Abstract
Immunodeficient mice play a critical role in hematology research as in vivo models of hematopoiesis and immunology. Multiple strains have been developed, but hematopoietic stem cell engraftment and immune reconstitution have not been methodically compared among them. Four mouse strains were transplanted with human fetal bone marrow or adult peripheral blood CD34+ cells: NSG, NSG-3GS, hSCF-Tg-NSG, and hSIRPα-DKO. Hematopoietic engraftment in the bone marrow, blood, spleen, and liver was evaluated by flow cytometry 12 weeks after transplant. The highest levels of human engraftment were observed in the liver, spleen, and bone marrow, whereas peripheral blood cell chimerism was notably less. The highest levels of tissue engraftment were in hSCF-Tg-NSG mice, but NSG mice exhibited the highest blood leukocyte engraftment. hSCF-Tg-NSG mice also exhibited the highest levels of CD133+CD34++ stem cells. hSIRPα-DKO engrafted poorly and exhibited poor breeding. Myelopoiesis was greatest in NSG-3GS mice, followed by hSCF-Tg-NSG and NSG mice, whereas B cell engraftment exhibited the opposite pattern. Engraftment of CD3+ T cells, CD3+CD161+ T cells, and CD3−CD56+ NK cells was greatest in NSG-3GS mice. Mast cell engraftment was highest in hSCF-Tg-NSG mice, but was also elevated in spleen and livers of NSG-3GS mice. Basophils were most abundant in NSG-3GS mice. Overall, hSCF-Tg-NSG mice are the best recipient mice for studies requiring high levels of human hematopoiesis, stem cell engraftment, and an intermediate level of myelopoiesis, whereas NSG and NSG-3GS mice offer select advantages in the engraftment of certain blood cell lineages.
Keywords: : bone marrow, hematopoiesis, HSC, CD34, CD133
Introduction
Humanized mouse models are an essential element for the study of development and function of the human immune and hematopoietic systems [1–3]. A fundamental key to achieving sufficient engraftment are mice lacking an adaptive immune system as well as NK cells. Furthermore, a crucial factor for enhancing human chimerism is having an in vivo environment favorable to transplanted human hematopoietic stem cells (HSCs). Most current efforts rely on one of two mouse strains to achieve robust engraftment: either the C;129S4-Rag2tm1.1Flv Il2rgtm1.1Flv/J (DKO) mice or NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice [4]. Hematopoietic cells and their precursors are transplanted by various routes, including intravenous, neonatal intrahepatic, or intrasplenic injection. Following transplantation into mice, the human HSCs can develop into most hematopoietic lineages, thus resulting in chimeric mice.
It has been noticed that altering the murine environment in favor of human hematopoiesis is beneficial to the output of HSCs and differentiated cells. Early efforts have employed injections of human cytokines such as interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), and erythropoietin (EPO) into immunodeficient mice to foster the growth and differentiation of human cells, measured as increased numbers of progenitors detected in vitro as colony-forming cells and long-term culture-initiating cells, as well as cells capable of multilineage engraftment in secondary recipients [5,6]. More recently, transgenic NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ (NSG-3GS, also known as NSG-SGM3 or NSGS) mice were developed that produce human IL-3, GM-CSF, and SCF, three human cytokines with minimal activity on mouse cells [7,8]. Irradiated NSG-3GS mice have been shown to have higher levels of human engraftment than NSG mice and, more specifically, increased human myelopoiesis and granulopoiesis with decreased erythropoiesis [8,9]. Additionally, it was observed that the presence of human cytokines do not effect homing efficiency [7], but promoted the mobilization of human progenitors leading to a reduction of more primitive progenitors, including HSCs capable of repopulating secondary recipients [9].
Because support of primitive HSCs is so important to the maintenance of long-term chimerism, NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(PGK1-KITLG*220)441Daw/SzJ (hSCF-Tg-NSG) mice and immunodeficient mice expressing human membrane-bound SCF on the NSG background, were developed. Investigation of these mice and their ability to support human hematopoiesis, led to the observation that the majority of the engrafted cells in the bone marrow were both immature and mature granulocytes, which is physiologically more similar to human bone marrow [10]. Furthermore, CD117 (c-Kit)+ mast cells were observed in these mice. Human chimerism levels in neonatal hSCF-Tg-NSG mice have been achieved at levels equal to NSG mice without preconditioning irradiation [11]. Interestingly, the same study found that the SCF transgene on a NOD.Cg-Rag1tm1MomIl2rgtm1Wjl/SzJ background was unable to engraft human cells in the absence of the preconditioning irradiation treatment, indicating that engraftment is heavily influenced by the genetic background of mice. Perhaps this is due to the fact that the Prkdc gene is involved in DNA repair and therefore widely expressed, whereas Rag1 and Rag2 expression is limited to hematopoietic cells and is involved solely in DNA recombination of T and B cell receptor genes, therefore, imparting a greater susceptibility to radiation-induced DNA damage in mice with the mutant Prkdc gene [1].
Other factors beyond host lymphocytes and growth factor incompatibilities limit the overall engraftment of human cells in mice. The C;129S4-Rag2tm1.1Flv Il2rgtm1.1Flv Tg(SIRPA)1Flv/J (hSIRPα-DKO) strain was created to evaluate the effects of transgenic expression of the human transmembrane, inhibitory receptor signal regulatory protein alpha (SIRPα). SIRPα is expressed by neurons, macrophages, dendritic cells, and neutrophils, and binds to the ligand CD47, which is ubiquitously expressed on human blood cells. Upon binding, phagocytosis is inhibited by phagocytic, SIRPα-expressing cells [12], resulting in an increased chance of human cell survival following transplantation. Elevated levels of human engraftment in the blood, bone marrow, spleen, and thymus of the hSIRPα-DKO mice were observed compared with NSG mice [4].
With the expanded number of immunodeficient mouse strains available to study human hematopoiesis or immune function, a direct comparison of hematopoietic engraftment and immune reconstitution is needed to determine the beneficial properties of each strain. NSG, NSG-3GS, hSCF-Tg-NSG, and hSIRPα-DKO mice were transplanted with human bone marrow and multilineage hematopoietic engraftment was compared in the bone marrow, blood, spleen, and liver.
Materials and Methods
Mice and animal husbandry
All breeder mice were obtained from Jackson Laboratories and bred at Blood Systems Research Institute. The described research was preformed with approval of the Institutional Animal Care and Use Committee at PMI Preclinical (San Carlos, CA). Mice were housed in a restricted access, pathogen-free vivarium in sterile, disposable microisolator cages, and maintained as previously detailed [13]. All animals were adults (≥ 8 weeks of age) at the time of transplantation. Recipients of fetal bone marrow cells were female, whereas recipients of adult blood cells were male.
Isolation of human fetal bone marrow
Human fetal long bones were obtained anonymously with the approval of the University of California San Francisco's Committee on Human Research in accordance with the amended Declaration of Helsinki. All donors gave written informed consent. Samples were 20 and 21 weeks’ gestation, estimated based on foot-length, obtained from elective abortions at San Francisco General Hospital. Central and endosteal bone marrow were isolated from long bones as previously described and cells pooled [14]. Washed cells were counted with a hemocytometer and either prepared directly for transplantation or suspended in 10% dimethyl sulfoxide (Sigma-Aldrich) and 40% fetal bovine serum (Life Technologies) and cryopreserved. Frozen cells were thawed, washed, and held on ice before transplantation.
Isolation of adult peripheral blood cells
Leukocyte-enriched blood was obtained from an anonymous blood donor by flushing residual blood from the discarded filter of a Trima Accel automated blood collection system (Terumo BCT). Blood was processed by a light-density step (Axis-Shield) followed by CD34+ bead selection (Invitrogen), according to the manufacturer's instructions, and then lineage depleted with BioLegend antibodies anti-human lineage cocktail fluorescein isothiocyanate (FITC; clones UCHT1, HCD14, HIB19, 2H7, and HCD56) and CD235a FITC (clone HI264) using BioMag Beads (Qiagen). After depletion, cells were counted and held on ice before transplantation.
Construction of humanized mice
Female or male mice were transplanted with total human fetal bone marrow or adult Lin−CD34+ cells to establish hematopoietic chimeras. NSG, NSG-3GS, hSCF-Tg-NSG, and hSIRPα-DKO, all from Jackson Laboratories, were transplanted intravenously with 5 × 105 total fetal bone marrow cells or 1.3 × 105 adult HSCs after 175 cGy using an RS2000 X-Ray Biological Irradiator (RAD Source Technologies, Inc.) 1–3 h before transplantation. Five mice of each strain received fetal cells and three mice of each strain received adult Lin−CD34+ cells.
Isolation of mouse tissues
Mice were sacrificed 12 weeks after transplant by cervical dislocation or inhalation anesthesia and retro-orbital enucleation to collect blood followed immediately by cervical dislocation. Tubes used to collect blood contained ethylenediaminetetraacetic acid powder (Eppendorf North America). Femoral central bone marrow, spleen, and liver were collected and prepared for antibody staining as previously described [13,14]. A small portion of blood collected was separated, added to blocking buffer, and reserved for antibody staining as whole blood. Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples by density separation. Bone marrow cell counts were preformed on a Cellometer Auto X4, version 1.1.1 (Nexcelom Bioscience). The total number of bone marrow cells in a mouse was estimated based on the bone marrow of two femurs representing 13% of total bone marrow cells [14].
Flow cytometric analysis
Prepared mouse tissues were suspended in blocking buffer consisting of PBS with 5% mouse serum, 0.01% NaN3, and supplemented with 2 μg/mL rat anti-mouse CD16/CD32 mAb (BioLegend). Samples were stained with monoclonal antibodies and live cells, identified based on a lack of propidium iodide staining, analyzed on a LSR II flow cytometer (BD Biosciences). Data were analyzed using FlowJo software, version 9.7 (Tree Star, Inc.)
The following FITC-labeled, phycoerythrin(PE)-labeled, allophycocyanin (APC)-labeled, PE-cyanine 7 (PE/Cy7)-labeled antibodies purchased from BioLegend or an otherwise stated vendor were used to stain human cells: β2-microglobulin FITC (clone 2 M2), CD3 PE/Cy7 (clone UCTH1), CD4 APC (clone RPA-T4), CD8 PE (clone RPA-T8; BD Pharmingen), CD14 APC or PE/Cy7 (clone HCD14), CD19 PE or PE/Cy7 or APC (clone HIB19), CD22 PE (clone S-HCL-1; BD Pharmingen), CD33 PE (clone P67.6; BD Pharmingen), CD33 APC (clone WM53), CD34 PE/Cy7 (clone 581), CD38 PE (clone HIT2), CD41 PE (clone HIP8), CD42b APC (clone HIP1), CD45 PE or APC or PE/Cy7 (clone HI30), CD56 APC (clone HCD56), CD71 APC (clone L01.1; BD Pharmingen), CD117 APC (clone 104D2), CD133 APC (clone AC133; Miltenyi Biotec, Auburn, CA), CD161 PE (clone HP-3G10), and CD203c PE (clone NP4D6) or CD235a PE (clone HI264). The following monoclonal antibodies were used to stain mouse cells: TER-119 (clone TER-119), CD45 (clone 30-F11), and H-2Kd (clone SF1-1.1), all pacific blue labeled.
Data presentation and statistical analysis
Statistical analysis and charting was performed using Aabel 4 software (Gigawiz Ltd. Co.). Data are presented using box and whisker plots, where the notch represents the median, the whiskers extend to the extreme data points, and mean is represented by diamonds on the plots. The frequency-specific subsets of human cells are presented as a frequency among all human cells, unless otherwise indicated. The significance of differences between groups was determined using the two-tailed Mann–Whitney U-test. A P-value of ≤0.05 is considered significant.
Results
Human engraftment
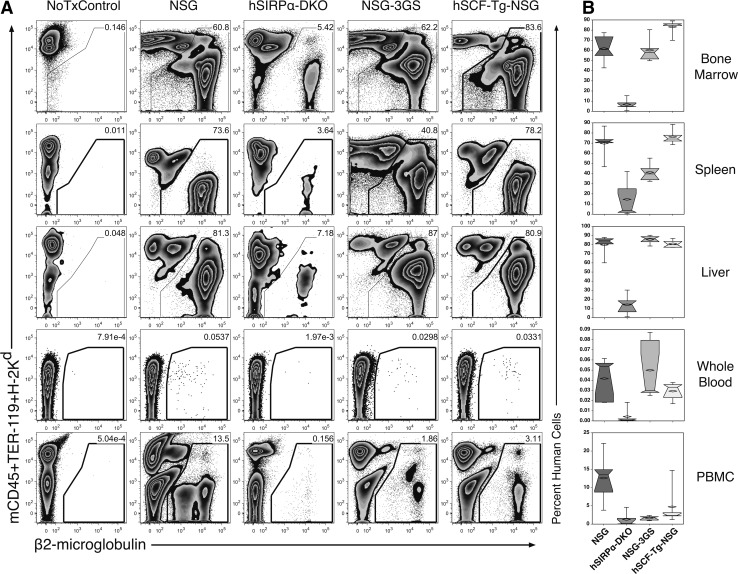
Total human engraftment was measured by expression of β2-microglobulin 84 days after transplantation (Fig. 1A). hSCF-Tg-NSG mice had significantly higher engraftment in the bone marrow (83.1%) than the other strains (P < 0.05; Fig. 1B). NSG and NSG-3GS mice have comparable levels of engraftment (61.9% and 60.3%, respectively) in the bone marrow. hSIRPα-DKO mice had significantly lower engraftment in the bone marrow (6.8%) than other strains (P < 0.05).
FIG. 1.
Human engraftment in murine hematopoietic tissues. Bone marrow, spleens, livers, whole blood, and PBMCs from NSG, hSIRPα-DKO, NSG-3GS and hSCF-Tg-NSG mice were stained with anti-mouse CD45, TER-119, and H-2Kd and anti-human β2-microglobulin. (A) Representative flow cytometric analysis for each strain is shown in comparison to a nontransplanted NSG mouse (NoTxControl). The complete gating strategy used for these analyses as well as for all other human cell populations analyzed are shown in Supplementary Fig. S4. (B) Box and whisker plots compare engraftment in the different strains for each tissue (n = 5). PBMC, peripheral blood mononuclear cell.
Human engraftment among the light-density cells of the spleen (Fig. 1B) was comparable and as high as 69.8% and 76.2% for NSG and hSCF-Tg-NSG, respectively. NSG-3GS mice had an average engraftment of 41.2%; significantly lower than NSG and hSCF-Tg-NSG mice, but significantly higher than hSIRPα-DKO (14.7%) (P < 0.05). Engraftment among light-density cells in the liver (Fig. 1B) was not significantly different among NSG, NSG-3GS, and hSCF-Tg-NSG (79.9%, 85.5%, and 80.3%, respectively), but all were significantly higher than hSIRPα-DKO (14.3%; P < 0.05).
Engraftment in the whole blood was much less than engraftment in the aforementioned tissues, but was similar to the liver in significance among strains. NSG, NSG-3GS, and hSCF-Tg-NSG (0.04%, 0.05%, and 0.03%, respectively) were comparable and had significantly more human cells than hSIRPα-DKO (0.004%; P < 0.05). The PBMC portion of the blood had higher percentages of human cells than the whole blood, but was still lower than the bone marrow, spleen, and liver. NSG mice had significantly higher engraftment (12.6%) than hSIRPα-DKO, NSG-3GS, and hSCF-Tg-NSG mice (1.3%, 1.7%, and 4.7%, respectively; P < 0.05), which did not have any significant differences among them.
The hSIRPα-DKO strain of mice was included in this study based on previous reports suggesting increased frequency of human cells in the peripheral blood of mice expressing human SIRPα [4]. As peripheral blood has the lowest levels of human chimerism among all the hematopoietic tissues analyzed, it was disappointing to have not observed higher levels of human peripheral blood cells in hSIRPα-DKO mice. As the blood chimerism levels may have been affected by the lower levels of hematopoietic engraftment in general, we performed a short-term human blood transfusion experiment in hSIRPα-DKO and NSG mice to compare the kinetics of cell clearance. The frequency of circulating human cells in hSIRPα-DKO mice was no higher than NSG mice (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). Additionally, we observed poor breeding behavior and a high tendency toward obesity in hSIRPα-DKO mice. For these reasons we excluded hSIRPα-DKO mice from further analysis.
Erythrocyte engraftment
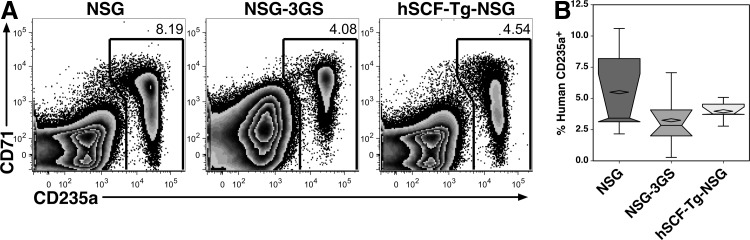
Human red blood cells (RBCs) in the bone marrow were measured based on the expression of CD71 and CD235a (Fig. 2A). Mean RBC levels were 5.5% in NSG mice, 3.3% in NSG-3GS mice, and 4% in hSCF-Tg-NSG mice with no significant differences among the three strains analyzed (Fig. 2B). Both immature erythrocytes (CD71++) and mature RBCs (CD71−CD235a+) were observed in the bone marrows. Spleens, livers, and PBMCs were excluded from erythrocyte analysis due to the light-density isolation procedure used to isolate cells from these tissues, greatly reducing the content of RBCs.
FIG. 2.
Erythropoiesis in the bone marrow of three mouse strains. (A) Representative flow cytometry plots of erythroid precursors in three strains of mice. (B) A box and whisker plot indicates similar levels of erythropoiesis among the strains.
Myeloid engraftment
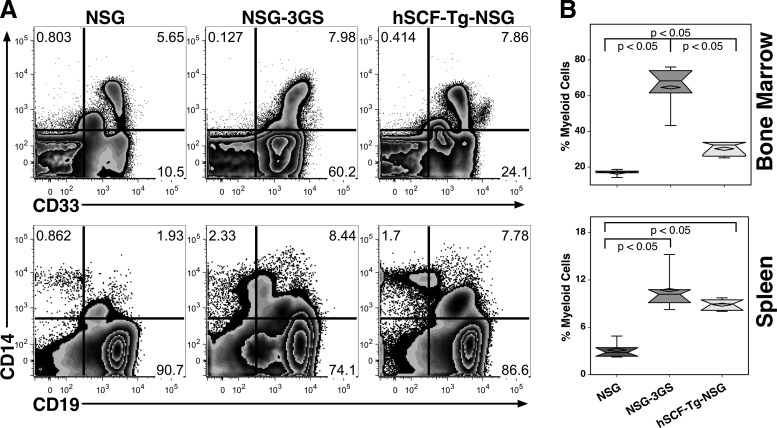
Myeloid cells in the bone marrow were measured using CD14 and CD33 (Fig. 3A). Most CD14+ cells coexpressed CD33, but not all CD33+ cells were positive for CD14. NSG-3GS mice had notably higher levels of CD33+ myeloid engraftment (64.6%) than hSCF-Tg-NSG mice (30.3%) and NSG mice (16.9%); NSG mice had significantly lower engraftment than the other strains (Fig. 3B).
FIG. 3.
Detection of myeloid cells in the bone marrow and spleen. (A) Representative flow cytometric data of myeloid engraftment. (B) Myeloid engraftment in the bone marrow is defined as cells positive for CD14 and/or CD33. In the spleen, myeloid cells are defined as cells positive for CD14 expression.
Myeloid cells in the spleen were measured by expression of CD14 (Fig. 3A). Like the bone marrow, NSG mice had significantly lower engraftment (3.1%) than the other strains of mice (Fig. 3B). Although NSG-3GS mice had the highest median and mean level of myeloid engraftment (10.7%), there was no significant difference between them and hSCF-Tg-NSG mice (8.9%).
In an additional experiment, we transplanted the three strains of mice (n = 3, each strain) with Lin−CD34+ cells from adult peripheral blood. Multilineage engraftment was not observed, but NSG-3GS mice had a slight, but significant (P < 0.05), higher level of β2-microglobulin expression (0.83%) than NSG mice (0.4%; data not shown). Only trace levels of engraftment were observed in NSG and hSCF-Tg-NSG (0.55%) strains, with no significant differences between NSG-3GS and hSCF-Tg-NSG mice or NSG and hSCF-Tg-NSG mice. The bulk of the human cell engraftment in NSG-3GS mice consisted of CD33+CD19low and CD33lowCD19− cells (7.82% and 8.41%, respectively) (Supplementary Fig. S2A) that were all but absent in NSG and hSCF-Tg-NSG mice. There was also a population of cells expressing very low levels of CD34 that were observed in the NSG-3GS mice (2.93%) that was mostly absent in NSG (0.3%) and hSCF-Tg-NSG (0.13%) mice (Supplementary Fig. S2B). Although the CD34+ cells were also CD38−, the complete lack of CD38 expression is inconsistent with engraftment patterns observed with active hematopoiesis and, therefore, these CD34+ cells are unlikely to represent HSCs.
B cell engraftment
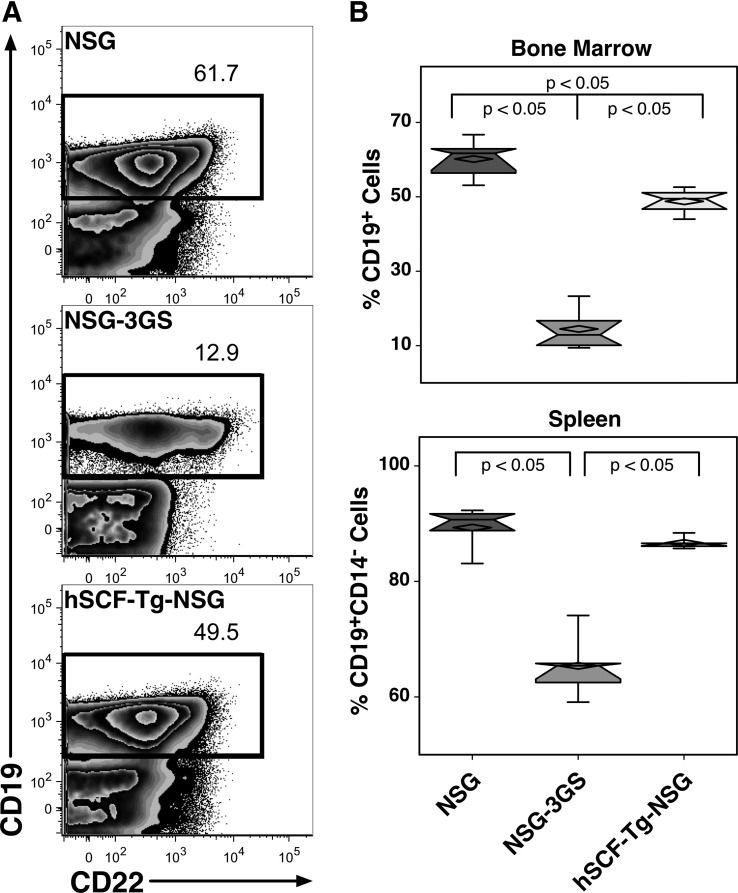
We use CD19 as a marker of B lymphocyte engraftment (Fig. 4A). In the bone marrow, NSG mice had the highest level of B cell engraftment (60.2%), followed by hSCF-Tg-NSG (48.8%), and NSG-3GS mice had the least amount (14.5%) (Fig. 4B). In the spleen, B cell engraftment was defined as CD19+CD14− cells (Fig. 3A). Engraftment patterns among the three strains was similar to the bone marrow; NSG mice had the highest engraftment (89.3%), followed by hSCF-Tg-NSG mice (86.7%), and NSG-3GS mice had the lowest level of engraftment (65.4%)
FIG. 4.
Detection of B lymphopoiesis in the bone marrow and spleen. (A) Representative flow cytometric data of B lymphoid engraftment. (B) B lymphopoiesis in the bone marrow includes all cells that are CD19+. In the spleen, B lymphopoiesis is defined as cells positive for CD19 and negative for CD14 expression (Fig. 3B).
HSC engraftment
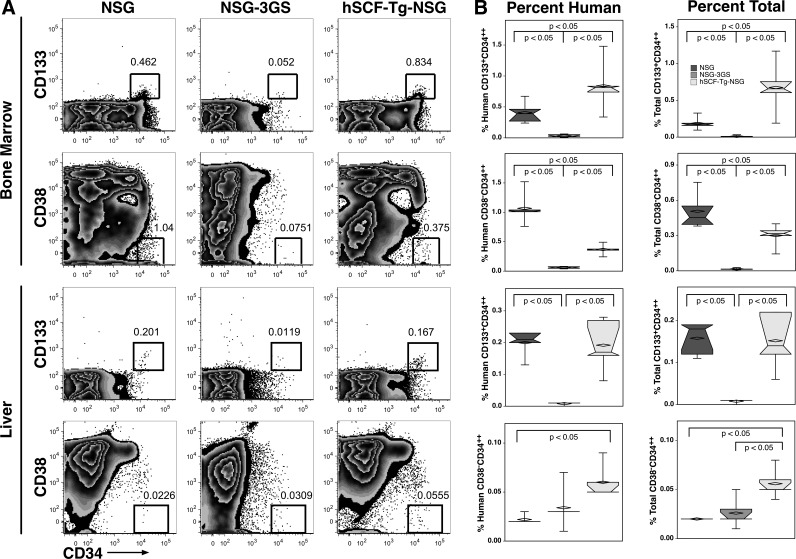
We sought to determine if the human cytokines present in the transgenic mice had an effect on self-renewing HSCs and early progenitors. CD133+CD34++ and CD38−CD34++ were analyzed in both the bone marrows and livers, but not the spleens, of transplanted mice with the NSG background (Fig. 5A). When comparing the CD133+CD34++ population in the bone marrow, we observed that hSCF-Tg-NSG mice had nearly twice the frequency of HSCs among human cells (0.84% and 0.41%, respectively) and nearly three times the frequency among total bone marrow cells (0.68% and 0.19%, respectively) (Fig. 5B) compared with NSG mice. However, NSG mice were found to have more CD38−CD34++ cells among human (1.07%) and total bone marrow cells (0.51%) compared with hSCF-Tg-NSG mice (0.37% and 0.30%, respectively). This finding was skewed by the presence of an unusual number of events in NSG mice displaying very low levels of CD38 that are sometimes observed in similar experiments. The NSG-3GS mice had significantly lower engraftment of both HSC populations among human (0.03%) and total bone marrow (0.01%) cells.
FIG. 5.
Human hematopoiesis in the bone marrow and liver. (A) Representative flow cytometric data of CD133+CD34++ and CD38−CD34++ among human cells in the BM and livers. (B) HSC engraftment patterns among human cells are similar to HSC engraftment patterns among total live single cells (including mouse cells). BM, bone marrow; HSC, hematopoietic stem cell.
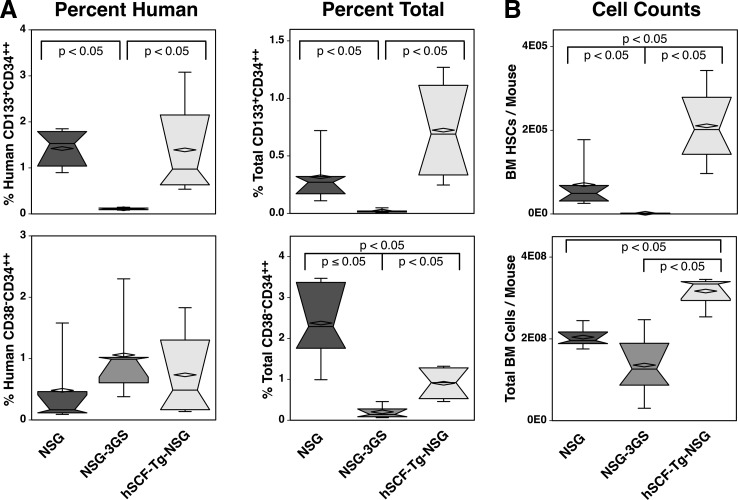
In a separate experiment we harvested bone marrow and found a similar pattern of CD133+CD34++ HSCs engraftment in the three strains of mice, but no significant differences between NSG (1.42% human and 0.32% total) and hSCF-Tg-NSG (1.4% human and 0.72% total) mice (Fig. 6A). There were no significant differences of percent human CD38−CD34++ HSCs between the strains, but among percent total CD38−CD34++ HSCs, all strains were significantly different with the NSG (2.38%) having the highest percent and NSG-3GS (0.2%) having the lowest percent (Fig. 6A). However, as mentioned above, there was a greater variability in the CD38−CD34++ phenotype than observed with the CD133+CD34++ phenotype, which favors the latter as a more reliable measure of the HSC compartment. In addition to these results, similar patterns of human multilineage engraftment were observed in this second cohort of mice (Supplementary Fig. S3) as presented in Figs. 1–4.
FIG. 6.
Quantification of HSC engraftment. Femoral bone marrow from three immunodeficient strains of mice, transplanted with 21 week's gestation human bone marrow, was harvested, counted, and analyzed by flow cytometry 12 weeks after transplantation. (A) CD133+CD34++ and CD38−CD34++ cell populations as a frequency of both human and all live, single cells are indicated. (B) The numbers of both CD133+CD34++ (HSCs) and total BM cells per mouse were calculated based on femoral bone marrow cell counts and the frequency of CD133+CD34++ cells measured by flow cytometry.
The number of bone marrow cells obtained from transplanted hSCF-Tg-NSG mice was significantly higher than the two other strains (Fig. 6B). Accordingly, the number of human CD133+CD34++ HSCs was also significantly higher in these mice.
We have previously shown that the murine liver supports a population of extramedullary human HSCs [13,15]. Unlike the bone marrow, nearly all of the light-density liver cells were human cells so the frequency of HSC among human and among total cells in the liver was similar. In this study, we show that the liver has a lower frequency of both HSC populations than the bone marrow and that there are no significant differences of CD133+CD34++ HSC engraftment between NSG and hSCF-Tg-NSG mice among human (0.2% and 0.19%, respectively) and total (0.16% and 0.15%, respectively) light-density liver cells. hSCF-Tg-NSG mice had about three times the mean frequency of liver CD38−CD34++ HSCs than NSG mice among both human (0.06% and 0.02%, respectively) and total (0.06% and 0.02%, respectively) cells. NSG-3GS mice had significantly less CD133+CD34++ HSCs in the liver than the other two strains among human (0.009%) and total (0.007%) cells. There was no difference in CD38−CD34++ HSCs between NSG-3GS and NSG and NSG-3GS and hSCF-Tg-NSG mice among human liver cells, or between NSG-3GS and NSG mice among total liver cells.
T and NK cell engraftment
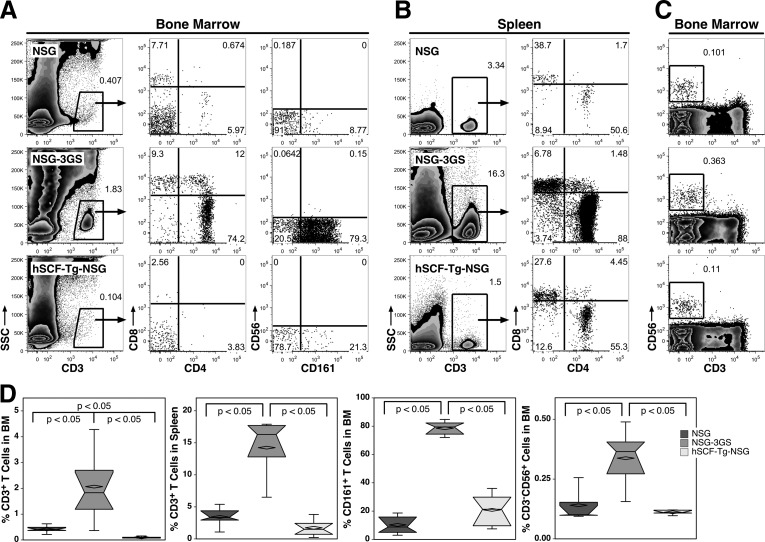
We measured engraftment of T cells in the bone marrow and spleen, as well as NK cells in the bone marrow. T cells were gated based on low side scatter and expression of CD3 (Fig. 7A). In the bone marrow, NSG-3GS mice had the highest frequency of CD3+ cells (2.06%), followed by NSG mice (0.41%) and hSCF-Tg-NSG (0.1%). Subsets of the CD3+ cells were examined based on CD4, CD8, and CD161 expression. CD4+CD8− and CD4−CD8+ single positive T cells were present in all of the strains examined. CD161+ T cells were present in all strains, with NSG-3GS mice having a significantly larger population (78.6%). CD3−CD56+ NK cells were observed in all strains, but were also most prevalent in NSG-3GS mice (0.34%; Fig. 7C).
FIG. 7.
Detection of human T and NK cells. (A) Bone marrow T cells were evaluated by gating on CD3+ cells with low side scatter and evaluating CD4, CD8, CD56, and CD161 staining. (B) Flow cytometric analyses of light-density spleen T cells are shown along with their characterization of CD4 and CD8 expression. (C) Bone marrow CD56+ NK cells are defined by lack of CD3 expression. (D) Box and whisker plots of lymphoid engraftment in bone marrow and spleen of three strains of mice.
T cells accounted for up to an average of 14.2% of the light-density spleen cells of NSG-3GS mice (Fig. 7B). Over four times higher than what was observed in NSG mice (3.43%) and about eight times what was observed in hSCF-Tg-NSG mice (1.74%). A majority of splenic CD3+ cells of NSG and NSG-3GS mice were either CD4+ or CD8+ single positive.
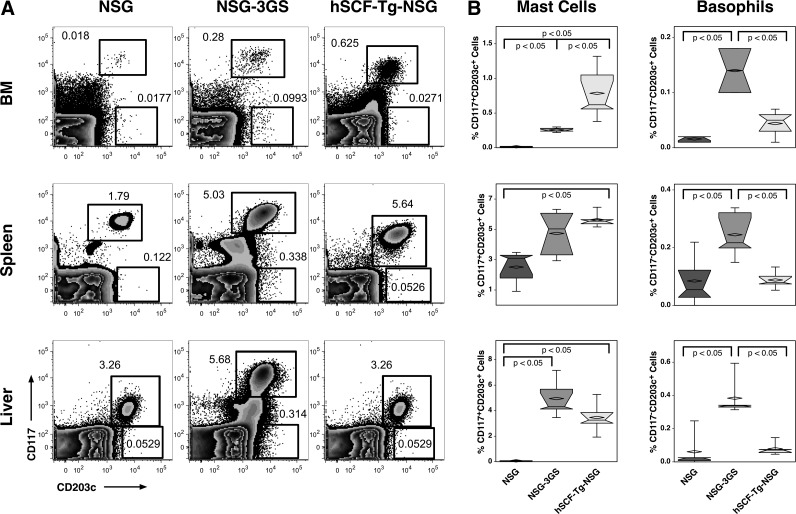
Mast cell and basophil engraftment
Mast cell and basophil engraftment in immunodeficient mice has had limited attention and has not been examined in NSG-3GS mice. We show mast cell engraftment in the bone marrow, spleen, and liver of all three strains (Fig. 8A). We used CD117 and CD203c coexpression to define mast cells and the CD117−CD203c+ phenotype to define basophils [16]. Higher mast cell engraftment was observed in the bone marrow of hSCF-Tg-NSG mice than the other strains (Fig. 8B). Mast cell frequencies in the spleen and the livers of NSG-3GS mice were similar to those of hSCF-Tg-NSG mice, both of which were higher than NSG mice. The NSG-3GS mice had the highest levels of basophil engraftment compared with the other two strains.
FIG. 8.
Mast cells and basophils in the bone marrow, spleens, and livers. (A) Representative flow cytometric analysis for the three strains and three tissues examined. Gates were adjusted for each strain due to variation of background brightness. (B) Mast cells are defined as CD117+CD203c+ and basophils are defined as CD203c+ cells lacking CD117 expression.
Discussion
Hematopoiesis is governed by environmental factors. Here, we have studied this by showing variations in both levels and output of hematopoietic engraftment in different strains of mice. hSCF-Tg-NSG mice had the highest overall chimerism. SCF is thought to assist in both directing HSCs to their stem cell niche and overall maintenance of the stem cell pool [17]. These mice had levels of CD133+ HSC engraftment higher than NSG mice, indicating that membrane-bound SCF had a positive influence on stem cell survival and growth. NSG-3GS mice exhibited notable levels of myelopoiesis, including the basophil and mast cell lineages, and T and NK lymphoid engraftment. As previously observed, the stem cell compartment was reduced in NSG-3GS mice compared with the other two strains [9,18]. The hSIRPα-DKO mice offered no particular advantage, most notably in a transfusion model that accessed the survival of circulating human cells, and were difficult to maintain as a breeding colony. Overall, this strain of mice offered no compelling advantage based on our experience.
Variations in hematopoietic output are directly effected by the balance of growth factors and inhibitory cytokines. Transgenes coding for human cytokines are responsible for the elevated levels of myelopoiesis observed in NSG-3GS and hSCF-NSG mice [8,19]. Conversely, the high frequency of B-lymphopoiesis in NSG mice was due to weaker myelopoiesis in these mice. Although robust engraftment was observed using fetal bone marrow cells, stem cells isolated from adult blood failed to produce multilineage engraftment. These results are consistent with the known proliferative advantages of fetal cells in vitro and in vivo [20–24]. Only in NSG-3GS mice was a clear population of human cells detected, which appeared to be all myeloid cells based on CD33 expression. Interestingly, a distinct subpopulation of these cells expressed CD19, generally considered to be a B cell marker, but which has been reported to be expressed on a subset of murine splenic dendritic cells [25,26]. The nature of the CD19+CD33+ cells observed in NSG-3GS mice needs further evaluation to assign a lineage to these cells.
Overall, human erythropoiesis is not strongly supported in any of the test mouse strains. hSCF-NSG mice engrafted with human cord blood cells have been reported to have improved erythropoiesis [11]. We did not observe a notable increased frequency of CD235a+ cells in these mice, but the overall increased human cell numbers in these mice point to higher red cell production. SCF is an important erythropoietic factor [27], yet the mice expressing this cytokine did not have an advantage over NSG mice in the production of CD235a+ cells. Fetal stem cells are more prone to make erythrocytes compared with cells derived from umbilical cord blood [24]. Thus, mice transplanted with umbilical cord blood cells may be affected more notably by the presence of human SCF even though SCF plays an important supportive role throughout human fetal erythropoiesis [28].
T and NK cells were observed in the bone marrow and spleens of all mouse strains after bone marrow transplantation, even without implantation of human thymic tissue [29]. Consistent with previous findings [18], there was an increase in CD3+ T cells in the bone marrow of the NSG-3GS mice and NSG mice. Similarly, NSG-3GS mice had over four-fold the amount of splenic T cell engraftment of NSG mice. Billerbeck et al. observed a notable increase in T regulatory cells in NSG-3GS mice [18]. These authors also noted that mature T cells did not appear to express any of the receptors for the human transgenes and, thus, their expansion was most likely due to indirect effects of the cytokines. Since we observed that T cell engraftment in hSCF-NSG mice was similar to that of NSG mice, with even a modest decrease observed in the bone marrow, SCF appears not to play an important role in the increase in T cells in NSG-3GS mice. NSG-3GS mice were also found to have a much higher frequency of CD161+ T cells than the other two mouse strains. CD161 is expressed by a variety of T cell subtype-expressing markers such as CD4, CD8, T cell receptor αβ, and T cell receptor γδ [30]. Moreover, CD161 is a marker of T cell that express interleukin 17, Th17 cells. CD161 expression has also been associated with memory T cells and mucosal associated invariant T cells. CD161 has been observed on fetal T cells [31] and, thus, these cells may have expanded within NSG-3GS mice rather than develop from immature T cells. NSG-3GS mice offer a promising in vivo model to study regulatory T cells and CD161+ T cells.
Human mast cells were detected in the bone marrow, spleens, and livers of the three strains of chimeric mice. Mast cells are identified by their high expression of CD117, the receptor for SCF, and SCF plays a key role in the development and expansion of mast cells [32]. Murine SCF also supports the growth of human cells [33], so even NSG mice lacking the human SCF transgene are capable of supporting mast cell development. Consistent with previous findings [10], hSCF-NSG mice had a higher frequency of mast cells in the bone marrow and spleen than NSG mice, and our observations extend these findings to the liver as well. We also observed higher levels of mast cells and basophils in NSG-3GS mice, which has not been previously reported and makes these mice suitable hosts for studying the development and function of these rare granulocyte populations.
Supplementary Material
Acknowledgments
The authors thank the staff and faculty at San Francisco General Hospital Women's Options Center for assistance in the collection of human fetal tissues and the administrative staff at Blood Systems Research Institute for their tremendous support.
The authors thank Betty Cheng and Ventura Mendoza for technical assistance in conducting the experiments. Ms. Cheng and Mr. Mendoza were supported by a Bridges to Stem Cell Training grant TB1-01188 from the California Institute of Regenerative Medicine.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health grant number P01DK088760. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Shultz LD, Ishikawa F. and Greiner DL. (2007). Humanized mice in translational biomedical research. Nat Rev Immunol 7:118–130 [DOI] [PubMed] [Google Scholar]
- 2.Garcia S. and Freitas AA. (2012). Humanized mice: current states and perspectives. Immunol Lett 146:1–7 [DOI] [PubMed] [Google Scholar]
- 3.Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA. and Manz MG. (2013). Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol 31:635–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, Eynon EE, Manz MG. and Flavell RA. (2011). Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A 108:13218–13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cashman JD, Lapidot T, Wang JC, Doedens M, Shultz LD, Lansdorp P, Dick JE. and Eaves CJ. (1997). Kinetic evidence of the regeneration of multilineage hematopoiesis from primitive cells in normal human bone marrow transplanted into immunodeficient mice. Blood 89:4307–4316 [PubMed] [Google Scholar]
- 6.Cashman JD. and Eaves CJ. (1999). Human growth factor-enhanced regeneration of transplantable human hematopoietic stem cells in nonobese diabetic/severe combined immunodeficient mice. Blood 93:481–487 [PubMed] [Google Scholar]
- 7.Wunderlich M, Chou FS, Link KA, Mizukawa B, Perry RL, Carroll M. and Mulloy JC. (2010). AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 24:1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller PH, Cheung AM, Beer PA, Knapp DJ, Dhillon K, Rabu G, Rostamirad S, Humphries RK. and Eaves CJ. (2013). Enhanced normal short-term human myelopoiesis in mice engineered to express human-specific myeloid growth factors. Blood 121:e1–e4 [DOI] [PubMed] [Google Scholar]
- 9.Nicolini FE, Cashman JD, Hogge DE, Humphries RK. and Eaves CJ. (2004). NOD/SCID mice engineered to express human IL-3, GM-CSF and Steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia 18:341–347 [DOI] [PubMed] [Google Scholar]
- 10.Takagi S, Saito Y, Hijikata A, Tanaka S, Watanabe T, Hasegawa T, Mochizuki S, Kunisawa J, Kiyono H, et al. (2012). Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood 119:2768–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brehm MA, Racki WJ, Leif J, Burzenski L, Hosur V, Wetmore A, Gott B, Herlihy M, Ignotz R, et al. (2012). Engraftment of human HSCs in nonirradiated newborn NOD-scid IL2rγ null mice is enhanced by transgenic expression of membrane-bound human SCF. Blood 119:2778–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okazawa H, Motegi S, Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O. and Matozaki T. (2005). Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol 174:2004–2011 [DOI] [PubMed] [Google Scholar]
- 13.Varga NL, Bárcena A, Fomin ME. and Muench MO. (2010). Detection of human hematopoietic stem cell engraftment in the livers of adult immunodeficient mice by an optimized flow cytometric method. Stem Cell Stud 1:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahajan MM, Cheng B, Beyer AI, Mulvaney US, Wilkinson MB, Fomin ME. and Muench MO. (2015). A quantitative assessment of the content of hematopoietic stem cells in mouse and human endosteal-bone marrow: a simple and rapid method for the isolation of mouse central bone marrow. BMC Hematol 15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muench MO, Beyer AI, Fomin ME, Thakker R, Mulvaney US, Nakamura M, Suemizu H. and Bárcena A. (2014). The adult livers of immunodeficient mice support human hematopoiesis: evidence for a hepatic mast cell population that develops early in human ontogeny. PLoS One 9:e97312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghannadan M, Hauswirth AW, Schernthaner GH, Müller MR, Klepetko W, Schatzl G, Sperr WR, Bühring HJ. and Valent P. (2002). Detection of novel CD antigens on the surface of human mast cells and basophils. Int Arch Allergy Immunol 127:299–307 [DOI] [PubMed] [Google Scholar]
- 17.Broudy VC. (1997). Stem cell factor and hematopoiesis. Blood 90:1345–1364 [PubMed] [Google Scholar]
- 18.Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM. and Ploss A. (2011). Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice. Blood 117:3076–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coughlan AM, Harmon C, Whelan S, O'Brien EC, O'Reilly VP, Crotty P, Kelly P, Ryan M, Hickey FB, O'Farrelly C. and Little MA. (2016). Myeloid engraftment in humanized mice: impact of granulocyte-colony stimulating factor treatment and transgenic mouse strain. Stem Cells Dev 25:530–541 [DOI] [PubMed] [Google Scholar]
- 20.Lansdorp PM, Dragowska W. and Mayani H. (1993). Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med 178:787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muench MO, Cupp J, Polakoff J. and Roncarolo MG. (1994). Expression of CD33, CD38, and HLA-DR on CD34+ human fetal liver progenitors with a high proliferative potential. Blood 83:3170–3181 [PubMed] [Google Scholar]
- 22.Rebel VI, Miller CL, Eaves CJ. and Lansdorp PM. (1996). The repopulation potential of fetal liver hematopoietic stem cells in mice exceeds that of their liver adult bone marrow counterparts. Blood 87:3500–3507 [PubMed] [Google Scholar]
- 23.Harrison DE, Zhong RK, Jordan CT, Lemischka IR. and Astle CM. (1997). Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp Hematol 25:293–297 [PubMed] [Google Scholar]
- 24.Nicolini FE, Holyoake TL, Cashman JD, Chu PP, Lambie K. and Eaves CJ. (1999). Unique differentiation programs of human fetal liver stem cells shown both in vitro and in vivo in NOD/SCID mice. Blood 94:2686–2695 [PubMed] [Google Scholar]
- 25.Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH. and Mellor AL. (2005). A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol 17:909–919 [DOI] [PubMed] [Google Scholar]
- 26.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ. and Munn DH. (2005). Cutting Edge: CpG Oligonucleotides Induce Splenic CD19+ Dendritic Cells to Acquire Potent Indoleamine 2,3-Dioxygenase-Dependent T Cell Regulatory Functions via IFN Type 1 Signaling. J Immunol 175:5601–5605 [DOI] [PubMed] [Google Scholar]
- 27.Nocka K, Buck J, Levi E. and Besmer P. (1990). Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J 9:3287–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muench MO. and Namikawa R. (2001). Disparate regulation of human fetal erythropoiesis by the microenvironments of the liver and bone marrow. Blood Cells Mol Dis 27:377–390 [DOI] [PubMed] [Google Scholar]
- 29.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT. and Garcia JV. (2006). Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12:1316–1322 [DOI] [PubMed] [Google Scholar]
- 30.Fergusson JR, Fleming VM. and Klenerman P. (2011). CD161-expressing human T cells. Front Immunol 2:Article 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanier LL, Chang C. and Phillips JH. (1994). Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol 153:2417–2428 [PubMed] [Google Scholar]
- 32.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, March CJ, Boswell HS, Gimpel SD. and Cosman D. (1990). Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell 63:235–243 [DOI] [PubMed] [Google Scholar]
- 33.Smith KA. and Zsebo KM. (2001). Measurement of human and murine stem cell factor (c-kit ligand). Curr Protoc Immunol Chapter 6:Unit 6.17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.