Abstract
Background
Obesity is a major clinical problem. The number of obese pregnant women is rising rapidly. The consequences of obesity are significant and affect every aspect of perinatal care for both the mother and the developing fetus. Adipose tissue may be responsible for chronic subclinical inflammation in obesity, being a source of inflammatory mediators. The study was designed to evaluate the analysis of the serum concentration of inflammatory mediators, including interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and adiponectin, in obese pregnant women at full-term pregnancies.
Material/Methods
The study included 40 women with body mass index (BMI) less than 30 and 24 pregnant women with BMI equal to or greater than 30, admitted to the Perinatology and Obstetrics Department of the University Hospital in Cracow in the first stage of labor. Blood samples were taken from patients to detect the serum concentration of cytokines. Ultrasound was used to evaluate the development of the fetus, including estimated fetal weight, Doppler flows, and the amount of amniotic fluid. We also included the history of chronic diseases and other complications of the pregnancy. A p-value <0.05 was considered significant.
Results
The level of adiponectin in obese patients as compared to controls was significantly lower. There was no statistically significant difference in either group when TNF-α and IL-6 were measured. The results of the survey are consistent with previous reports.
Conclusions
The exact role of inflammation in pregnancy is not well understood. Determining the exact functions of the different cytokines in physiological pregnancy and pregnancy complicated by obesity requires further study.
MeSH Keywords: Adiponectin, Adipose Tissue, Interleukin-6, Obesity, Pregnancy Complications, Tumor Necrosis Factor-alpha
Background
Obesity is a major clinical problem, affecting a large number of people. Overweight is determined by the World Health Organization as one of the greatest global threats, affecting over 39% of the world population, and up to 13% of people are obese [1,2] Overweight is defined as BMI of 25 to 29.9, and obesity is defined as a BMI of 30 or higher [3].
The number of obese pregnant women is growing rapidly, and excessive weight is one of the most important risk factors for pregnancy complications. The consequences of obesity are significant, affecting every aspect of perinatal care for both the mother and the developing fetus, including the long-term effects. Complications of obesity include fetal macrosomia, gestational hypertension, pre-eclampsia, infections of the genitourinary tract, and premature birth. Obesity may be an independent risk factor for perinatal deaths of women [4]. Most pregnancies of overweight women end with cesarean section, and vaginal births are complicated by shoulder dystocia. In addition, there is a greater loss of blood during the labor and greater risk of infectious complications in the puerperium [5]. It is also noted that women with a BMI >30 kg/m2 are more likely to give birth to children with defects of the nervous system and gastrointestinal tract, heart defects, and umbilical hernia [3]. Moreover, excessive maternal weight is a risk factor for long-term complications in the newborn [6,7].
The main known role of adipose tissue is as energy storage. Nevertheless, it mediates many inflammatory and immune reactions. Adipose tissue is the largest metabolically active endocrine structure in the human body. Adipocytes produce and secrete a number of biologically active substances, including cytokines, enzymes, growth factors, and hormones. These compounds are involved in the regulation of nutrition and maintaining the body’s energy balance, insulin sensitivity, metabolism of carbohydrates and lipids, hemostasis, angiogenesis, blood pressure, and immune and inflammatory processes. Results from clinical studies in recent years indicate that adipocytokines may be responsible for chronic subclinical inflammation in obesity. Adipose tissue can be the source of pro-inflammatory mediators that can directly cause lesions in the vascular endothelium, insulin resistance, and, consequently, the development of atherosclerosis. Such effects may be caused by TNF-α, IL-1, IL-6, CRP, leptin, resistin, PAI-1, and angiotensinogen. Other substances produced by adipocytes, such as nitric oxide and adiponectin, have anti-inflammatory effects and reduce insulin resistance [8].
Normal pregnancy is associated with a highly regulated inflammatory response and there is a balance between the anti-inflammatory and inflammatory factors. The inflammatory response is essential for proper implantation, trophoblast invasion, and the correct functioning of the placenta. In contrast, the post-implantation period is associated with immunosuppression to prevent immune rejection of the fetus. Maternal obesity is associated with disproportion of inflammatory mediators, which ultimately can lead to disorders specific to pregnancies complicated by excessive maternal weight [9].
In this study, we analyzed the level of inflammatory mediators, including IL -6, tumor necrosis factor alpha (TNF-α), and adiponectin, among obese pregnant patients at full-term pregnancies.
Material and Methods
This was an observational study, conducted in a group of 64 adult women in the third trimester of pregnancy during the perinatal period, admitted to the Department of Obstetrics and Perinatology University Hospital in Cracow in the period from August 2013 to June 2014. The inclusion criteria were 37–41 weeks pregnancy and in the first stage of labor. The exclusion criteria were: multiple pregnancy, diseases associated with chronic inflammation, or symptoms of severe infection at admission.
On admission, with patient’s written consent, a blood sample was taken for the assessment of venous blood serum concentrations of biochemical markers IL-6, TNF-α, and adiponectin. The ultrasound examination was performed to evaluate the development of the fetus, including growth profile, evaluation of Doppler flow in the umbilical artery, Doppler flow in the middle cerebral artery with calculation of the cerebroplacental ratio (CPR), Doppler flow in uterine arteries, and the amniotic fluid index. The body mass index (BMI) was calculated. The medical interview provided information on pregnancy complications such as gestational diabetes, hypertension, fetal macrosomy or small for gestational age (SGA), and history of miscarriage. Data, including mode of delivery and neonatal outcomes (fetal birth weight and Apgar score), were statistically analyzed using Statistica v10.0.
We investigated the relations between the inflammatory mediators excreted by adipose tissue and BMI. Linear regression analysis was used to detect the association. We used the t test for average concentration in both groups: BMI less than 30 and BMI equal to or above 30. The p-values below 0.05 were considered to be statistically significant. Multiple regression analysis was used to access the effect of age, week of pregnancy, and previous miscarriages on the level of inflammatory mediators.
The study was approved by our Ethics Committee (No. KBET/81/B/2013).
Results
The study included women at 37–41 weeks of pregnancy (median 39 weeks), ages 19–47 years (median 31 years), who had singleton pregnancies. Obesity (BMI at least 30) was observed in 37% of cases. Median BMI for the group was 28. Approximately 43% of the population were primiparous and 57% were multiparous. Gestational diabetes and hypertension were more common in the obesity group (33.3% vs. 10.3% for diabetes and 16.6% vs. 0% for hypertension), but the group was too small to show statistical significance. In our study, more newborns were macrosomic in the obese group than in the non-obese group (8.7% vs. 2.6%, respectively), but without statistical significance. Over 73% of patients had cesarean section. The most common indications were previous cesarean section (24%) and threatening fetal asphyxia (18%) (Table 1).
Table 1.
The characteristics of the study group.
| Total pregnant women N (%) |
BMI <30 n (%) |
BMI ≥30 n (%) |
|
|---|---|---|---|
| BMI | |||
| <30 | 40 (63) | ||
| ≥30 | 24 (37) | ||
| Parity | |||
| Primipara | 27 (43) | 19 (47.5) | 8 (34.8) |
| Multipara | 36 (57) | 21 (52.5) | 15 (65.2) |
| History of miscarriage (at least one) | 18 (29) | 13 (32.5) | 5 (21.7) |
| Small for gestational age | 8 (13) | 7 (17.5) | 1 (4.2) |
| Macrosomy | 3 (5) | 1 (2.6) | 2 (8.7) |
| Hypertension | |||
| Chronic | 2 (3) | 0 | 2 (8.3) |
| Pregnancy induced | 2 (3) | 0 | 2 (8.3) |
| Gestational diabetes | |||
| Chronic | 3 (5) | 0 | 3 (12.5) |
| Pregnancy induced | 9 (14) | 4 (10.3) | 5 (20.8) |
| Mode of delivery | |||
| Vaginal delivery | 17 (27) | 12 (30) | 5 (23) |
| Cesarean section | 45 (73) | 28 (70) | 17 (77) |
The average level of adiponectin in the obese group was 6211 ng/ml (95% CI 5262–7160 ng/ml) and was significantly lower than the 7782 ng/ml in patients with normal BMI (95% CI 6905–8657 ng/ml) p-value=0.0216) (Figures 1, 2). The average level of TNF-α in the obese patients was 2.15 pg/ml (95% CI 1.94–2.35 pg/ml) and was higher than in patients with normal BMI (2.13 pg/ml (95% CI 1.94-2.32 pg/ml), but the difference was not significant (p-value=0.5465). The average level of IL-6 in the obese patients was 1.37 pg/ml (95% CI 1.64–1.10 pg/ml) and was slightly lower than the 1.52 pg/ml found in patients with normal BMI (95% CI 1.30–1.75 pg/ml), but without statistical significance (p-value=0.3922).
Figure 1.
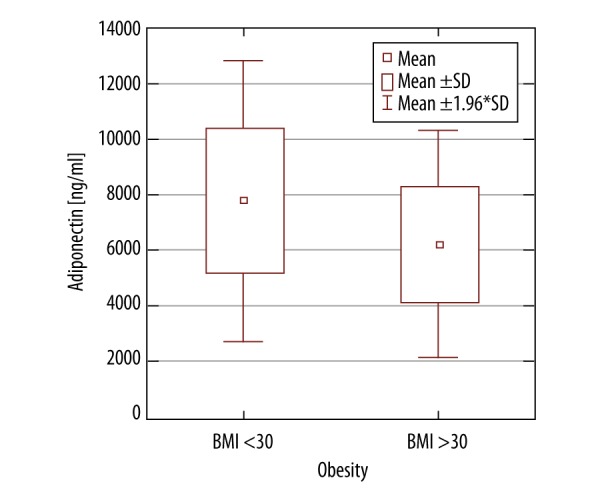
The average level of adiponectin in a group of obese patients versus non-obese patients.
Figure 2.
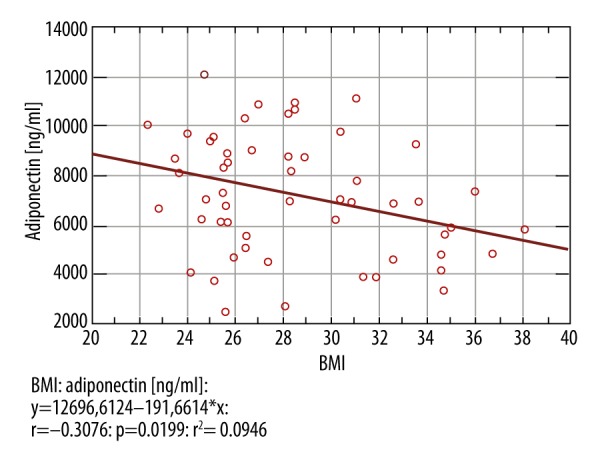
The average level of adiponectin depending on the BMI.
There was a statistically significant negative correlation between patient age and the levels of Interleukin-6 and TNF-α: a decline of 0.053pg/ml (p-value 0.0372) (Figure 3) and the 0.039 pg/ml (p-value 0.0127) (Figure 4), respectively, for each year of life of the patient.
Figure 3.
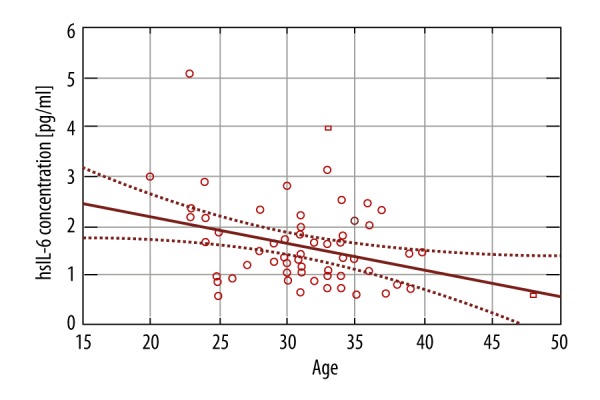
IL-6 concentration and maternal age.
Figure 4.
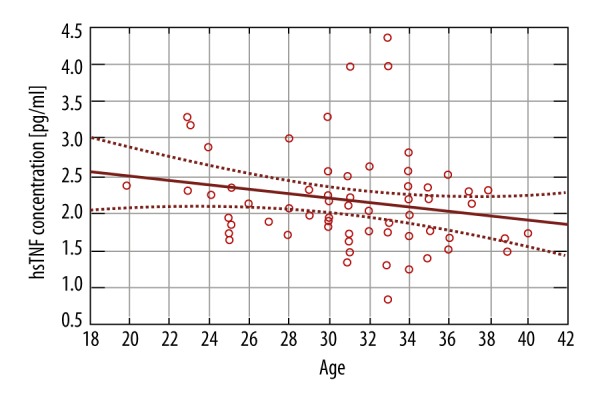
TNF-α concentration and maternal age.
In addition, higher levels of TNF-α occurred in patients who had a miscarriage in the past; the average level of TNF-α was 2.395 (95% CI 1.885–2.9055), while in the group with no history of miscarriage it was 2.0886 (95% CI 1.947–2.229), which is a statistically significant difference (p-value 0.0361).
We found an association between the TNF-α level and the amount of amniotic fluid. When TNF-α increased by 1 pg/ml, the amount of amniotic fluid, measured as amniotic fluid index (AFI), increased by 23 mm (p-value 0.0062). There was no statistically significant relationship between IL-6 (p-vale 0.5991) and adiponectin (p-value 0.9632) (Figure 5). We also found no association between the obese and non-obese groups in serum levels TNF-α, IL-6, adiponectin, and AFI. This suggests that BMI probably does not influence this relationship.
Figure 5.
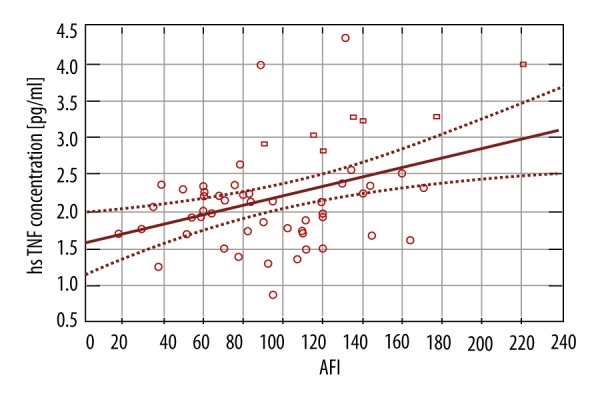
TNF-α concentration and AFI.
Using regression analysis, we found no correlation between the cerebroplacental ratio (the ratio of resistance in the middle cerebral artery of the fetus to the resistance in the umbilical artery of the fetus) and the markers of inflammation.
In our study, the mode of delivery did not differ significantly between the 2 groups. For the whole population, 73% had delivery by cesarean section (77% in obese patients vs. 70% in non-obese patients).
For the neonatal outcomes, there were 2 factor taken into consideration: Apgar score and fetal birth weight. As most of the newborns were delivered in good general condition, there were no statistically significant differences between the 2 groups. We performed regression analysis of the effect of inflammatory markers on fetal birth weight, which showed there was a significant effect of IL-6 level (p-value 0.0010) and BMI (p-value 0.0026) on birth weight, but not for TNF-α (p-value 0.4868) and adiponectin (p-value 0.0675) (Figures 6, 7).
Figure 6.
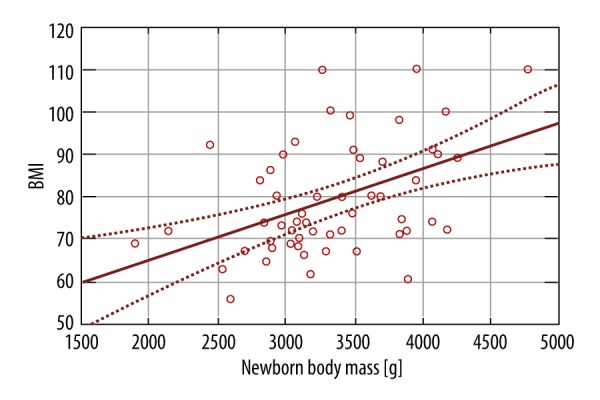
The effect of BMI on birth weight of the fetal birth weight.
Figure 7.
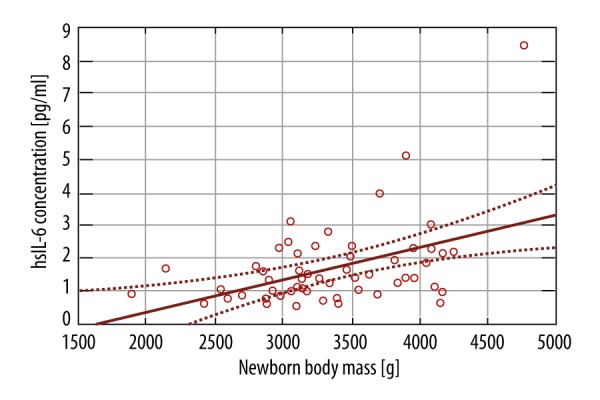
The effect of IL-6 level on the fetal birth weight.
Discussion
In the study, we showed the dependence of declining values of adiponectin on increasing values of BMI. The level of adiponectin was significantly lower in obese patients than in patients with normal BMI. Adiponectin is a cytokine specific for adipose tissue. It has anti-inflammatory and antiatherogenic effects and increases insulin sensitivity. Unlike other cytokines, its concentration decreases in obesity, insulin resistance, or cardiovascular diseases [10]. It appears that the placenta has many receptors for the cytokine. It was shown that in obesity, there is reduced adiponectin expression due to increased DNA methylation and negative feedback signals transmitted through the adiponectin receptors in various tissues, including the placenta [11].
In our study, gestational diabetes and hypertension were more common in the obesity group (33.3% vs. 10.3% for diabetes and 16.6% vs. 0% for hypertension), but the group was too small to show statistical significance. Low level of adiponectin is an independent predictor of gestational diabetes in obese women [12,13], but there is no clear data on the role of adiponectin in the pathogenesis of pre-eclampsia or intrauterine growth restriction of the fetus [10]. A study reported a significant negative correlation between oxidative stress markers and adiponectin, suggesting a relationship between antioxidant levels and this adipokine in healthy pregnancies, which is altered in patients with gestational hypertension or pre-eclampsia [14]. Nevertheless, another study on the role of adiponectin, leptin, and resistin in onset of pre-eclampsia showed only the influence of leptin and resistin [15].
It is assumed that in case of inflammation, the level of inflammatory cytokines is increased. In our study, the average concentration of TNF-α was higher in obese women, but this difference was not significant. On the contrary, the trend in level of IL-6 was unexpected, with lower levels in obese women, but this difference was not statistically significant. The results of previous reports show that in obese patients, serum levels of these adipocytokines are increased during pregnancy due to the influence of increased BMI [16–18]. One study showed that maternal adiposity was associated with significantly higher circulatory levels of these markers, but at the end of pregnancy, the BMI-dependent increase was not as evident as at the first or second trimester of pregnancy [19]. The available literature does not provide clear data on the role of these cytokines during pregnancy. Chronic inflammation that occurs in the fat tissue may increase the risk of insulin resistance. Monocytes and macrophages in adipose tissue producing inflammatory cytokines may eventually lead to altered glucose absorption. It has been shown that TNF-α is an independent predictor of gestational diabetes [10]. Furthermore, it was shown that inflammatory cytokines, including IL-6 and TNF-α, may play an important role in pre-eclampsia [20,21]. Increased concentration of TNF-α was similarly described in the intrauterine growth restriction, but only with placental insufficiency [22].
In our material, we observed a positive correlation between level of TNF-α and the number of previous miscarriages; this association is probably not influenced by BMI, and a similar relation is described in the literature. Immune disorders modulated by cytokines play a major role in the pathogenesis of recurrent miscarriage [23,24]. The levels of TNF-α are higher in patients with a miscarriage compared to those with a successful pregnancy, suggesting that it might have harmful effects for pregnancy [25,26]. A meta-analysis evaluating the association between TNF-α genetic polymorphisms and recurrent pregnancy loss risk demonstrated that 308G/A polymorphism might be a risk factor for miscarriage [27].
In this study, we did not show a correlation between cerebroplacental ratio and the markers of inflammation, and the literature does not provide any objective evidence of this.
Our results show a positive correlation between amniotic fluid index and TNF-α serum level. Interestingly, a significant correlation between maternal serum level of IL-6 and AFI values but not TNF-α was reported [28]. Moreover, previous studies revealed higher AFI in obese women when compared with non-obese women [29].
The mode of delivery did not differ significantly between the 2 groups in our study. For the whole study population, 73% delivered by cesarean section, slightly more in obese women, but without statistical significance. On contrary, data from the present study indicate that obesity is associated with an increased risk of cesarean section [30–32].
We found a statistically significant positive effect of IL-6 level and BMI on fetal birth weight. This is consistent with previous reports, where infants of women with overweight or obesity were more likely to be macrosomic [29–31].
The exact role of inflammation in pregnancy is not well understood. A pregnant woman’s immune system is in a specific balance between inflammatory and anti-inflammatory agents in favor of immunosuppression, due to the protection against rejection of the embryo. During each trimester, there may be various factors that modulate inflammatory reactions, where in the initial stage of pregnancy the pro-inflammatory effect is dominant, and in the second and third trimesters it evolves towards an anti-inflammatory state. The trajectories of cytokines are not well known. Moreover, it is not well known to what extent excessive body weight, activating the process of chronic subclinical inflammation, can modify these trajectories. Therefore, better understanding of the effect of obesity on pregnancy is of great clinical importance.
The results presented in this study are mostly consistent with previous reports [33]. Unfortunately, due to the small size of our groups, the study failed to demonstrate statistical significance for all outcomes. Moreover, the extension of inclusion criteria to patients that delivered before 37 weeks of pregnancy must be taken into consideration. Measuring other inflammatory markers, such as leptin or resistin, may also be helpful, as there are data showing an important correlation with outcomes in pregnancies complicated by obesity [34,35].
Conclusions
The role of inflammatory processes during pregnancy complicated by obesity should be emphasized. Currently, there are no reference levels of cytokines for either physiological pregnancy or pregnancy in obese patients. Circulating cytokines do not necessarily represent the actual severity of inflammation, and there is no evidence that inflammation in the mother increases inflammatory mediators in the fetus. The exact role of inflammatory processes in pregnancy complicated by obesity, in particular the impact on fetal tissue and long-term effects for the mother and child, requires further study.
Footnotes
Source of support: Departmental sources
References
- 1.World Health Organization Media Centre Factsheets, factsheet 311: obesity and overweight. 2013. [accessed May 2016]. URL: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Szostak-Węgierek D, Waśkiewicz A. Metabolic disorders in women at procreative age living in Warsaw. Rocz Panstw Zakl Hig. 2015;66(3):245–51. [PubMed] [Google Scholar]
- 3.Lim CC, Mahmood T. Obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29(3):309–19. doi: 10.1016/j.bpobgyn.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Saving mothers’ lives: reviewing maternal deaths to make motherhood safer 2003e2005. London: CEMACH; 2007. The Seventh Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom Confidential enquiry into maternal and child health. [Google Scholar]
- 5.Mission JF, Marshall NE, Caughey AB. Obesity in pregnancy: A big problem and getting bigger. Obstet Gynecol Surv. 2013;68:389–99. doi: 10.1097/OGX.0b013e31828738ce. [DOI] [PubMed] [Google Scholar]
- 6.Santangeli L, Sattar N, Huda SS. Impact of maternal obesity on perinatal and childhood outcomes. Best Pract Res Clin Obstet Gynaecol. 2015;29(3):438–48. doi: 10.1016/j.bpobgyn.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf) 2013;78:9–16. doi: 10.1111/cen.12055. [DOI] [PubMed] [Google Scholar]
- 8.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 9.Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709–15. doi: 10.1016/j.placenta.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briana DD, Malamitsi-Puchner A. Reviews: Adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16(10):921–37. doi: 10.1177/1933719109336614. [DOI] [PubMed] [Google Scholar]
- 11.Haghiac M, Basu S, Presley L, et al. Patterns of adiponectin expression in term pregnancy: impact of obesity. J Clin Endocrinol Metab. 2014;99(9):3427–34. doi: 10.1210/jc.2013-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ianniello F, Quagliozzi L, Caruso A, Paradisi G. Low adiponectin in overweight/obese women: Association with diabetes during pregnancy. Eur Rev Med Pharmacol Sci. 2013;17(23):3197–205. [PubMed] [Google Scholar]
- 13.Doruk M, Uğur M, Oruç AS, et al. Serum adiponectin in gestational diabetes and its relation to pregnancy outcome. J Obstet Gynaecol. 2014;34(6):471–75. doi: 10.3109/01443615.2014.902430. [DOI] [PubMed] [Google Scholar]
- 14.Eleuterio NM, Palei AC, Machado JS, et al. Role of adiponectin on antioxidant profile: evaluation during healthy and hypertensive disorders of pregnancy. Blood Press. 2016;25(4):241–43. doi: 10.3109/08037051.2015.1134550. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Gao J, Qu Y, et al. Serum levels of leptin, adiponectin and resistin in relation to clinical characteristics in normal pregnancy and preeclampsia. Clin Chim Acta. 2016;458:133–37. doi: 10.1016/j.cca.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Verhaeghe J, van Bree R, Lambin S, Caluwaerts S. Adipokine profile and C-reactive protein in pregnancy: Effects of glucose challenge response versus body mass index. J Soc Gynecol Investig. 2005;12(5):330–34. doi: 10.1016/j.jsgi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Stewart FM, Freeman DJ, Ramsay JE, et al. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007;92(3):969–75. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- 18.Sen S, Iyer C, Meydani SN. Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J Perinatol. 2014;34(2):105–11. doi: 10.1038/jp.2013.153. [DOI] [PubMed] [Google Scholar]
- 19.Friis CM, Paasche Roland MC, Godang K, et al. Adiposity-related inflammation: Effects of pregnancy. Obesity (Silver Spring) 2013;21(1):E124–30. doi: 10.1002/oby.20120. [DOI] [PubMed] [Google Scholar]
- 20.Udenze I, Amadi C, Awolola N, Makwe CC. The role of cytokines as inflammatory mediators in preeclampsia. Pan Afr Med J. 2015;20:219. doi: 10.11604/pamj.2015.20.219.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Wang Y, Ding X, et al. Serum Levels of TNF-α and IL-6 are associated with pregnancy-induced hypertension. Reprod Sci. 2016;23(10):1402–8. doi: 10.1177/1933719116641760. [DOI] [PubMed] [Google Scholar]
- 22.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine. 2014;70(2):134–40. doi: 10.1016/j.cyto.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piosik ZM, Goegebeur Y, Klitkou L, et al. Plasma TNF-α levels are higher in early pregnancy in patients with secondary compared with primary recurrent miscarriage. Am J Reprod Immunol. 2013;70(5):347–58. doi: 10.1111/aji.12135. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med. 2006;11(5):302–8. doi: 10.1016/j.siny.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Calleja-Agius J, Jauniaux E, et al. Investigation of systemic inflammatory response in first trimesterpregnancy failure. Hum Reprod. 2012;27:349–57. doi: 10.1093/humrep/der402. [DOI] [PubMed] [Google Scholar]
- 26.Hua F, Li CH, Wang H, Xu HG. Relationship between expression of COX-2, TNF-α, IL-6 and autoimmune-type recurrent miscarriage. Asian Pac J Trop Med. 2013;6:990–94. doi: 10.1016/S1995-7645(13)60178-9. [DOI] [PubMed] [Google Scholar]
- 27.Li HH, Xu XH, Tong J, et al. Association of TNF-α genetic polymorphisms with recurrent pregnancy loss risk: A systematic review and meta-analysis. Reprod Biol Endocrinol. 2016;14:6. doi: 10.1186/s12958-016-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poniedziałek-Czajkowska E, Leszczyńska-Gorzelak B, Oleszczuk J. The relationship between the levels of proinflammatory cytokines, and AFI value in pregnancies complicated by premature rupture of membranes. Ginekol Pol. 2001;72(12):1163–69. [PubMed] [Google Scholar]
- 29.Calderon AC, Quintana SM, Marcolin AC, et al. Obesity and pregnancy: A transversal study from a low-risk maternity. BMC Pregnancy Childbirth. 2014;14:249. doi: 10.1186/1471-2393-14-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Hakmani FM, Al-Fadhil FA, Al-Balushi LH, et al. The effect of obesity on pregnancy and its outcome in the population of Oman, Seeb Province. Oman Med J. 2016;31(1):12–17. doi: 10.5001/omj.2016.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Der Linden EL, Browne JL, Vissers KM, et al. Maternal body mass index and adverse pregnancy outcomes: A ghanaian cohort study. Obesity (Silver Spring) 2016;24(1):215–22. doi: 10.1002/oby.21210. [DOI] [PubMed] [Google Scholar]
- 32.Beaudrot ME, Elchert JA, DeFranco EA. Influence of gestational weight gain and BMI on cesarean delivery risk in adolescent pregnancies. J Perinatol. 2016;36(8):612–17. doi: 10.1038/jp.2016.61. [DOI] [PubMed] [Google Scholar]
- 33.Deshmukh VL, Jadhav M, Yelikar K. Impact of HIGH BMI on pregnancy: Maternal and foetal outcome. J Obstet Gynaecol India. 2016;66(Suppl 1):192–97. doi: 10.1007/s13224-015-0825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castellano Filho DS, do Amaral Correa JO, Dos Santos Ramos P, et al. Body weight gain and serum leptin levels of non-overweight and overweight/obese pregnant women. Med Sci Monit. 2013;19:1043–49. doi: 10.12659/MSM.884027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lepsch J, Farias DR, dos Vaz JS, et al. Serum saturated fatty acid decreases plasma adiponectin and increases leptin throughout pregnancy independently of BMI. Nutrition. 2016;32(7–8):740–47. doi: 10.1016/j.nut.2016.01.016. [DOI] [PubMed] [Google Scholar]


