Abstract
This randomized controlled crossover-trial compared cycling versus continuous programming in women with OAB treated with sacral neuromodulation. At six months, treatment order significantly affected OABq-SF symptom scores; the cycling followed by continuous stimulation group had superior OABq-SF scores (p=.02).
Keywords: sacral neuromodulation, programming, OAB-q SF, OAB (overactive bladder)
Introduction
In 1997, the FDA approved the sacral nerve stimulator as a treatment for refractory urgency urinary incontinence (Burks et al., 2008). Sacral Neuromodulation works via stimulation of the sacral nerve roots, S-2, S-3 and S-4. Although the exact mechanism of action is unknown, it is believed that sacral neuromodulation stimulates S2–S4 afferent nerves originating from the pelvic organs to the spinal cord. This can activate inhibitory reflexes that promote bladder storage that ultimately restricts involuntary detrusor contractions. (Noblett et al., 2013) While implanted electronic stimulators have increasingly been used in the last twenty years, little investigation has focused on stimulator programming. The purpose of this research was to evaluate the effect of cycling versus continuous neuromodulator programming on overactive bladder symptoms by comparing cycling to continuous neuromodulator stimulation for women with refractory overactive bladder symptoms.
Overactive bladder (OAB) is defined by the International Continence Society (ICS) as urgency, with or without urgency incontinence, usually accompanied by frequency and nocturia in the absence of urinary tract infection or other obvious pathology (Haylen et al., 2009). OAB prevalence is approximately 15% in the general population, and among those with OAB, 11% have urgency urinary incontinence, or leakage of urine with urge symptoms (Hartmann et al., 2009). First and second line treatments include medications, behavioral and physical therapy (Gormley et. al 2012). Women with overactive bladder who are refractory to first line treatments are eligible for a trial placement of a neuromodulator. The modulators are placed in two stages; in stage one, a temporary lead with four electrodes is placed in the 3rd sacral foramen. During placement, the electrodes are tested for stimulation of the “bellows” reflex as well as the “downward toe” reflex. Ideally, patients exhibit both reflexes for all 4 electrodes. In addition, the lead is tested to make sure that patients do not have pain with stimulation. Finally, impedance between electrodes is checked to confirm whether or not all electrode circuits are working. The patient will generally return to clinic one week following stage 1 for a post-op evaluation. If the patient reports a 50% improvement in urinary symptoms as measured on a 3-day voiding diary, the modulator is permanently implanted. This is stage 2. After placement, the modulator is programmed in the recovery room with the goal being a sensation in the perineum that is comfortable. Each program differs in electrode combination, pulse width, amplitude and rate as well as direction of electrical current. Finally, patients can either have their modulator set on a cycling or continuous mode which controls whether or not the stimulation is provided continuously or intermittently. Typically, cycling means that the neuromodulator stimulation is on for 16 seconds and off for 8 seconds. Continuous means the patient receives constant stimulation. Currently, decisions regarding programming are made in an ad hoc manner by whoever is assessing the patient and are not data driven.
Literature Review
Literature investigating neuromodulator programming is limited. We performed a literature search in Cochrane, PubMed MEDLINE, Web of Science and CINAHL with the following key words, “sacral neuromodulation”, “programming”, “overactive bladder” that identified 40 articles. After review of the article abstracts, two papers were selected as relevant to programming of neuromodulators. A single retrospective case review described parameters for programming of the modulator for treatment of OAB (Bin Mahfooz et al., 2004). The study described 67 patients who had good response to neuromodulation. The average starting amplitude was 1.462 volts, the average pulse width was 204.090 usec, and average rate was 9.018 pulse/second. The investigators found that increasing amplitude was required in the long-term management of modulator patients. In this study, the majority of women were on the cycling mode (91%) and it was noted that the cycling mode was beneficial in maintaining the patient’s sense of awareness of the pelvic floor. In the second paper, Rittenmeyer (2008) states, “The ultimate goal is to manipulate parameters to optimize the neurostimulator (battery) life. This is accomplished by using the cycling mode (20 seconds on, 8 seconds off); keeping the amplitude, rate, and pulse width values as low as effectively possible; avoiding unipolar (case positive) stimulation; and using the minimum number of electrodes for effective therapy”.
Purpose
Given the sparse data in the literature regarding use and efficacy of the different programming modalities, our objective in the current study was to compare OAB improvement as measured by the symptom portion of the Overactive Bladder Questionnaire Short Form (OAB-q SF) in women who received continuous mode versus cycling mode in neuromodulator programming. We hypothesized that women on cycling programs would report better OAB-q SF symptom scores than women on continuous programs. In addition, we sought to compare urinary frequency, leakage and pad counts as recorded on 3 day voiding diaries between women placed on continuous versus cycling stimulation of their neuromodulator. We hypothesized that women on cycling programs would report fewer episodes of urinary frequency, incontinence episodes, and pad usage as measured on 3 day voiding diaries (VD) than women on continuous programming. Finally, we compared women’s global impression of improvement between those placed on cycling versus those on continuous programs, and again hypothesized that those women on cycling mode would have greater improvement on their Patient Global Impression of Improvement (PGI-I) scores.
Methods
Our primary outcome measure was the OAB-q-SF symptom, a validated measure score developed to assess symptom bother among patients with either continent or incontinent overactive bladder. The short form version, the OAB-q SF, was derived to provide a “quick” assessment of symptom bother. It consists of a 6-item symptom bother scale (Coyne, et al., 2002). Our secondary outcome measures were PGI-I and voiding diaries. The PGI-I scale is a patient global impression index that may be used to rate the response of a condition to a therapy. It is a transition scale that uses a single question asking the patient to rate their urinary tract condition now, as compared to how it was prior to treatment on a 7 point scale that ranges from ”Very much better” to “ Very much worse” (Yalcin, et al., 2003). Voiding Diaries (VD) were used in this study because they are one of the most common outcome measure used in studies of urinary incontinence and other forms of lower urinary tract dysfunction. The International Continence Society (ICS) recommends diary duration of two to three days when collecting clinical data (Haylan, et al., 2010). Patients did not need to record volumes or types of fluids ingested in this trial. Pad type was likewise not differentiated.
Study subjects were recruited from the Urogynecology clinic at the University of New Mexico Hospital from November 2012 to October 2014. We conducted a randomized controlled crossover clinical trial after receiving IRB approval (IRB # 12-133). This trial was registered with Clinicaltrials.gov (NCT #02551822). Women who were eligible for neuromodulation surgery and were being scheduled for surgery were approached by study personnel during their clinic visit or by a phone call to determine if they were interested in participating in the programming study. Women were screened as possible study subjects based on the following inclusion criteria; women over 21 years old, not currently pregnant or planning on becoming pregnant, English speaking, and willing as well as mentally and physically able to participate in the study. Women were allowed to participate in other studies as long as there was no change to neuromodulator programming or new therapies or use of OAB medications. After written consent, demographic variables and patient characteristics were collected including; patient age, parity, BMI, ethnicity/race, history of prior incontinence surgery, history of prior prolapse surgery, history of hysterectomy, and current or past use of anti-cholinergic medications. Women taking anticholinergic therapy, performing pelvic floor exercises, or pursuing other therapies for OAB were not required to stop therapies, however, for the six month duration of the study, women were asked not to start any new therapies. Women completed the OAB-q SF symptom questionnaire, a 3-day voiding diary, which included average urgency urinary incontinence episodes and voids per day, as well as pad usage.
Randomization occurred after successful Stage 2 implantation, when the modulator was being programmed in the recovery room. Randomization was performed using a random numbers table based on permutated blocks of four, to ensure that equal numbers of women were assigned to each group. Assignments were placed in sealed opaque envelopes by study personnel otherwise un-involved in patient interactions and were opened in sequential order once women underwent successful Stage 2 implantation. Women assigned to cycling for the first three months followed by continuous stimulation the second three months were assigned to Group A; Group B participants were first randomized to continuous and then to cycling stimulation. Patients were blinded to their randomization assignment as were study personnel who were assessing the primary and secondary outcome measures. Between one and two weeks, patients returned to clinic for a routine post-operative wound check. Their neuromodulator was checked to assure that it was on. Patients were strongly encouraged not to make program changes on their own and were asked to return to the clinic if they felt they needed program changes. Patients were given 3-day voiding diaries to fill out prior to their three month visit. At three months, women reported for their second postoperative visit. At this visit, women turned in their voiding diaries, completed the OAB-q SF symptoms form as well as the PGI-I and were queried regarding any complications since the start of the use of their modulator. At this time, women who had been assigned to continuous stimulation were switched to cycling stimulation and women who had been assigned to cycling stimulation were switched to continuous stimulation. Women were asked to follow-up for a six months post-op check with a completed 3-day voiding diary. At this clinic visit, women once again turned in their voiding diaries and were asked to complete the OAB-q SF and PGI-I and a complications form. Study subjects were compensated for their time with twenty-five dollars at the three-month and six-month clinic follow up appointments.
Statistical Methods
Descriptive statistics were used to describe patient characteristics. The analysis of the change in OAB-q SF scores in this 2 group, 2 period (2×2) crossover design was a repeated measure (RM) ANOVA with the treatments (cycling versus continuous) as a grouping factors and the two periods (3 and 6 months) as a repeated factor. The cross over effect was accounted for in the analysis by an order variable which was equal to 1 if cycling was first and equal to 0 if continuous was first.
Significance was set at p = 0.05.
Power analysis
In our cross over randomized controlled trial we assumed that a clinically important difference between changes in OAB-q SF scores between women on cycling versus continuous stimulation, with a standard deviation of the paired differences of 15 points between groups with an alpha error of 0.05 and power of 80%. Twenty women were needed to be randomized into equal sized groups. We assumed that 15% of women will be lost to follow-up. Therefore, we recruited 23 women to the study.
Results
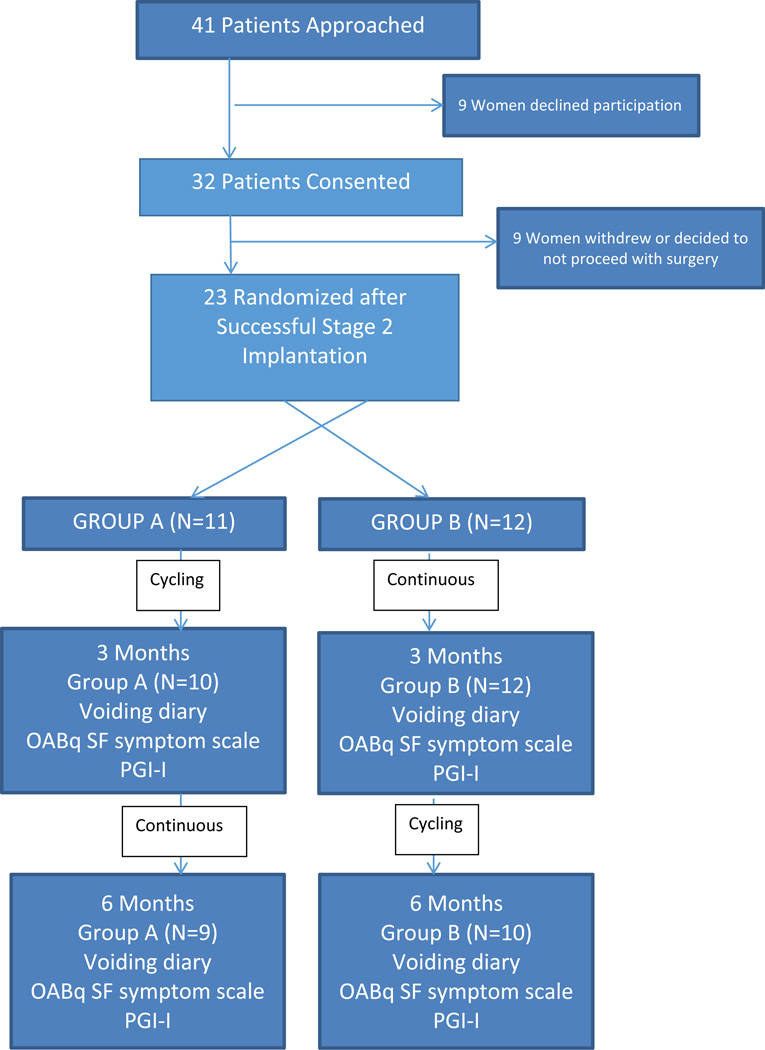
Forty one women were approached to participate in the study (Figure 1). Thirty two women were enrolled; 9 of whom withdrew because they either did not have a successful stage 2 implantation or elected to withdraw from the trial. By chance, the women who enrolled but were not randomized were all White, but did not differ in other patient characteristics (Table 1). Twenty-three women were randomized into Group A (cycling mode first 3 months) or Group B (continuous mode first three months). Our study population was made up of Hispanics, Whites, Blacks and American Indian women all of whom were in their mid-60’s, were overweight and parous. The majority of women had undergone prior anti-incontinence procedures and or prolapse repair. Women in Group B were less likely to have had a prior prolapse procedure. Seventy five percent of both Group A and B had comorbid conditions including diabetes, hypertension, and a history of coronary artery disease or stroke. Other characteristics between groups were not different. (Table1)
Figure 1.
Consort Diagram
Table1.
Patient Characteristics by Randomization Group
| Characteristic | Group A* (N=11) |
Group B** (N=12) |
P value |
Randomized (N=23) |
Not-randomized (N=9) |
P |
|---|---|---|---|---|---|---|
| Age (Mean +/− SD) | 69.8 +/− 10.4 | 63.4 +/− 13.1 | .21 | 66.5 +/−12.0 | 65.0 +/− 12.9 | .77 |
| BMI # (Kg/m2) | 29.9 +/− 10.2 | 34.3 +/− 19.2 | .49 | 32.2 +/− 15.4 | 35.6 +/− 9.5 | .46 |
| Parity | 4.2 +/− 1.2 | 3.0 +/− 2.1 | .11 | 3.6 +/− 1.8 | 2.3 +/− 1.2 | .03 |
| Race (%) | .07 | .007 | ||||
| Hispanic Latina | 5(45) | 5(42) | 10(43) | 0 | ||
| White | 6(55) | 2(17) | 8(35) | 9(100) | ||
| Black | 0 | 3(25) | 3(13) | 0 | ||
| American Indian | 0 | 2(16) | 2(9) | 0 | ||
| Menopausal | 11(100) | 10(83) | .48 | 21(91) | 7(78) | .56 |
| Hormone Replacement Therapy (%) | .86 | .22 | ||||
| None | 5(45) | 3(30) | 8(38) | 5(71) | ||
| Patch or pill | 2(18) | 3(30) | 5(24) | 0 | ||
| Vaginal estrogen | 4(36) | 4(40) | 8(38) | 2(28) | ||
| Prior Urinary incontinence procedures (%) |
6(55) | 5(42) | .68 | 11(48) | 3(33) | .69 |
| Prior prolapse procedures (%) | 7(64) | 2(17) | .04 | 9(39) | 3(33) | 1.0 |
| Hysterectomy (%) | 9(82) | 7(58) | .37 | 16(69) | 7(78) | 1.0 |
| Comorbid conditions (Diabetes, Hypertension, Coronary artery disease, stroke, Multiple sclerosis) (%) |
8(73) | 9(75) | 1.0 | 17(74) | 7(78) | 1.0 |
| Smoking (%) | 2(18) | 1(8) | .59 | 3(13) | 0 | .54 |
| Alcohol (%) | 4(36) | 3(25) | .67 | 7(30) | 3(33) | 1.0 |
| Baseline OAB-q SF*** Symptom score |
68.3 +/−22.3 | 66.3 +/−27.0 | .84 | |||
| Average Baseline number of leaks per day on 3 day voiding diary (Mean +/−SD) |
6.9 +/− 3.4 | 8.1 +/− 6.5 | .59 | |||
| Average Baseline number of voids per day on a 3 day voiding diary (Mean +/− SD) |
12.8 +/− 6.6 | 15.1 +/− 9.0 | .49 | |||
| Average Baseline number of Pads used per day over 3 days (Mean +/− SD) |
5.3 +/− 3.6 | 6.9 +/− 7.04 | .55 | |||
Group A: Cycling followed by continuous stimulation
Group B: Continuous followed by cycling stimulation
OAB-qSF- Overactive bladder questionnaire short form
BMI Body Mass Index
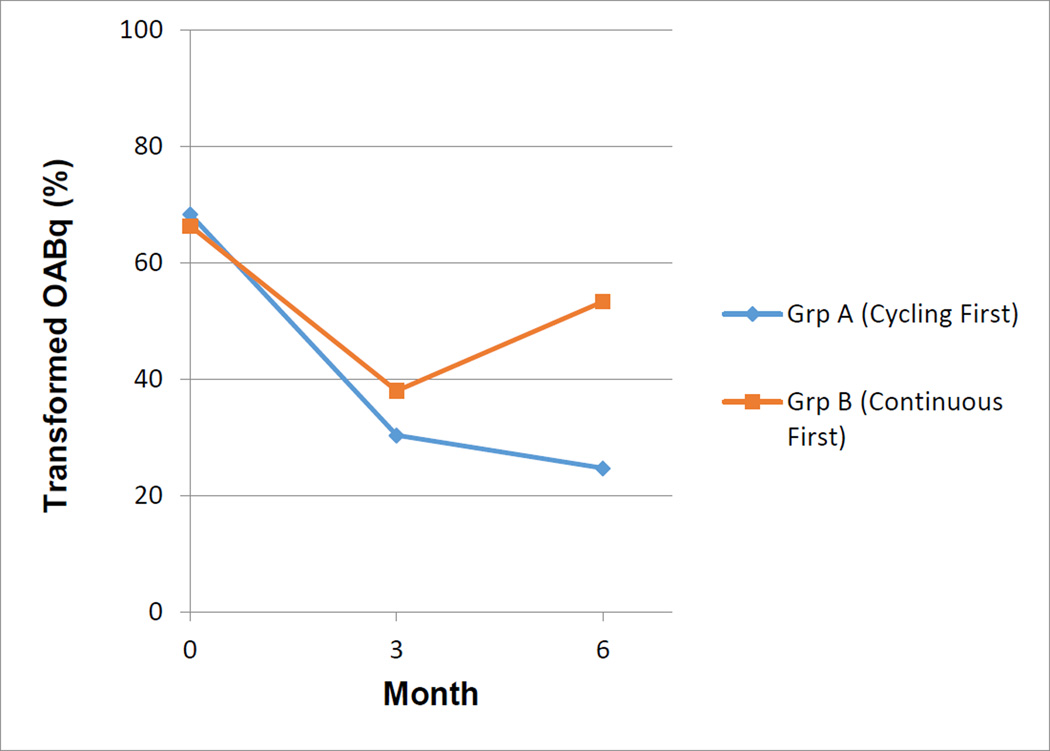
At baseline, no differences were found in OABqSF Symptoms score between groups [68.3 +/−22.3 (Group A) 66.3 +/−27.0 (Group B)]. Our results showed that at 3 months 22/23 (96%) of women followed up; at 6 months 19/23(82%) of women followed up. Using a mixed analysis of variance model with order of treatment (Group A versus Group B) and the treatments as grouping variables, and time as a repeated factor, we found that the order of treatments significantly affected our primary outcome measure, the OABq-SF symptom scores. (Figure 2) At three months, OABq-SF Symptoms Scores improvement did not differ between groups [30.3 +/−20.7 (Group A) 38.0 +/−22.0 (Group B), P=0.41]. At 6 months, Group A had superior improvement in the OABq-SF symptom scores compared to Group B (p=.02). Our secondary outcomes evaluated 3-day voiding diaries which measured number of leaks, voids, and pads per day. PGI-I scores were also calculated. Neither group demonstrated the order effect (all P>0.05). (Table 2) In conclusion, the order effect, cycling followed by continuous mode stimulation resulted in greater OABq-SF Symptom improvement than continuous followed by cycling mode stimulation. Importantly, all subjects perceived that they were at least “much better” on the PGI-I score (2.5 +/− 1.5 (Group A) 2.7 +/−1.7 (Group B), P=0.85) regardless of group assignment.
Figure 2.
Comparison of groups at baseline, 3 and 6 months on the symptom portion of the Overactive Bladder Questionnaire Short Form (OAB-qSF)
Table 2.
Comparison of the symptom portion of the Overactive Bladder Questionnaire Short Form (OAB-qSF), Patient Global Impression of Improvement (PGI-I) and voiding diary outcomes between groups at 3 and 6 months
| Group A (N=10) | Group B (N=12) | P | |
|---|---|---|---|
| 3 Months | |||
| OAB-qSF* symptom scores (Means +/− SD) |
30.3 +/−20.7 | 38.0 +/−22.0 | .41 |
| PGI – I** scores (Means +/− SD) | 2.6 +/− 1.3 | 2.6 +/− .79 | .97 |
| Number of leaks per day on 3 day voiding diary (Mean +/−SD) |
5.5 +/− 5.2 | 5.3 +/− 3.8 | .88 |
| Number of voids per day on a 3 day voiding diary (Mean +/− SD) |
13.6 +/− 7.3 | 8.7 +/− 1.9 | .049 |
| Number of pads used per day over 3 days (Mean +/−SD) |
3.5 +/− 4.1 | 2.4 +/− 1.9 | .46 |
| 6 Months | |||
| Group A (N=9) | Group B (N=10) | ||
| OAB-qSF* symptom scores (Mean +/− SD) |
24.7 +/−20.7 | 53.3 +/−27.0 | .02 |
| PGI – I** scores (Mean +/− SD) | 2.5 +/− 1.5 | 2.7 +/− 1.7 | .85 |
| Number of leaks per day on 3 day voiding diary (Mean +/−SD) |
6.3 +/− 4.4 | 5.6 +/− 4.8 | .74 |
| Number of voids per day on a 3 day voiding diary (Mean +/− SD) |
10.4 +/− 3.7 | 8.9 +/− 2.0 | .27 |
| Number of pads used per day over 3 days (Mean +/−SD) |
3.7 +/− 3.4 | 3.4 +/− 2.6 | .80 |
OAB-qSF- Overactive bladder questionnaire short form
PGI-I - Patient Global impression of improvement
Adverse event data were collected at 3 and 6 months and were largely unrelated to study intervention. These included; recent falls, surgeries (cataract operations, hemorrhoidectomy) received post neuromodulator, urinary tract infections, antibiotic use, and changes in bowel movements. No differences were found between Group A and Group B. Although there was no statistical significance in falls between the groups, we found the number of falls (4/22, or 18%, in the first 3 months and 9/21, or 42%, in the second 3 months) to be high. We felt these events, as well as one AED unrelated to the study protocol, were most likely reflective of the high number (75%) of women with co-morbid conditions in our population. In regard to bowel changes, this question was added with IRB approval due to the FDA’s approval (April, 2011) of neuromodulator use for the treatment of fecal incontinence. Twenty percent of subjects in Group A reported bowel changes at 3 and 6 months. In Group B, 33 % noted bowel changes at 3 months and 36% at 6 months. We did not have baseline data to compare to the collected data and therefore was not part of the analysis for this study.
Study Strengths and Limitations
The strengths of our study were its crossover randomized design, the fact that it was double blinded (subjects and research personnel collecting data) and high patient compliance with program mode and follow-up. Some limitations were noted in our study. While our study population included diverse ethnic backgrounds, it was limited due to small sample size. Also, the average age of our population was 67 and may under-represent younger women receiving neuromodulation for overactive bladder.
Discussion and Nursing Implications
This study was initiated as a result of conflicting advice and opinions from neuromodulator representatives and industry experts regarding cycling and continuous modes in programming. We were unable to find evidence in the literature regarding cycling and continuous modes and how they affect patient satisfaction with regard to overactive bladder symptoms and whether or not one mode was superior in the treatment of OAB. It was our goal to find evidence that could support guidelines in the programming of neuromodulators in patients with OAB that could be applied in the clinical setting. We set out to determine if the cycling mode of neuromodulator programming resulted in superior OAB-q SF symptom scores improvement, fewer episodes of urinary frequency, incontinence episodes and pad usage measured on a 3 day voiding diary, and greater improvement on PGI-I scores versus the continuous mode.
In our 2 group, 2 period crossover study, we found that the order of treatments, cycling mode followed by continuous mode significantly affected the OAB-q SF symptom scores improvement. It is unclear why this happens. We do not know if patients become refractory to the cycling mode and then have improved results with constant stimulation or whether the continuous mode is more reflective of normal stimulation. We also do not know if patients need to be switched from the cycling mode to continuous in the absence of worsening symptoms. This evidence however, suggests that when patients are initially being programmed they should be started on the cycling mode and then switched to the continuous mode. Also, while it is important to be mindful of battery life, symptom improvement must be the driving force when programming.
We question the practical use of voiding diaries as an objective measure. While 3 day voiding diaries are a standard measure in Urology research, they proved quite onerous and cumbersome for our study population, especially for those who had severe refractory OAB. All of our patients perceived improvement despite lesser improvement in objective measures. It is our belief that women are not concerned about how many times they leak or how often they run to the bathroom or even what is causing them to leak. The impact on the quality of their lives and the simple fact that they are wet and do not have control of their leaking is what causes them to seek treatment. Perhaps subjective scores, such as the OABq-SF symptoms scores and the PGI-I, reflect patient centered outcomes more accurately and should therefore be used more frequently to measure patients’ perceptions of outcome of treatment. At the very least, more research needs to be done in finding a less challenging tool to measure a patients voiding history.
We did find that a large number of our population reported falls following implantation. We do not feel that the falls were caused by the implant, but rather are reflective of the fragile health of many women with refractory urgency incontinence. Nonetheless, falls can affect the functioning of an implanted modulator, and this common adverse event should be taken into consideration when implantation is considered.
The use of neuromodulators in the treatment of a wide variety of diseases is expanding. In order for nurses and practitioners to provide the best treatment outcomes for patients, further research and evidence are needed to manage the programming of these neuromodulators. This study is only a first step in guiding practitioners when programming neuromodulators for OAB.
Conclusion
The primary objective of this study was to compare OAB improvement as measured by the symptoms portion of the Overactive Bladder Questionnaire Short Form (OAB-q SF) in women who received continuous versus cycling mode in neuromodulator programming. Using a mixed analysis of variance model with order of treatment as a grouping variable, and treatment group as a repeated factor, we found that the order of treatments significantly affected our primary outcome measure, the OABq-SF symptom scores. The cycling mode followed by the continuous mode resulted in greater OABq-SF Symptom improvement than continuous followed by cycling mode stimulation. Importantly, all subjects perceived that they were at least “much better” on the PGI-I score regardless of group assignment.
Supplementary Material
Table 3.
Difference in the symptom portion of the Overactive Bladder Questionnaire Short Form (OAB-qSF) and voiding diary outcomes within groups between baseline to 3 months and baseline to 6 months
| Baseline |
Change to 3 Months |
P |
Change to 6 months |
P | |
| OABq – SF* Symptom Scores | |||||
| (N=11) Group A (cycling) |
68.3 +/−22.3 | (N=10) −35.0 +/−23.0 |
.001 | (N=9) −40.3 +/−26.3 |
.002 |
| (N=12) Group B (continuous) |
66.3 +/−27.0 | (N=12) −28.3 +/−29.7 |
.007 | (N=10) −8.7 +/−15.7 |
.12 |
| Number of leaks per day on 3 day voiding diary (Mean +/−SD) | |||||
| (N=10) Group A (cycling) |
6.9 +/− 3.4 | −1.3 +/− 4.5 | .35 | −.35 +/− 5.7 | .85 |
| (N=12) Group B (continuous) |
8.1 +/− 6.5 | −2.9 +/− 7.4 | .22 | −2.5 +/− 6.3 | .22 |
| Number of voids per day on a 3 day voiding diary (Mean +/− SD) | |||||
| (N=10) Group A (cycling) |
12.8 +/− 6.6 | +.86 +/− 4.2 | .51 | −2.7 +/− 7.3 | .26 |
| (N=12) Group B (continuous) |
15.1 +/− 9.0 | −6.4 +/− 10.4 | .06 | −4.6 +/− 7.3 | .08 |
| Number of Pads used per day over 3 days (Mean +/−SD) | |||||
| (N=10) Group A (cycling) |
5.3 +/− 3.7 | −1.44 +/− 2.8 | .15 | −1.0 +/− 1.9 | .14 |
| (N=12) Group B (continuous) |
6.9 +/− 6.0 | −3.8 +/− 6.4 | .11 | −3.5 +/− 5.23 | .10 |
| 3 Months Post Implant | |||||
| Complications | Group A (N=10) | Group B (N=12) | P value | ||
| Recent Falls (%) | 1(10) | 3(25) | .59 | ||
| Surgery post neuromodulator placement (%) |
2(20) | 1(8) | .57 | ||
| Current UTI (%) | 0 | 0 | 1.0 | ||
| Current antibiotics (%) | 0 | 0 | 1.0 | ||
| Changes in bowel movements (%) | 2(20) | 4(33) | .65 | ||
| Constipation | 1 | 2 | |||
| Diarrhea | 0 | 1 | |||
| Both | 0 | 1 | |||
| Other | 1 | 0 | |||
| New treatments for OAB (newtreatment_3mo) |
0 | 0 | 1.0 | ||
| Stopped medications for your OAB (stopmeds_3mo) |
0 | 0 | 1.0 | ||
| 6 Months Post Implant | |||||
| Complication | Group A (N=10) | Group B (N=11) | P value | ||
| Recent Falls (%) | 5(50) | 4(36) | .67 | ||
| Surgery post neuromodulator placement (%) |
2(20) | 2(18) | 1.0 | ||
| Current UTI (%) | 1(11) | 2(18) | 1.0 | ||
| Current antibiotics (%) | 1 | 1 | 1.0 | ||
| Changes in bowel movements (%) | 2(20) | 4(36) | .64 | ||
| Constipation | 0 | 0 | |||
| Diarrhea | 0 | 2 | |||
| Both | 0 | 0 | |||
| Other | 2 | 2 | |||
| New treatments for OAB (newtreatment_3mo) |
3(30) | 1(9) | .31 | ||
| Stopped medications for your OAB (stopmeds_3mo) |
0 | 2(18) | .48 | ||
OAB-qSF- Overactive bladder questionnaire short form
Acknowledgments
We would like to thank Dr. Yuko Komesu and Dr. Rebecca Rogers for their guidance and mentorship during this study. We would also like to acknowledge Dr. Clifford Qualls for his help with the statistical analysis of these data.
Biographies
Ms. Beer has been a nurse specialist in urogynecology for the past 15 years, and has participated in the care of women with all forms of pelvic floor dysfunction. She was responsible for protocol development, recruitment of patients, analysis of data and manuscript writing.
Ms. Gurule has been a nurse specialist in urogynecology for the past 23 years, and has participated in the care of women with all forms of pelvic floor dysfunction. She was responsible for protocol development, recruitment of patients, analysis of data and manuscript writing.
Dr. Komesu is a fellowship trained, board certified urogynecologist with a special interest in overactive bladder treatments. She was responsible for protocol development, recruitment of patients, analysis of data and manuscript writing.
Dr. Qualls was responsible for statistical development and analysis of data.
Dr. Rogers is a fellowship trained, board certified urogynecologist. She was responsible for protocol development, recruitment of patients, analysis of data and manuscript writing.
Contributor Information
Gwendolyn M. Beer, Urogynecology Outpatient Clinic University of New Mexico Hospital, Albuquerque, NM.
Margaret M. Gurule, Outpatient Clinic University of New Mexico Hospital, Albuquerque, NM.
Yuko M. Komesu, Department of Obstetrics and Gynecology, Albuquerque, New Mexico.
Clifford R. Qualls, Faculty, Clinical and Translational Science Center, Office of Research University of New Mexico.
Rebecca G. Rogers, Division Chief of Urogynecology, Vice Chair of Research Department of Obstetrics and Gynecology, Albuquerque, New Mexico.
References
- Burks F, Diokno A, Lajiness M, Ibrahim I, Peters K. Sacral neuromodulation reprogramming: is it an office burden? International Urogynecology Journal. 2008 Feb 27;19:1137–1140. doi: 10.1007/s00192-008-0601-3. [DOI] [PubMed] [Google Scholar]
- Bin Mahfooz A, Elmayergi N, Abdelhady M, Wang Y, Hassouna M. Parameters of successful sacral root neuromodulation of the pelvic floor: a retrospective study. The Canadian Journal of Urology. 2004 Jun;11(3):2303–2308. [PubMed] [Google Scholar]
- Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover H, Kurth H. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: The OAB-q. Quality of Life Research. 2002;11:563–574. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- Gormley E, Lightner D, Burgio K, Chai T, Clemens J, Kas A, Foster H, Jr, Scarpero H, Tessier C, Vasavada S. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. The Journal of Urology. 2012;188:2455–2063. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- Hartmann K, McPeeter M, Biller D. Treatment of overactive bladder in women. Evidence Report/Technology Assessment No. 187. Rocville, MD: Agency For Healthcare Research and Quality; 2009. AHRQ Publication No. 09-E017 PMID: 19947666. [Google Scholar]
- Haylan B, De Ridder D, Freeman R, Swift S, Berghmans J, Monga A, Petri E, Rizk D. An international urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourology and Urodynamics. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- Noblett K, Cadish L. Sacral nerve stimulation for the treatment of refractory voiding and bowel dysfunction. American Journal of Obstetrics and Gynecology. 2014 Feb;:99–106. doi: 10.1016/j.ajog.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Rittenmeyer H. Sacral nerve neuromodulation (Interstim) Part II: Review of programming. Urologic Nursing. 2008 Feb;28(1):21–25. [PubMed] [Google Scholar]
- Yalcin I, Bump R. Validation of two global impression questionnaires for incontinence. American Journal of Obstetrics and Gynecology. 2003;189:98–101. doi: 10.1067/mob.2003.379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.