Abstract
Hypophosphatasia (HPP) is an inherited skeletal and dental disease caused by loss-of-function mutations in the gene that encodes tissue-nonspecific alkaline phosphatase (TNALP). The major symptoms of severe forms of the disease are bone defects, respiratory insufficiency, and epileptic seizures. In 2015, enzyme replacement therapy (ERT) using recombinant bone-targeted TNALP with deca-aspartate (D10) motif was approved to treat pediatric HPP patients in Japan, Canada, and Europe. However, the ERT requires repeated subcutaneous administration of the enzyme because of the short half-life in serum. In the present study, we evaluated the feasibility of neonatal ex vivo gene therapy in TNALP knockout (Akp2−/−) HPP mice using lentivirally transduced bone marrow cells (BMC) expressing bone-targeted TNALP in which a D10 sequence was linked to the C-terminus of soluble TNALP (TNALP-D10). The Akp2−/− mice usually die within 20 days because of growth failure, epileptic seizures, and hypomineralization. However, an intravenous transplantation of BMC expressing TNALP-D10 (ALP-BMC) into neonatal Akp2−/− mice prolonged survival of the mice with improved bone mineralization compared with untransduced BMC-transplanted Akp2−/− mice. The treated Akp2−/− mice were normal in appearance and experienced no seizures during the experimental period. The lentivirally transduced BMC were efficiently engrafted in the recipient mice and supplied TNALP-D10 continuously at a therapeutic level for at least 3 months. Moreover, TNALP-D10 overexpression did not affect multilineage reconstitution in the recipient mice. The plasma ALP activity was sustained at high levels in the treated mice, and tissue ALP activity was selectively detected on bone surfaces, not in the kidneys or other organs. No ectopic calcification was observed in the ALP-BMC-treated mice. These results indicate that lentivirally transduced BMC can serve as a reservoir for stem cell-based ERT to rescue the Akp2−/− phenotype. Neonatal ex vivo gene therapy thus appears to be a possible treatment option for treating severe HPP.
Introduction
Hypophosphatasia (HPP) is an inherited skeletal and dental disease caused by mutations in the tissue-nonspecific alkaline phosphatase (TNALP) gene (ALPL).1,2 TNALP is a cell surface enzyme abundantly localized on the outer cell membrane of osteoblasts and chondrocytes and on the surface of their released matrix vesicles.3 The absence of functional TNALP in HPP results in extracellular accumulation of its natural substrates, which include inorganic pyrophosphate (PPi), pyridoxal 5′-phosphate and phosphoethanolamine.4 In addition, TNALP deficiency also leads to a hypomineralization of bones and teeth because of strong inhibition of hydroxyapatite crystal growth caused by accumulation of extracellular PPi.2–4
All forms of HPP are usually diagnosed by first assaying serum alkaline phosphatase (ALP) activity, at least at the beginning of the diagnostic work-up. TNALP is thought to be released into the blood stream through phosphatidylinositol-specific phospholipase-catalyzed cleavage of its TNALP-glycosylphosphatidyl-inositol (GPI) anchoring motif.2,5 The clinical manifestations of HPP vary widely, and the disease was recently classified into six forms based on its severity and age at diagnosis.1 The perinatal and infantile forms of HPP are severe and often fatal. The major symptoms of severe HPP are bone defects, respiratory insufficiency, and epileptic seizures.6,7 The impaired bone mineralization caused by TNALP deficiency leads to bone defects that make these patients prone to bone fracture, and the most severely affected patients endure intubation and mechanical ventilation to relieve respiratory insufficiency because of rib cage dysplasia. In addition, pyridoxine-responsive seizures often occur in infantile HPP.8
Clinical studies of transplantation therapy for severe HPP have been conducted using allogeneic bone marrow cells (BMC), mesenchymal stem cells (MSC), and cultured osteoblasts.9–12 The rationale of these protocols is that donor cells may distribute and engraft in the skeletal microenvironment to differentiate into osteoprogenitor cells synthesizing physiologically active TNALP. With these approaches, therapeutic benefits such as improved bone mineralization were observed, but the biochemical markers, including serum ALP activity, were not corrected.9–12 Further studies aimed at further improving the therapeutic effect are thus needed.
Millán and colleagues reported that enzyme replacement therapy (ERT) using recombinant bone-targeted TNALP with the Fc region of human IgG and a bone targeting deca-aspartate (D10) motif at the enzyme's C-terminus prevented disease in the TNALP knockout (Akp2−/−) mouse model of infantile HPP.13 These findings underpinned the phase I/II clinical trials of ERT using the recombinant bone-targeted TNALP (asfotase alfa; Alexion Pharmaceuticals) to treat perinatal and infantile HPP patients.14 Subsequently, asfotase alfa was approved for treatment of pediatric HPP in Japan, Canada, and Europe. Other potential ERTs using GPI-anchorless TNALP15 or intestinal-like chimeric alkaline phosphatase16 to prevent HPP in Akp2−/− mice have been reported. However, because these biologics have a short half-life in serum, ERT for HPP requires repeated subcutaneous injections of the enzyme.14
Taking a different approach to treating HPP, we demonstrated that a single intravenous or intraperitoneal injection of a lentiviral or adeno-associated viral (AAV) vector encoding bone-targeted TNALP in which D10 was linked adjacent to the C-terminus of soluble TNALP (TNALP-D10) resulted in sustained expression of TNALP-D10 and prevention of the disease phenotype in Akp2−/− mice.17–19 Viral vector-mediated ERT should be more practical and economical than classic ERT, which requires repeated injection of purified recombinant enzyme. There are several concerns with this in vivo gene therapy, however, including the risk of inadvertent germline gene transfer20,21 and induction of immune responses to the viral vector peptide or transgene product22,23 after systemic or tissue-specific administration of the high-dose viral vector needed to achieve therapeutic benefits.
An alternative gene therapy approach is BMC-mediated ex vivo gene therapy. Up to now, no ex vivo gene therapy protocols have been approved; however, several clinical trials of BMC-mediated ex vivo gene therapy have been conducted to treat not only hematological diseases24,25 but also inherited lysosomal storage disorders.26–28 Lysosomal enzymes are secreted and recaptured by the surrounding cells. Accordingly, the goal of this approach is to provide a reservoir of lysosomal enzyme in vivo. Lentivirally transduced BMC showed robust, long-term expression of the transgene, and provided the therapeutic enzyme in appreciable levels for correction of the symptoms.26–28 In addition, with ex vivo gene therapy the risks of incidental germline gene transfer and induction of an immunoreaction against the viral vector are eliminated.29 Although the oncogenicity of conventional integrating viral vectors used for ex vivo gene therapy is a concern, the genotoxiciy is now minimized through several safety modifications.29,30 In that context, we reasoned that HPP could also be treated by BMC-mediated ex vivo gene therapy. Moreover, these strategies would be applicable not only to inherited bone disorders, including HPP, but also to other diseases that could be treated using ERT.
In the present study, therefore, we examined the feasibility of neonatal ex vivo gene therapy using genetically modified BMC as an approach to treatment of HPP. On day 2 after birth, we performed a single systemic transplantation of lentivirally transduced BMC expressing TNALP-D10 to treat the lethal phenotype of Akp2−/− mice.
Materials and Methods
Mice
Akp2−/− mice, which phenotypically mimic infantile HPP, were created as previously described.31 These mice usually appear normal at birth but die within 20 days while exhibiting serious growth failure and skeletal hypomineralization.32 The major cause of death is apnea, most likely resulting from their severe epileptic seizures. Breeding heterozygous (Akp2+/−) pairs, their pups, and weanlings were fed a rodent diet supplemented with 325 ppm pyridoxine (Oriental Yeast Co., Ltd., Tokyo, Japan). The pyridoxine supplementation delayed the onset of epileptic attacks and extended their survival.32 Akp2 genotyping was done using PCR with primers 5′-AGTCCGTGGGCATTGTGACTA-3′ and 5′-TGCTGCTCCACTCACGTCGAT-3′.17 B6.CD45.1 (Akp2+/+) mice were purchased from Sankyo Labo Service Co., Inc. (Tokyo, Japan) and used as donors for bone marrow transplantation. The haplotypes of the Ly5 antigens of Akp2−/− and B6.CD45.1 mice are Ly5.2 and Ly5.1, respectively. All animal experiments were carried out with approval from the Nippon Medical School Animal Ethics Committee.
Cell culture
The 293T and HeLa cell lines were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin, and 50 μg/ml streptomycin under an atmosphere enriched with 5% CO2.
BMC preparation was performed as described previously.33 In brief, BMC were harvested from the femurs and tibias of 8–12-week-old B6.CD45.1 mice. Lineage-negative (Lin−) BMC were enriched using a Mouse Hematopoietic Progenitor (Stem) Cell Enrichment Set (BD Biosciences, Franklin Lakes, NJ) following manufacturer's instructions. The Lin− BMC were then prepared at the density of 1 × 106 cells/ml in X-Vivo medium (X-Vivo15; Takara Bio Inc., Shiga, Japan) supplemented with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO), 0.1 mM β-mercaptoethanol (Invitrogen, Life Technology Japan, Tokyo, Japan), 2 mM L-glutamine (Invitrogen), 100 units/ml penicillin, 100 μg/ml streptomycin (Invitrogen), 50 ng/ml murine stem cell factor (R&D Systems, Minneapolis, MN), 10 ng/ml murine interleukin-3 (R&D Systems), and 50 ng/ml human interleukin-6 (R&D Systems).
Lentiviral vector preparation and transduction
The lentiviral vector plasmids carrying cDNA for TNALP-D1019 or enhanced green fluorescent protein (EGFP)34 have been described previously. The lentiviral genome contains a reverse-oriented, 0.25 kb insulator element from the 5′ hypersensitive site 4 (5′HS4) of the chicken β-globin gene (cHS4 insulator) within the U3 region of the self-inactivating long terminal repeat (SIN-LTR), which blocks unintended enhancer effects of the internal promoter. Expression of TNALP-D10 or EGFP was under the control of the U3 region of the murine stem cell virus LTR (MSCV-U3) acting as an internal promoter. The lentiviral vector was prepared by transient transfection of 293T cells as previously described.34 The titer of the vector stock was determined in HeLa cells by measuring the copy number of the integrated vector genome using real-time PCR (7500 Fast; Applied Biosystems, Carlsbad, CA), and was expressed as transducing units per milliliter (TU/ml). Thereafter, 1 × 106 Lin− BMC were transferred to Retronectin (Takara Bio Inc.)-coated 24-well plates and transduced with the lentiviral vector carrying the TNALP-D10 gene (ALP-BMC) or EGFP gene (GFP-BMC) by incubation for 20 hr at 37°C at a multiplicity of infection of 50.
Colony-forming unit assay
Colony-forming unit (CFU) assay was performed as described previously.33 In brief, 2 × 103 transduced or untransduced Lin− BMC in 1 ml of MethoCult M3534 (Stem Cell Technologies, Vancouver, BC, Canada) were seeded into 6-well culture plates. CFU-macrophage (CFU-M) and CFU-granulocyte-macrophage (CFU-GM) colonies were then scored on day 7. All assays were performed in triplicate.
BMC transplantation
Four hours before transplantation on postnatal day 2, neonatal Akp2−/− mice were conditioned with a single 4 Gy dose of total body irradiation (TBI) using an X-ray machine (MBR-1505R2, Hitachi Medical Co., Tokyo, Japan). The mice were then transplanted with 1 × 106 transduced or untransduced Lin− BMC intravenously via the jugular vein or superficial temporal vein using a 29-gauge insulin syringe.
Engraftment and lineage analysis of peripheral blood
Using a heparinized hematocrit capillary tube (Terumo Corp., Tokyo, Japan), peripheral blood (PB) was collected from the retro-orbital plexus of recipient mice approximately every 30 days following transplantation. The erythrocytes were then lysed with NH4Cl, and the remaining cells were washed once with PBS. To then measure the donor cell engraftment rate, PB mononuclear cells (PBMC) were resuspended in PBS containing 5% FBS and incubated first with biotin-conjugated antibodies directed against murine CD45.1 (Ly5.1) for 30 min at 4°C and then with Streptavidin-FITC. In addition, PE-conjugated antibodies directed against murine Mac-1 (monocytes), Gr-1 (granulocytes), B220 (B-cells), and CD3e (T-cells) were used together to analyze multilineage potency.33 All monoclonal antibodies used were purchased from BD Biosciences. The cells were then subjected to FACS analysis using a FACScalibur flow cytometer (BD Biosciences).
Colorimetric ALP activity assay
ALP activity was determined as previously described.17 One unit (U) was defined as the amount of enzyme needed to catalyze production of 1 μmol of p-nitrophenol per minute, and ALP activity in plasma and cell culture supernatants was calculated as U/ml. PB was collected 1 week after transplantation via the tail vein. In addition, the brain, heart, liver, spleen, kidney, and bone (a mixture of bone and bone marrow) were harvested after perfusing mice with 20 ml of PBS containing 10 U/ml of heparin under deep anesthesia. The organs were then homogenized using the Percellys-24 bead-beating homogenizer according to the company's protocol (Bertin Technologies, Paris, France), and the supernatants of homogenates were used to measure tissue ALP activity. After measuring the protein concentration using a DC protein assay kit (Bio-Rad, Hercules, CA), ALP activity was expressed per milligram (mg) protein.
Biodistribution of lentiviral vector
Genomic DNA was extracted from tissue homogenates using a Gentra Puregene Kit (Qiagen, Venlo, The Netherlands) and then subjected to real-time PCR to estimate the vector distribution. The primer/probe set LentiP.F (5′- CAG GAC TCG GCT TGC TGA AG −3′), LentiP.R (5′- TCC CCC GCT TAA TAC TGA CG −3′) and TaqMan probe LentiP.P (5′- FAM - CGC ACG GCA AGA GGC GAG G - TAMRA −3′) was used to detect the lentiviral vector provirus, as described previously.35 A TaqMan Copy Number Reference Assay, Mouse, Tfrc (transferrin receptor) VIC-TAMRA probe (Applied Biosystems) was used to quantify the genomic DNA. To estimate the vector distribution, genomic DNA from wild-type (Akp2+/+) mice spiked with plasmid DNA was used as a standard, and the average copy number per diploid was determined. Assays were performed in triplicate on an ABI 7500Fast Real-Time PCR System (Applied Biosystems) using the following protocol: 1 cycle of 10 min at 95°C followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C.
Histochemical ALP activity staining
Knee joints were removed and embedded in SCEM compound (Leica Microsystems, Tokyo, Japan) without fixation or decalcification and stored at −80°C. For study, sections (7 μm thick) were cut using the Kawamoto film method36 and fixed with 4% paraformaldehyde. ALP activity was assayed by incubating the tissue in 20 ml of 0.1 M Tris-HCl buffer (pH 8.5) containing 0.1 mg/ml naphthol AS-MX phosphate, which served as the substrate, and 0.3 mg/ml fast blue BB salt for 15 min at 37°C, as described previously.17 After washing away excess buffer with distilled H2O, the tissue sections were mounted on silane-coated slides (Muto Pure Chemicals, Ltd., Tokyo, Japan) and examined under a light microscope (BX 60; Olympus Ltd., Tokyo, Japan).
X-ray analysis
Radiographic images were acquired using a μFX-1000 X-ray unit (Fujifilm, Tokyo, Japan) and analyzed with an FLA-7000 image reader (Fujifilm). The X-ray energy level was 25 kV, and the exposure time was 30 sec.17
Microcomputed tomography
To analyze the femoral bone morphology, high-resolution microcomputed tomography (μCT) was carried out using a HMX-225 Actis4 system (Tesco Co., Tokyo, Japan).37 The samples were moistened with PBS and scanned at 15× magnification using a slice width of 50 μm, matrix size of 512 × 512, slice pitch of 50 μm, and voxel size of 23 × 23 × 50 μm, applying an energy level of 120 kV and intensity of 83 μA. The digital images obtained were processed to reconstruct 3D structures using reconstruction software (VG Studio; Volume Graphics Co. Ltd., Heidelberg, Germany). The 3D structure of trabecular bone was evaluated in a 0.5-mm-thick section of the distal metaphysis region adjacent to the growth plate. The bone morphometric parameters were calculated using TRI/3D-BON software (Ratoc System Engineering Inc., Tokyo, Japan).
Statistical analysis
Data are presented as mean ± standard deviation (SD). Differences between means were tested for statistical significance using Student's t-test. Values of p < 0.05 were considered significant. Survival rates were analyzed using the Kaplan–Meier method, and a log-rank test was used to assess the survival differences. Statistical analyses were performed with IBM SPSS Statistics Version 20 (IBM Corp., Tokyo, Japan).
Results
Prolonging survival of Akp2−/− mice through neonatal transplantation of BMC expressing TNALP-D10
In this study, we compared the therapeutic effects of transplantation of lentivirally transduced BMC expressing TNALP-D10 (ALP-BMC) and untransduced BMC (mock-BMC), which were harvested from B6.CD45.1 (Akp2+/+, Ly5.1) mice, for the treatment of neonatal Akp2−/− mice. According to the titration studies, we determined 4 Gy of TBI as the sublethal dose for the neonatal transplantation. Neonatal mice irradiated with 4 Gy showed a moderate reduction in body weight compared with unirradiated neonatal mice, but no symptoms of acute radiation syndrome such as inhibition of hair growth, cataracts, or marked growth retardation (data not shown).
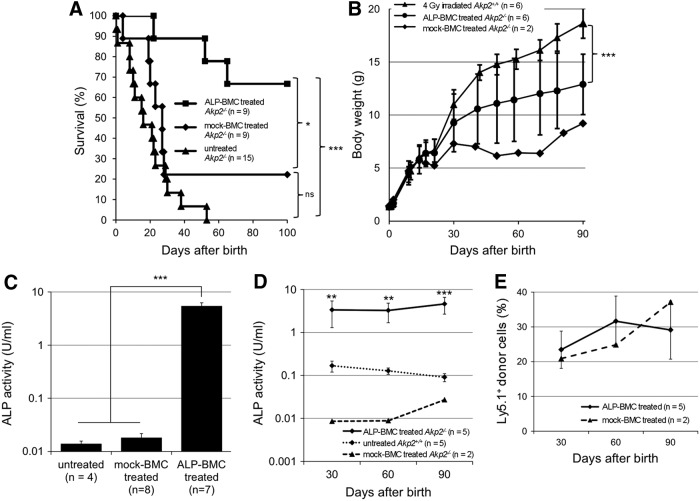
Following injection of mock-BMC into irradiated neonatal Akp2−/− mice on day 2 after birth (mock-BMC-treated Akp2−/− mice), seven of nine mice died within 1 month (Fig. 1A). The survival was narrowly prolonged in the remaining two mock-BMC-treated Akp2−/− mice; however, this was not a significant effect, as compared with untreated Akp2−/− mice (p = 0.14). By contrast, survival was significantly prolonged in neonatal Akp2−/− mice receiving ALP-BMC (ALP-BMC-treated Akp2−/− mice), as compared with untreated Akp2−/− mice (p < 0.001) and mock-BMC-treated Akp2−/− mice (p < 0.05). The ALP-BMC-treated Akp2−/− mice were normal in appearance and experienced no seizures during the experimental period. In addition, the growth curve for ALP-BMC-treated Akp2−/− mice was significantly improved over that obtained with mock-BMC-treated Akp2−/− mice (Fig. 1B). However, the body weight increase in ALP-BMC-treated Akp2−/− mice did not reach the level seen with age-matched 4 Gy-irradiated Akp2+/+ mice (Fig. 1B).
Figure 1.
Therapeutic effects of ex vivo gene therapy in neonatal Akp2−/− mice. (A) Survival curves for ALP-BMC-treated, mock-BMC-treated, and untreated Akp2−/− mice. *p < 0.05, ALP-BMC-treated vs. mock BMC-treated Akp2−/− mice; ***p < 0.001, ALP-BMC-treated vs. untreated Akp2−/− mice; ns, not significant (p = 0.14, mock BMC-treated vs. untreated Akp2−/− mice). (B) Comparison of average body weights among Akp2+/+ mice irradiated at a dose of 4 Gy, ALP-BMC-treated Akp2−/− mice (4 Gy irradiation), and mock-BMC-treated Akp2−/− mice (4 Gy irradiation). ***p < 0.001, ALP-BMC-treated vs. 4 Gy-irradiated Akp2+/+ mice on day 90. (C) Comparison of plasma ALP activity 1 week after treatment among untreated (n = 4), mock-BMC-treated (n = 8), and ALP-BMC-treated (n = 7) Akp2−/− mice. ***p < 0.001 vs. ALP-BMC-treated Akp2−/− mice. (D) Time course of the changes in plasma ALP activity in ALP-BMC-treated Akp2−/− mice, untreated Akp2+/+ mice, and mock-BMC-treated Akp2−/− mice. **p < 0.01, ***p < 0.001, ALP-BMC-treated vs. untreated Akp2+/+ mice. (E) Long-term engraftment of Ly5.1+ donor cells in ALP-BMC-treated and mock-BMC-treated Akp2−/− mice. ALP, alkaline phosphatase; BMC, bone marrow cells.
Long-term enzyme supplementation from BMC expressing TNALP-D10
To evaluate TNALP-D10 expression in the ALP-BMC-treated Akp2−/− mice, plasma ALP activity was measured 1 week and every 30 days after BMC transplantation. The plasma ALP activity rapidly increased in ALP-BMC-treated Akp2−/− mice and was approximately 400 times higher than in untreated Akp2−/− mice within 1 week after transplantation (5.391 ± 2.285 vs. 0.014 ± 0.004 U/ml) (Fig. 1C). The plasma ALP activity of mock-BMC-treated Akp2−/− mice (0.018 ± 0.011 U/ml) was essentially the same as in untreated Akp2−/− mice. In addition, the plasma ALP activity in ALP-BMC-treated Akp2−/− mice was sustained at significantly higher level than was seen in untreated Akp2+/+ mice throughout the experimental period (Fig. 1D). This is noteworthy, as the plasma ALP level was sufficient to obtain therapeutic benefits without adverse effects, as we reported previously.17–19 In both ALP-BMC-treated Akp2−/− mice and the two surviving mock-BMC-treated Akp2−/− mice, the engraftment of donor cells was maintained at approximately 30% throughout the experimental period (Fig. 1E), though only the ALP-BMC-treated Akp2−/− mice experienced a therapeutic effect. These results indicate that transplantation of native BMC is not adequate to treat Akp2−/− mice; genetic modification of the donor BMC is necessary for continuous supplementation of TNALP-D10 at a level sufficient to obtain therapeutic effects.
Overexpression of TNALP-D10 does not influence multilineage reconstitution in ALP-BMC-treated Akp2−/− mice
The effect of TNALP-D10 overexpression on the multilineage differentiation potential of lentivirally transduced BMC was assessed using CFU assays (Table 1). The numbers of CFU-M and CFU-GM did not differ among untreated BMC, ALP-BMC, and GFP-BMC. Transduction efficiency, based on the fraction of GFP-positive colonies among the total colonies formed by GFP-BMC, was 73.9 ± 20.9%. The in vivo multilineage reconstitution of hematopoiesis (myeloid, B, and T-cells) in the ALP-BMC-treated Akp2−/− mice was also assessed by FACS analysis on day 90 after transplantation (Table 2). Upon reconstitution, the lineage profile in PB from ALP-BMC-treated Akp2−/− mice was similar to that in mock-BMC-treated Akp2+/+ mice. There was no disturbance in the numbers of circulating monocytes, neutrophils, or B and T lymphocytes, although the exogenous TNALP-D10 was manufactured in the transduced bone marrow precursor cells. Moreover, the ratios of donor BMC in each lineage were similar between ALP-BMC-treated Akp2−/− mice and mock-BMC-treated Akp2+/+ mice. These results indicate that long-term overexpression of TNALP-D10 does not affect donor cell engraftment or multilineage reconstitution in the recipient mice.
Table 1.
Colony-forming unit assay of bone marrow cells transduced by lentiviral vectors
| Transduction | CFU-M | CFU-GM | Total |
|---|---|---|---|
| UT-BMC | 11.7 ± 2.1 | 9.7 ± 3.2 | 21.3 ± 4.7 |
| ALP-BMC | 12.0 ± 1.7 | 9.3 ± 2.5 | 21.3 ± 3.2 |
| GFP-BMC | 12.7 ± 1.5 | 10.7 ± 5.9 | 23.3 ± 4.6 |
ALP, alkaline phosphatase; BMC, bone marrow cells; CFU, colony-forming unit; GFP, green fluorescent protein; GM, granulocyte macrophage; M, macrophage; UT, untransduced.
CFU colonies in methylcellulose culture were evaluated at day 7 (n = 3). Data are presented as mean ± SD. No significant differences were observed.
Table 2.
Multilineage reconstitution in the peripheral blood of the transplanted mice
| % in total PBMC | |||
|---|---|---|---|
| Myeloid cell | B-cell | T-cell | |
| ALP-BMC-treated Akp2−/− (n = 5) (% donor in each lineages) | 11.9 ± 4.2 | 33.6 ± 20.7 | 59.0 ± 17.3 |
| (38.5 ± 7.5) | (21.7 ± 9.9) | (42.0 ± 9.6) | |
| Mock-BMC-treated Akp2+/+ (n = 3) (% donor in each lineages) | 9.6 ± 0.9 | 32.9 ± 8.4 | 55.7 ± 14.8 |
| (49.3 ± 19.4) | (29.1 ± 14.3) | (57.1 ± 27.4) | |
PBMC, peripheral blood mononuclear cells.
Myeloid cells, B-cells, and T-cells were identified by PE-conjugated anti-mouse Mac-1 + Gr-1, B220, and CD3e antibodies, respectively, on day 90 after transplantation. Donor Ly5.1+ cells were identified by FITC-conjugated anti-mouse CD45.1 antibody. Data are presented as the mean ± SD. No significant differences were observed.
Accumulation of TNALP-D10 on the bone surface in ALP-BMC-treated Akp2−/− mice
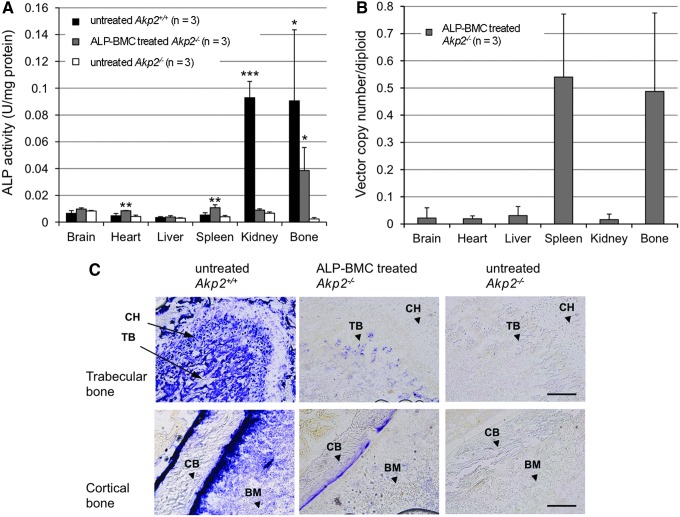
To examine ALP expression in the organs, selected tissues were harvested from untreated Akp2+/+ mice and untreated and ALP-BMC-treated Akp2−/− mice on day 30 after transplantation. Colorimetric assays of ALP activity in the tissue homogenates revealed that bone ALP activity was significantly increased in ALP-BMC-treated Akp2−/− mice, compared with untreated Akp2−/− mice (Fig. 2A). The renal ALP activity, which was abundant in Akp2+/+ mice, was not restored in the ALP-BMC-treated Akp2−/− mice. In the Akp2+/+ mice, native form of TNALP exists on the cell membrane surface, anchored by a GPI anchor motif. However, TNALP-D10 secreted from the transduced cells has a high affinity for hydroxyapatite, and so the circulating TNALP-D10 is thought to accumulate selectively on bone surfaces in ALP-BMC-treated Akp2−/− mice, not in the kidneys. In addition, splenic ALP activity was only slightly elevated from the basal level, though the same high copy number of the lentiviral vector genome present in bone was also detected in the spleen (Fig. 2B). Furthermore, histological detection showed apparent ALP activity on the surface of trabecular and cortical bones in the distal femoral metaphysis of 30-day-old ALP-BMC-treated Akp2−/− mice, whereas no ALP signal was detected in the corresponding area in untreated Akp2−/− mice (Fig. 2C). No ectopic calcification was observed within internal organs or muscle upon macroscopic diagnosis or X-ray examination. These results indicate that bone-targeted TNALP-D10 selectively accumulated on the bone surface, in agreement with its high affinity for hydroxyapatite,3,17 although the accumulated levels were much lower than those found in the Akp2+/+ mice (Fig. 2C).
Figure 2.
Tissue ALP activity and lentiviral vector distribution in ALP-BMC-treated Akp2−/− mice. (A) Comparison of tissue ALP activity. ALP activities were measured in the supernatants from the indicated tissue homogenates using a colorimetric assay. Tissue samples were collected from untreated Akp2+/+ mice, ALP-BMC-treated Akp2−/− mice, and untreated Akp2−/− mice 30 days after transplantation (n = 3, respectively). *p < 0.05, **p < 0.01, ***p < 0.001 vs. untreated Akp2−/− mice. (B) Distribution of the lentiviral vector genome in the tissues of ALP-BMC-treated Akp2−/− mice (n = 3). The presence of integrated vector genome was detected using real-time PCR with a lentiviral vector-specific TaqMan probe. Vector copy numbers were normalized to the mTfrc gene. (C) Histochemical staining of ALP activity in the femurs of 30-day-old untreated Akp2+/+ mice, ALP-BMC-treated Akp2−/− mice, and untreated Akp2−/− mice. ALP activity is reflected by blue staining on the surface of the trabecular and cortical bone. BM, bone marrow; CB, cortical bone; CH, chondrocyte; TB, trabecular bone. Scale bar: trabecular bone (upper panels), 200 μm; cortical bone (lower panels), 100 μm.
Morphological analysis of bone structure in ALP-BMC-treated Akp2−/− mice
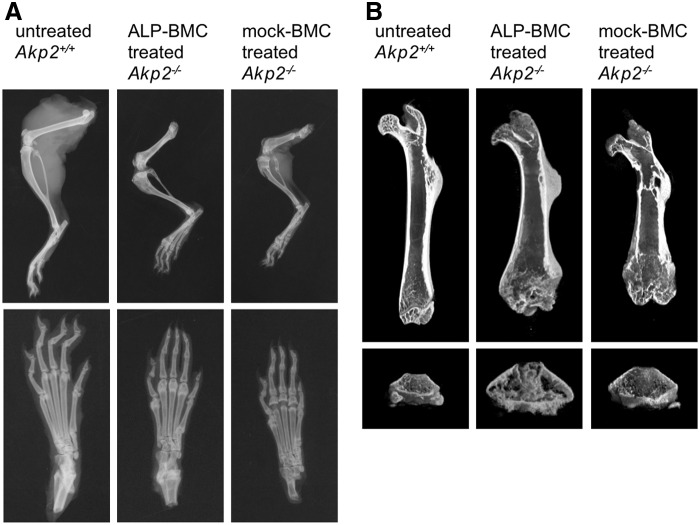
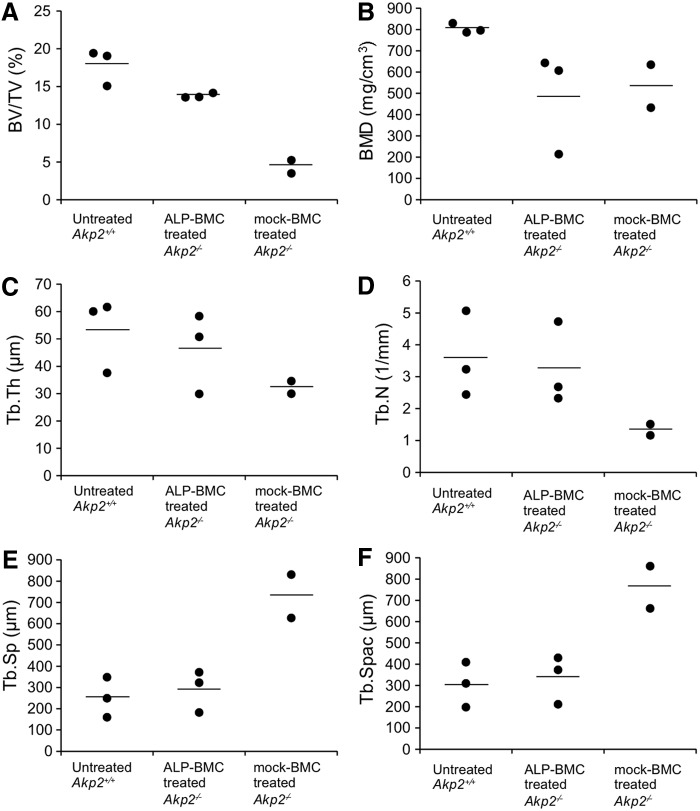
The efficacy of ex vivo gene therapy for correction of bone hypomineralization was evaluated through X-ray and μCT examination of the hind limbs of 100-day-old untreated Akp2+/+ and ALP-BMC-treated and mock-BMC-treated Akp2−/− mice. Bone formation in ALP-BMC-treated Akp2−/− mice was improved, as compared with mock-BMC-treated Akp2−/− mice (Fig. 3A and B). The lack of bone formation in the proximal femoral diaphysis and secondary ossification centers in the hind paw was only seen in the mock-BMC-treated Akp2−/− mice, although the lengths of the femur and tibia were nearly the same in ALP-BMC-treated and mock-BMC-treated Akp2−/− mice (Fig. 3A). On the other hand, incomplete osteogenesis, such as a widened metaphysis, which is frequently observed in the HPP patients,38,39 persisted in both ALP-BMC-treated and mock-BMC-treated Akp2−/− mice (Fig. 3B). Nonetheless, μCT analysis indicated that the bone morphology of ALP-BMC-treated Akp2−/− mice was markedly better than that of mock-BMC-treated Akp2−/− mice, and was similar to that in Akp2+/+ mice (Fig. 4A and C–F), except for the bone mineral density (BMD) (Fig. 4B). These data indicate that bone structural development is improved in ALP-BMC-treated mice, although accumulation of minerals such as calcium remains insufficient.
Figure 3.
Hind limb bone morphology. Representative bone morphology in 100-day-old untreated Akp2+/+ mice, ALP-BMC-treated Akp2−/− mice and mock-BMC-treated Akp2−/− mice. (A) X-ray radiographs of hind limbs (upper panels) and paws (lower panels). (B) μCT 3D images of femurs (upper panels) and distal femoral metaphysis (lower panels). μCT, micro-computed tomography.
Figure 4.
μCT analysis of femoral bones. The trabecular structural parameters were calculated for 100-day-old untreated Akp2+/+ mice (n = 3), ALP-BMC-treated Akp2−/− mice (n = 3), and mock-BMC-treated Akp2−/− mice (n = 2). BMD, bone mineral density; BV/TV, bone volume/total volume; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Spac, trabecular spacing; Tb.Th, trabecular thickness. Horizontal bars represent the mean values for each group.
Discussion
In this study, we examined the feasibility of neonatal ex vivo gene therapy using lentivirally transduced BMC expressing TNALP-D10 to rescue Akp2−/− mice, a model of infantile HPP. We found that gene therapy prevented the epileptic seizures otherwise were seen in Akp2−/− mice and significantly prolonged the survival of the mice, which also exhibited improved weight gain (Fig. 1A and B). In response to gene therapy, plasma ALP activity rapidly increased to therapeutically effective levels (Fig. 1C) and was sustained at the higher levels for more than 3 months (Fig. 1D). Collectively, these results indicate that a single neonatal transplantation of BMC expressing TNALP-D10 provides significant therapeutic benefits and significantly prolongs the survival of Akp2−/− mice.
Seven of the nine mock-BMC-treated Akp2−/− mice died within 1 month (Fig. 1A), and the remaining two mock-BMC-treated Akp2−/− mice narrowly survived but with significant growth impairment (Fig. 1B). This may reflect a benefit provided by the transplanted mock-BMC12 or by other phosphatases related to bone mineralization, such as PHOSPHO1 and nucleotide pyrophosphatase/phospho-diesterase-1, which could act to compensate for the absence of endogenous TNALP40 and contribute to the relief of symptoms in the mock-BMC-treated Akp2−/− mice. In this experiment, we used B6.CD45.1 (Akp2+/+, Ly5.1) mice as a donor for bone marrow transplantation because of difficulty of collecting BMC from Akp2−/− newborn mice and the utility of the Ly5.1 marker for detection of engrafted donor cells. Additional experiments using Akp2−/− BMC as donor cells may be needed to clarify the efficacy of bone marrow transplantation and ex vivo gene therapy for the treatment of HPP. On the other hand, the therapeutic efficacy of MSC and osteoblast transplantation has been reported.9–12 In addition, the differentiation of a patient's MSC into osteoblasts after transduction with a retroviral vector encoding native form of TNALP has also been reported.41 Based on those findings, we suggest that co-transplantation of genetically modified BMC and MSC could be an effective approach to treating HPP. Further studies will be needed to determine whether BMC expressing TNALP-D10 for systemic supplementation of soluble TNALP and MSC expressing native form of TNALP for differentiation into physiologically active osteoblasts can provide synergistic effects in the treatment of HPP.
There is no established regimen of conditioning for BMT of neonatal mice, although lethal irradiation is the most widely used in mouse BMT experiments. We gave 4 Gy of TBI to neonatal Akp2−/− mice in this study. In recipient mice, the engraftment of donor cells was maintained at approximately 30% throughout the experimental period (Fig. 1E). However, even low-dose irradiation may be toxic especially in the neonatal period and associated with risks of growth impairment (Fig. 1B) and/or neural cell damage.42 Recently, it was reported that various genetic diseases can be successfully treated by hematopoietic stem cell-based ex vivo gene therapy in which the immunosuppressive drug busulfan or melphalan was used for the preconditioning.26,43–45 Thus, immunosuppressive drugs may also be applicable for the preconditioning before ex vivo gene therapy for HPP.
The transplanted ALP-BMC in the recipient Akp2−/− mice showed stable engraftment (Fig. 1E) with normal multilineage reconstitution (Tables 1 and 2). This suggests that the overexpression of TNALP-D10 did not affect reconstitution by the donor BMC. It has also been reported that overexpression of a therapeutic enzyme in lentivirally transduced BMC does not affect the hematopoietic system during ex vivo gene therapy in metachromatic leukodystrophy26 or Pompe46 and Fabry47 disease. We therefore suggest that lentivirally transduced BMC can serve as an applicable reservoir for a safe, long-term supply of TNALP-D10 with normal hematopoietic reconstitution. The treated Akp2−/− mice were normal in appearance, and no ectopic calcification was observed within internal organs. However, careful follow-up (e.g., monitoring of vascular calcification) is needed to assess the efficacy and safety of the stem cell-based ERT in future experiments.
Analysis of tissue ALP activity in ALP-BMC-treated Akp2−/− mice revealed that, whereas similar amounts of lentiviral vector genome were present in spleen and bone, TNALP-D10 localized exclusively in bone tissue (Fig. 2A and B). Additionally, histochemical detection of ALP activity in femurs revealed targeted accumulation of TNALP-D10 on the surface of trabecular and cortical bone in the ALP-BMC-treated Akp2−/− mice (Fig. 2C). These findings suggest that the TNALP-D10 produced by transduced BMC accumulates on the surface of bone due to its high affinity for hydroxyapatite.3,17 On the other hand, virtually no localization of TNALP-D10 was detected on growth plate chondrocytes in ALP-BMC-treated Akp2−/− mice, whereas the growth plate chondrocytes of Akp2+/+ mice express abundant TNALP during bone development (Fig. 2C). X-ray and μCT examination of the skeleton showed that bone formation was much improved in ALP-BMC-treated Akp2−/− mice (Fig. 3A and B) and that the trabecular bone structure in ALP-BMC-treated Akp2−/− mice was restored to levels similar to that in Akp2+/+ mice (Fig. 4). Nonetheless, immature bone formation, including a widened metaphysis, persisted in ALP-BMC-treated Akp2−/− mice (Fig. 3A and B). These results indicate that the moderate accumulation of TNALP-D10 on the bone surface was sufficient to enhance bone mineralization, but was not sufficient to mediate the formation of a normal metaphysis. Proliferative growth plate chondrocytes are reportedly abnormal in the articular and epiphyseal cartilage of Akp2−/− mice treated with recombinant GPI-anchorless TNALP, though survival of these animals was prolonged over 6 months by the ERT.15 Thus, accumulation of TNALP on the surface of both bone and growth plate chondrocytes in the epiphyses is essential for normal bone formation.
Perinatal HPP is the most severe form of the disease, and efficacious treatment as early as possible is required for these patients. With development of prenatal diagnosis through the use of echography, computed tomography, and genetic diagnosis, perinatal HPP can be correctly diagnosed in the fetus before birth.48,49 To develop a fetal treatment for perinatal HPP, we previously examined the effect of transuterine intraperitoneal injection of an AAV vector harboring the TNALP-D10 construct into fetal Akp2−/− mice on day 15 of gestation.18 The fetal treatment provided preferential transduction of growth plate chondrocytes by AAV vector in the epiphysis of the treated Akp2−/− mice. In utero transplantation of paternal hematopoietic stem cells was successfully applied to treat X-linked severe combined immunodeficiency.50,51 In addition, in utero transplantation of genetically modified BMC was also determined to be an applicable strategy for treating inherited disease model mice.52,53 If transplanted BMC migrate to nearby primordial cartilage in fetal bone in Akp2−/− mice, it may be that TNALP-D10 could be safely supplied at levels sufficient for chondrocyte differentiation early during fetal development. The possibility of in utero transplantation of BMC expressing TNALP-D10 would be examined to determine whether genetically modified BMC can protect against the skeletal abnormalities and rescue perinatal Akp2−/− mice.
In summary, we successfully applied stem cell-based ERT to treat the lethal phenotype of Akp2−/− mice through a single systemic transplantation of lentivirally transduced BMC on day 2 after birth. Plasma ALP activity in the treated Akp2−/− mice rapidly increased following transplantation and was maintained at a level sufficient to prolong survival with improved weight gain and bone mineralization. To achieve greater therapeutic efficacy leading to normal bone development, there will need to be improvements to the engraftment regimen and strategy for local delivery of TNALP onto the surface of bone matrix and growth plate chondrocytes. However, neonatal ex vivo gene therapy using BMC expressing TNALP-D10 appears to be a potentially effective and beneficial means of continuously supplying TNALP-D10 for the treatment of infantile HPP.
Acknowledgments
We thank Dr. Seiko Yamamoto at the Nihon University Graduate School of Dentistry at Matsudo, and Dr. Tae Matsumoto and Dr. Hanako Sugano-Tajima at Nippon Medical School for valuable advice and support. This work was supported by JSPS KAKENHI Grant Number 25461564.
Author Disclosure
J.L.M. is a consultant for Alexion Pharmaceuticals (Cheshire, CT) and AM Pharma (Bunnik, Netherlands). The other authors have no competing financial interests.
References
- 1.Mornet E. Hypophosphatasia. Orphanet J Rare Dis 2007;2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whyte MP. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann N Y Acad Sci 2010;1192:190–200 [DOI] [PubMed] [Google Scholar]
- 3.Millán JL. The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int 2013;93:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whyte MP, Landt M, Ryan LM, et al. Alkaline phosphatase: Placental and tissue-nonspecific isoenzymes hydrolyze phosphoethanolamine, inorganic pyrophosphate, and pyridoxal 5'-phosphate. Substrate accumulation in carriers of hypophosphatasia corrects during pregnancy. J Clin Invest 1995;95:1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedde KN, Lane CC, Whyte MP. Alkaline phosphatase is an ectoenzyme that acts on micromolar concentrations of natural substrates at physiologic pH in human osteosarcoma (SAOS-2) cells. Arch Biochem Biophys 1988;264:400–409 [DOI] [PubMed] [Google Scholar]
- 6.Kozlowski K, Sutcliffe J, Barylak A, et al. Hypophosphatasia. Review of 24 cases. Pediatr Radiol 1976;5:103–117 [DOI] [PubMed] [Google Scholar]
- 7.Whyte MP, Zhang F, Wenkert D, et al. Hypophosphatasia: Validation and expansion of the clinical nosology for children from 25 years experience with 173 pediatric patients. Bone 2015;75:229–239 [DOI] [PubMed] [Google Scholar]
- 8.Demirbilek H, Alanay Y, Alikaşifoğlu A, et al. Hypophosphatasia presenting with pyridoxine-responsive seizures, hypercalcemia, and pseudotumor cerebri: Case report. J Clin Res Pediatr Endocrinol 2012;4:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill RA, Wenkert D, Perlman SA, et al. Infantile hypophosphatasia: Transplantation therapy trial using bone fragments and cultured osteoblasts. J Clin Endocrinol Metab 2007;92:2923–2930 [DOI] [PubMed] [Google Scholar]
- 10.Tadokoro M, Kanai R, Taketani T, et al. New bone formation by allogeneic mesenchymal stem cell transplantation in a patient with perinatal hypophosphatasia. J Pediatr 2009;154:924–930 [DOI] [PubMed] [Google Scholar]
- 11.Taketani T, Oyama C, Mihara A, et al. Ex vivo expanded allogeneic mesenchymal stem cells with bone marrow transplantation improved osteogenesis in infants with severe hypophosphatasia. Cell Transplant 2015;24:1931–1943 [DOI] [PubMed] [Google Scholar]
- 12.Whyte MP, Kurtzberg J, McAlister WH, et al. Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res 2003;18:624–636 [DOI] [PubMed] [Google Scholar]
- 13.Millán JL, Narisawa S, Lemire I, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res 2008;23:777–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whyte MP, Greenberg CR, Salman NJ, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med 2012;366:904–913 [DOI] [PubMed] [Google Scholar]
- 15.Oikawa H, Tomatsu S, Haupt B, et al. Enzyme replacement therapy on hypophosphatasia mouse model. J Inherit Metab Dis 2014;37:309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasque KC, Foster BL, Kuss P, et al. Improvement of the skeletal and dental hypophosphatasia phenotype in Alpl-/- mice by administration of soluble (non-targeted) chimeric alkaline phosphatase. Bone 2015;72:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto T, Miyake K, Yamamoto S, et al. Rescue of severe infantile hypophosphatasia mice by AAV-mediated sustained expression of soluble alkaline phosphatase. Hum Gene Ther 2011;22:1355–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugano H, Matsumoto T, Miyake K, et al. Successful gene therapy in utero for lethal murine hypophosphatasia. Hum Gene Ther 2012;23:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto S, Orimo H, Matsumoto T, et al. Prolonged survival and phenotypic correction of Akp2(-/-) hypophosphatasia mice by lentiviral gene therapy. J Bone Miner Res 2011;26:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porada CD, Park PJ, Tellez J, et al. Male germ-line cells are at risk following direct-injection retroviral-mediated gene transfer in utero. Mol Ther 2005;12:754–762 [DOI] [PubMed] [Google Scholar]
- 21.Schuettrumpf J, Liu JH, Couto LB, et al. Inadvertent germline transmission of AAV2 vector: Findings in a rabbit model correlate with those in a human clinical trial. Mol Ther 2006;13:1064–1073 [DOI] [PubMed] [Google Scholar]
- 22.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu T, Töpfer K, Lin SW, et al. Self-complementary AAVs induce more potent transgene product-specific immune responses compared to a single-stranded genome. Mol Ther 2012;20:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginn SL, Alexander IE, Edelstein ML, et al. Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 2013;15:65–77 [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee S, Thrasher AJ. Gene therapy for PIDs: Progress, pitfalls and prospects. Gene 2013;525:174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biffi A, Montini E, Lorioli L, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013;341:1233158. [DOI] [PubMed] [Google Scholar]
- 27.Hofling AA, Devine S, Vogler C, et al. Human CD34+ hematopoietic progenitor cell-directed lentiviral-mediated gene therapy in a xenotransplantation model of lysosomal storage disease. Mol Ther 2004;9:856–865 [DOI] [PubMed] [Google Scholar]
- 28.Yoshimitsu M, Higuchi K, Ramsubir S, et al. Efficient correction of Fabry mice and patient cells mediated by lentiviral transduction of hematopoietic stem/progenitor cells. Gene Ther 2007;14:256–265 [DOI] [PubMed] [Google Scholar]
- 29.Scaramuzza S, Biasco L, Ripamonti A, et al. Preclinical safety and efficacy of human CD34(+) cells transduced with lentiviral vector for the treatment of Wiskott-Aldrich syndrome. Mol Ther 2013;21:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persons DA, Baum C. Solving the problem of gamma-retroviral vectors containing long terminal repeats. Mol Ther 2011;19:229–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narisawa S, Fröhlander N, Millán JL. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn 1997;208:432–446 [DOI] [PubMed] [Google Scholar]
- 32.Narisawa S, Wennberg C, Millán JL. Abnormal vitamin B6 metabolism in alkaline phosphatase knock-out mice causes multiple abnormalities, but not the impaired bone mineralization. J Pathol 2001;193:125–133 [DOI] [PubMed] [Google Scholar]
- 33.Miyake N, Brun AC, Magnusson M, et al. HOXB4-induced self-renewal of hematopoietic stem cells is significantly enhanced by p21 deficiency. Stem Cells 2006;24:653–661 [DOI] [PubMed] [Google Scholar]
- 34.Hanawa H, Yamamoto M, Zhao H, et al. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element. Mol Ther 2009;17:667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charrier S, Dupré L, Scaramuzza S, et al. Lentiviral vectors targeting WASp expression to hematopoietic cells, efficiently transduce and correct cells from WAS patients. Gene Ther 2007;14:415–428 [DOI] [PubMed] [Google Scholar]
- 36.Kawamoto T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol 2003;66:123–143 [DOI] [PubMed] [Google Scholar]
- 37.Kinoshita H, Nakahara K, Matsunaga S, et al. Association between the peri-implant bone structure and stress distribution around the mandibular canal: A three-dimensional finite element analysis. Dent Mater J 2013;32:637–642 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura-Utsunomiya A, Okada S, Hara K, et al. Clinical characteristics of perinatal lethal hypophosphatasia: A report of 6 cases. Clin Pediatr Endocrinol 2010;19:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shohat M, Rimoin DL, Gruber HE, et al. Perinatal lethal hypophosphatasia; clinical, radiologic and morphologic findings. Pediatr Radiol 1991;21:421–427 [DOI] [PubMed] [Google Scholar]
- 40.Ciancaglini P, Yadav MC, Simão AM, et al. Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res 2010;25:716–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsube Y, Kotobuki N, Tadokoro M, et al. Restoration of cellular function of mesenchymal stem cells from a hypophosphatasia patient. Gene Ther 2010;17:494–502 [DOI] [PubMed] [Google Scholar]
- 42.Igarashi T, Miyake K, Hayakawa J, et al. Apoptotic cell death and regeneration in the newborn retina after irradiation prior to bone marrow transplantation. Curr Eye Res 2007;32:543–553 [DOI] [PubMed] [Google Scholar]
- 43.Candotti F, Shaw KL, Muul L, et al. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: Clinical comparison of retroviral vectors and treatment plans. Blood 2012;120:3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009;326:818–823 [DOI] [PubMed] [Google Scholar]
- 45.Hacein-Bey-Abina S, Hauer J, Lim A, et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2010;363:355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Til NP, Stok M, Aerts Kaya FS, et al. Lentiviral gene therapy of murine hematopoietic stem cells ameliorates the Pompe disease phenotype. Blood 2010;115:5329–5337 [DOI] [PubMed] [Google Scholar]
- 47.Pacienza N, Yoshimitsu M, Mizue N, et al. Lentivector transduction improves outcomes over transplantation of human HSCs alone in NOD/SCID/Fabry mice. Mol Ther 2012;20:1454–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mornet E, Hofmann C, Bloch-Zupan A, et al. Clinical utility gene card for: Hypophosphatasia—update 2013. Eur J Hum Genet 2014;22:e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe A, Yamamasu S, Shinagawa T, et al. Prenatal genetic diagnosis of severe perinatal (lethal) hypophosphatasia. J Nippon Med Sch 2007;74:65–69 [DOI] [PubMed] [Google Scholar]
- 50.Flake AW, Roncarolo MG, Puck JM, et al. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med 1996;335:1806–1810 [DOI] [PubMed] [Google Scholar]
- 51.Wengler GS, Lanfranchi A, Frusca T, et al. In-utero transplantation of parental CD34 haematopoietic progenitor cells in a patient with X-linked severe combined immunodeficiency (SCIDXI). Lancet 1996;348:1484–1487 [DOI] [PubMed] [Google Scholar]
- 52.Rio P, Martinez-Palacio J, Ramirez A, et al. Efficient engraftment of in utero transplanted mice with retrovirally transduced hematopoietic stem cells. Gene Ther 2005;12:358–363 [DOI] [PubMed] [Google Scholar]
- 53.Meza NW, Alonso-Ferrero ME, Navarro S, et al. Rescue of pyruvate kinase deficiency in mice by gene therapy using the human isoenzyme. Mol Ther 2009;17:2000–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]