Abstract
Background: In allergen-induced asthma, activated mast cells start the lung inflammatory process with degranulation, cytokine synthesis, and mediator release. Bruton's tyrosine kinase (Btk) activity is required for the mast cell activation during IgE-mediated secretion.
Methods: This study characterized a novel inhaled Btk inhibitor RN983 in vitro and in ovalbumin allergic mouse models of the early (EAR) and late (LAR) asthmatic response.
Results: RN983 potently, selectively, and reversibly inhibited the Btk enzyme. RN983 displayed functional activities in human cell-based assays in multiple cell types, inhibiting IgG production in B-cells with an IC50 of 2.5 ± 0.7 nM and PGD2 production from mast cells with an IC50 of 8.3 ± 1.1 nM. RN983 displayed similar functional activities in the allergic mouse model of asthma when delivered as a dry powder aerosol by nose-only inhalation. RN983 was less potent at inhibiting bronchoconstriction (IC50(RN983) = 59 μg/kg) than the β-agonist salbutamol (IC50(salbutamol) = 15 μg/kg) in the mouse model of the EAR. RN983 was more potent at inhibiting the antigen induced increase in pulmonary inflammation (IC50(RN983) = <3 μg/kg) than the inhaled corticosteroid budesonide (IC50(budesonide) = 27 μg/kg) in the mouse model of the LAR.
Conclusions: Inhalation of aerosolized RN983 may be effective as a stand-alone asthma therapy or used in combination with inhaled steroids and β-agonists in severe asthmatics due to its potent inhibition of mast cell activation.
Key words: : dust feed, jet mill, inhaled therapy, lung function, pharmacokinetics, pre-clinical
Introduction
Protein kinases constitute one of the largest families of enzymes and regulate many different signaling processes by adding phosphate groups to proteins.(1) Bruton's Tyrosine Kinase (Btk) is a member of the Tec family of tyrosine kinases and is involved in signal transduction downstream of the B-cell receptor and the high-affinity receptor for IgE (FcɛR) on mast cells and basophils. The use of Btk inhibitor drugs have primarily focused on diseases involving B-cells.(2) The link between Btk and B-cells comes from human genetics, as X-linked agammaglobulineima (XLA) patients have a loss of functional mutation of Btk and an immunocompromised phenotype with impaired maturation of B-cells.(3)
Asthmatics may also benefit from Btk inhibitors and aerosol administration to the lungs could avoid systemic immunosuppression and accumulation of circulating IgE.(4) Btk is expressed by mast cells and mast cells deficient in Btk(5) or depleted of Btk by siRNA(6) demonstrate impaired antigen-induced degranulation. This suggests that Btk inhibitors could reduce the early asthmatic response (EAR). The EAR response is a type I hypersensitivity reaction where inhaled allergen interacts with mast cell-bound IgE to trigger the release of histamine that causes bronchoconstriction and increases airflow resistance through airway smooth muscle contraction.(7) The EAR is significantly but incompletely reduced by mast cell stabilizers(8) and anti-IgE antibodies,(9) and is currently treated mainly by inhaled β-agonist bronchodilators. Of note, there are species-specific differences in how mast cell-dependent bronchoconstriction develops.(10,11) Instead of histamine, 5-HT is the predominant mediator released from mast cells in mice and drives bronchoconstriction through a central nervous system reflex instead of a direct effect on smooth muscle.(11) That said, the primary mechanisms driving the EAR are similar in mouse and man. The response in both species has been shown to be IgE- and mast cell mediator-dependent.(10–12)
Pulmonary inflammation is a component of the late asthmatic response (LAR) and currently treated with inhaled corticosteroids. Btk inhibitors could also dampen the allergic inflammation in asthma by inhibiting the release of mast cell secreted proinflammatory and vasoactive mediators including histamine, leukotrienes, and cytokines. The absence of Btk has been shown to severely impair FcɛRI-dependent mast cell production of cytokines and degranulation.(13,14) It has also been shown that Btk was required for IgE-mediated activation of human basophils.(15) Collectively, these data suggest a Btk inhibitor could be useful to treat pathological mast cell responses present in asthma.
In this study, ovalbumin-sensitized mice were given RN983 by the nose-only inhalation route of administration to assess its effects on bronchoconstriction and inflammatory cell influx into the bronchoalveolar lavage fluid induced by antigen challenge. We show that RN983 is a potent and selective Btk inhibitor that inhibits bronchoconstriction and pulmonary inflammation in the antigen-induced ovalbumin mouse model of asthma.
Materials and Methods
Enzymatic activity and selectivity
A FRET (fluorescence resonance energy transfer) assay was used to determine the potency of RN983 to competitively inhibit binding of a substrate to Btk. It was adapted from a standard “one step, mix-and-read, time-resolved FRET binding assay” from Invitrogen (Carlsbad, CA). In brief, Btk-BioEase, a biotinylated Btk, was conjugated to a FRET donor, europium, through an incubation with Eu-streptavidin (PerkinElmer Life and Analytical Sciences, Waltham, MA) for 1 h in a buffer containing 20 mM HEPES, pH 7.15, 0.1 mM dithiothreitol, 10 mM MgCl2, and 0.5 mg/mL bovine serum albumin. Btk-Eu conjugate (0.1 nM) was then incubated with KT-178, a kinase tracer and FRET acceptor from Invitrogen, and RN983 or vehicle (DMSO) overnight at 15°C. Photons emitted from both the donor (620 nm) and acceptor (665 nm) were measured by using a BMG Pherastar Fluorescent plate reader (BMG Labtech Gmbh, Offenberg, Germany). The ratio between the 620- and 665-nm signals was calculated to determine Btk-substrate binding and its inhibition by RN983.
Selectivity of RN983 for Btk over other kinases was determined by testing the compound at a single 10−6 M concentration against a panel of 451 kinases at DiscoveRx (San Diego, CA) by using a high-throughput Kinomescan based on ATP free competitive binding.(16) Results for kinase binding interactions were reported as percent competition.
Surface plasmon resonance (SPR) binding kinetics assay
Avi-tagged Btk was captured on Biacore streptavidin sensor chips (Series S) at densities of 10000 resonance units (1 ru = 1 pg protein/mm2). RN983 dissolved in DMSO was tested at 8 concentrations (100 nM down to 0.8 nM, two-fold dilutions). RN983 was injected for 100 s association time, and dissociation was followed for 20 min. Experiments were performed at 25°C. The buffer consisted of 50 mM Hepes pH 7.2, 150 mM NaCl, 10 mM MgCl2, 2 mM MnCl2, 1 mM TCEP, 1% PEG 3350, 5% DMSO. Kinetic analysis was performed using Biacor BIAevaluation software using a simple model for 1:1 (Langmuir) binding.
In vitro studies
B cells
Human total B cells were enriched with RosetteSep human B cell enrichment cocktail (#28921, Vancouver, BC) from buffy coat leukocyte packs (New York Blood Center) following manufacturer's protocol. Enriched B cell purity (around 80%) was checked by FACS with CD19+ staining. B cells were suspended (0.1 million cells/well/100 μL) in RPMI-1640 based conditional medium (50 ng/mL IL-2, 50 ng/mL IL-10, 1 μg/mL anti-IgD for the activation of B cells to produce IgG) together with RN983. Cells were cultured for 10 days at 37°C in 5% CO2 incubator. Culture supernatants were collected for IgG analysis following Bethyl Laboratory's protocol (#E80-104, Montgomery, TX).
Mast cells
One million human cord blood derived CD34+ hematopoietic stem cells (HSCs) from different donors (AllCells #CB008F-S, Emeryville, CA) were cultured for 8 weeks in a serum-free complete medium (StemPro-34 with supplements; Invitrogen, Carlsbad, CA), with recombinant h-SCF (100 ng/mL) and h-IL6 (50 ng/mL). During the first week of culturing, recombinant h-IL3 (10 ng/mL) was also included to support HSCs differentiation. After 8 weeks of culture, cells were stimulated with recombinant h-IL-4 (10 ng/mL) for 5 days. Confirmation of the mast cell differentiation process was routinely done by FACS to check for c-kit and FcɛRI expression; differentiated cells were routinely more than 90% c-kit positive, FcɛRI positive. Differentiated mast cells were sensitized with 0.1 μg/mL anti-NP IgE (Serotec, Raleigh, NC) overnight at 37°C. Cells were washed and then treated with RN983 for 1 h at 37°C. After treatment, cells were cross-linked with 1 μg/mL NP(30)-BSA (Biosearch Technologies, Novato CA) for 30 min. Culture supernatants were collected and assayed for PGD2 (Cayman Chemical Company, Ann Arbor MI) release as per kits' instructions.
Aerosol formulations
Test compounds were micronized (MC One Jet Mill, Jetpharma USA Inc., South Plainfield, NJ) and blended by a Turbula Mixer (GlenMills Inc., Clifton, NJ) with micronized lactose (Lactohale 200, DFE Pharma, Goch, Germany) if required. Dry powder aerosol was generated using a Wright dust feed dry powder aerosol generator. The micronized drug/lactose powder was packed into cylindrical reservoirs using a hydraulic press at approximately 1000 psi to produce compacted cakes of powder used as input by the Wright dust feed aerosol generator.(17)
The aerosol passed through a sonic nozzle for de-agglomeration and into a cyclone to remove non-respirable particles and agglomerates. RN983 (6-tert-Butyl-8-fluoro-2-(3-hydroxymethyl-4-[1-methyl-5-(1′-methyl-1′,2′,3′,4′,5′,6′-hexahydro-[3,4′-6-ylamino)-6-oxo-1,6-dihydro-pyridazin-3-yl]-pyridin-2-yl)-2H-phthalazine-1-one) was synthesized at Hoffmann-La Roche in Nutley, NJ. The chemical structure of RN983 is shown in Figure 1. Salbutamol (AL156) and budesonide (B1595) were purchased from Spectrum Chemicals (New Brunswick, NJ).
FIG. 1.
Chemical structure of inhaled Btk-inhibitor RN983.
In vivo studies
Mouse allergen-induced bronchoconstriction model of the early asthmatic response (EAR)
Adult male Balb/c mice (greater than 8 weeks of age) were sensitized and boosted by intraperitoneal (i.p.) injection of 0.2 mL of 2% aluminum hydroxide (ALUM) gel (Serva Electrophoretics, 12261, Heidelberg, Germany) containing 10 μg of ovalbumin (OVA) antigen (Worthington Biochemical Corporation, LS003054, Lakewood, NJ) on days 0 and 14. The i.p. injection solution was prepared by dissolving 2.55 mg OVA in one mL of 0.9% saline, then adding it to 50 mL of ALUM gel, yielding a final concentration of 50 μg OVA/mL ALUM gel.
Once animals were sensitized, nebulized OVA was inhaled to evoke antigen-induced lung inflammation and expand mast cell population in the lungs. For nebulized OVA challenge, mice were placed in a plexiglass box, and aerosolized OVA was nebulized into the box by a nebulizer (PARI Respiratory Equipment, LC STAR nebulizer and Proneb Ultra II compressor, Midlothian, VA) filled with 1% ovalbumin in saline (0.01 g/mL) for 20 min on days 21, 22, and 23. On day 26, a 20 minute nebulized OVA challenge with 5% ovalbumin in saline was conducted. Animal care and experimental procedures used in this study were approved by the Roche Animal Care and Use Committee (RACUC, Nutley, NJ, USA), which is a facility accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC).
Dry powder nose-only inhalation drug dosing
In four separate experiments (100% RN983, 30% RN983 q.s. lactose, 100% salbutamol, and 30% budesonide q.s. lactose), three groups of 8 mice were exposed to drug aerosol for 5, 15, or 45 minutes. Two groups of 8 control mice (saline and ovalbumin control groups) were exposed to filtered air. RN983 was dosed once a day for 6 days (Days 21, 22, 23, 26, 27, and 28), salbutamol was dosed once 1 h before bronchoconstriction measurements on day 28, and budesonide was dosed once a day for 3 days (Days 26, 27, and 28). The cyclone output from the aerosol generator passed into the Nose-Only Exposure Inhalation Unit (TSE systems, Bad Homburg, Germany)(18). The Inhalation Unit had 32 ports for animals and 3 sampling ports for sampling: real time aerosol concentration (Microdust pro, Casella, Buffalo, NY), gravimetric aerosol concentration (absolute filter), and particle size (cascade impactor). The output of the aerosol generator was set to supply 0.5 liters/minute to each animal port.
During exposure, the animals were restrained in glass tubes designed for the anatomy of mice. The exposure system was qualified for spatial uniformity and temporal stability by an air mass balance. The air flow to each port and the port to port flow rates varied by less than 2% with the input flow rate set at 17 liters per minute. The particle size distribution was evaluated by collecting aerosol samples with an eight stage cascade impactor (In-Tox Products, Moriarty, NM). Aerosol was sampled by the cascade impactor at an airflow rate of 1 liter per minute for 45 minutes. HPLC was used to separate the lactose from drug and determine the amount of drug collected on each stage. Impactor data were mathematically evaluated to determine the Mass Median Aerodynamic Diameter (MMAD) and Geometric Standard Deviation (GSD) of drug or lactose using graphical analysis of an assumed lognormal distribution.
Aerosol dose calculations
The deposited dose(19) in mg/Kg was calculated by multiplying the concentration of the drug in the aerosol (mg/L) x minute ventilation (L/min) x duration of exposure (min) x inhalable fraction x pulmonary deposition fraction and dividing by the body weight (Kg). The aerosol concentration (mg/L, Table 1) was measured gravimetrically and calculated by dividing the absolute filter weight (mg) by the air flow rate through the filter (L/min) multiplied by the sampling time (min). The mouse's minute ventilation was estimated as 0.026 liters per minute,(20) the inhalable fraction was 1 as the aerosol was passed through a cyclone to remove non-respirable particles, and the pulmonary deposition fraction (Table 1) was determined from the drug aerosol MMAD using experimental calibration curves from monodisperse aerosols.(21)
Table 1.
Summary of Drug Aerosol Properties and Deposited Dose for in Vivo Experiments
| Average daily deposited dose (μg Drug /kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug (Study endpoint) | Formulation (wt% drug) | MMAD drug (μm) | Deposition fraction | Aerosol concentration (μg /L Air) | Post-cyclone fraction of drug in aerosol | Days of dosing | 5 minute group | 15 minute group | 45 minute group |
| RN983 (Resistance) | 100 | 1.4 | 0.034 | 184 ± 31 | 1 | 6 | 30 ± 6 | 100 ± 20 | 300 ± 50 |
| Salbutamol (Resistance) | 100 | 0.8 | 0.036 | 82 | 1 | 1 | 15 | 50 | 140 |
| RN983 (Inflammation) | 30 | 1.8 | 0.037 | 115 ± 14 | 0.39 ± 0.1 | 6 | 3 ± 1 | 10 ± 2 | 30 ± 5 |
| Budesonide (Inflammation) | 30 | 1.7 | 0.036 | 105 ± 7 | 0.64 ± 0.2 | 3 | 12 ± 1 | 40 ± 2 | 110 ± 10 |
Endpoint measurements (Day 28)
One hour after drug dosing, mice were surgically prepared while under anesthesia (100 mg/kg pentobarbital, i.p.) for cannulation of the trachea. The anterior cervical skin was opened and tracheostomy performed with an 18 gauge Angiocath (Becton, Dickinson and Company, 381144, Sandy, UT). The animals were then subject to respiratory system resistance measurements or pulmonary inflammation measurements.
Respiratory system resistance measurements
The animals were connected to the flexiVent system (SCIREQ Scientific Respiratory Equipment, Inc., Montreal, Canada). The flexiVent system contains a rodent respirator (Tidal volume = 10 mL/kg, Respiratory rate = 150 breaths/min, 3 cmH2O positive end expiratory pressure), under computer control. Once trained to artificial ventilation (not breathing against the ventilator), a deep inflation maneuver was performed by inflating the lungs to total lung capacity (30 cmH2O) to open up atelectic regions of the lung and normalize the lung volume. Then, sinusoidal volume forced 150 Hz oscillation waveforms up to a tidal volume of 10 mL/kg (SnapShot-150 perturbation) were applied to the lungs while pressure measurements were recorded. The volume and pressure data were fit to the single-compartment model and respiratory system resistance (R) and elastance (E) were calculated.
Baseline lung function measurements were repeated five times over 30 seconds. Bronchoconstriction was then induced by a 30 mg/kg injection (100 μL of volume) of OVA in saline into the tail vein. OVA causes bronchoconstriction by inducing mast cell degranulation in OVA sensitized mice, different from agents such as methacholine and histamine that directly contract airway smooth muscle. Lung function was measured continuously every 10 sec over the next 10 min. The percent increase in R for each animal, calculated as the maximum R over the 10 min interval after i.v. OVA injection (Rmax) minus the average R value of the 5 baseline measurements (Rave) divided by Rave (% increase in R = (Rmax-Rave)/Rave), was used to quantitate the bronchoconstriction.
Pulmonary inflammation measurements
The lungs were lavaged four times with 0.3 mL of phosphate buffered saline (PBS). The saline was instilled into the lungs and allowed to equilibrate for at least 30 s before recovery. The four aliquots were pooled and total recovery volume per mouse was approximately 0.9 mL. Total leukocyte number per μL was determined by a coulter counter from a 100 μL bronchoalveolar lavage fluid (BALF) sample by diluting into 10 mL diluent buffer with 100 μL of erythrocyte lysing reagent (Zap-Oglobin II, Beckman Coulter, Fullerton, CA, USA). For differential leukocyte counts, cytospin slides (Shandon Scientific Ltd., Runcorn, UK) were prepared using 100 μL of the BALF sample diluted with 100 μL PBS and centrifuged onto a microscope slide at 1300 rpm for 5 min. The deposited cells were then fixed to the microscope slides and stained with the Hemacolor stain set (EMD Chemicals Inc., Gibbstown, NJ, USA). Cellular staining and standard morphological characteristics were used to determine the percentage of neutrophils, eosinophils, macrophages, and lymphocytes from a microscopic count of 200 cells as described previously.(22)
Data analysis
All data were summarized as group mean ± standard error of the mean. ANOVA with Dunnett's multiple comparisons test was performed to determine whether differences between groups vs OVA control group were significant. The null hypothesis was rejected at *p < 0.05.
Results
Inhibition of Btk and selectivity
RN983 demonstrated an IC50 of 0.40 nM in the Btk enzymatic activity FRET assay and 0.41 nM affinity (KD) measured in the SPR assay (data not shown). RN983 demonstrated an off-rate (Kd) of 3.6 × 10−4 (1/s) against Btk in a surface plasmon resonance assay. RN983 maintained its nanomolar potency in whole cell assays, inhibiting IgG production in human B-cells with an IC50 of 2.5 ± 0.7 nM and PGD2 production from human mast cells with an IC50 of 8.3 ± 1.1 nM (Fig. 2).
FIG. 2.
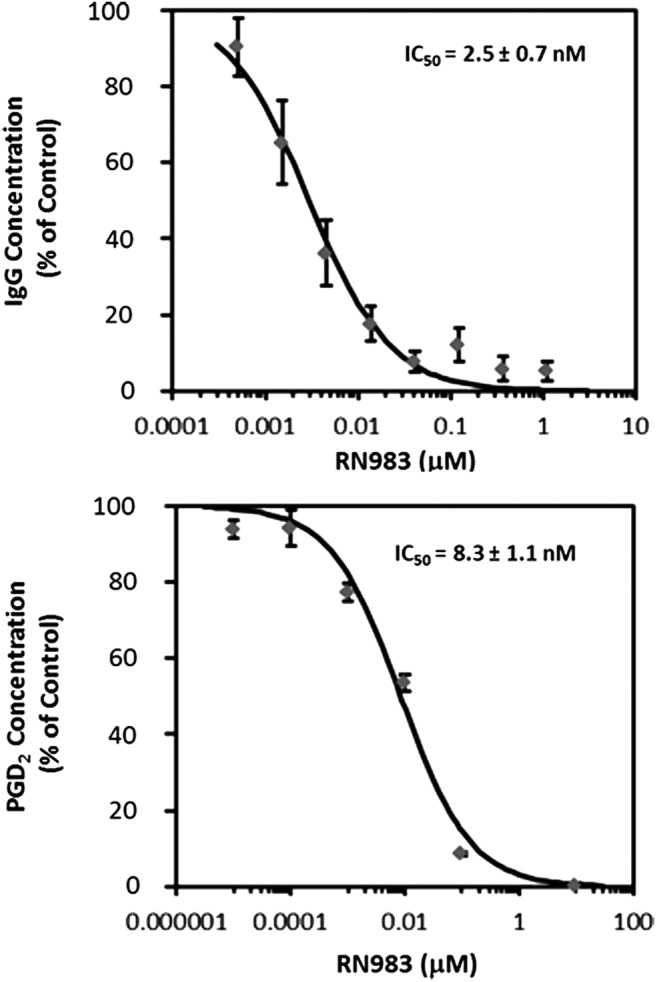
RN983 inhibited IgG production from human B-cells (top panel) and PGD2 production from human mast cells (bottom panel) with single digit nanomolar potency.
RN983 was highly selective with 98% competition against Btk at 1 μM and having between 76% to 88% competition with only 7 of the other 450 kinases screened (scanMAX KINOMEscan, DiscoveRx, San Diego, CA). The specificity of RN983 was also tested at 10 μM in the high-throughput profile screen (Cerep Inc., Redmond, WA), demonstrating less than 80% inhibition of the control compounds specific binding for the 80 receptors, ion channels, and transporters tested (data not shown).
Inhalation dosing
Aerosol deposited dose values and properties (concentration and particle size) from samples of aerosol collected from the animal exposure chamber during dosing of the four in vivo studies are summarized for drug (Table 1) and lactose (Table 2). The geometric standard deviation of the aerosol mass median aerodynamic diameter ranged from 1.7 to 2.2 micron for these studies.
Table 2.
Summary of Lactose Aerosol Properties and Deposited Dose for in Vivo Experiments
| Average daily deposited dose (μg lactose/kg) | |||||||
|---|---|---|---|---|---|---|---|
| Drug (Study endpoint) | Formulation (wt% lactose) | MMAD Lactose (μm) | Deposition fraction | Aerosol concentration (μg lactose/L Air) | 5 minute group | 15 minute group | 45 minute group |
| RN983 (Inflammation) | 70 | 1.9 | 0.038 | 70 ± 6 | 14 ± 2 | 42 ± 5 | 125 ± 14 |
| Budesonide (Inflammation) | 70 | 2.2 | 0.043 | 38 ± 4 | 8 ± 1 | 25 ± 2 | 74 ± 5 |
Inhibition of bronchoconstriction
RN983 inhibited i.v. OVA induced bronchoconstriction in the OVA sensitized and challenged BALB/c mouse model of the early asthmatic response (EAR). OVA increased R within minutes of intravenous injection (Fig. 3) and OVA-sensitized mice developed acute bronchoconstriction in a dose-dependent manner (Fig. 3). Immediately after antigen (i.v. OVA) induced bronchoconstriction, the percent increase in respiratory system resistance (R) was inhibited in a dose-dependent manner by inhaled dry powder doses (q.d. for 6 d.) of RN983. The percent increase in R was inhibited by 44 ± 24%, 59 ± 16%, and 86 ± 21% (Fig. 4) at average daily deposited doses of 30 ± 6, 100 ± 20, and 300 ± 50 μg/kg (Table 1); respectively.
FIG. 3.
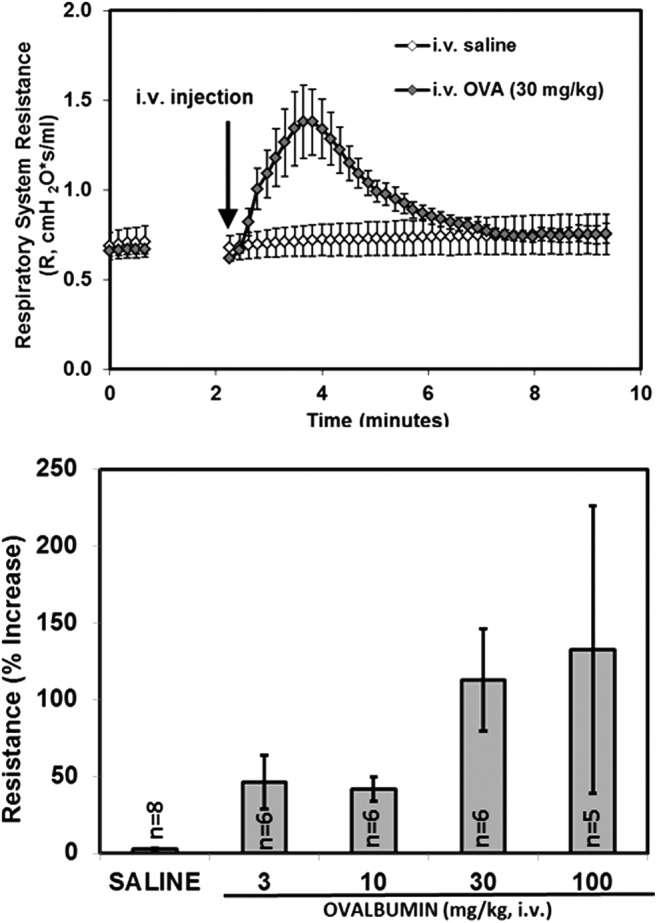
An example of the rapid temporal increase in respiratory system resistance induced by intravenous ovalbumin (30 mg/kg) administered to ovalbumin-sensitized mice (n = 6–8 per group, top panel). The percent peak increase over baseline in respiratory system resistance (bronchoconstriction) to increasing doses of intravenous ovalbumin administered to ovalbumin-sensitized mice (bottom panel).
FIG. 4.
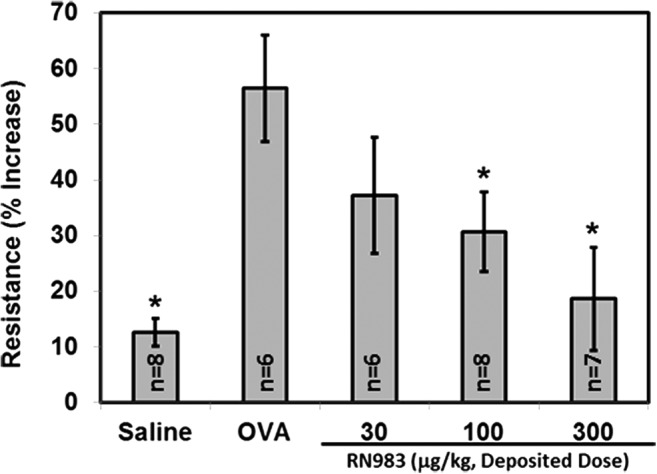
RN983 delivered by nose-only inhalation inhibited the ovalbumin-induced increase in respiratory system resistance (bronchoconstriction) in ovalbumin-sensitized mice, *p < 0.05 vs. OVA.
The pharmacokinetic profile of RN983 after a single deposited dose of 290 μg/kg was measured in the lungs and blood of a satellite group of mice (two per timepoint) at 5 timepoints (15 min, 1, 2, 6, and 24 h) and found to maintain a greater than 100-fold higher concentration in the lungs over the 24 h measurement period (Fig. 5). The calculated deposited dose value of 290 μg/kg for RN983 after 45 minutes of aerosol inhalation in the study where R was measured was comparable to amount of drug that could be extracted (17 ± 2 μg/g) from the lungs (lung weight = 0.216 ± 0.012 g) immediately after the exposure period (151 ± 25 μg/kg) from two mice (Fig. 5). RN983 was less potent at inhibiting bronchoconstriction (IC50(RN983) = 59 μg/kg) than the β-agonist salbutamol (IC50(salbutamol) = 15 μg/kg) in the mouse model of the EAR (Fig. 6).
FIG. 5.
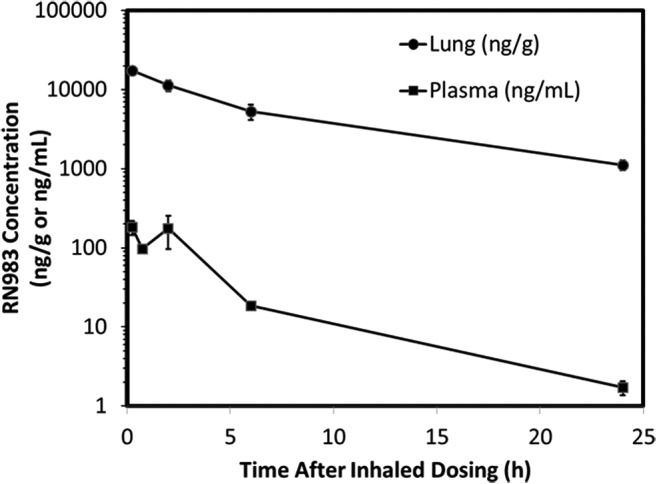
Pharmacokinetic profile in the lung and plasma after a single dose (290 μg/kg deposited) of RN983 delivered by nose-only inhalation. Average values from two mice per data point.
FIG. 6.
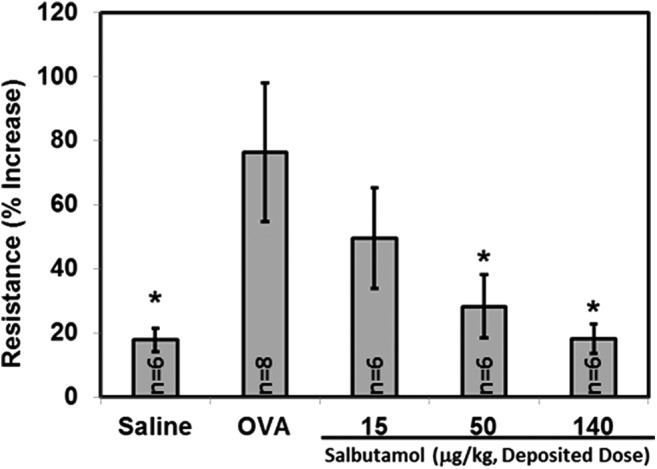
The β-agonist salbutamol sulfate delivered by nose-only inhalation inhibited the ovalbumin-induced increase in respiratory system resistance (bronchoconstriction) in ovalbumin-sensitized mice, *p < 0.05 vs. OVA.
Inhibition of pulmonary inflammation
RN983 significantly inhibited the total number of recoverable leukocytes in the bronchoalveolar lavage fluid (BAL) by 96 ± 9%, 106 ± 12%, and 102 ± 12% (Fig. 7) at all doses tested (3 ± 1, 10 ± 2, and 30 ± 5 μg/kg), respectively. The inhaled corticosteroid budesonide (Fig. 8) was less potent (IC50(budesonide) = 27 μg/kg) inhibitor of the antigen-induced increase in pulmonary inflammation (total leukocytes) than RN983 (IC50(RN983) = <3 μg/kg).
FIG. 7.
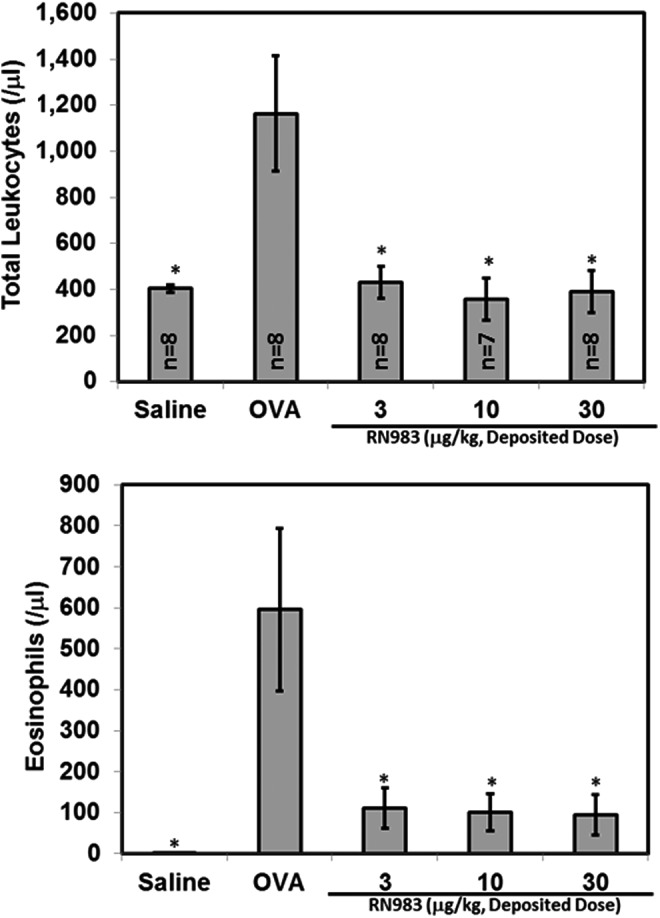
RN983 delivered by nose-only inhalation inhibited the ovalbumin-induced increase in the number of recoverable cells in the bronchoalveolar lavage (pulmonary inflammation) from ovalbumin-sensitized and nebulized ovalbumin-exposed mice (top panel). The increase in total leukocytes is primarily due to appearance of eosinophils in the BAL (bottom panel), *p < 0.05 vs. OVA.
FIG. 8.
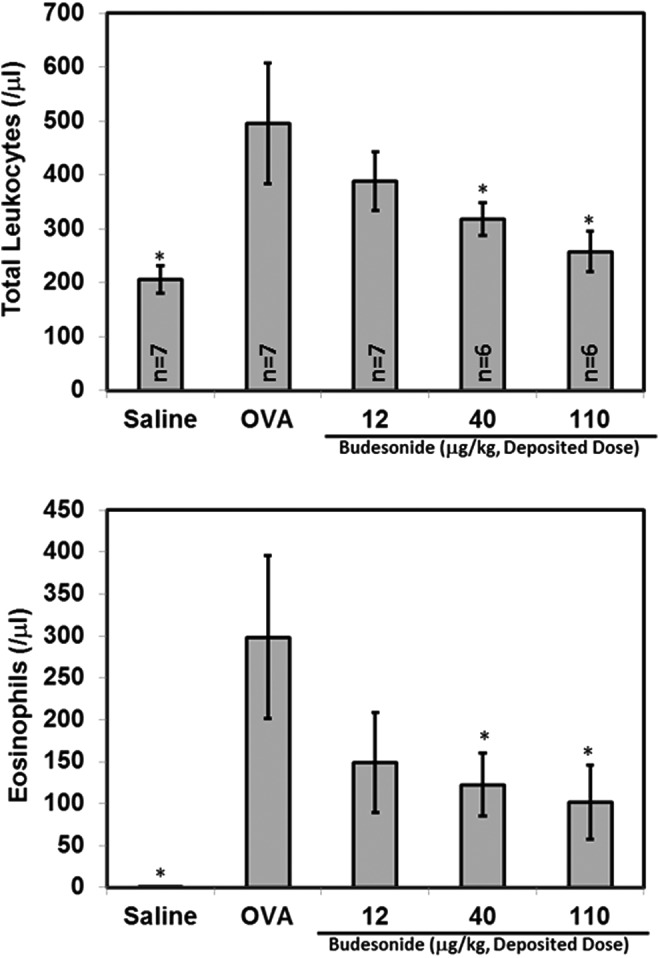
The corticosteroid budesonide delivered by nose-only inhalation inhibited the ovalbumin-induced increase in the number of recoverable cells in the bronchoalveolar lavage (pulmonary inflammation) from ovalbumin-sensitized and nebulized ovalbumin-exposed mice (top panel). The increase in total leukocytes is primarily due to appearance of eosinophils in the BAL (bottom panel), *p < 0.05 vs. OVA.
Discussion
Orally bioavailable Btk inhibitors are known. The irreversible orally-administered Btk inhibitor Ibrutinib does inhibit mast cell degranulation(23) and is currently approved for some B-cell malignancies. Ibrutinib has many side effects,(24) some due to lack of selectivity,(25) with an acceptable side effect profile for cancer indications, but not for asthma. It remains to be seen in the clinic whether orally available, selective, reversible Btk inhibitors such as RN486(2) will have better side effect profiles.
This is the first study to report the effects of an inhaled Btk inhibitor. Aerosol administration of RN983 to the lungs is expected to avoid the systemic immunosuppression(26) and accumulation of circulating IgE(4) which may be expected from orally available Btk inhibitors, while maintaining efficacy (inhibition of mast cells). Drugs that inhibit molecular targets upstream of Btk (SYK, PI3K) are also in development for asthma, allergic rhinitis, and rheumatoid arthritis, and have been recently reviewed.(27)
RN983 is a potent (single digit nanomolar, Fig. 2), reversible, and selective Btk inhibitor. RN983 significantly inhibits the allergen-induced acute bronchoconstriction in a mouse model of the early asthmatic response (EAR), with an IC50 of 59 μg/kg deposited dose (Fig. 4) when delivered by nose-only inhalation. A submaximal (30 mg/kg, i.v.) dose of OVA was used to elicit the EAR (Fig. 3). We have previously demonstrated that the mouse EAR model is antigen dependent as non-OVA sensitized mice do not bronchoconstrict to i.v. OVA.(28) Inhaled RN983 was found to maintain a greater than 100-fold higher concentration in the lungs than in the blood, decreasing the chances of observing systemic side effects (Fig. 5). The calculated deposited dose value of 290 μg/kg for RN983 after 45 minutes of aerosol inhalation in the study where R was measured was greater than the 151 ± 25 μg/kg (n = 2) dose of drug that could be calculated from the amount of drug extracted from the lungs immediately after the exposure period, due in part to absorption of drug from the lungs into the blood over the 45 minute exposure period.
RN983 had a similar potency to inhaled salbutamol (IC50 = 15 μg/kg deposited dose, Fig. 6) in the EAR model where submaximal dose combinations of RN983 and β-agonists would be expected to work in synergy (or at least additively) due to their different mechanisms of action. RN983 also significantly inhibited the antigen-induced pulmonary inflammation (total leukocytes in the BAL fluid) in a mouse model of the late asthmatic response (LAR), with an IC50 of less than 3 μg/kg deposited dose (Fig. 7) and more potently than the inhaled steroid budesonide (IC50 = 27 μg/kg deposited dose, Fig. 8). This suggests that asthmatics may benefit from an inhaled Btk inhibitor alone or it could also be a complementary therapy to β-agonists and corticosteroids that only partially inhibit mast cell activation and the number of mast cells in the lungs.
All compounds were delivered by passive nose-only inhalation to conscious mice. This technique requires hundreds of milligrams of micronized drug, but is the only technique that provides a uniform drug distribution throughout the lungs.(29–32) Uniform lung distribution is possible as a high quality respirable aerosol is generated and maintained for a period long enough to allow the mice to inhale an efficacious deposited dose.(20) The deposited dose can be utilized to calculate a therapeutic index,(33) ensuring that the drug has an appropriate exposure multiple (usually >10) present to rule out the manifestation of any systemic side effects and is also useful when planning inhaled toxicology studies.
When combined with animal disease models and pharmacodynamic measurements, nose-only inhalation dosing not only provides information on the efficacious deposited dose, but also the duration of action required to maintain the targeted efficacy; both helpful for dose translation to humans. When the amount of drug is limited, direct administration methods are an option but all suffer from non-homogeneous deposition, with more drug concentrated along the central airways and less well represented in the parenchymal/alveolar regions.(29,30) Direct instillation methods including intranasal(34) or intratracheal liquid(31,32) and spray instillation(35) or dry-powder insufflation,(36,37) can be used as a screening tool to determine the approximate dose range for later inhalation studies, or to determine the ranking of efficacy/toxicity for a series of structurally similar drugs.(38)
The current standard of care in asthma, inhaled β-agonist and steroid combination treatments, have a beneficial inhibition of the early asthmatic response (EAR) and the late asthmatic response (LAR), respectively.(39) Steroids predominantly dampen inflammation in the lungs by repressing the transcription of inflammatory mediators(40) that are important in the LAR,(41) and have little effect on the EAR.(42) β-Agonists predominantly relax airway smooth muscle and can reverse bronchoconstriction present in the EAR, but chronic use can exacerbate the decrements in lung function during the LAR.(43) Btk inhibitors inhibit mast cells from generating a diverse number of mediators(13,44,45) that induce symptoms and the pathogenesis of asthma.(27)
Both β-agonists and steroids are also known to have effects on mast cells. β-Agonists inhibit the release/generation of mast cell mediators,(46) but suffer from desensitization/internalization(39,46,47) and submaximal/partial inhibition of mast cell exocytosis.(46) Steroids only partially decrease mast cell numbers in the airways.(48) Therefore, the potent inhibition of mast cell exocytosis and synthesis of eicosanoids and cytokines by the inhaled Btk inhibitor RN983 may be effective as a stand-alone asthma therapy or used in combination with inhaled steroids and β-agonists in severe asthmatics.
Acknowledgments
The authors thank Achal Pashine, Jie Dai, Mark Zhang, and Katherine Tang for help with in vitro assays, Mingyan Zhou for help with the PK study, and Raman Iyer and Shridhar Hegde for help with compound micronization.
Author Disclosure Statement
All authors were employed by Hoffmann-La Roche Inc. when experiments were performed.
References
- 1.Hunter T: A thousand and one protein kinases. Cell. 1987;50:823–829 [DOI] [PubMed] [Google Scholar]
- 2.Xu D, Kim Y, Postelnek J, Vu MD, Hu DQ, Liao C, Bradshaw M, Hsu J, Zhang J, Pashine A, Srinivasan D, Woods J, Levin A, O'Mahony A, Owens TD, Lou Y, Hill RJ, Narula S, DeMartino J, and Fine JS: RN486, a selective Bruton's tyrosine kinase inhibitor, abrogates immune hypersensitivity responses and arthritis in rodents. J Pharmacol Exp Ther. 2012;341:90–103 [DOI] [PubMed] [Google Scholar]
- 3.Rosen FS, Cooper MD, and Wedgwood RJP: The primary immunodeficiencies. N Engl J Med. 1995;333:431–440 [DOI] [PubMed] [Google Scholar]
- 4.Greer AM, Wu N, Putnam AL, Woodruff PG, Wolters P, Kinet J-P, and Shin J-S: Serum IgE clearance is facilitated by human FcɛRI internalization. J Clin Invest. 2014;124:1187–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, and Gilfillan AM: Btk plays a crucial role in the amplification of Fc epsilonRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–40270 [DOI] [PubMed] [Google Scholar]
- 6.Heinonen JE, Smith CI, and Nore BF: Silencing of Bruton's tyrosine kinase (Btk) using short interfering RNA duplexes (siRNA). FEBS Lett. 2002;527:274–278 [DOI] [PubMed] [Google Scholar]
- 7.Palm NW, Rosenstein RK, and Medzhitov R: Allergic host defences. Nature. 2012;484:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youngchaiyud P, and Lee TB: Effect of nedocromil sodium on the immediate response to antigen challenge in asthmatic patients. Clin Allergy. 1986;16:129–134 [DOI] [PubMed] [Google Scholar]
- 9.Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, Fick RB, Jr., and Boushey HA: The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–1834 [DOI] [PubMed] [Google Scholar]
- 10.Eum SY, Norel X, Lefort J, Labat C, Vargaftig BB, and Brink C: Anaphylactic bronchoconstriction in BP2 mice: Interactions between serotonin and acetylcholine. Br J Pharmacol. 1999;126:312–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigand LA, Myers AC, Meeker S, and Undem BJ: Mast cell-cholinergic nerve interaction in mouse airways. J Physiol. 2009;587:3355–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haile S, Lefort J, Eum SY, Dumarey C, Huerre M, Heusser C, and Vargaftig BB: Suppression of immediate and late responses to antigen by a non-anaphylactogenic anti-IgE antibody in a murine model of asthma. Eur Respir J. 1999;13:961–969 [DOI] [PubMed] [Google Scholar]
- 13.Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, Maeda-Yamamoto M, Miura T, Han W, Hartman SE, Yao L, Nagai H, Goldfeld AE, Alt FW, Galli SJ, Witte ON, and Kawakami T: Involvement of Bruton's tyrosine kinase in FcepsilonRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer AS, Morales JL, Huang W, Ojo F, Ning G, Wills E, Baines JD, and August A: Absence of Tec family kinases interleukin-2 inducible T cell kinase (Itk) and Bruton's tyrosine kinase (Btk) severely impairs Fc epsilonRI-dependent mast cell responses. J Biol Chem. 2011;286:9503–9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacGlashan D, Jr., Honigberg LA, Smith A, Buggy J, and Schroeder JT: Inhibition of IgE-mediated secretion from human basophils with a highly selective Bruton's tyrosine kinase, Btk, inhibitor. Int Immunopharmacol. 2011;11:475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, and Lockhart DJ: A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336 [DOI] [PubMed] [Google Scholar]
- 17.Wright BM: A new dust-feed mechanism. J Sci Inst. 1950;27:12–15 [Google Scholar]
- 18.Oldham MJ, Phalen RF, and Budiman T: Comparison of predicted and experimentally measured aerosol deposition efficiency in BALB/c mice in a new nose-only exposure system. Aerosol Sci Technol. 2009;43:970–977 [Google Scholar]
- 19.Forbes B, Asgharian B, Dailey LA, Ferguson D, Gerde P, Gumbleton M, Gustavsson L, Hardy C, Hassall D, Jones R, Lock R, Maas J, McGovern T, Pitcairn GR, Somers G, and Wolff RK: Challenges in inhaled product development and opportunities for open innovation. Adv Drug Deliv Rev. 2011;63:69–87 [DOI] [PubMed] [Google Scholar]
- 20.Alexander DJ, Collins CJ, Coombs DW, Gilkison IS, Hardy CJ, Healey G, Karantabias G, Johnson N, Karlsson A, Kilgour JD, and McDonald P: Association of Inhalation Toxicologists (AIT) working party recommendation for standard delivered dose calculation and expression in non-clinical aerosol inhalation toxicology studies with pharmaceuticals. Inhal Toxicol. 2008;20:1179–1189 [DOI] [PubMed] [Google Scholar]
- 21.Hsieh TH, Yu CP, and Oberdorster G: Deposition and clearance models of Ni compounds in the mouse lung and comparisons with the rat models. Aerosol Sci Technol. 1999;31:358–372 [Google Scholar]
- 22.Natiello M, Kelly G, Lamca J, Zelmanovic D, Chapman R, and Phillips J: Manual and automated leukocyte differentiation in bronchoalveolar lavage fluids from rodent models of pulmonary inflammation. Comp Clin Pathol. 2009;18:101–111 [Google Scholar]
- 23.Soucek L, Buggy JJ, Kortlever R, Adimoolam S, Monclus HA, Allende MT, Swigart LB, and Evan GI: Modeling pharmacological inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia. 2011;13:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, Devereux S, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Pocock C, Thornton P, Caligaris-Cappio F, Robak T, Delgado J, Schuster SJ, Montillo M, Schuh A, de Vos S, Gill D, Bloor A, Dearden C, Moreno C, Jones JJ, Chu AD, Fardis M, McGreivy J, Clow F, James DF, and Hillmen P: Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC, Mendonca RV, Sweeney MD, Scott KC, Grothaus PG, Jeffery DA, Spoerke JM, Honigberg LA, Young PR, Dalrymple SA, and Palmer JT: Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem. 2007;2:58–61 [DOI] [PubMed] [Google Scholar]
- 26.Benson MJ, Rodriguez V, von Schack D, Keegan S, Cook TA, Edmonds J, Benoit S, Seth N, Du S, Messing D, Nickerson-Nutter CL, Dunussi-Joannopoulos K, Rankin AL, Ruzek M, Schnute ME, and Douhan J: Modeling the clinical phenotype of Btk inhibition in the mature murine immune system. J Immunol. 2014;193:185–197 [DOI] [PubMed] [Google Scholar]
- 27.Harvima IT, Levi-Schaffer F, Draber P, Friedman S, Polakovicova I, Gibbs BF, Blank U, Nilsson G, and Maurer M: Molecular targets on mast cells and basophils for novel therapies. J Allergy Clin Immunol. 2014;134:530–544 [DOI] [PubMed] [Google Scholar]
- 28.Phillips JE, Peng R, Harris P, Burns L, Renteria L, Lundblad LK, Fine JS, Bauer CM, and Stevenson CS: House dust mite models: will they translate clinically as a superior model of asthma? J Allergy Clin Immunol. 2013;132:242–244 [DOI] [PubMed] [Google Scholar]
- 29.Zecchi R, Trevisani M, Pittelli M, Pedretti P, Manni ME, Pieraccini G, Pioselli B, Amadei F, Moneti G, and Catinella S: Impact of drug administration route on drug delivery and distribution into the lung: An imaging mass spectrometry approach. Eur J Mass Spectrom (Chichester, Eng). 2013;19:475–482 [DOI] [PubMed] [Google Scholar]
- 30.Leong BK, Coombs JK, Sabaitis CP, Rop DA, and Aaron CS: Quantitative morphometric analysis of pulmonary deposition of aerosol particles inhaled via intratracheal nebulization, intratracheal instillation or nose-only inhalation in rats. J Appl Toxicol. 1998;18:149–160 [DOI] [PubMed] [Google Scholar]
- 31.Brain JD, Knudson DE, Sorokin SP, and Davis MA: Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ Res. 1976;11:13–33 [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Li W, Pauluhn J, Trubel H, and Wang C: Lipopolysaccharide-induced acute lung injury in rats: Comparative assessment of intratracheal instillation and aerosol inhalation. Toxicology. 2013;304:158–166 [DOI] [PubMed] [Google Scholar]
- 33.Biju P, McCormick K, Aslanian R, Berlin M, Solomon D, Chapman R, McLeod R, Prelusky D, Eckel S, Kelly G, Natiello M, House A, Fernandez X, Bitar R, Phillips J, and Anthes J: Steroidal C-21 mercapto derivatives as dissociated steroids: discovery of an inhaled dissociated steroid. Bioorg Med Chem Lett. 2011;21:6343–6347 [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui S, Morris J, Avery N, Wyand S, Rood D, and Silbart LK: Pulmonary eosinophilia correlates with allergen deposition to the lower respiratory tract in a mouse model of asthma. Clin Exp Allergy. 2008;38:1381–1390 [DOI] [PubMed] [Google Scholar]
- 35.Bivas-Benita M, Zwier R, Junginger HE, and Borchard G: Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur J Pharm Biopharm. 2005;61:214–218 [DOI] [PubMed] [Google Scholar]
- 36.Morello M, Krone CL, Dickerson S, Howerth E, Germishuizen WA, Wong YL, Edwards D, Bloom BR, and Hondalus MK: Dry-powder pulmonary insufflation in the mouse for application to vaccine or drug studies. Tuberculosis (Edinb). 2009;89:371–377 [DOI] [PubMed] [Google Scholar]
- 37.Guillon A, Montharu J, Vecellio L, Schubnel V, Roseau G, Guillemain J, Diot P, and de Monte M: Pulmonary delivery of dry powders to rats: Tolerability limits of an intra-tracheal administration model. Int J Pharm. 2012;434:481–487 [DOI] [PubMed] [Google Scholar]
- 38.Pauluhn J. and Mohr U: Inhalation studies in laboratory animals–Current concepts and alternatives. Toxicol Pathol. 2000;28:734–753 [DOI] [PubMed] [Google Scholar]
- 39.Cockcroft DW. and Murdock KY: Comparative effects of inhaled salbutamol, sodium cromoglycate, and beclomethasone dipropionate on allergen-induced early asthmatic responses, late asthmatic responses, and increased bronchial responsiveness to histamine. J Allergy Clin Immunol. 1987;79:734–740 [DOI] [PubMed] [Google Scholar]
- 40.Barnes PJ: Glucocorticosteroids: Current and future directions. Br J Pharmacol. 2011;163:29–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diamant Z, Gauvreau GM, Cockcroft DW, Boulet LP, Sterk PJ, de Jongh FH, Dahlen B, and O'Byrne PM: Inhaled allergen bronchoprovocation tests. J Allergy Clin Immunol. 2013;132:1045–1055.e1046. [DOI] [PubMed] [Google Scholar]
- 42.Ravensberg AJ, Luijk B, Westers P, Hiemstra PS, Sterk PJ, Lammers JW, and Rabe KF: The effect of a single inhaled dose of a VLA-4 antagonist on allergen-induced airway responses and airway inflammation in patients with asthma. Allergy. 2006;61:1097–1103 [DOI] [PubMed] [Google Scholar]
- 43.Gauvreau GM, Jordana M, Watson RM, Cockcroft DW, and O'Byrne PM: Effect of regular inhaled albuterol on allergen-induced late responses and sputum eosinophils in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:1738–1745 [DOI] [PubMed] [Google Scholar]
- 44.Luskova P, and Draber P: Modulation of the Fcepsilon receptor I signaling by tyrosine kinase inhibitors: Search for therapeutic targets of inflammatory and allergy diseases. Curr Pharm Des. 2004;10:1727–1737 [DOI] [PubMed] [Google Scholar]
- 45.Ellmeier W, Abramova A, and Schebesta A: Tec family kinases: Regulation of FcepsilonRI-mediated mast-cell activation. FEBS J. 2011;278:1990–2000 [DOI] [PubMed] [Google Scholar]
- 46.Scola AM, Loxham M, Charlton SJ, and Peachell PT: The long-acting beta-adrenoceptor agonist, indacaterol, inhibits IgE-dependent responses of human lung mast cells. Br J Pharmacol. 2009;158:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volovyk ZM, Wolf MJ, Prasad SV, and Rockman HA: Agonist-stimulated beta-adrenergic receptor internalization requires dynamic cytoskeletal actin turnover. J Biol Chem. 2006;281:9773–9780 [DOI] [PubMed] [Google Scholar]
- 48.James A, Gyllfors P, Henriksson E, Dahlen SE, Adner M, Nilsson G, and Dahlen B: Corticosteroid treatment selectively decreases mast cells in the smooth muscle and epithelium of asthmatic bronchi. Allergy. 2012;67:958–961 [DOI] [PubMed] [Google Scholar]


