Abstract
Background
Parkinson disease with orthostatic hypotension (PD + OH) and the parkinsonian form of multiple system atrophy (MSA-P) can be difficult to distinguish clinically. Recent studies indicate that PD entails a vesicular storage defect in catecholaminergic neurons. Although cardiac sympathetic neuroimaging by 18F-dopamine positron emission tomography can identify decreased vesicular storage, this testing is not generally available. We assessed whether plasma biomarkers of a vesicular storage defect can separate PD + OH from MSA-P.
Methods
We conceptualized that after F-dopamine injection, augmented production of F-dihydroxyphenylacetic acid (F-DOPAC) indicates decreased vesicular storage, and we therefore predicted that arterial plasma F-DOPAC would be elevated in PD + OH but not in MSA-P. We measured arterial plasma F-DOPAC after 18F-dopamine administration (infused i.v. over 3 min) in patients with PD + OH (N = 12) or MSA-P (N = 21) and in healthy control subjects (N = 26). Peak F-DOPAC:dihydroxyphenylglycol (DHPG) was also calculated to adjust for effects of denervation on F-DOPAC production.
Results
Plasma F-DOPAC accumulated rapidly after initiation of 18F-dopamine infusion. Peak F-DOPAC (5–10 min) in PD + OH averaged three times that in MSA-P (P < 0.0001). Among MSA-P patients, none had peak F-DOPAC > 300 nCi-kg/cc-mCi, in contrast with 7 of 12 PD + OH patients (χ2 = 16.6, P < 0.0001). DHPG was lower in PD + OH (3.83 ± 0.36 nmol/L) than in MSA-P (5.20 ± 0.29 nmol/L, P = 0.007). All MSA-P patients had peak F-DOPAC:DHPG < 60, in contrast with 9 of 12 PD + OH patients (χ2 = 17.5, P < 0.0001). Adjustment of peak F-DOPAC for DHPG increased test sensitivity from 58 to 81 % at similar high specificity.
Interpretation
After F-dopamine injection, plasma F-DOPAC and F-DOPAC:DHPG distinguish PD + OH from MSA-P.
Keywords: Parkinson disease, Biomarker, Multiple system atrophy, Orthostatic hypotension, Autonomic, Sympathetic, Fluorodopamine
Introduction
Parkinson disease with orthostatic hypotension (PD + OH) can be difficult to distinguish clinically from the parkinsonian form of multiple system atrophy (MSA-P), since both diseases typically involve parkinsonism and neurogenic OH. Both also are alpha-synucleinopathies, but with alpha-synuclein deposited in Lewy bodies in PD + OH and in glial cytoplasmic inclusions in MSA-P. Cardiac sympathetic neuroimaging by 18F-dopamine positron emission tomographic (PET) scanning or by 123I-metaiodobenzylguanidine single photon emission tomographic (SPECT) scanning aids the differential diagnosis [5, 13] but is expensive, involves radioactivity exposure, and is not available for this purpose at most centers in the United States. Recent studies indicate that PD + OH entails a vesicular storage defect in sympathetic noradrenergic nerves, whereas MSA does not [6, 9].
In this study we tested whether, after i.v. injection of F-dopamine, arterial plasma levels of F-dihydroxyphenylacetic acid (F-DOPAC) provide a biomarker of a vesicular storage defect that distinguishes PD + OH from MSA-P.
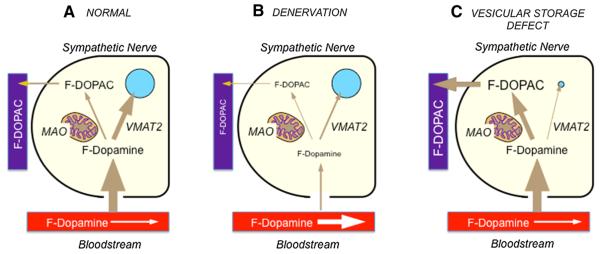
The diagram in Fig. 1 shows the concept underlying the study. After F-dopamine administration, the tracer is taken up rapidly by sympathetic noradrenergic nerves via the cell membrane norepinephrine transporter (NET). F-dopamine in the neuronal cytoplasm has two fates. The main intraneuronal fate of cytoplasmic F-dopamine is uptake into storage vesicles via the type 2 vesicular monoamine transporter (VMAT2), after which intra-vesicular F-dopamine is converted slowly to F-norepinephrine, is released by exocytosis, or leaks back into the cytoplasm. A minor fate of cytoplasmic F-dopamine is enzymatic deamination catalyzed by monoamine oxidase (MAO) in the outer mitochondrial membrane, to form F-DOPAC, which rapidly exits the nerve terminals and enters the plasma. As shown in Fig. 1b, if there were denervation, then plasma F-DOPAC would be decreased. Thus, chemical sympathectomy by 6-hydroxydopamine and NET blockade by desipramine both substantially decrease plasma F-DOPAC levels [1, 3]. As shown in Fig. 1c, if there were a vesicular storage defect, then plasma F-DOPAC would be increased. Thus, VMAT blockade by reserpine increases plasma F-DOPAC levels [3].
Fig. 1.
Concept diagrams for the fate of F-dopamine (F-DA) in sympathetic noradrenergic nerves. a Normal; b denervation or blockade of neuronal uptake; c decreased vesicular storage. VMAT2 type 2 vesicular monoamine transporter; MAO monoamine oxidase; F-DOPAC F-dihydroxyphenylacetic acid
Although 18F-dopamine was administered for PET scanning, the assays were done on plasma samples after complete radioactive decay of the tracer. On a mass basis, only a tiny fraction of the administered drug was 18F-dopamine; most was non-radioactive 19F-dopamine.
To take into account the potentially offsetting effects of denervation and decreased vesicular sequestration, we also evaluated the ratio of peak F-DOPAC:3,4-dihydroxyphenylglycol (DHPG). DHPG is the main neuronal metabolite of norepinephrine [2]. Peak F-DOPAC:DHPG might therefore provide a more sensitive measure of a vesicular storage defect by taking into account the effect of noradrenergic denervation on F-DOPAC production.
Methods
Subjects
The Intramural Research Board of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health approved the protocols for this study. All subjects gave informed written consent before the procedures.
This was a cross-sectional study. Patients were referred to the NIH and carried an admission diagnosis of PD, MSA, or PAF. Categorization in terms of occurrence of OH and diagnoses of PD or MSA were based on previously published consensus criteria [12] supplemented by data about plasma levels of catechols [8], septal myocardial 18F-derived radioactivity [7], and the University of Pennsylvania Smell Identification Test [5].
MSA patients were grouped clinically in terms of parkinsonian or cerebellar subtypes (MSA-P and MSA-C). MSA patients with both parkinsonism and cerebellar functional abnormalities were considered to have MSA-P.
In all patients with OH, the OH was determined to be neurogenic based on of abnormal responses of beat-to-beat blood pressure associated with the Valsalva [10] or attenuated orthostatic increments in plasma norepinephrine levels [14].
The study included 12 PD + OH patients, 21 MSA-P patients, and 26 control subjects. Among the controls, 25 were healthy volunteers and one was a referred patient who had no evidence of OH or central neurodegeneration. For comparison purposes, data were also obtained in 5 MSA-C, 11 PAF, and 7 PD No OH patients.
All patients were off levodopa, carbidopa, and entacapone and were not taking medications known to affect neuronal uptake or intra-neuronal metabolism of catecholamines.
Research procedures
In each subject, a brachial or radial artery catheter was placed after local anesthesia of the overlying skin. 18F-Dopamine (usually 1 mCi, specific activity about 800 mCi/mmole) was infused i.v. using an infusion pump over 3 min. In most subjects, blood samples were drawn through the arterial catheter at baseline and at various time points up to 180 min, for assays of plasma catechols.
Plasma concentrations of catechols including F-DOPAC and DHPG were assayed by batch alumina extraction followed by liquid chromatography with electrochemical detection, as described previously [4, 11].
The amount of radioactivity in the interventricular septal myocardium was measured by positron emission tomography at 8 min after initiation of 18F-dopamine administration (by which time the sympathetic noradrenergic neurons are loaded), and the mono-exponential slope (k) of decline in radioactivity between 8 and 25 min was calculated.
Data analysis and statistics
Whereas 1.0 mCi of 18F-dopamine was administered for PET scanning, the total amount of F-dopamine depended on the specific activity of the tracer. In order to obtain data that could be compared across subjects, the plasma 6F-DOPAC level in each subject was adjusted for the dose per kg of body mass. Since by definition the specific activity of plasma 18F-DOPAC was the same as that of 18F-dopamine, we made this adjustment by converting the assayed 6F-DOPAC concentrations (in units of pg/mL) to concentrations in units of nCi/cc adjusted for the dose per kg, or nCi-kg/cc-mCi. Plasma DHPG levels were expressed in units of nmol/L.
Mean values for peak plasma F-DOPAC and peak F-DOPAC:DHPG were compared across groups by factorial analysis of variance, with post hoc group comparisons using the Fisher’s Protected Least Significant Difference test. The graphics and statistical package was Kaleida-Graph 4.01 (Synergy Software, Reading, PA). Mean values in the two main subject groups (e.g., PD + OH vs. MSA-P) were compared using independent means t tests. Pearson correlation coefficients were calculated for scatter plots (e.g., peak F-DOPAC:DHPG vs. k). For frequency comparisons between groups, χ2 was calculated.
For each subject, myocardial radioactivity at 8 min was used as a measure of cardiac neuronal uptake. As a measure of loss of intra-neuronal catecholamine, we used the slope of the mono-exponential line of best fit for the relationship of the log of radioactivity with time during the 8–25 min period. In a small number of subjects in whom radioactivity did not decrease during this interval, the data about slope were excluded.
Receiver operating characteristic (ROC) curves were generated for (A) peak arterial plasma F-DOPAC and (B) peak arterial F-DOPAC:DHPG, to distinguish PD + OH from MSA-P. For (A), data points corresponded to peak F-DOPAC levels of <100, 100–199, 200–299, 300–399, 400–499, 500–599, 600–699, 700–799, 800–899, 900–999, and >1000 nCi-kg/cc-mCi. For (B), data points corresponded to F-DOPAC:DHPG ratios of <25, 25–49, 50–74, 75–99, 100–149, 150–199, 200–299, 300–399, and >400 (nCi-kg/cc-mCI)/nmol/L.
All clinical, neuroimaging, and neurochemical mean values were expressed ±1 SEM.
Results
Clinical characteristics of the subject groups are summarized in Table 1.
Table 1.
Clinical characteristics of the compared groups
| Variable | PD + OH | PD No OH | MSA-C | MSA-P | PAF | Controls |
|---|---|---|---|---|---|---|
| Age (years) | 69 ± 2 | 59 ± 6 | 55 ± 2 | 63 ± 2 | 57 ± 4 | 51 ± 4 |
| Sex, M/F | 8/4 | 3/4 | 3/2 | 14/7 | 6/5 | 19/7 |
| Race, %Cauc. | 100 | 71 | 100 | 95 | 91 | 81 |
| Age at Onset (years) | 65 ± 2 | 53 ± 6 | 50 ± 4 | 57 ± 2 | 52 ± 3 | |
| Levodopa (%) | 75 | 57 | 0 | 24 | 0 | |
| MAOI (%) | 0 | 14 | 0 | 0 | 0 | |
| Fludrocort. (%) | 25 | 0 | 20 | 19 | 86 | |
| Midodrine (%) | 42 | 0 | 0 | 14 | 43 |
In all subjects, levodopa was discontinued before the PET scanning, while fludrocortisone and midodrine were allowed. Percents are the numbers with positive results divided by the total number of subjects (i.e., if no data then counted as negative)
Mean values expressed ± 1 SEM
%Cauc. percent light-skinned; Fludrocort. fludrocortisone, MAOI monoamine oxidase-B inhibitor; y years
As shown in Fig. 2, arterial plasma F-DOPAC levels increased remarkably rapidly after initiation of 3-min i.v. infusion of F-dopamine. Peak F-DOPAC levels were attained at about 5–10 min and thereafter declined slowly mono-exponentially. At all time points after F-dopamine administration, the PD + OH group had higher mean arterial plasma F-DOPAC levels than did the MSA-P or healthy control groups.
Fig. 2.
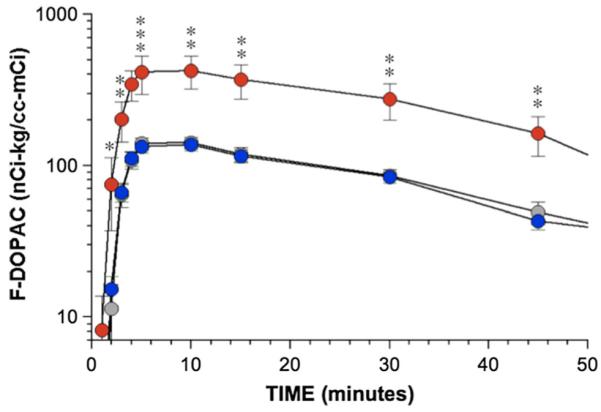
Mean (± SEM) values for arterial plasma F-DOPAC as a function of time from initiation of 3-min F-dopamine injection in Parkinson disease with orthostatic hypotension (PD + OH, red), the parkinsonian form of multiple system atrophy (MSA-P, blue), and normal control subjects (gray). Single asterisks PD + OH different from MSA-P, p < 0.05; double asterisks PD + OH different from MSA-P, p < 0.01; triple asterisks PD + OH different from MSA-P, p < 0.001
Table 2 and Fig. 3 summarize peak arterial plasma F-DOPAC levels in PD + OH, MSA-P, normal control subjects, and other comparison patient groups. The mean value for peak plasma F-DOPAC was highly significantly greater in PD + OH than in MSA-P, normal control subjects, and all the other patient groups.
Table 2.
Results of one-way ANOVA for peak F-DOPAC in PD + OH, MSA-P, control, PAF, PD No OH, and MSA-C groups
| Source | df | SS | MS | F | p |
|---|---|---|---|---|---|
| Total | 81 | 2,893,093 | 35,717 | ||
| Group | 5 | 979,345 | 195,869 | 7.778 | <0.0001 |
| Error | 76 | 1,913,748 | 25,181 |
| Comparison | Mean difference | ∣ t ∣ | p |
|---|---|---|---|
| PD + OH vs. MSA-P | 299.833 | 5.2214 | < 0.0001 |
| PD + OH vs. Control | 298.09 | 5.3827 | < 0.0001 |
| PD + OH vs. PAF | 308.894 | 4.6633 | < 0.0001 |
| PD + OH vs. PD No OH | 348.881 | 4.6228 | < 0.0001 |
| PD + OH vs. MSA-C | 316.167 | 3.7431 | 0.0004 |
| Control vs. PD No OH | 50.7912 | 0.7517 | 0.4546 |
| MSA-P vs. PD No OH | 49.0476 | 0.7082 | 0.481 |
| PAF vs. PD No OH | 39.987 | 0.5212 | 0.6038 |
| MSA-C vs. PD No OH | 32.7143 | 0.3521 | 0.7258 |
| Control vs. MSA-C | 18.0769 | 0.2333 | 0.8162 |
| MSA-P vs. MSA-C | 16.3333 | 0.2068 | 0.8367 |
| Control vs. PAF | 10.8042 | 0.1893 | 0.8504 |
| MSA-P vs. PAF | 9.06061 | 0.1534 | 0.8785 |
| PAF vs. MSA-C | 7.27273 | 0.085 | 0.9325 |
| Control vs. MSA-P | 1.74359 | 0.0375 | 0.9702 |
Group comparisons used Fisher’s Least Significant Difference method
Fig. 3.
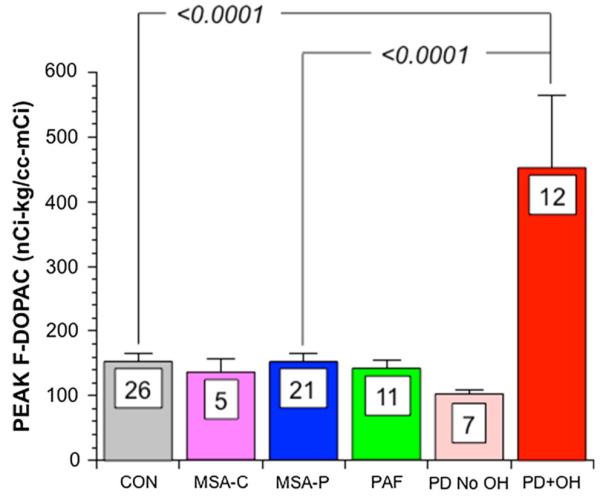
Mean (± SEM) values for peak arterial plasma F-DOPAC as a function of subject groups (PD + OH, red; MSA-P, blue; normal controls (CON, gray), cerebellar MSA (MSA-C, purple; pure autonomic failure (PAF, green); PD without OH (PD No OH, pink). Values for p are for post hoc comparisons by Fisher’s PLSD method after factorial analysis of variance. Numbers are numbers of subjects in each group
Individual values for peak F-DOPAC among PD + OH and MSA-P patients and control subjects were positively correlated with values for k (the slope of mono-exponential decline in septal myocardial 18F-dopamine-derived radioactivity 8′–25′ after initiation of 18F-dopamine infusion) as seen in both Fig. 4 and Table 2.
Fig. 4.
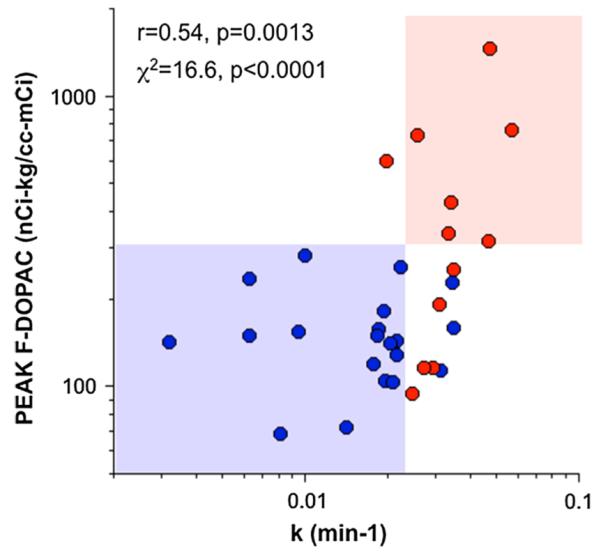
Individual values for peak arterial plasma F-DOPAC as a function of k, the slope of mono-exponential decline of 18F-dopamine-derived radioactivity, in PD + OH (red) and MSA-P (blue). Correlation coefficient (r) and statistical significance are across all displayed data points. χ2 value and associated p value are for the data in the shaded boxes. Note complete separation of PD + OH (pink box) from MSA-P (light blue box)
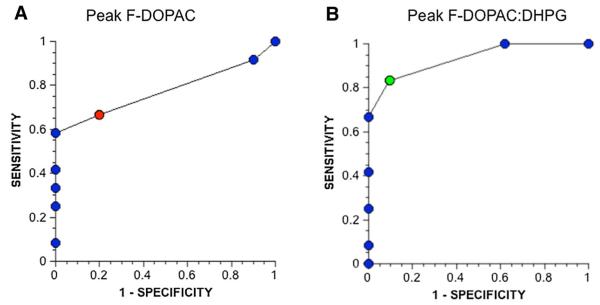
A receiver operating characteristic (ROC) curve for peak F-DOPAC to distinguish PD + OH from MSA-P indicated a sensitivity of 67 % at a specificity of 80 %, when peak F-DOPAC was between 200 and 299 nCi-kg/cc-mCi (red circle in Fig. 5a). Analogously, the ROC curve for peak F-DOPAC:DHPG indicated a sensitivity of 83 % at a specificity of 89 % when peak F-DOPAC:DHPG was between 50 and 74 (nCi-kg/cc-mCi)/nmol/L (green circle in Fig. 5b).
Fig. 5.
Receiver operating characteristic curves for a peak arterial plasma F-DOPAC and b peak arterial F-DOPAC:DHPG to distinguish PD + OH from MSA-P. Red circle in a, corresponds to peak F-DOPAC of 20-299 nCi-kg/cc-mCi. Green circle in b corresponds to Peak F-DOPAC:DHPG of 50-74 (nCi-kg/cc-mCi)/nmol/L
Three of the PD + OH patients and 6 of the PAF patients had normal peak F-DOPAC and low DHPG levels. When peak F-DOPAC:DHPG was evaluated as a diagnostic test to distinguish PD + OH from MSA-P, the ROC curve indicated a sensitivity of 83 % at a specificity of 91 % (Fig. 5b).
Discussion
In this study, beginning almost immediately after initiation of a 3-min i.v. infusion of F-dopamine and for at least 60 min thereafter, patients with PD + OH had on average about 3 times higher arterial plasma F-DOPAC levels than did patients with MSA-P or healthy control subjects. Importantly, none of the MSA-P patients had elevated F-DOPAC levels, indicating a high degree of specificity of a positive test result. Plasma F-DOPAC levels therefore seem to provide an efficient plasma biomarker that distinguishes between the two diseases. Based on the known sources of circulating F-DOPAC, this distinction is likely to reflect a vesicular storage defect in sympathetic nerves in PD + OH but not in MSA-P.
Only about 60 % of PD + OH patients had elevated plasma F-DOPAC levels, meaning that measurement of plasma F-DOPAC alone is not a sensitive test for a vesicular storage defect. F-DOPAC is produced within sympathetic nerves after neuronal uptake of F-dopamine via the cell membrane norepinephrine transporter. Denervation or interference with the transporter could have attenuated F-DOPAC production and blunted plasma F-DOPAC responses in some patients. Consistent with this explanation, among the six patients with PD + OH who did not have elevated plasma F-DOPAC, three had markedly decreased plasma levels of DHPG, a neurochemical biomarker of sympathetic noradrenergic denervation [8]. Also consistent with this explanation, the majority of patients with PAF, which involves more severe or widespread noradrenergic denervation than does PD + OH, did not have elevated F-DOPAC but did have low DHPG levels, suggesting offsetting effects of denervation and decreased vesicular storage on plasma F-DOPAC levels. Thus, a ROC curve based on F-DOPAC:DHPG indicated higher sensitivity in distinguishing PD + OH from MSA-P, without reducing specificity.
Nevertheless, three of the PD + OH patients had both normal F-DOPAC and normal DHPG levels, so that even F-DOPAC:DHPG does not provide a highly sensitive differential diagnostic test for PD + OH versus MSA-P. Further research is called for to identify a more sensitive plasma biomarker to screen for a vesicular storage defect. Although, theoretically, concurrent measurement of F-DOPAC and F-DHPG would provide such a biomarker, we have not detected either F-DHPG or its O-methylated metabolite F-methoxyhydroxyphenylglycol at any time point after F-dopamine administration (unpublished observations).
Across individual PD + OH and MSA-P patients and controls there was a positive correlation between peak F-DOPAC and the rate constant (k) for the mono-exponential decline of interventricular septal 18F-dopamine-derived radioactivity. The positive correlation suggests that elevated plasma F-DOPAC indicates a vesicular storage defect.
It is important to note that the approach presented here for differentiating PD + OH from MSA-P does not require synthesis or injection of 18F-dopamine, exposure to radioactivity, or positron emission tomography. We envision that this testing could be done at any of a variety of collaborating centers, after due consideration of the limitations discussed in the next section.
We previously reported evidence for a vesicular storage defect in both PAF and PD + OH [6]. In the present study, why did PAF patients not also have high F-DOPAC levels? A potential answer is that sympathetic noradrenergic denervation may be more severe or more generalized in PAF than in PD + OH. If so, then in PAF, decreased F-DOPAC production due to decreased neuronal uptake of 6F-dopamine could offset increased F-DOPAC production due to a vesicular storage defect. Our previously reported finding of lower plasma DHPG in PAF than in PD + OH fits with this explanation [8].
Study limitations
Medications, individual differences in catechol metabolism, and heterogeneity among organs in the extent of noradrenergic denervation might influence circulating F-DOPAC levels for a given amount of decrease in vesicular storage. In this study, all patients were off levodopa, carbidopa, and entacapone and were not taking medications known to affect neuronal uptake or intra-neuronal metabolism of catecholamines. One may question the practicality of this approach in a more typical clinical setting.
F-DOPAC produced from F-dopamine in non-neuronal cells is converted efficiently to F-homovanillic acid by catechol-O-methyltransferase, so that low catechol-O-methyltransferase activity might build up plasma F-DOPAC; however, we have not detected F-homovanillic acid in plasma at any time point after F-dopamine administration (unpublished observations), so that this does not seem to be an important limitation.
In conclusion, in the differential diagnosis of PD + OH vs. MSA-P, the finding of elevated plasma F-DOPAC and elevated F-DOPAC:DHPG effectively rules out MSA-P.
Acknowledgments
Authors acknowledge the support of the intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke.
Abbreviations
- 6F-DOPAC
6-Fluorodihydroxyphenylacetic acid
- DHPG
3,4-Dihydroxyphenylglycol
- MAO
Monoamine oxidase
- OH
Orthostatic hypotension
- MSA
Multiple system atrophy
- PAF
Pure autonomic failure
- PD
Parkinson disease
- ROC
Receiver operating characteristic
- VMAT2
Type 2 vesicular monoamine transporter
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
Contributor Information
David S. Goldstein, Clinical Neurocardiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health (NINDS, NIH), 10 Center Drive MSC-1620, Building 10 Room 5N220, Bethesda, MD 20892-1620, USA
Irwin J. Kopin, Clinical Neurocardiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health (NINDS, NIH), 10 Center Drive MSC-1620, Building 10 Room 5N220, Bethesda, MD 20892-1620, USA
Yehonatan Sharabi, Chaim Sheba Medical Center, Tel Aviv University, Tel-Hashomer, Israel.
Courtney Holmes, Clinical Neurocardiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health (NINDS, NIH), 10 Center Drive MSC-1620, Building 10 Room 5N220, Bethesda, MD 20892-1620, USA.
References
- 1.Goldstein DS, Eisenhofer G, Dunn BB, Armando I, Lenders J, Grossman E, Holmes C, Kirk KL, Bacharach S, Adams R, et al. Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18F]fluorodopamine: initial findings in humans. J Am Coll Cardiol. 1993;22:1961–1971. doi: 10.1016/0735-1097(93)90786-z. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DS, Grossman E, Tamrat M, Chang PC, Eisenhofer G, Bacher J, Kirk KL, Bacharach S, Kopin IJ. Positron emission imaging of cardiac sympathetic innervation and function using 18F-6-fluorodopamine: effects of chemical sympathectomy by 6-hydroxydopamine. J Hypertens. 1991;9:417–423. doi: 10.1097/00004872-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DS, Holmes C. Metabolic fate of the sympathoneural imaging agent 6-[18F]fluorodopamine in humans. Clin Exp Hypertens. 1997;19:155–161. doi: 10.3109/10641969709080812. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, Imrich R, Conant S, Eldadah BA. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord. 2008;14:600–607. doi: 10.1016/j.parkreldis.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein DS, Holmes C, Kopin IJ, Sharabi Y. Intra-neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Investig. 2011;121:3320–3330. doi: 10.1172/JCI45803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology. 2003;60:1327–1332. doi: 10.1212/01.wnl.0000058766.46428.f3. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem. 2013;126:591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res. 2000;10:285–291. doi: 10.1007/BF02281111. [DOI] [PubMed] [Google Scholar]
- 11.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 13.Orimo S, Suzuki M, Inaba A, Mizusawa H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2012;18:494–500. doi: 10.1016/j.parkreldis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler MG, Lake CR, Kopin IJ. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med. 1977;296:293–297. doi: 10.1056/NEJM197702102960601. [DOI] [PubMed] [Google Scholar]