Abstract
The United States is in the midst of an opiate epidemic, with abuse of prescription and illegal opioids increasing steadily over the past decade. While it is clear that there is a genetic component to opioid addiction, there is a significant portion of heritability that cannot be explained by genetics alone. The current study was designed to test the hypothesis that maternal exposure to opioids prior to pregnancy alters abuse liability in subsequent generations. Female adolescent Sprague Dawley rats were administered morphine at increasing doses (5–25 mg/kg, s.c.) or saline for 10 days (P30–39). During adulthood, animals were bred with drug-naïve colony males. Male and female adult offspring (F1 animals) were tested for morphine self-administration acquisition, progressive ratio, extinction, and reinstatement at three doses of morphine (0.25, 0.75, 1.25 mg/kg/infusion). Grandoffspring (F2 animals, from the maternal line) were also examined. Additionally, gene expression changes within the nucleus accumbens were examined with RNA deep sequencing (PacBio) and qPCR. There were dose- and sex-dependent effects on all phases of the self-administration paradigm that indicate decreased morphine reinforcement and attenuated relapse-like behavior. Additionally, genes related to synaptic plasticity, as well as myelin basic protein (MBP), were dysregulated. Some, but not all, effects persisted into the subsequent (F2) generation. The results demonstrate that even limited opioid exposure during adolescence can have lasting effects across multiple generations, which has implications for mechanisms of the transmission of drug abuse liability in humans.
Keywords: opiate, epigenetic, self-administration, transgenerational, RNA sequencing, myelin basic protein
1. Introduction
The cost of opiate addiction is exceedingly high to individuals and society (Li and Burmeister, 2009); however, the effect of widespread exposure to opiates on future generations is unknown. There is a growing body of evidence demonstrating that experiences of one generation can have lasting effects on subsequent generations. For example, inter-generational effects have been documented in animal models following variations in stress, diet, as well as toxin and drug exposures (Skinner, 2015). Following exposure to drugs of abuse animal studies have found both increases and decreases in offspring propensity towards addiction-like behaviors (Szutorisz et al., 2014; Vassoler et al., 2013; Vassoler et al., 2016) that indicate alterations within the reward pathway that may increase vulnerability in certain populations. There remains a paucity of studies examining transgenerational effects following exposure to drugs of abuse.
Transgenerational epigenetics refers to the transmission of a phenotype across multiple generations of a species in the absence of changes in the DNA sequence (Bohacek et al., 2013; Franklin and Mansuy, 2010; Gapp et al., 2014; Skinner, 2015; Yohn et al., 2015). The term is limited to effects that extend to a generation that was not exposed to the initial environmental manipulation. Thus, in the absence of in utero exposure, grandoffspring of female animals that show an effect are demonstrating transgenerational epigenetic inheritance. There is mounting evidence that supports the hypothesis that preconception drug use has effects on subsequent generations (Vassoler et al., 2014a; Yohn et al., 2015). However, this distinction should not downplay the significance of preconception drug exposure on the F1 generation, as these also have significant implications for public health.
In the current set of studies we tested the hypothesis that female adolescent morphine exposure, terminating several weeks prior to pregnancy, would increase opiate abuse liability in offspring and grandoffspring. Further, based on other studies examining sex differences in opiate self-administration, we hypothesized that females will take more morphine than males (Cicero et al., 2003). Finally, we hypothesized that the effect would be diminished in the F2 generation as there was no direct exposure in F2 animals (Dunn and Bale, 2011). Voluntary responding of male and female F1 and F2 animals was measured during morphine self-administration acquisition, PR, extinction and drug-primed reinstatement, as these model distinct phases of substance use (i.e. addiction/reinforcement, withdrawal/rehabilitation, and relapse). We then used deep sequencing to identify pathways of gene expression changes in the nucleus accumbens in F1 animals. The nucleus accumbens was chosen based on its role in addiction and on our previous work demonstrating transgenerational effects on receptor expression within the accumbens using this model (Byrnes et al., 2013). Based on sequencing data, high level targets related to neuroplasticity were identified. The genes were examined using qPCR in the nucleus accumbens of both F1 and F2 animals of both sexes.
The data revealed significant decreases in the acquisition, extinction, and reinstatement of morphine self-administration that were sex- and dose-dependent. A number of effects were observed in both F1 and F2 animals demonstrating transgenerational epigenetic inheritance as a function of maternal drug history. Taken together, we show that female adolescent morphine exposure, in the absence of any direct fetal exposure, induces sex-specific transgenerational epigenetic effects that span at least two generations. These findings suggest the inheritance of homotypic drug resistant phenotypes following parental exposure to drugs of abuse.
2. Materials and Methods
2.1 Animals and housing
For all experiments, post-natal day 23 (PND23) female Sprague-Dawley rats [Crl:CD(SD)BR] were purchased from Charles River Breeding Laboratories. All animals were housed in standard acrylic laboratory cages at Cummings School for Veterinary Medicine at Tufts University. Animals were maintained on a 12-hour light/dark cycle with lights on at 7:00 am and all procedures were performed during the light phase. Food and water was available ad libitum, unless otherwise stated. All procedures were approved by the Institutional Animal Care and Use Committee of Tufts University, and were carried out in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals.
2.2 Generation of F0, F1, and F2 animals
All animals were housed 3–4 per cage. The adolescent exposure is described in Vassoler et al., 2015. Briefly, beginning at PND30 females (n=36) were injected (s.c.) once daily with morphine sulfate (MS) for a total of 10 days using an increasing dosing regimen with doses increased every other day (5, 5, 10, 10, 15, 15, 20, 20, 25, 25 mg/kg). Age-matched control animals (n=36) received saline injections (s.c) with volumes adjusted to match those of drug-treated females. On PND 60–80 (3 to 6 weeks after their final injection), females were mated with drug-naïve colony males. Each male was placed with 4 age-matched females (2 morphine treated and 2 saline treated). Once an animal was visibly pregnant (approximately E16–E20) she was housed singly. On PND1 (day of birth = PND0) all litters were culled to ten pups (5 male, 5 female) (Kosten et al., 2014). The weight of the pups and the dam was recorded. All litters were weighed and weaned on PND21 and housed with same-sex littermates. No differences in bodyweights were observed at either time point (data not shown). To generate F2 animals (grandoffspring), naïve, adult, female F1 animals were mated with drug-naïve colony males in the same way as F0 animals (ie. 4 females per male, two MOR-F1 and two SAL-F1). Offspring were culled to ten pups (5 male, 5 female) on PND1. All litters were weighed and weaned on PND21 and housed with same sex littermates. It is important to note that F1 female animals used to generate F2 grandoffspring did not receive any drug or behavioral manipulations. Offspring of morphine-exposed females are designated Mor-F1. Offspring of saline controls are designated Sal-F1. Grandoffspring are designated Mo-F2 and Sal-F2, respectively. All testing was conducted once F1 and F2 animals were at least 60 days of age. In all of the reported findings only one offspring per litter was used in any individual treatment group. The following table lists the n’s for each experiment.
Table 1.
F1 and F2 animals used in experiments
| Experiment | F1 | F2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Sal-F1 | Mor-F1 | Sal-F2 | Mor-F2 | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 0.25 mg/kg/infusion SA | 10 | 11 | 11 | 11 | - | - | - | - |
| 0.75 mg/kg/infusion SA | 8 | 8 | 8 | 9 | 12 | 12 | 12 | 12 |
| 1.25 mg/kg/infusion SA | 7 | 9 | 9 | 9 | - | - | - | - |
| Sucrose Pellet SA | 6 | 6 | 6 | 6 | - | - | - | - |
| Sequencing | 6 | 6 | 6 | 6 | - | - | - | - |
| PCR | 8 | 8 | 7 | 7 | 8 | 8 | 8 | 8 |
2.3 Morphine Self-Administration
2.3.1 Catheterization Surgery
Animals were anesthetized using a ketamine/xylazine cocktail (80 mg/kg and 8 mg/kg, respectively). The catheter (CamCaths, Cambridge, UK) was composed of silastic tubing that was fed into the right external jugular vein and routed to a mesh backmount platform secured subcutaneously between the shoulder blades. Catheters were flushed daily with an antibiotic (Cefazolin, 0.02 mg/ml) dissolved in heparinized saline and sealed with plastic obturators when not in use.
2.3.2 Apparatus
Self-administration was conducted in operant chambers housed within sound-attenuating cubicles (MedAssociates, St. Albans, VT). Chambers were equipped with house lights, cue lights, and two retractable levers (one active; one inactive). Active lever pressing initiated the activation of the syringe pump (MedAssociates) to deliver a drug infusion (rate 60 µl in 5 s, 15 s post-infusion timeout). Drug delivery and data collection were controlled by MedAssociates software (MedPCIV). Following catheterization surgery, animals were recovered for 1 week prior to initiation of self-administration.
2.3.3 Acquisition/Progressive Ratio
Animals were allowed to self-administer morphine (0.25, 0.75, or 1.25 mg/kg/infusion; the dose was a between subjects variable thus different animals were used for each dose) for 15 days on a fixed ratio 1 (FR1; one lever press = one infusion) schedule of reinforcement. Each session was 2 hours long. A cue light was illuminated over the active lever during the infusion for FR1 only, there were no cues associated with FR5, PR, extinction, or reinstatement. There was a 10 second time out following an infusion. Responding that is presented is from the active phase and does not include the time out period. Following 15 days of FR1, the animals were switched to a FR5 (five lever presses = one infusion) schedule for 5 days. This allowed for a greater response output in the animals. After the fifth day on FR5 animals were tested on a progressive ratio schedule of reinforcement. Under a PR schedule the response requirement for each subsequent drug delivery was increased until the subject failed to meet a requirement. The response requirement for the ith reinforcement was given by R(i)=[5e0.2i−5] and the session expired when an animal took more than 60 minutes to receive an injection. The breakpoint was operationally defined as the number of responses prior to the termination of the session. The following day, the animals entered into the extinction phase.
2.3.4 Extinction and Reinstatement
The FR5 schedule was utilized throughout extinction and reinstatement testing. Additionally, saline was available in the syringe pump during these phases. Extinction testing was conducted for 2 hours/day for 10 days. All animals reached extinction criteria (<15% of last day of self-administration) by 10 days of extinction. The animals then underwent reinstatement. All animals were tested with saline first (i.p.). One extinction day was used between the saline and morphine reinstatement tests. On the morphine reinstatement day, morphine (1 mg/kg, i.p.) was administered immediately prior to the reinstatement session. Active and inactive lever presses as well as infusions were recorded during these sessions.
2.4 Sucrose pellet self-administration was performed similar to that of morphine self-administration
Animals were mildly food restricted (~95% free feeding bodyweight) and allowed to self-administer sucrose pellets (Bioserve, Flemington, NJ) for 10 days on an FR1 schedule. They were then switched to an FR5 schedule for 5 days. All sessions were 1 hour long. Following this acquisition phase, animals were tested on a PR schedule (see description above). They then underwent extinction for 10 days prior to reinstatement. On the reinstatement test day, animals were given non-contingent sucrose pellets, one pellet every two minutes for the first 10 minutes via remote administration. Responses were recorded.
2.5 Brain dissection and tissue preparation
Adult (PND60-72), naïve, F1 animals were utilized for deep sequencing, while both F1 and F2 naïve adults were used for follow-up qRT-PCR analysis. Animals were decapitated, and brains were rapidly removed, frozen in methylbutane, and stored at −80°C. To collect samples for analysis of gene expression, frozen brains were mounted on a cryostat and bilateral micropunches were taken from the NAc (2 mm; starting at: +2.5 mm A/P, +/− 1.5 mm M/L, −7 mm D/V, a region encompassing both core and shell) according to Paxinos and Watson (Paxinos and Watson, 1997).
2.6 Sequencing and analysis
2.6.1 Sequencing
For each group, accumbal tissue from 6 animals was pooled for sample preparation. Total RNA was extracted using the Qiagen midi RNeasy kit (Valencia, CA, USA). Ribosomal RNA was depleted using the Ribo-Zero kit according to manufacturer’s protocols (epicenter). Double stranded cDNA was synthesized with the superscript dscDNA kit (ThermoFisher, Waltham, MA). In between the first and second strand synthesis, the PreCR repair Mix was utilized to fill in nicks in the DNA (New England Biolabs, Ipswich, MA). The samples were then checked for integrity using the bioanalyzer (Agilinent Technologies, Santa Clara, CA). Samples were run through the Pacific Bio Sequencer at the University of Massachusetts School of Medicine Core Facility (Pacific Biosciences RSII Instrument; P4/C2 version run chemistry with 120-minute data collection times and smrtportal data analysis).
2.6.2 Analysis of sequencing
Pac-Bio reads were aligned to the Rat genome downloaded from UCSC ftp://hgdownload.cse.ucsc.edu/goldenPath/rn5/bigZips/rn5.fa.gz using two separate aligners (GMAP (Wu and Watanabe, 2005) and STAR (Dobin et al., 2013)), in order to validate read mapping.. Output SAM files were further processed with Samtools (Li et al., 2009) to filter reads that failed to map to the genome. Finally, reads were counted for each gene using featureCounts (Liao et al., 2014) in R (RCoreTeam, 2015). Expression change estimates were generated with edgeR (Robinson et al., 2010), and reads were prioritized based upon log-fold change in genes with greater expersssion levels than 10 log counts/million. Gene lists were then inputed into gene ontology program, GeneMANIA as well as into the gene list enrichment analysis tool, Enrichr (Chen et al., 2013; Warde-Farley et al., 2010).
2.7 Quantitative Reverse Transcriptase Polymerase Chain Reaction (qrtPCR) and quantification
Tissue punches were homogenized in lysis buffer and total RNA extracted using the RNeasy kit from Qiagen (Valencia, CA, USA) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized using the RETROscript kit from Applied Biosystems (Carlsbad, CA, USA). PCR was performed using an AB 7500 (Applied Biosystems) under standard amplification conditions: 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 15 s at 95 °C and 60 s at 60 °C. All PCR primers were TaqMan® Gene Expression Assays purchased from Applied Biosystems. The amplification efficiency of each of these assays has been validated by Applied Biosystems and averages 100% (± 10). Assay ID and accession numbers were as follows: S16:Rn01476520_g1, MBP:RN01399619_m1,, Gria2:Rn00568514_m1, zwint:Rn00595851_m1, snap25:Rn00578534_m1, ApoE: Rn00593680_m1, TMSB4X: Rn00821311_g1.
2.7.1 Quantification of gene transcription
Final quantification of mRNA was obtained using the comparative cycle threshold (CT) method (User Bulletin #2, Applied Biosystems). All samples were run in duplicates and the average CT value was used. Data are reported as relative transcription or the N-fold difference relative to a calibrator cDNA. In brief, the housekeeping gene for the rat brain tissue, S16, was used as an internal control against which each target signal was normalized; this is referred to as the ΔCT. Validation studies confirmed that the raw CT values of S16 did not vary by treatment group, confirming S16 as an appropriate housekeeping gene. The ΔCT was then normalized against a calibrator (i.e. the lowest expressing value for the target gene in each brain region) (ΔΔCT). The data are expressed as fold change from the control group (Sal-F1 or Sal-F2 of appropriate sex) (2ΔΔCT).
2.8 Statistical analyses
F1 acquisition and PR data were analyzed with two-way ANOVAs with maternal treatment and dose as factors. Extinction data were analyzed with repeated measures two-way ANOVA with day as the repeated measure and maternal treatment as the between subjects factor. All reinstatement data were analyzed with a two-way repeated measures ANOVA with maternal treatment as a between subjects factor and dose as a within subjects factor. F2 (except extinction and reinstatement), and qPCR data were analyzed using student’s t-test. Sidak’s multiple comparison post hocs were used when appropriate. Significance was defined as p<0.05. In all experiments data for each sex was analyzed separately. This was determined a priori due to the complexity of the design and the questions that we wanted to address.
3. Results
3.1 F1 morphine self-administration acquisition, progressive ratio, extinction, and reinstatement
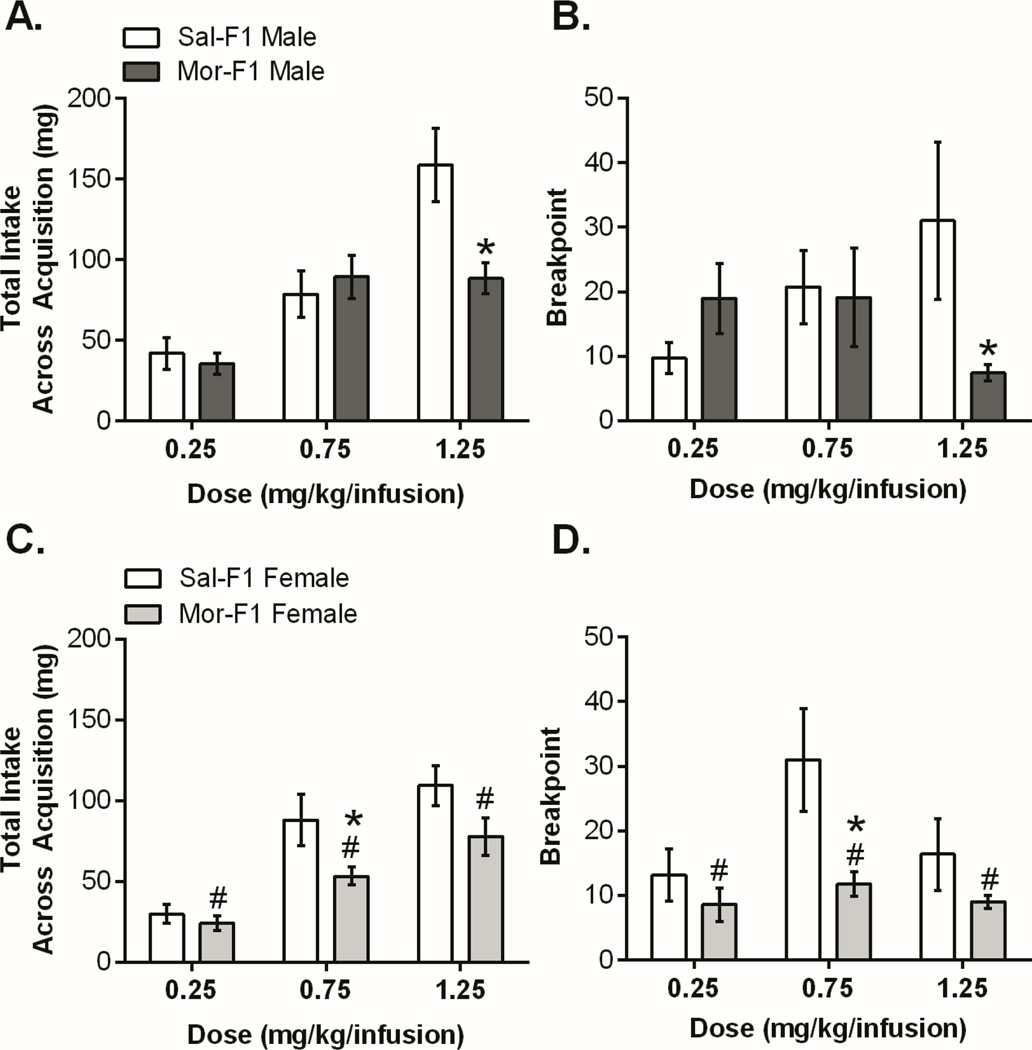
Male and female F1 animals were trained to self-administer morphine at one of 3 doses (0.25, 0.75, or 1.25 mg/kg/infusion, separate animals for each dose) during adulthood. The paradigm included 4 phases: acquisition, PR, extinction, and reinstatement. Figure 1 shows the acquisition and PR data. In males, there was a significant decrease in morphine intake by Mor-F1 males (Figure 1A) as well as a decrease in breakpoint (Figure 1B) at the highest dose compared with Sal-F1 males. In females, there was blunted responding with decreases in acquisition intake (Figure 1C) and a main effect of decreased breakpoint on PR (Figure 1D).
Figure 1.
Morphine F1 Self-Administration Acquisition and Progressive Ratio. For all panels, data is presented as mean +/− SEM. For the male acquisition (1A) there was a significant main effect of dose [F(2,47)=24.24, p<0.0001], a main effect of maternal treatment [F(1,47)=4.59, p<0.05] as well as an interaction [F(2,47)=5.45, p<0.01]. Sidak’s post hoc analysis revealed that Mor-F1 males took significantly less morphine than Sal-F1 males at the highest dose (p=0.001). Analysis of the PR data (1B) also showed a significant interaction [F(2,47)=3.63, p<0.05] but no main effect of dose or maternal treatment. Sidak’s post hoc analysis showed that, similar to the acquisition, Mor-F1 males had a significantly lower breakpoint than Sal-F1 males at the high dose. Analysis of female acquisition (1C) revealed a main effect of dose [F(2,51)=27.51, p<0.0001] with Mor-F1 animals showing blunted acquisition [main effect of maternal treatment: F(1,51)=9.69, p<0.01]. Sidak’s showed a significant decrease at the middle dose (p<0.05). Likewise, for PR there was a main effect of dose [F(2,47)=3.57, p<0.05] and maternal treatment [F(1,47)=9.3, p<0.01] with Mor-F1 females showing decreased breakpoint compared with Sal-F1 females (1D). Sidak’s showed a significant decrease at the middle dose (p<0.01). (n=7–11, see supplement for detailed n) # main effect p<0.05, *p<0.05 compared to Sal-F1 of same group.
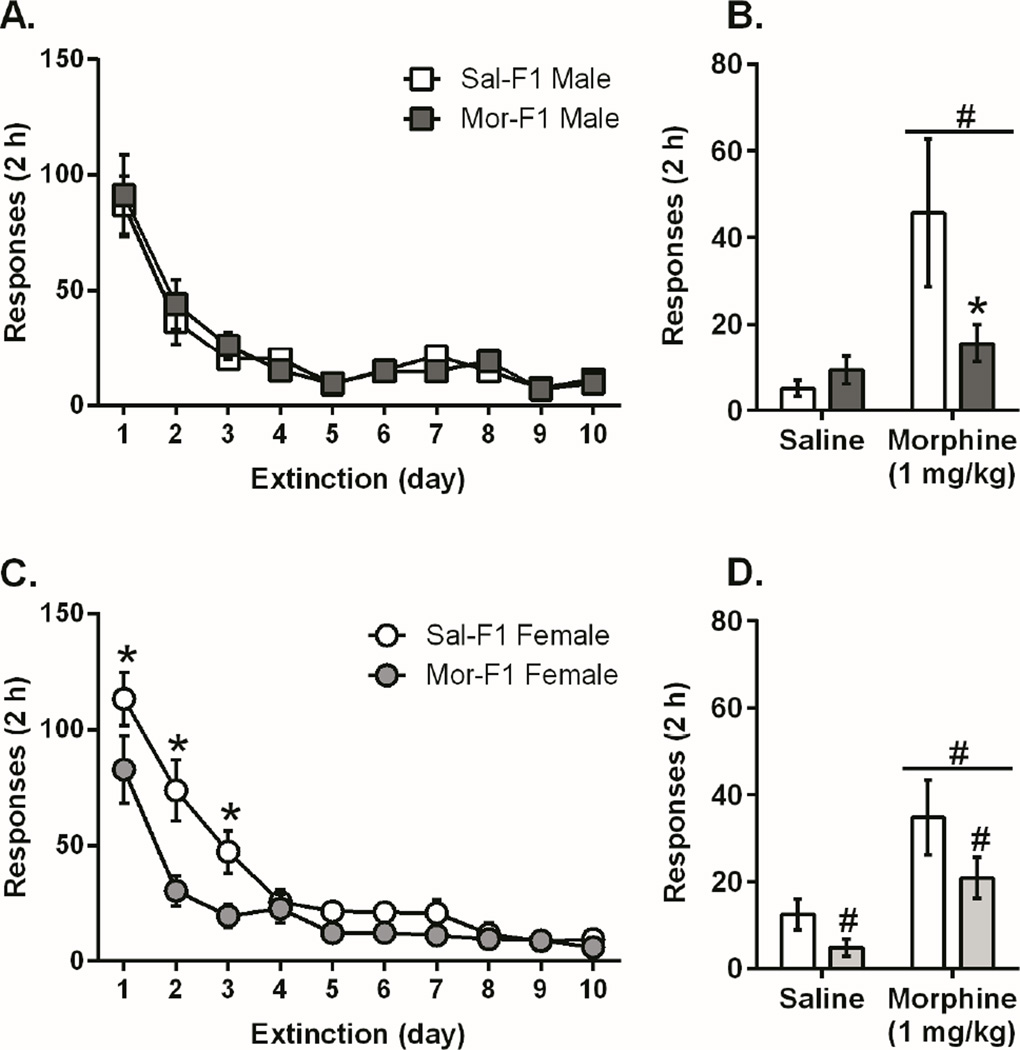
Next we examined the extinction and reinstatement data following the 0.75 mg/kg dose (Figure 2). While there was no difference between Sal- and Mor-F1 males at this dose during extinction, there was a significant attenuation of priming induced reinstatement (Figure 2A, 2B). In contrast, the females Mor-F1 animals, similar to their responding during acquisition and PR, demonstrated decreased responding during extinction and reinstatement (Figure 2C, 2D). There were no differences in extinction or reinstatement between Sal- and Mor-F1 animals at either of the other two doses (Figure S1). The effects observed during morphine self-administration behavior in the F1 animals were not due to a general operant learning deficit as there were no differences in acquisition, PR, extinction, or reinstatement when sucrose pellets were used as the reinforcer in place of morphine (Figure S2).
Figure 2.
Extinction and Reinstatement in Male and Female F1 Animals. For all panels, data is presented as mean +/− SEM. For the male animals, there were no differences in extinction responding between Mor-F1 and Sal-F1, although there was a main effect of day indicating that the responding in both groups was significantly decreased at the end of extinction [F(9, 126) = 28.7; P<0.0001] (2A). However, analysis of the reinstatement data showed a main effect of dose on reinstatement behavior (2B), [F(1,28)=6.8; p<0.05] demonstrating that both groups reinstated to a priming injection of morphine. There was also a significant interaction [F(1,28)=5.9; p<0.05]. Sidak’s post hoc analysis revealed that Mor-F1 animals responded significantly less than Sal-F1 animals following a morphine priming injection (p<0.05). Female animals also demonstrated extinction with a significant main effect of day [F(9, 135) = 49.36; p<0.0001] but the female Mor-F1 animals also showed blunted responding compared to Sal-F1 females (2C, main effect of maternal treatment [F(1, 15)=6.7; p<0.05]; significant interaction [F(9, 135) = 3.5; p<0.001] with Sidak’s multiple comparisons test showing a decrease in responding on days 1, 2, and 3 (p<0.05). In addition, there was also an effect on reinstatement (2D; main effect of dose [F (1,30)=13.9, p<0.001] indicating that all animals reinstated and main effect of maternal treatment [F(1,30)=4.4, p<0.05] indicating that Mor-F1 females had blunted responding on both reinstatement days). (n=8–9/group, see supplement for detailed n) # main effect p<0.05, *p<0.05 compared to Sal-F1 of same group.
3.2 F2 morphine self-administration acquisition, progressive ratio, extinction, reinstatement
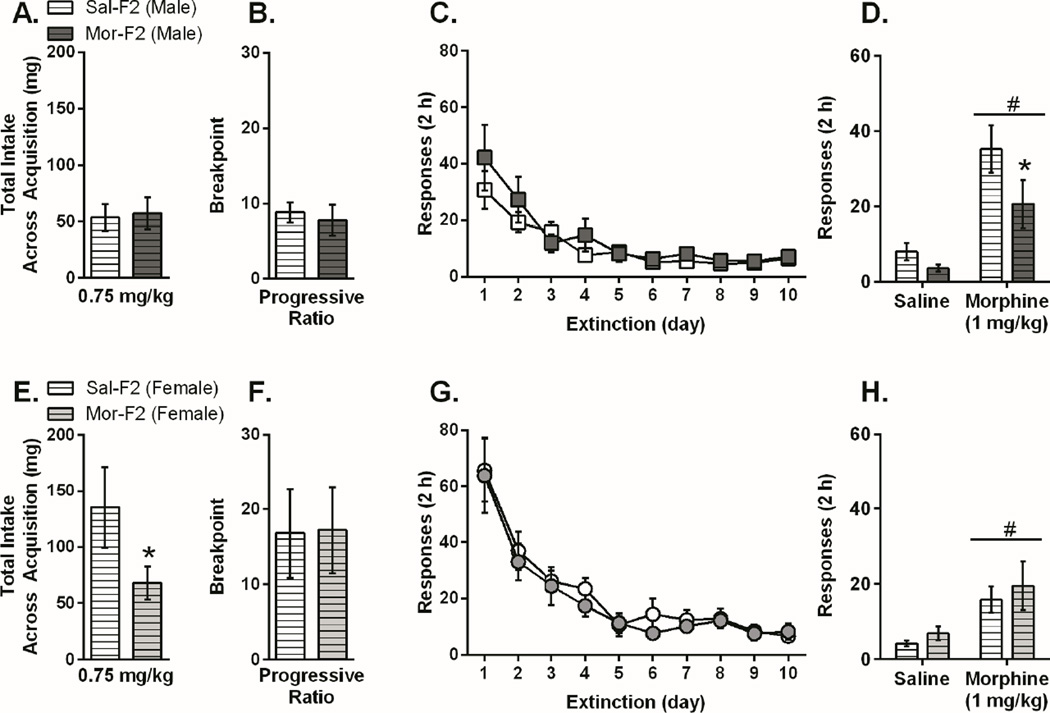
Based on the results from the F1 generation, we chose to test F2 animals using the middle (0.75 mg/kg/infusion) dose. F2 animals (maternal line) were tested during adulthood using the same self-administration paradigm and dose as that used for F1 animals. The pattern of behavior observed in the male F1 animals was also observed in the male F2 animals. Thus, there were no differences in self-administration acquisition, PR, or extinction between Sal-F2 and Mor-F2 males. However, Mor-F2 males had significantly attenuated morphine-primed reinstatement when compared with Sal-F2 males (Figure 3A–3D). In females, the blunted responding during acquisition was maintained in the F2 animals while no other differences were observed (Figure 3E–3H).
Figure 3.
Morphine Self-Administration Acquisition, Progressive Ratio, Extinction, and Reinstatement in Male and Female F2 Animals. For all panels, data is presented as mean +/− SEM. There were no differences between Mor-F2 and Sal-F2 male animals in acquisition (3A), progressive ratio (3B), or extinction (3C). There was a main effect of dose on reinstatement behavior (3D), [F(1,17)=22.2; p<0.05] demonstrating that both groups reinstated to a priming injection of morphine. Based on the F1 data, we had the a priori hypothesis that reinstatement would be blunted and therefore conducted a post hoc analysis, which revealed that Mor-F2 animals responded significantly less than Sal-F2 animals following a morphine priming injection (p<0.05). There was a significant decrease in total intake during acquisition (3E) for Mor-F2 females compared with Sal-F2 females [t=1.736, p<0.05] but no differences during, progressive ratio (3F), extinction (3G), or reinstatement (3H), although both groups did reinstate to a morphine prime [main effect of dose: F(1,20)=14.68, p<0.001]. #p<0.05 main effect of dose, *p<0.05 (n=12/group)
3.3 Gene expression changes in the nucleus accumbens of F1 and F2 animals
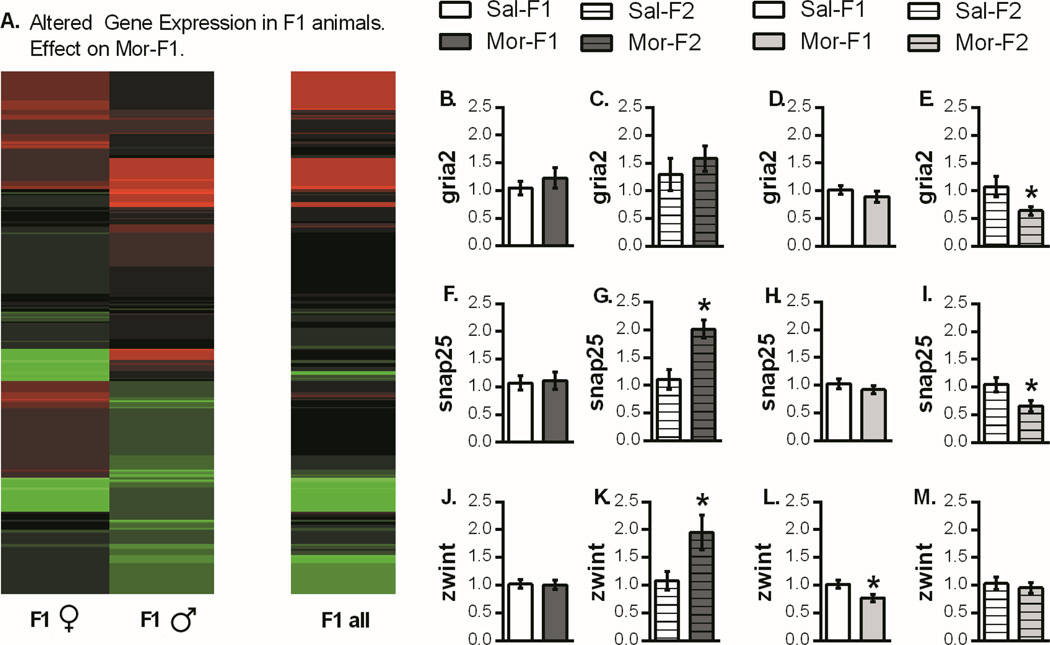
Gene expression in the nucleus accumbens of male and female F1 adults was analyzed using RNA sequencing. Using expression change analysis of PacBio sequencing data, genes differentially expressed between Mor-F1 and Sal-F1 groups were identified (Figure 4A). RNA-sequencing results were interrogated using gene ontology analysis (PANTHER). This highlighted differences in a set of genes related to synaptic function and plasticity, which were identified for follow up qPCR analysis. These included AMPA glutamate receptor type 2 (Gria2), synaptosomal-associated protein of 25kDa (Snap25) and ZW10 interacting kinetochore protein (Zwint), which is involved in kinetochore function and interacts directly with Snap25. Myelin basic protein (Mbp), a developmental gene critical for myelination, was also measured.
Figure 4.
Analysis of Pac-Bio sequencing data and synaptic plasticity genes. (A) Differential expression analysis produced a list of genes whose expression was > 10 counts per million. These genes were used to generate a heat map showing the gene expression changes in Mor-F1 animals relative to Sal-F1 animals. qPCR analysis of synaptic plasticity genes are presented as fold change relative to control (mean +/− SEM). There was no change in Gria2 expression in Mor-F1 males or females (4B, 4D) or in Mor-F2 males (4C). However, there was a decrease in expression in Mor-F2 females compared with Sal-F2 females (4E). For Snap25, there was no change in either sex in the F1 generation (4F, 4H). However, in the F2 animals there was increased expression in Mor-F2 males (4G) and decreased expression in Mor-F2 females (4I). Finally, for Zwint, there were no differences in F1 males (4J) but there was an increase in Mor-F2 males (4K). There was also a decrease in Mor-F1 females (4L) but no change in Mor-F2 females (4M). *p<0.05 detected with a two tailed student’s t-test. n=8 for all groups except Mor-F1 males (n=7) and Mor-F1 females (n=7)
3.4 qPCR analysis of target genes
In F1 animals, there were no significant differences in Gria2 or Snap25 as a function of maternal morphine history in either sex, but there was a significant decrease in the expression of Zwint in the Mor-F1 females (Figure 4L). In F2 animals, however, there were significant changes in all three of these genes, and these effects were sex-specific. Both Gria2 and Snap25 had lower expression in Mor-F2 females compared with Sal-F2 females, and both Snap25 and Zwint had higher levels of expression in Mor-F2 males compared with Sal-F2 males (Figure 4).
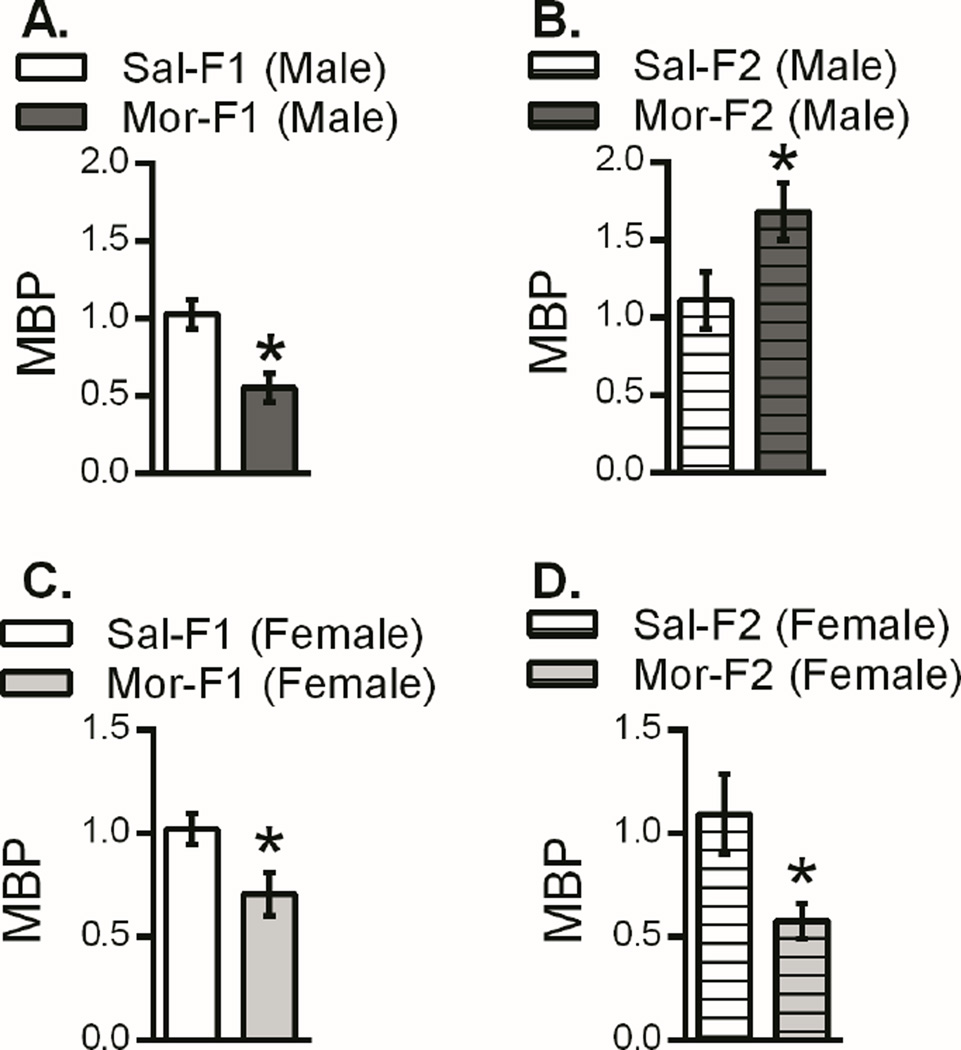
With regard to Mbp there was decreased expression in Mor-F1 males compared with Sal-F1 males. However, the opposite pattern was observed in F2 males. Thus, increased expression was observed in Mor-F2 males when compared with Sal-F2 males (Figure 5). In contrast, Mor-F1 and Mor-F2 females expressed lower levels of Mbp in the nucleus accumbens when compared to their appropriate control (Figure 5). Two additional genes identified via sequencing were also examined, however, no differences in either apolipoprotein E (ApoE) or thymosin beta-4, x-linked (Tmsb4x) as a function of maternal morphine history in either generation were observed (data not shown).
Figure 5.
Expression of myelin basic protein in the nucleus accumbens of male and female F1 and F2 animals. Data are presented as fold change relative to control (mean +/− SEM). In both Mor-F1 males and females there was a down regulation of Mbp relative to the respective Sal-F1 control (5A, 5C). In the F2 generation, the Mor-F2 females continued to show this down regulation of Mbp (5D) while Mor-F2 males showed an increase in expression levels (5B). *p<0.05 detected with a two-tailed student’s t-test. n=8 for all groups except Mor-F1 males (n=7) and Mor-F1 females (n=7)
4. Discussion
The results of the present study demonstrate transgenerational effects in progeny of females exposed to morphine during adolescence. Overall, Mor-F1 animals showed decreased levels of morphine intake and attenuated relapse-like behavior, with female offspring profoundly affected across all phases. In addition, genes involved with synaptic plasticity and CNS development were dysregulated within the NAc. Some, but not all, of these effects persisted into the second generation (F2). These findings reveal that limited exposure to opioids during adolescence, even in the absence of continued use or in utero exposure, have lasting effects that persist across generations.
4.1 Mor-F1 and F2 males have blunted reinstatement behavior
In males examined at the 0.75 mg/kg dose, there were no significant differences in the rate or intake of morphine during acquisition, and no change in motivated responding during PR or extinction. However, when tested for morphine priming-induced reinstatement there was a significant decrease in drug-seeking behavior in the Mor-F1 compared to Sal-F1 males. In humans, addiction is a chronic relapsing disease characterized by compulsive drug seeking followed by periods of abstinence and relapse to drug-seeking behavior. Reinstatement is an animal model of relapse consistently characterized in the literature as representative of a propensity towards relapse ((Ahmed and Koob, 1998). Therefore, the decreased reinstatement behavior observed in these animals could indicate a reduced susceptibility to relapse-like behavior. As there were no differences in any other aspect of self-administration behavior at this dose, the diminished relapse-like behavior was not due to a general decrease in responding or drug intake, suggesting a selective effect on neural systems that mediate drug-primed reinstatement. Additionally, there were no changes in reinstatement behavior observed when the reinforcer was sucrose rather than morphine, indicating that these effects were not due to a general deficit in learning and/or memory. It does suggest changes in the drug memory or drug craving in these animals, resulting in decreased levels of responding during priming-induced reinstatement. Alterations that we observed in genes associated with synaptic plasticity within the NAc, a critical brain region mediating drug-primed reinstatement, support this hypothesis. Future studies are needed to determine whether these modifications in genes associated with synaptic plasticity, where were observed in non-drug exposed MOR-F1 males, mediate the observed changes in reinstatement. These data, however, provide evidence that a brief exposure to opiates during adolescence can have lasting consequences in future generations.
While examination of a higher and lower dose yielded similar patterns (i.e. decreased responding in Mor-F1 animals); 0.75 mg/kg/infusion was the only dose that produced blunted reinstatement. Therefore, this dose was examined in the F2 generation to determine whether a similar selective effect on drug-primed reinstatement could be discerned. As with F1 males, decreased drug-primed reinstatement behavior in Mor-F2 males was also observed in the absence of any changes in acquisition, PR, or extinction. While the current study examined drug priming-induced reinstatement, future studies should examine cue or stress induced reinstatement as both modes contribute significantly to opioid relapse. Moreover, the mechanisms underlying reinstatement are divergent, and the effects observed utilizing these models may be different (Lai et al., 2013; Wang et al., 2006) which may provide more specific molecular targets.
While the mechanism of transmission to the F2 remains unclear, it does not appear to be solely due to direct effects of morphine as there was never any exposure to F2 germ cells. A number of the behavioral effects were less robust in the F2 generation compared to the F1 generation, which may implicate a role for direct exposure. In addition, exposure to opioids during the adolescent window may impact the development of both endocrine and neural circuits, which could contribute to the F1 effects to a greater extent than the F2 effect (Cicero et al., 1991; Hofford et al., 2012; Kennedy et al., 2011). Moreover, it is not uncommon nor unexpected to see effects in only one generation, effects that skip a generation, or effects that weaken with subsequent generations (Daxinger and Whitelaw, 2012; Dunn and Bale, 2011; Dunn et al., 2011; van Otterdijk and Michels, 2016; Vassoler et al., 2014a; Yohn et al., 2015). Additional mechanisms of transmission may include incorporation of epigenetic marks, alterations in miRNA, behavioral/physiological changes in the F1 dam, or a combination (Rodgers et al., 2015; Vassoler and Sadri-Vakili, 2013). Previous findings using this model also documented behavioral and molecular effects in MOR-F1 males that persist into the F2 generation and implicate transgenerational modifications within the NAc (Byrnes et al., 2013). We have assessed maternal behavior using this model and showed minimal differences between Sal-F0 and Mor-F0 dams; limited to slight decreases in time spent on nest during the dark cycle (Johnson et al., 2011). Additional pre- and/or postnatal factors may still play a role in the etiology of the observed effects. Nonetheless, overall these data support the hypothesis that additional mechanisms of inheritance, other than Mendelian genetics, play a role in modulating patterns of substance use across multiple generations.
4.2 Decreased Responding for Morphine in Mor-F1 and F2 females
In Mor-F1 females, there was an overall decrease in responding at all phases of the paradigm. Lower levels of morphine self-administration on a FR1 schedule is commonly interpreted as higher drug sensitivity (Arnold and Roberts, 1997; Carroll and Lac, 1997; Doherty et al., 2009; Koob et al., 1984). This is consistent with recent results using this same adolescent exposure regimen that showed increased place conditioning at low doses of morphine in a conditioned place preference paradigm (Vassoler et al., 2015). However, it could also be interpreted as a decrease in motivation to obtain morphine, which is more consistent with the PR, extinction, and reinstatement data. Previously, we reported increased kappa opioid receptor gene expression in Mor-F1 and F2 males (Byrnes et al., 2013). As activity at kappa opioid receptors is thought to be largely responsible for negative effects of morphine, increased expression could result in augmented withdrawal symptoms, leading to reduced motivated responding.
Mor-F2 females also demonstrated blunted intake during the acquisition phase, although this effect did not extend to PR, extinction, or reinstatement. This change in phenotype may indicate that the initial response to morphine in Mor-F2 females is altered. The changes in gene expression related to synaptic plasticity (particularly in the F2 animals) support this notion. However, the change in phenotype from the F1 animals indicates that decreased responding observed in Mor-F1 females may partially result from direct exposure of the oocytes to morphine or to alterations in the perinatal environment in Mor-F0 dams. Additionally, in many transgenerational effects, females are less affected than males (Biglarnia et al., 2013; Timar et al., 2010; Vassoler et al., 2013).
Recently, it was shown that offspring of cocaine experienced sires had decreased self-administration behavior (Vassoler et al., 2013). These data are similar to those reported in the current set of studies, suggesting decreased reinforcing effectiveness of a drug in the offspring of parents exposed to the same drug prior to conception. The observed reduction in self-administration behaviors, however, should not necessarily be interpreted as a demonstration of a “protective effect” on offspring and grandoffspring with regard to addiction liability. Indeed, other data from our lab suggest additional behavioral and molecular modifications in these animals that would be expected to increase their vulnerability to addiction under various conditions (Byrnes, 2005a, b, 2008; Byrnes et al., 2011; Byrnes et al., 2013; Johnson et al., 2011; Vassoler et al., 2014b; Vassoler et al., 2015). Thus, what we suggest is that maternal exposure to morphine during adolescence alters the neurodevelopment of future offspring that impacts how they respond to drugs of abuse, which may be dependent upon a host of environmental and genetic factors.
4.3 Dysregulation of synaptic plasticity genes
It is clear that continued drug use induces adaptive genomic changes in the central nervous system that lead to drug dependence (Koob and Kreek, 2007). Further, epigenetic adaptations in transcriptional regulation are likely part of the observed alterations in addiction-related phenotypes in the F1 and F2 generations. Indeed, results from Pac-Bio sequencing of the NAc of F1 animals indicated changes in several genes involved in the regulation of neuronal plasticity, suggesting functional circuitry changes. Using this initial unbiased screen, we selected three genes for follow up, which showed significant differential expression and are known to regulate synaptic plasticity; Gria2, Snap25, and Zwint.
Gria2 codes for GluA2, which is implicated in the reinstatement of drug-seeking behavior for multiple drugs of abuse including cocaine and morphine (Briand et al., 2014; Dias et al., 2012; Hearing et al., 2016; LaCrosse et al., 2015; Schmidt et al., 2015). While the sequencing results indicated that we would see changes in the F1 animals, there were no differences observed in the qPCR experiments. This discrepancy may be due to higher sensitivity of PacBio sequencing and analysis. However, when we examined the F2 animals, there was a significant decrease in Gria2 in Mor-F2 compared with Sal-F2 females. This pattern of effects observed in F2 but not F1 animals occurred throughout our analysis of gene expression changes. This may be due to compensatory changes as the behavioral differences observed in morphine self-administration were decreased in the F2 animals, or the changes may produce a deficit in a behavior paradigm that we have not yet tested. In either case, we are observing a sex-specific addiction-related molecular phenotype in F1 and F2 generations in response to opioid exposure limited to the adolescent period. Changes in AMPA receptor expression in the accumbens can affect many aspects of brain state including learning and memory, reward, and plasticity. Snap25 is involved in vesicle fusion and neurotransmitter release and thereby plays a role in synaptic plasticity. Additionally, Snap25 is down regulated in the hippocampus following morphine treatment (Xu et al., 2004). However, similar to the effect with Gria2, Snap25 was not regulated in the F1 animals yet affected the F2 generation with an up regulation in Mor-F2 males and down regulation in Mor-F2 females. Zwint interacts directly with Snap25 and also plays a role in synaptic plasticity. Zwint is a relatively understudied gene but has been implicated in cancer (Endo et al., 2012; Miller, 2010). There was no change in Zwint in the F1 males, but a down regulation in the Mor-F1 females, animals that were mated to produce F2 animals. In this next generation, an increase in the level of Zwint was observed in Mor-F2 males while expression had normalized in F2 females. Dysregulation of genes associated with synaptic plasticity may partly underlie impaired drug memory or drug seeking behavior observed in the F1 and F2 animals. Importantly, the observed changes were all at baseline and multiple exposures to opiates may alter how these genes are regulated (Byrnes et al., 2013).
4.4 Mbp is dysregulated in both F1 and F2 animals
Recently, myelin has come into focus because of its potential role in psychiatric diseases, including addiction (Bora et al., 2012). Indeed, myelin and oligodendrocytes that produce myelin are proving to be important regulators in development as well as neuroplasticity, remodeling, and addiction (Bruce et al., 2010). Additionally, oligodendrocytes express opiate receptors and produce endogenous opioids in a developmentally specific manner (Knapp et al., 2001; Knapp et al., 1998), which would allow for exogenous opioids to have pronounced effects. Indeed, changes in Mbp have been shown following drug exposure in both rodents and humans; single nucleotide polymorphisms (Snp) in the Mbp gene have been associated with cocaine, heroin and alcohol in humans as well as alcohol in rats (Levey et al., 2014; Levran et al., 2015). In addition, prenatal and perinatal exposures to nicotine or opiates have caused alterations in expression of Mbp in both male and female offspring in rats (Cao et al., 2013; Sanchez et al., 2008). Moreover, in humans white matter changes have been observed in children prenatally exposed to opiates (Walhovd et al., 2010). Finally, prolonged self-administration of cocaine causes a down regulation in MBP protein expression in the precommisural striatum in both non-human primates as well as in humans (Bannon et al., 2005; Smith et al., 2014). Here, we report that exposure to opiates during adolescence has effects on Mbp which persist for multiple generations. We found down regulation of Mbp in both Mor-F1 males and females. This effect extended to F2 females and reversed in the F2 males. Mbp is particularly well situated to play a significant role in the transmission of opiate related neurodevelopmental changes because of its role in development. It is regulated by opioids, has developmentally mediated expression patterns, and has been linked with addiction in multiple animal models as well as in humans (Cao et al., 2013; Levey et al., 2014; Levran et al., 2015; Sanchez et al., 2008).
4.5 Conclusions
These data are the first to demonstrate transgenesrational epigenetic effects on motivated responding for drugs of abuse. The majority of previous studies on transgenerational effects of drugs of abuse have only documented effects in the F1 generation. Moreover, the molecular changes clearly indicate the persistence of epigenetic modifications across two generations. Perhaps even more intriguing, they suggest the emergence of novel molecular effects in the F2 generation which cannot be predicted based on F1 effects.
While these results may appear contradictory from data suggesting increased risk of drug addiction in the children of substance abusers (Levran et al., 2012), to date there have been no studies in human populations that examine the generational effect of discreet adolescent exposure to opioids. Moreover, studies of this nature would be unfeasible due to the numerous genetic and environmental factors that contribute to abuse liability in people. From an evolutionary perspective, these effects may represent an epigenetic mechanism preparing progeny for an environment in which increased opioid receptor activation is possible. Indeed, this would not be the first example of a hypothetical “protective” effect as a consequence of parental drug exposure (Vassoler et al., 2013). However, under certain conditions this “protective” effect may be detrimental. For example, the observed changes may be indicative of a blunted reward system or a depressive-like phenotype, both of which are known to increase the risk of addictive behaviors in humans. Regardless of the direction of the effect, what these data represent is that maternal adolescent exposure to opioids in the absence of in utero exposure has lasting effects on addictive-like behaviors in offspring and grandoffspring. In human populations, the interaction between these epigenetic developmental changes, genetics, and environment may mitigate or exacerbate addictive behaviors in offspring and may provide a mechanism underlying variability in human phenotypes.
Supplementary Material
Figure S1. Extinction and Reinstatement Following the Low (0.25 mg/kg/infusion) and High (1.25/mg/kg/infusion) Dose of Morphine in Male and Female F1 Animals. For all panels, data is presented as mean +/− SEM. There were no differences between Mor-F1 and Sal-F1 in extinction or reinstatement following the low dose of morphine in either males or females (S1A, B, C, D). Although, all animals showed a significant main effect of dose following a morphine prime indicating reinstatement [males: F(1,18)=10.06, p<0.05; females: F(1,21)=5.65, p<0.05]. (n=10–11). Similarly, there werer no differences between Mor-F1 and Sal-F1 in extinction or reinstatement following the high dose of morphine in either males or females (S1E, F, G, H). Although, all animals showed a significant main effect of dose following a morphine prime indicating reinstatement [males: F(1,12)=9.97; p<0.05; females: F(1,13)=5.49, p<0.5]. (n=10-11) #p<0.05 main effect of dose.
Figure S2. Sucrose Self-Administration Acquisition, Progressive Ratio, Extinction, and Reinstatement in Male and Female F1 Animals. For all panels, data is presented as mean +/− SEM. There were no differences between Mor-F1 and Sal-F1 male animals in acquisition (S1A), progressive ratio (S1B), extinction (S1C), or reinstatement (S1D). There was a main effect of day on reinstatement behavior (S1D), [F(1,7)=7.3; p<0.05] demonstrating that both groups reinstated sucrose-seeking behavior. There were no differences between the Mor-F1 and Sal-F1 females during acquisition (S1E), progressive ratio (S1F), extinction (S1G), or reinstatement (S1H), although both groups did reinstate [main effect of day: F(1,10)=13.37, p<0.05]. (n=7–9)
Highlights.
Adolescent opiate exposure decreases morphine self-administration in offspring
Male offspring and grandoffspring demonstrate decreased reinstatement behavior
Genes related to synaptic plasticity were dysregulated in F1 and F2 animals
Myelin basic protein is dysregulated in male and female F1 and F2 animals
Acknowledgments
We would like to thank Dr. John Byrnes, Dr. Chris Pierce, and Dr. Lisa Briand for their careful reading and insightful comments on this manuscript. Additionally, we would like to thank the University of Massachusetts Medical School Deep Sequencing Core Laboratories and Pacific Biosciences Core Enterprise for providing Next-Generation sequence support and services for this project.
Funding
This work was generously supported by grants from the National Institutes of Health (NIH) R01 DA025674, R03 DA034886 and the Tufts University Provost Office Tufts Collaborates Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have no financial disclosures and declare no conflict of interest.
Contributions: FMV conceived and designed experiments, collected data, analyzed data, and wrote the manuscript; DJO analyzed data, wrote and edited the manuscript; CW collected data and edited the manuscript; AB collected data and edited the manuscript; MS analyzed the data and approved the manuscript; JRT analyzed data, designed analyses, wrote and edited the manuscript; EMB conceived and designed the experiments, collected data, analyzed data, wrote and edited the manuscript.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Bannon M, Kapatos G, Albertson D. Gene expression profiling in the brains of human cocaine abusers. Addict Biol. 2005;10:119–126. doi: 10.1080/13556210412331308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglarnia M, Karami M, Hafshejani ZK. Differences in morphine-induced antinociception in male and female offspring born of morphine exposed mothers. Indian J Pharmacol. 2013;45:227–231. doi: 10.4103/0253-7613.111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Gapp K, Saab BJ, Mansuy IM. Transgenerational epigenetic effects on brain functions. Biol Psychiatry. 2013;73:313–320. doi: 10.1016/j.biopsych.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, Lubman DI. White matter microstructure in opiate addiction. Addict Biol. 2012;17:141–148. doi: 10.1111/j.1369-1600.2010.00266.x. [DOI] [PubMed] [Google Scholar]
- Briand LA, Kimmey BA, Ortinski PI, Huganir RL, Pierce RC. Disruption of glutamate receptor-interacting protein in nucleus accumbens enhances vulnerability to cocaine relapse. Neuropsychopharmacology. 2014;39:759–769. doi: 10.1038/npp.2013.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CC, Zhao C, Franklin RJ. Remyelination - An effective means of neuroprotection. Horm Behav. 2010;57:56–62. doi: 10.1016/j.yhbeh.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Chronic morphine exposure during puberty decreases postpartum prolactin secretion in adult female rats. Pharmacol Biochem Behav. 2005a;80:445–451. doi: 10.1016/j.pbb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005b;182:537–544. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Chronic morphine exposure during puberty induces long-lasting changes in opioid-related mRNA expression in the mediobasal hypothalamus. Brain Res. 2008;1190:186–192. doi: 10.1016/j.brainres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res. 2011;218:200–205. doi: 10.1016/j.bbr.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Carini LM, Byrnes EM. Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology (Berl) 2013;227:263–272. doi: 10.1007/s00213-012-2960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Wang J, Dwyer JB, Gautier NM, Wang S, Leslie FM, Li MD. Gestational nicotine exposure modifies myelin gene expression in the brains of adolescent rats with sex differences. Transl Psychiatry. 2013;3:e247. doi: 10.1038/tp.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl) 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Adams ML, Giordano A, Miller BT, O'Connor L, Nock B. Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring. J Pharmacol Exp Ther. 1991;256:1086–1093. [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- Dias C, Wang YT, Phillips AG. Facilitated extinction of morphine conditioned place preference with Tat-GluA2(3Y) interference peptide. Behav Brain Res. 2012;233:389–397. doi: 10.1016/j.bbr.2012.05.026. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav. 2009;92:164–172. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–2236. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Morgan CP, Bale TL. Sex-specificity in transgenerational epigenetic programming. Horm Behav. 2011;59:290–295. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Endo H, Ikeda K, Urano T, Horie-Inoue K, Inoue S. Terf/TRIM17 stimulates degradation of kinetochore protein ZWINT and regulates cell proliferation. J Biochem. 2012;151:139–144. doi: 10.1093/jb/mvr128. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Mansuy IM. Epigenetic inheritance in mammals: evidence for the impact of adverse environmental effects. Neurobiol Dis. 2010;39:61–65. doi: 10.1016/j.nbd.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Gapp K, von Ziegler L, Tweedie-Cullen RY, Mansuy IM. Early life epigenetic programming and transmission of stress-induced traits in mammals: how and when can environmental factors influence traits and their transgenerational inheritance? Bioessays. 2014;36:491–502. doi: 10.1002/bies.201300116. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, Schmidt C, Larson EB, Thomas MJ. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci U S A. 2016;113:757–762. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Wellman PJ, Eitan S. Morphine alters the locomotor responses to a D2/D3 dopamine receptor agonist differentially in adolescent and adult mice. J Psychopharmacol. 2012;26:1355–1365. doi: 10.1177/0269881112443741. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry. 2011;2:29. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BC, Panksepp JB, Wong JC, Krause EJ, Lahvis GP. Age-dependent and strain-dependent influences of morphine on mouse social investigation behavior. Behav Pharmacol. 2011;22:147–159. doi: 10.1097/FBP.0b013e328343d7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp PE, Itkis OS, Zhang L, Spruce BA, Bakalkin G, Hauser KF. Endogenous opioids and oligodendroglial function: possible autocrine/paracrine effects on cell survival and development. Glia. 2001;35:156–165. doi: 10.1002/glia.1080. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: mu and kappa opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Pettit HO, Ettenberg A, Bloom FE. Effects of opiate antagonists and their quaternary derivatives on heroin self-administration in the rat. J Pharmacol Exp Ther. 1984;229:481–486. [PubMed] [Google Scholar]
- Kosten TA, Huang W, Nielsen DA. Sex and litter effects on anxiety and DNA methylation levels of stress and neurotrophin genes in adolescent rats. Dev Psychobiol. 2014;56:392–406. doi: 10.1002/dev.21106. [DOI] [PubMed] [Google Scholar]
- LaCrosse AL, Hill K, Knackstedt LA. Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. Eur Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Chen W, Zhu H, Zhou X, Liu H, Zhang F, Zhou W. Low dose risperidone attenuates cue-induced but not heroin-induced reinstatement of heroin seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2013;16:1569–1575. doi: 10.1017/S1461145712001563. [DOI] [PubMed] [Google Scholar]
- Levey DF, Le-Niculescu H, Frank J, Ayalew M, Jain N, Kirlin B, Learman R, Winiger E, Rodd Z, Shekhar A, Schork N, Kiefer F, Wodarz N, Muller-Myhsok B, Dahmen N, Consortium G, Nothen M, Sherva R, Farrer L, Smith AH, Kranzler HR, Rietschel M, Gelernter J, Niculescu AB. Genetic risk prediction and neurobiological understanding of alcoholism. Transl Psychiatry. 2014;4:e391. doi: 10.1038/tp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Peles E, Randesi M, Correa da Rosa J, Ott J, Rotrosen J, Adelson M, Kreek MJ. Synaptic Plasticity and Signal Transduction Gene Polymorphisms and Vulnerability to Drug Addictions in Populations of European or African Ancestry. CNS Neurosci Ther. 2015;21:898–904. doi: 10.1111/cns.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Yuferov V, Kreek MJ. The genetics of the opioid system and specific drug addictions. Hum Genet. 2012;131:823–842. doi: 10.1007/s00439-012-1172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nat Rev Genet. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Miller WR. Clinical, pathological, proliferative and molecular responses associated with neoadjuvant aromatase inhibitor treatment in breast cancer. J Steroid Biochem Mol Biol. 2010;118:273–276. doi: 10.1016/j.jsbmb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- RCoreTeam. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56:1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, McFarland KN, Darnell SB, Huizenga MN, Sangrey GR, Cha JH, Pierce RC, Sadri-Vakili G. ADAR2-dependent GluA2 editing regulates cocaine seeking. Mol Psychiatry. 2015;20:1460–1466. doi: 10.1038/mp.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK. Environmental Epigenetics and a Unified Theory of the Molecular Aspects of Evolution: A Neo-Lamarckian Concept that Facilitates Neo-Darwinian Evolution. Genome Biol Evol. 2015;7:1296–1302. doi: 10.1093/gbe/evv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Nader MA, Porrino LJ. Regionally-specific alterations in myelin proteins in nonhuman primate white matter following prolonged cocaine self-administration. Drug Alcohol Depend. 2014;137:143–147. doi: 10.1016/j.drugalcdep.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, Ren Y, Miller ML, Blitzer RD, Hurd YL. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology. 2014;39:1315–1323. doi: 10.1038/npp.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timar J, Sobor M, Kiraly KP, Gyarmati S, Riba P, Al-Khrasani M, Furst S. Peri, pre and postnatal morphine exposure: exposure-induced effects and sex differences in the behavioural consequences in rat offspring. Behav Pharmacol. 2010;21:58–68. doi: 10.1097/FBP.0b013e3283359f39. [DOI] [PubMed] [Google Scholar]
- van Otterdijk SD, Michels KB. Transgenerational epigenetic inheritance in mammals: how good is the evidence? FASEB J. 2016;30:2457–2465. doi: 10.1096/fj.201500083. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology. 2014a;76(Pt B):269–275. doi: 10.1016/j.neuropharm.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Johnson-Collins NL, Carini LM, Byrnes EM. Next generation effects of female adolescent morphine exposure: sex-specific alterations in response to acute morphine emerge before puberty. Behav Pharmacol. 2014b;25:173–181. doi: 10.1097/FBP.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Wright SJ, Byrnes EM. Exposure to opiates in female adolescents alters mu opiate receptor expression and increases the rewarding effects of morphine in future offspring. Neuropharmacology. 2015;103:112–121. doi: 10.1016/j.neuropharm.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Wright SJ, Byrnes EM. Exposure to opiates in female adolescents alters mu opiate receptor expression and increases the rewarding effects of morphine in future offspring. Neuropharmacology. 2016;103:112–121. doi: 10.1016/j.neuropharm.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Moe V, Slinning K, Due-Tonnessen P, Bjornerud A, van der Kouwe A, Dale AM, Fjell AM. White matter characteristics and cognition in prenatally opiate- and polysubstance-exposed children: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2010;31:894–900. doi: 10.3174/ajnr.A1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TD, Watanabe CK. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics. 2005;21:1859–1875. doi: 10.1093/bioinformatics/bti310. [DOI] [PubMed] [Google Scholar]
- Xu NJ, Yu YX, Zhu JM, Liu H, Shen L, Zeng R, Zhang X, Pei G. Inhibition of SNAP-25 phosphorylation at Ser187 is involved in chronic morphine-induced down-regulation of SNARE complex formation. J Biol Chem. 2004;279:40601–40608. doi: 10.1074/jbc.M406896200. [DOI] [PubMed] [Google Scholar]
- Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol. 2015;118:21–33. doi: 10.1016/j.pbiomolbio.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Extinction and Reinstatement Following the Low (0.25 mg/kg/infusion) and High (1.25/mg/kg/infusion) Dose of Morphine in Male and Female F1 Animals. For all panels, data is presented as mean +/− SEM. There were no differences between Mor-F1 and Sal-F1 in extinction or reinstatement following the low dose of morphine in either males or females (S1A, B, C, D). Although, all animals showed a significant main effect of dose following a morphine prime indicating reinstatement [males: F(1,18)=10.06, p<0.05; females: F(1,21)=5.65, p<0.05]. (n=10–11). Similarly, there werer no differences between Mor-F1 and Sal-F1 in extinction or reinstatement following the high dose of morphine in either males or females (S1E, F, G, H). Although, all animals showed a significant main effect of dose following a morphine prime indicating reinstatement [males: F(1,12)=9.97; p<0.05; females: F(1,13)=5.49, p<0.5]. (n=10-11) #p<0.05 main effect of dose.
Figure S2. Sucrose Self-Administration Acquisition, Progressive Ratio, Extinction, and Reinstatement in Male and Female F1 Animals. For all panels, data is presented as mean +/− SEM. There were no differences between Mor-F1 and Sal-F1 male animals in acquisition (S1A), progressive ratio (S1B), extinction (S1C), or reinstatement (S1D). There was a main effect of day on reinstatement behavior (S1D), [F(1,7)=7.3; p<0.05] demonstrating that both groups reinstated sucrose-seeking behavior. There were no differences between the Mor-F1 and Sal-F1 females during acquisition (S1E), progressive ratio (S1F), extinction (S1G), or reinstatement (S1H), although both groups did reinstate [main effect of day: F(1,10)=13.37, p<0.05]. (n=7–9)