Endogenous Antioxidant Enzyme Activity
LG BAEC and HG-BAEC were treated with nitrones for 24 h, washed twice with PBS and then scraped and homogenized in ice cold 50 mM phosphate buffer with 2 mM EDTA, pH 7.4. Protein content was quantified using the BCA assay. SOD and CAT activities were measured spectrophotometrically. Results were normalized to control means and expressed as folds of units of activity per minute per mg of protein. NTB reduction by O2•− anion was used to determine SOD activity [28]. Briefly, 30 μL of cell extract was added to 240 μL of a reaction mixture (0.13 mg/mL bovine serum albumin, 0.2 mM xanthine, 1.33 mM DTPA, 50 μM bathocuproinedisulfonic acid, 70 μM nitro blue tetrazolium, and 1 U/mL CAT in 50 mM phosphate buffer at pH 7.8. The mixture was then incubated for 5 min at 37 degrees. The reaction was initiated by adding 30 μL of xanthine oxidase (0.1 U/mL) and monitored for 10 min at 560 nm at 37 °C. H2O2 decomposition was used to determine CAT activity [29]. Briefly, 20 μL of cell extract was added to 100 μL of 50 mM phosphate buffer at pH 7. The reaction was initiated by adding 180 μL of 30 mM H2O2 and was monitored at 240 nm for 10 min in a 96-well quartz plate. All reagents for these experiments were purchased through Sigma Aldrich.
2.11. Intracellular Thiol Content
Reduced (GSH) and oxidized (GSSG) content was spectrophotometrically determined by monitoring the formation of TNB for 10 min at 415 nm [31]. Briefly, 20 μL of cell extract was added to a reaction mixture (0.84 mM DTNB, 0.28 mM NADPH, 5 mM EDTA, in 100 mM phosphate buffer pH 7.5. Reaction was initiated by adding 30 μL of glutathione reductase (20U/mL).
Oxidized glutathione (GSSG) content was determined by the addition of 2M 4 vinyl pyridine to block present GSH from reacting with DNTB. Reduced GSH was found by subtracting total gsh from GSSG. Ratio of GSH/GSSG was determined by dividing calculated GSH of sample by the experimentally determined GSSG of sample. Treatments were standardized by concurrently running a glutathione curve (0.1–50 μM). Results were expressed as μM of glutathione per mg of protein. All reagents for these experiments were purchased through Sigma Aldrich.
Total cellular redox state was measured by quantifying the intensity ratio of sharp and broad peaks of the reduced thiol sensitive probe, RSSR, using EPR spectroscopy [30]. Briefly, LG-BAEC and HG-BAEC were treated with nitrones for 24 h, washed twice with PBS and then scraped and homogenized in ice cold 5% metaphosphoric acid. A 20 μL of 0.1 mM RSSR in 0.1M PBS was mixed with 30 μL of cell extract, and transferred to a capillary tube for EPR analysis using the following parameters: microwave frequency, 9.8 GHz; center field, 3513.76 G; modulation amplitude, 0.2 G; microwave power, 13 mW; conversion time: 40.96 ms; time constant 81.92 ms; sweep width, 60 G; receiver gain 1× 103; total number of scans, 1. The 1-D spectra were baseline corrected using Bruker WinEPR Data Processing Software.
2.12 Statistical Analysis
Experimental data were expressed as mean ± standard error mean (SEM). Data analysis was performed using GraphPad Prism 4 software. For statistical analysis one way-ANOVA with Student-Newman-Keuls post test was used with *p <0.05 being statistically significant vs. untreated LG-BAEC control, and **p <0.05 being statistically significant vs. untreated HG-BAEC.
3. Results
3.1. Nitrone Toxicity and Cytoprotection
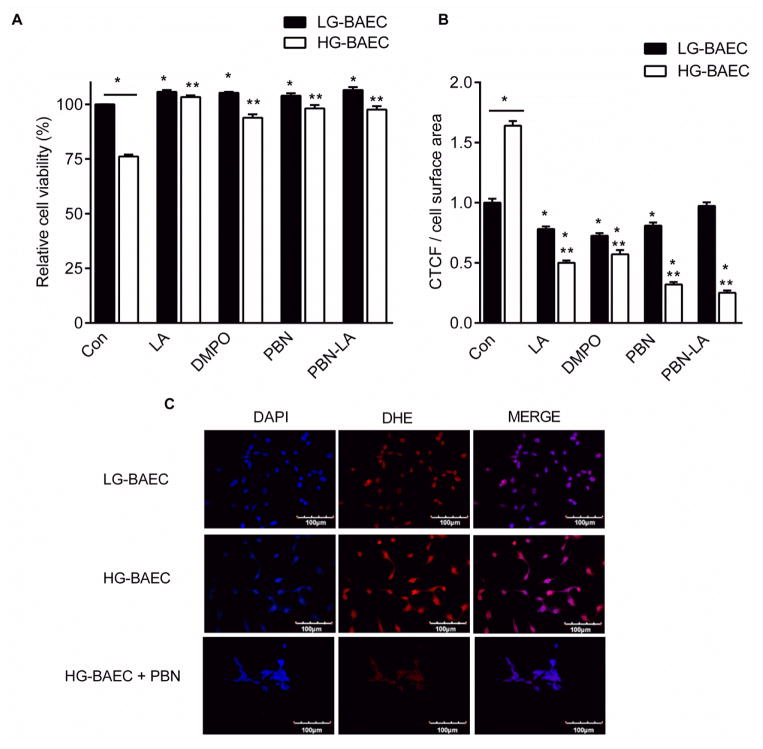
A 25% decrease in cell viability was observed in HG-BAEC compared to LG-BAEC (Fig. 1A). Treatment of HG-BAEC with PBN, LA, DMPO or PBN-LA for 24 h protected cells from hyperglycemia-mediated cytotoxicity with a maximum of ~7% decrease in cell viability was observed.. Since both dosages (i.e., 25 μM and 50 μM) exhibited no cytotoxicity and both inhibited significant viability decreases in HG-BAEC, 50 μM concentration was used for the remainder of the study.
Figure 1.
Effect of nitrones on hyperglycemia-induced cell death and ROS generation. (A) MTT assay showing cell viability of LG-BAEC and HG-BAEC media after 24 hr treatment with nitrones (n = 3). (B) Average DHE fluorescent intensity of LG-BAEC and HG-BAEC treated nitrones (50 μM) (C) Confocal micrographs of O2•− generation as detected by DHE of HG-BAEC treated with nitrones for 24 h (at least 50 cells/treatment, n = 2). All data represented as the mean ± SEM, *p < 0.05 vs. untreated LG-BAEC, **p < 0.05 vs. untreated HG-BAEC.
3.2. Intracellular O2•− Concentration
HG-BAEC had 60% higher DHE fluorescence than LG-BAEC. Only PBN-LA did not significantly decrease DHE fluorescence in LG-BAEC (Fig 1B). All nitrone treatments as well as LA significantly (p < 0.001) reduced the fluorescence intensity of DHE of HG-BAEC (Fig. 1B and 1C)
3.3. NO Bioavailability, Restoration of BH4 and p-eNOS content
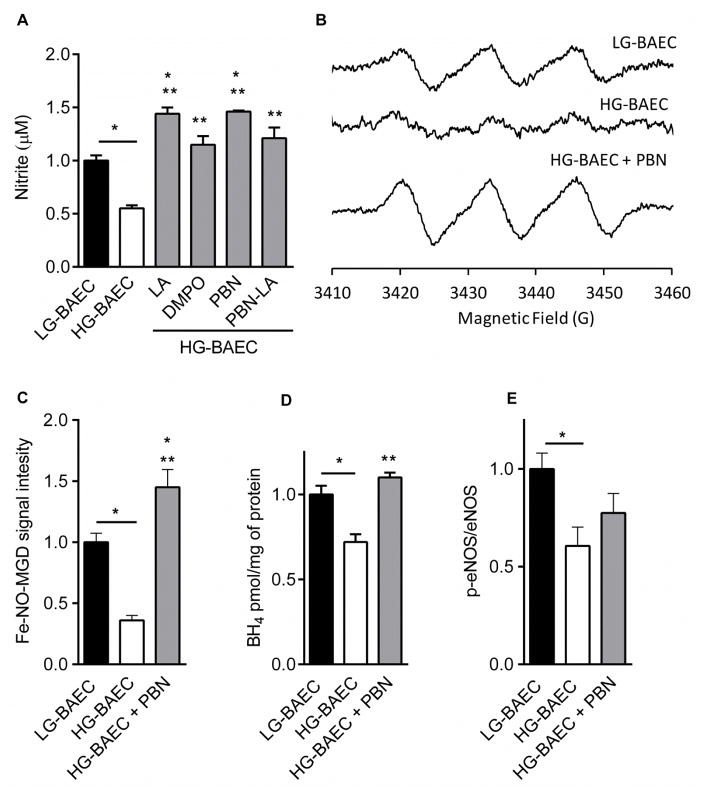
Using Greiss assay results show that the media of HG-BAEC contained approximately 50% less nitrite content than that of LG-BAEC (i.e, 22 μM and 41 μM, respectively). Nitrone-treated HG-BAEC has at least approximately 50% more nitrite content than the untreated HG-BAEC. PBN treatment of HG-BAEC gave the highest nitrite content of all the tested nitrones, and its effect was comparable to that of LA (Fig. 2A). Detection of NO through EPR spin trapping was carried out to further validate Griess assay data. As shown in Figures 2B and 2C, the intensity of Fe[NO(MGD)2] was roughly 64% higher for untreated LG-BAEC compared to untreated HG-BAEC, and that treatment of HG-BAEC with PBN (50 μM) for 24 h gave a higher EPR signal when compared to the basal NO levels observed in LG-BAEC. The trend in NO production using EPR spin trapping is in agreement with the Griess assay results for nitrite formation. To further investigate whether the increase in NO production was BH4 dependent, BH4 level was measured using HPLC analysis. Acid and alkali oxidization of BH4 and 7,8-dihydrobiopterin (BH2) from LG-, HG- and PBN-treated HG-BAEC show a marked increase in the actual BH4 levels by 14% (p < 0.009) in PBN-treated HG-BAEC compared to untreated HG-BAEC (Fig. 2D). This same trend was observed when comparing p-eNOS of untreated LG-BAEC, HG-BAEC and HG-BAEC treated with PBN for 24 h (Fig 2E). However, statistical analysis showed no significant difference between HG-BAEC and HG-BAEC treated with PBN (P > 0.05).
Figure 2.
Effect of nitrones on NO production from cells. (A) Relative nitrite content (μM) after 8 h of cell stimulation with CaI in the medium of untreated LG-BAEC and HG-BAEC, and in medium of HG-BAEC treated with nitrones (50 μM, n = 3). (B) Relative EPR spectrums of Fe-NO-(MGD)2 signal intensity after 36 min stimulation of the cells with CaI in the medium of untreated LG-BAEC and HG-BAEC, and HG-BAEC treated with PBN (50 μM, n = 3). (C) Plots of the relative EPR signal intensity of Fe-NO-(MGD)2 (Same as B). (D) Graph of relative BH4 content per mg of protein in untreated LG-BAEC and HG-BAEC, HG-BAEC treated for 24 h with PBN (50 μM, n = 3). (E) Graph of relative p-eNOS/t-eNOS per mg of protein in untreated LG-BAEC and HG-BAEC, and HG-BAEC treated with PBN for 24 h (50 μM; n=3). All data represented as the mean ± SEM, *p < 0.05 vs. untreated LG-BAEC, **p < 0.05 vs. untreated HG-BAEC.
3.4. Reduction of Mitochondrial Membrane Potential
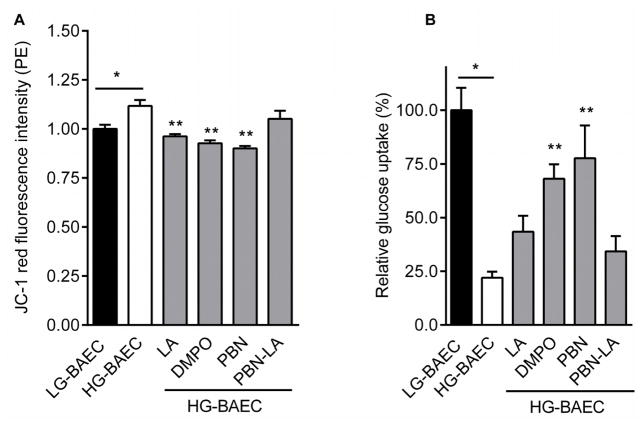
Membrane potential (ΔPsi;m) in HG-BAEC is increased by 11% compared to LG-BAEC (see Figure 3A). While the PBN-LA conjugate was less effective in decreasing ΔPsi;m, HG-BAEC treated with PBN or DMPO further reduced the ΔPsi;m by roughly 22% (Fig. 3A). The effect of PBN and DMPO on ΔPsi;m were comparable to that of LA alone suggesting that the nitrones, PBN and DMPO, decrease the hyperglycemia-induced membrane polarization.
Figure 3.
Effect of nitrones on glucose transport and mitochondrial depolarization. (A) Plot of relative JC-1 dye intensity after HG-BAEC were treated with nitrones for 24 h (50 μM, n = 3). (B) Plot of relative glucose uptake of LG-BAEC, HG-BAEC and HG-BAEC treated with nitrones 24 h (50 μM, n = 3). All data represented as the mean ± SEM, *p < 0.05 vs. untreated LG-BAEC, **p < 0.05 vs. untreated HG-BAEC.
3.5. Up-Regulation of Glucose Uptake
The amount of fluorescent-labeled glucose found within HG-BAEC was substantially lower (~78%, p < 0.001) than those of LG-BAEC. HG-BAEC treated with nitrones for 24 h had at least a 30% increase in glucose uptake. Additionally, nitrones PBN and DMPO were roughly 20% more effective than LA at increasing glucose uptake (Fig. 3B).
3.6. Endogenous Antioxidant Enzyme Activity
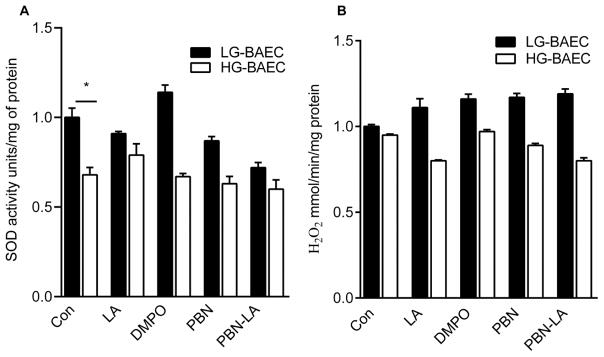
Figure 4A shows that the SOD activity is decreased in HG-BAEC by approximately 30% (p = 0.04), but there was no significant change in CAT activity of HG-BAEC when compared to LG-BAEC (p = 0.673) (Fig. 4B). Nitrone treatments had little effect on both SOD and CAT activities (Fig. 4A–B).
Figure 4.
Effect of nitrones on endogenous antioxidant activity of LG-BAEC and HG-BAEC treated with nitrones for 24 h (50 μM, n = 3). Relative (A) SOD activity via NTB reduction measured spectrophotometrically at 560 nm. (B) CAT activity via H2O2 decomposition measured spectrophotometrically at 240 nm. All data represented as the mean ± SEM, *p < 0.05 vs. untreated LG-BAEC, **p < 0.05 vs. untreated HG-BAEC.
3.7. GSH, GSSG and Reduced Thiol levels (RSH/RSSR)
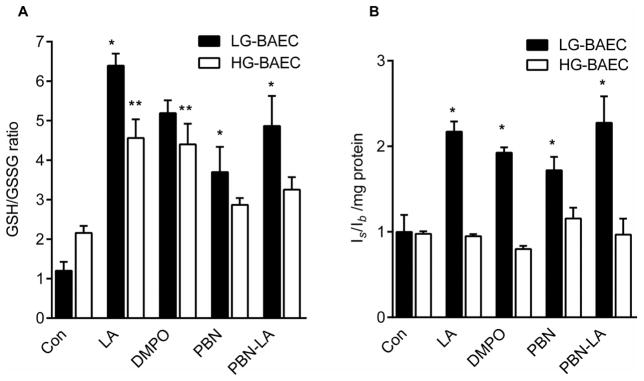
Nitrone treatments significantly increased GSH (Fig. S2A) and generally decreased GSSG (Fig. S2B) in LG-BAEC. Compared to LG-BAEC, HG-BAEC contained both increased levels of GSH and GSSG. Nevertheless, the ratio of GSH/GSSG in untreated LG-BAEC and HG-BAEC was not significantly different (Fig. 5A). HG-BAEC treated with nitrones showed a trend of decreased GSSG levels, and only PBN-LA was found to be statistically significant (Figure S1C). On the other hand, only LA and DMPO significantly increased GSH levels in HG-BAEC (Fig. S2B). Additionally, data show that HG-BAEC treated with nitrones generally resulted in increased GSH/GSSG ratios, as compared to the controls, with LA and DMPO having a statistically significant effect (Fig. 5A). As shown in Fig. 5B, there was no significant difference in the ratio of RSH/RSSR levels of LG-BAEC and HG-BAEC (p>0.05). Upon nitrone treatments, reduced thiol levels increased significantly in LG-BAEC. However, the nitrones had no significant effect on the levels of reduced thiols in HG BAEC.
Figure 5.
Effect of nitrones on cellular redox state of LG-BAEC and HG-BAEC treated with nitrones for 24 h (50 μM). (A) GSH, (B) GSSG, and (C) GSH/GSSG ratio, measured using TNB formation using glutathione reductase (n = 3). (D) Relative EPR signal intensity ratios (Is/Ib = intensity of sharp versus broad peak) of reduced and oxidized intracellular thiols using RSSR probe (n = 3). All data represented as the mean ± SEM, *p < 0.05 vs. untreated LG-BAEC, **p < 0.05 vs. untreated HG-BAEC.
4. Discussion
Hyperglycemia-induced oxidative stress is characterized by inactivation of the mitochondrial electron transport chain [3], NOX enzyme activation [32], and uncoupled eNOS generating O2•− [8], and subsequently other ROS [6]. Increased ROS production results in decrease in NO bioavailability due to its sequestration by O2•− and/or decreased expression of eNOS [33], eNOS uncoupling (look for a good reference of hypergluycemia and eNOS uncoupling) impaired glucose metabolism and insulin resistance in metabolic tissues [34], increased advanced glycation product formation in tissues [35], inflammation [36], hypertension [37], and apoptosis [38]. Cardiometabolic disease exhibits the same phenotypes [39]. In this study, ROS production is increased in HG-BAEC compared to LG-BAEC and this is paralleled by a decrease in NO levels and decreased nitrite formation.. Hyperglycemic conditions cause dynamic changes in mitochondrial morphology leading to mitochondrial fragmentation, increased respiration and overproduction of ROS [40]. The observed hyperpolarization of the ΔPsi;m in this study is consistent with overproduction of ROS in hyperglycemic conditions [3, 40]. As a consequence of increased O2•− production, its reaction with NO results in ONOO− formation that can trigger eNOS uncoupling via BH4 oxidation or downregulation of Akt-mediated eNOS phosphorylation, therefore, exacerbating O2•− production [16, 41]. The observed lower glucose transporter activity in HG condition is consistent with those previously reported for BAEC and retinal endothelial cells and is ROS-mediated [42, 43]. Gene expression of various enzymes including that of CAT and SOD after 24 h of exposure to HG has been reported and showed to be cell-line dependent, that is, downregulation of both enzymes in human umbilical vein endothelial cells; while upregulation in human microvascular endothelial cells [44]. In this study, lower SOD activity but negligible change in CAT activity were observed in HG-BAEC compared to LG-BAEC which parallels previous study showing significant gene polymorphism in T1DM, T2DM compared to normal patients for SOD1 and SOD2 but not for CAT with decreased serum SOD activity in T1DM and T2DM subjects [45]. The unchanged ratio of reduced thiol to oxidized thiol (RSH/RSSR) from LG-BAEC to HG-BAEC indicates that in chronic conditions, oxidized thiols may be reduced back to RSH as part of antioxidant defense mechanism as also evident by the increased synthesis of GSH under HG conditions. However, nitrones are only effective in increasing reduced thiol levels and total GSH level at LG but not in HG. Observations made in both endothelial cells under HG condition [46] and red blood cells in diabetic patients [47] show decrease in GSH levels. This suggests that in HG conditions, nitrones antioxidant effect is independent to that of thiol regulation.
In general, nitrone, LA or PBN-LA treatment of HG-BAEC showed improved cell viability, lowered ROS production, increased NO and BH4 level (for PBN only), decrease mitochondrial membrane potential polarization, improved glucose uptake, and higher reduced-thiol levels but gave no significant effect overall on improving SOD and catalase activity under HG condition. Although LA alone gave significant protection, PBN and DMPO impart the highest improvement in glucose uptake. Due to nitrones’ chemical properties and biological activity [14], nitrones have been widely used as pharmacological agents against oxidative stress-mediated diseases such as cancer, stroke and neurodegeneration [15]. In relation to cardioprotection, nitrones have been shown to protect against ischemia-reperfusion injury [48] using isolated rat heart, and in-vitro models, nitrones protect cells from endothelial dysfunction by reversing eNOS dysfunction [16] and bolstering phase II enzymes via Nrf-2 nuclear translocation [18]. In neuronal NOS knockout cardiomyocytes, esterified nitrone corrects nitroso-redox imbalance and increase cell contractility via increase sarcoplasmic reticulum Ca2+ handling [49]. There is a strong correlation between impaired glucose metabolism and the development and progression of cardiovascular disease [50] whose risk factors include high blood pressure, abnormally high glucose, elevated triglycerides, etc., which in combination, lead to an increased risk of developing T2DM and cardiovascular disease. Benefits of LA with respect to glycemic control, improved insulin sensitivity, oxidative stress, and neuropathy in diabetic patients have been promising [51] through reduced NF-κB activation in human monocytic cells and reduced inflammation [52], induce phase 2 enzymes via Nrf-2 nuclear translocation [12]. Since insulin resistance and T2DM are prime risk factors for cardiovascular diseases, therapeutic interventions that may have synergistic effects that could improve insulin sensitivity and vascular functions targeting CMD is important. However, studies on the effects of co-supplementation of LA with other antioxidants have limited benefits that address complication due to CVD [13]. Additionally, LA may not address a number of other abnormalities. In mouse model, PBN has been shown to protect against hyperglycemia in both alloxan- and streptozotocin-induced diabetes [19] and given nitrones’ ability to ameliorate ischemia and reperfusion injury as well as endothelial and cardiomyocyte contractile dysfunctions, nitrones could have potential therapeutic benefits against CMD.
Both the linear nitrone, PBN [15], and the cyclic nitrone, DMPO [14], are employed as therapeutic agents but the former has been the gold standard in nitrone therapeutics. DMPO reacts with O2•− twice as fast relative to PBN and at least two orders of magnitude faster with HO2• making DMPO an ideal parent compound for therapeutic applications [53]. Also, the opportunity for biconjugation for improved bioavailability and target specificity to cellular compartments could make these nitrone-analogues effective therapeutic agents. Surprisingly, while both PBN and LA were effective at increasing NO bioavailability and glucose transport (for PBN alone), the PBN-LA conjugate did not show any increased in NO formation nor improved glucose uptake, indicating no significant synergistic effect between the two antioxidants when conjugated together. Previously, effect of amphiphilic nitrones on mitochondrial function was investigated [54] and data shows that amide derivatives showed the highest protection compared to other PBN-analogues while we previously showed that DMPO can preserve mitochondrial electron transport by scavenging mitochondrial ROS and increased mitochondrial biogenesis [48].
Current therapeutic strategies for CMD involve ameliorating hyperglycemia-induced endothelial dysfunction by targeting eNOS and Nrf-2 signalling [55], mitochondria [56] and by restoring redox balance for improved heart contractility [57]. Since nitrones have been employed to target all eNOS, Nrf-2, mitochondria and nitroso-redox balance [16, 18, 48, 49] under acute and/or chronic conditions, nitrone therapeutics have the potential to modulate several of these pathways in improving cardiovascular function in T2DM. The lack of multifunctional antioxidants that can ameliorate cardiovascular disease is a key factor limiting the application of antioxidants as therapeutic agents. Nitrone therapeutics as applied to the treatment of cardiovascular diseases, with the goal of ameliorating, reversing or preventing the effect of oxidative injury, has yet to be fully explored due to lack of innovative nitrone designs that can increase bioavailability and target the specific subcellular effector compartments. Plasma membrane (PM)-localized eNOS is more sensitive to Ca2+ mobilizing agents in producing NO compared to Golgi-localized eNOS [58]. Furthermore, the degree of phosphorylation/S-nitrosylation has significant effects on the activity on these localized eNOS since NOS activity is also regulated by [Ca2+] release from ER by activation of the Ca2+-calmodulin. Therefore, in addition to the mitochondria, targeting Golgi [59], ER [60], and PM (through NAPDH oxidases) [32] with nitrones as sources of RS, and with nitrones exhibiting controlled-delivery of NO where they can concentrate NO at these organelle sites would be desirable. Future aims must involve innovative nitrone design that targets the post-translational regulation of NOS activity and/or suppression of signal transduction and gene induction processes in subcellular locations of NOS (i.e., plasma membrane, sarcoplasmic (endoplasmic) reticulum, or Golgi), therefore, salvaging eNOS from dysfunction or reverse eNOS uncoupling where it is most susceptible – a potentially a powerful pharmacological strategy.
This work demonstrates that nitrones, PBN and DMPO, were effective at improving endothelial cell viability, lowering intracellular ROS concentrations, improving eNOS function, NO bioavailability and mitochondrial function, as well as up-regulating glucose transport under chronic hyperglycemic condition. Therefore, therapies that not only directly scavenge ROS and increase NO bioavailability, but can also indirectly regulate ROS production via induction of antioxidant enzyme activity, reverse eNOS uncoupling, suppress maladaptive signal transduction processes and prevent eNOS dysfunction may represent promising drug leads for the prevention of cardiometabolic complications arising from cardiovascular diseases and T2DM.
Supplementary Material
Acknowledgments
This research was supported by the Department of Pharmacology Research Incentive Funds (F.A.V.) and The Ohio State University Arts and Sciences Undergraduate Research Award (CAH). Award Number 20020728 from American Egg Board, Award Number 10040042 from Novo Nordisk Pharmaceuticals as well as Award Number R21OD017244 and UL1RR025755 (OSUCCC) from the National Center for Research Resources, funded by the Office of the Director (OD), National Institutes of Health and supported by the NIH Roadmap for Medical Research (O.Z., D.D.). Dustin Perth is acknowledged for technical assistance. Prof. Jeffrey Deiuliis of School of Medicine, University of Maryland, for helpful suggestions.
Abbreviations
- AGE
advanced glycation end products
- LA
alpha lipoic acid
- BAEC
bovine aortic endothelial cell
- BCA
bicinchoninic acid
- BH4
tetrahydrobiopterin
- CaI
calcium ionophore
- CAT
catalase
- CMD
cardiometabolic disease
- CVD
cardiovascular disease
- DAPI
4′,6-diamidino-2-phenylindole
- DHE
dihydroethidium
- DMEM
dulbecco’s modified eagle’s medium
- DTNB
5-(3-Carboxy-4-nitrophenyl)disulfanyl-2-nitrobenzoic acid
- DTPA
diethylene triamine penta-acetic acid
- ED
endothelial dysfunction
- EDTA
ethylenediaminetetra acetic acid
- eNOS
endothelial nitric oxide synthase
- EPR
electron paramagnetic resonance
- GPx
glutathione peroxidase
- GR
glutathione reductase
- HG
high glucose
- HPLC
high pressure liquid chromatography
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
- JNK
c-Jun N-terminal kinase
- LG
low glucose
- MAPK
mitogen-activated protein kinases
- MGD
N-methyl-D-glucamine
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NaOH
sodium hydroxide
- 2-NBDG
2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose
- NEAA
Non essential amino acids
- NF-kB
nuclear factor kappa beta
- NO
nitric oxide
- NOX
NADPH oxidase
- NrF-2
nuclear factor erythroid 2
- ONOO−
peroxynitrite
- PBN
α-phenyl-n-t-butylnitrone
- PBN-LA
α-phenyl-n-t-butylnitrone-lipoic acid
- PBS
phosphate-buffered saline
- PKC
protein kinase C
- PM
plasma membrane
- ROS
reactive oxygen species
- RS
reactive species
- SEM
standard error mean
- SIN-1
3-morpholinosydnonimine
- SOD
superoxide dismutase
- T1DM
type I diabetes mellitus
- T2DM
type II diabetes mellitus
Footnotes
Authorship contribution statement:
CA Headley aided in the conceptualization this study, designed the experiments; performed and analyzed data; drafted the manuscript.
D DiSilvestro performed and analyzed data for glucose uptake
C Hemann gave substantial contribution to the acquisition and analysis of BH4; contributed in revising the manuscript
KE Bryant performed western blot procedures
C Chen gave substantion contribution to the analysis of p-eNOS protein; contributed in revising the manuscript
A Das provided supporting data pertaining to eNOS protein and eNOS activity.
O Ziouzenkova gave critically important intellectual input on glucose uptake; contributed in revising the manuscript
G Durand gave substantial contribution by synthesizing the PBN-lipoic acid conjugate; contributed in revising the manuscript
FA Villamena conceptualized this study; interpreted data; revised and gave final approval of the version to be published.
References
- 1.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 2.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 3.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–41. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, et al. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42:80–9. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 6.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Victor VM, Rocha M, Sola E, Banuls C, Garcia-Malpartida K, Hernandez-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 8.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 9.Zamora PL, Villamena FA. Pharmacological approaches to the treatment of oxidative stress-induced cardiovascular dysfunctions. Future Med Chem. 2013;5:465–78. doi: 10.4155/fmc.13.15. [DOI] [PubMed] [Google Scholar]
- 10.Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes--part I: pathways of vascular disease in diabetes. Vascul Pharmacol. 54:68–74. doi: 10.1016/j.vph.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Ghibu S, Richard C, Vergely C, Zeller M, Cottin Y, Rochette L. Antioxidant properties of an endogenous thiol: Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol. 2009;54:391–8. doi: 10.1097/fjc.0b013e3181be7554. [DOI] [PubMed] [Google Scholar]
- 12.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–60. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadi G, Yilmaz O, Guray T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu-Zn SOD and catalase. Mol Cell Biochem. 2008;309:109–16. doi: 10.1007/s11010-007-9648-6. [DOI] [PubMed] [Google Scholar]
- 14.Villamena FA, Das A, Nash KM. Potential implication of the chemical properties and bioactivity of nitrone spin traps for therapeutics. Future Med Chem. 2012;4:1171–207. doi: 10.4155/fmc.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floyd RA, Kopke RD, Choi CH, Foster SB, Doblas S, Towner RA. Nitrones as therapeutics. Free Radic Biol Med. 2008;45:1361–74. doi: 10.1016/j.freeradbiomed.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das A, Gopalakrishnan B, Druhan LJ, Wang TY, De Pascali F, Rockenbauer A, et al. Reversal of SIN-1-induced eNOS dysfunction by the spin trap, DMPO, in bovine aortic endothelial cells via eNOS phosphorylation. Br J Pharmacol. 2010 doi: 10.1111/bph.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durand G, Prosak RA, Han Y, Ortial S, Rockenbauer A, Pucci B, et al. Spin trapping and cytoprotective properties of fluorinated amphiphilic carrier conjugates of cyclic versus linear nitrones. Chem Res Toxicol. 2009;22:1570–81. doi: 10.1021/tx900114v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das A, Gopalakrishnan B, Voss OH, Doseff AI, Villamena FA. Inhibition of ROS-induced apoptosis in endothelial cells by nitrone spin traps via induction of phase II enzymes and suppression of mitochondria-dependent pro-apoptotic signaling. Biochem Pharmacol. 84:486–97. doi: 10.1016/j.bcp.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho E, Chen G, Bray TM. Alpha-phenyl-tert-butylnitrone (PBN) inhibits NFkappaB activation offering protection against chemically induced diabetes. Free Radic Biol Med. 2000;28:604–14. doi: 10.1016/s0891-5849(99)00271-3. [DOI] [PubMed] [Google Scholar]
- 20.Villamena FA. Superoxide radical anion adduct of 5,5-dimethyl-1-pyrroline N-oxide. 5. Thermodynamics and kinetics of unimolecular decomposition. J Phys Chem A. 2009;113:6398–403. doi: 10.1021/jp902269t. [DOI] [PubMed] [Google Scholar]
- 21.Locigno EJ, Zweier JL, Villamena FA. Nitric oxide release from the unimolecular decomposition of the superoxide radical anion adduct of cyclic nitrones in aqueous medium. Organic & biomolecular chemistry. 2005;3:3220–7. doi: 10.1039/b507530k. [DOI] [PubMed] [Google Scholar]
- 22.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 23.Clerk A, Fuller SJ, Michael A, Sugden PH. Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J Biol Chem. 1998;273:7228–34. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- 24.Durand G, Polidori A, Salles JP, Prost M, Durand P, Pucci B. Synthesis and antioxidant efficiency of a new amphiphilic spin-trap derived from PBN and lipoic acid. Bioorg Med Chem Lett. 2003;13:2673–6. doi: 10.1016/s0960-894x(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 25.Gopalakrishnan B, Nash KM, Velayutham M, Villamena FA. Detection of nitric oxide and superoxide radical anion by electron paramagnetic resonance spectroscopy from cells using spin traps. J Vis Exp. 2012:e2810. doi: 10.3791/2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriounov D, Schircks B, et al. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab. 2004;81:45–51. doi: 10.1016/j.ymgme.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Duarte S, Arango D, Parihar A, Hamel P, Yasmeen R, Doseff AI. Apigenin protects endothelial cells from lipopolysaccharide (LPS)-induced inflammation by decreasing caspase-3 activation and modulating mitochondrial function. International journal of molecular sciences. 2013;14:17664–79. doi: 10.3390/ijms140917664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mockett RJ, Bayne AC, Sohal BH, Sohal RS. Biochemical assay of superoxide dismutase activity in Drosophila. Methods in enzymology. 2002;349:287–92. doi: 10.1016/s0076-6879(02)49343-3. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Schellhorn HE. Rapid kinetic microassay for catalase activity. J Biomol Tech. 2007;18:185–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Bobko AA, Eubank TD, Voorhees JL, Efimova OV, Kirilyuk IA, Petryakov S, et al. In vivo monitoring of pH, redox status, and glutathione using L-band EPR for assessment of therapeutic effectiveness in solid tumors. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2012;67:1827–36. doi: 10.1002/mrm.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–65. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 32.Drummond GR, Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab. 2014;25:452–63. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Ding Y, Vaziri ND, Coulson R, Kamanna VS, Roh DD. Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am J Physiol Endocrinol Metab. 2000;279:E11–17. doi: 10.1152/ajpendo.2000.279.1.E11. [DOI] [PubMed] [Google Scholar]
- 34.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 35.Negre-Salvayre A, Salvayre R, Auge N, Pamplona R, Portero-Otin M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 36.Ceriello A, Novials A, Ortega E, La Sala L, Pujadas G, Testa R, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes. 2012;61:2993–7. doi: 10.2337/db12-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong WT, Tian XY, Huang Y. Endothelial dysfunction in diabetes and hypertension: cross talk in RAS, BMP4, and ROS-dependent COX-2-derived prostanoids. J Cardiovasc Pharmacol. 2013;61:204–14. doi: 10.1097/FJC.0b013e31827fe46e. [DOI] [PubMed] [Google Scholar]
- 38.Liu TS, Pei YH, Peng YP, Chen J, Jiang SS, Gong JB. Oscillating high glucose enhances oxidative stress and apoptosis in human coronary artery endothelial cells. J Endocrinol Invest. 2014;37:645–51. doi: 10.1007/s40618-014-0086-5. [DOI] [PubMed] [Google Scholar]
- 39.Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–8. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Triggle CR, Ding H. A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens. 2010;4:102–15. doi: 10.1016/j.jash.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Alpert E, Gruzman A, Riahi Y, Blejter R, Aharoni P, Weisinger G, et al. Delayed autoregulation of glucose transport in vascular endothelial cells. Diabetologia. 2005;48:752–5. doi: 10.1007/s00125-005-1681-y. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes R, Hosoya K, Pereira P. Reactive oxygen species downregulate glucose transport system in retinal endothelial cells. Am J Physiol Cell Physiol. 2011;300:C927–36. doi: 10.1152/ajpcell.00140.2010. [DOI] [PubMed] [Google Scholar]
- 44.Patel H, Chen J, Das KC, Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol. 2013;12:142. doi: 10.1186/1475-2840-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flekac M, Skrha J, Hilgertova J, Lacinova Z, Jarolimkova M. Gene polymorphisms of superoxide dismutases and catalase in diabetes mellitus. BMC Med Genet. 2008;9:30. doi: 10.1186/1471-2350-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Recchioni R, Marcheselli F, Moroni F, Pieri C. Apoptosis in human aortic endothelial cells induced by hyperglycemic condition involves mitochondrial depolarization and is prevented by N-acetyl-L-cysteine. Metabolism. 2002;51:1384–8. doi: 10.1053/meta.2002.35579. [DOI] [PubMed] [Google Scholar]
- 47.Dincer Y, Akcay T, Alademir Z, Ilkova H. Effect of oxidative stress on glutathione pathway in red blood cells from patients with insulin-dependent diabetes mellitus. Metabolism. 2002;51:1360–2. doi: 10.1053/meta.2002.35192. [DOI] [PubMed] [Google Scholar]
- 48.Zuo L, Chen YR, Reyes LA, Lee HL, Chen CL, Villamena FA, et al. The radical trap 5,5-dimethyl-1-pyrroline N-oxide exerts dose-dependent protection against myocardial ischemia-reperfusion injury through preservation of mitochondrial electron transport. J Pharmacol Exp Ther. 2009;329:515–23. doi: 10.1124/jpet.108.143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traynham CJ, Roof SR, Wang H, Prosak RA, Tang L, Viatchenko-Karpinski S, et al. Diesterified nitrone rescues nitroso-redox levels and increases myocyte contraction via increased SR Ca(2+) handling. PLoS One. 2012;7:e52005. doi: 10.1371/journal.pone.0052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44:369–74. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- 51.Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004;11:1135–46. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- 52.Lee HA, Hughes DA. Alpha-lipoic acid modulates NF-kappaB activity in human monocytic cells by direct interaction with DNA. Exp Gerontol. 2002;37:401–10. doi: 10.1016/s0531-5565(01)00207-8. [DOI] [PubMed] [Google Scholar]
- 53.Durand G, Choteau F, Pucci B, Villamena FA. Reactivity of superoxide radical anion and hydroperoxyl radical with alpha-phenyl-N-tert-butylnitrone (PBN) derivatives. J Phys Chem A. 2008;112:12498–509. doi: 10.1021/jp804929d. [DOI] [PubMed] [Google Scholar]
- 54.Durand G, Poeggeler B, Böker J, Raynal S, Polidori A, Pappolla MA, et al. Fine-tuning the amphiphilicity: A crucial parameter in the design of potent alpha-phenyl-N-tert-butylnitrone analogues. J Med Chem. 2007;50:3976–9. doi: 10.1021/jm0706968. [DOI] [PubMed] [Google Scholar]
- 55.Ramprasath T, Kumar PH, Puhari SS, Murugan PS, Vasudevan V, Selvam GS. L-Arginine ameliorates cardiac left ventricular oxidative stress by upregulating eNOS and Nrf2 target genes in alloxan-induced hyperglycemic rats. Biochem Biophys Res Commun. 2012;428:389–94. doi: 10.1016/j.bbrc.2012.10.064. [DOI] [PubMed] [Google Scholar]
- 56.Apostolova N, Rocha M, Rovira-Llopis S, Banuls C, Falcon R, Castello R, et al. Mitochondria-targeted antioxidants as a therapeutic strategy for protecting endothelium in cardiovascular diseases. Curr Med Chem. 2014;21:2989–3006. doi: 10.2174/0929867321666140601200416. [DOI] [PubMed] [Google Scholar]
- 57.Bhatt NM, Aon MA, Tocchetti CG, Shen X, Dey S, Ramirez-Correa G, et al. Restoring redox balance enhances contractility in heart trabeculae from type 2 diabetic rats exposed to high glucose. Am J Physiol Heart Circ Physiol. 2015;308:H291–302. doi: 10.1152/ajpheart.00378.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, et al. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem. 2004;279:30349–57. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- 59.Jiang Z, Hu Z, Zeng L, Lu W, Zhang H, Li T, et al. The role of the Golgi apparatus in oxidative stress: is this organelle less significant than mitochondria? Free Radic Biol Med. 2011;50:907–17. doi: 10.1016/j.freeradbiomed.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 60.van der Vlies D, Woudenberg J, Post JA. Protein oxidation in aging: endoplasmic reticulum as a target. Amino Acids. 2003;25:397–407. doi: 10.1007/s00726-003-0025-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.