Abstract
Background
Curcumin has well-known, explicit biological anti-tumor properties. The Wnt/β-catenin signaling pathway plays a central role in tumor cell proliferation and curcumin can regulate the Wnt/β-catenin signaling pathway of several carcinomas. The aim of this study was to investigate the impact of curcumin on the Wnt/β-catenin signaling pathway in human gastric cancer cells.
Material/Methods
We used 3 gastric cancer cell lines: SNU-1, SNU-5, and AGS. Research methods used were MTT assay, flow cytometry, clonogenic assay, annexin V/PI method, Western blotting analysis, tumor formation assay, and in vivo in the TUNEL assay.
Results
Curcumin markedly impaired tumor cell viability and induced apoptosis in vitro. Curcumin significantly suppressed the levels of Wnt3a, LRP6, phospho-LRP6, β-catenin, phospho-β-catenin, C-myc, and survivin. Xenograft growth in vivo was inhibited and the target genes of Wnt/β-catenin signaling were also reduced by curcumin treatment.
Conclusions
Curcumin exerts anti-proliferative and pro-apoptotic effect in gastric cancer cells and in a xenograft model. Inhibition of the Wnt/β-catenin signaling pathway and the subsequently reduced expression of Wnt target genes show potential as a newly-identified molecular mechanism of curcumin treatment.
MeSH Keywords: Apoptosis, Curcumin, Stomach Neoplasms, Wnt Signaling Pathway
Background
Despite significant advances in cancer therapy, gastric carcinoma (GC) is still one of the deadliest cancers worldwide. GC occurs as a localized disease but may quickly spread to distant sites through invasion or migration [1]. Various therapeutic strategies are currently available for GC patients, including surgery, chemotherapy, and radiotherapy. Chemotherapy remains the main therapeutic approach for GC patients by preventing tumor invasion and metastasis. However, the mortality rates of GC still remain high because of the failure to effectively kill tumor cells by use of chemotherapy agents. Furthermore, chemotherapy has severe residual chemotherapy-related adverse effects. Finding effective and safe preventive agents to suppress tumor invasion and metastasis is a critical approach to cure the disease or improve quality of life for GC patients.
Recently, biologically active anti-cancer agents from natural products have been a focus of research attention, and many reports have indicated that these agents from natural resources are suitable alternatives for controlling cancers [2–8]. Curcumin is a polyphenol compound extracted from the root of Curcuma longa [9]. Curcumin has well-known, explicit biological anti-tumor properties. Many studies have shown that curcumin inhibited the growth of various cancer cells and prevented invasion and metastasis through modulating the activity of oncogenes and tumor-suppressor genes, as well as through signaling pathways. Curcumin can inhibit tumor cell proliferation and induce apoptosis in head and neck squamous cell cancer, breast cancer, prostate cancer, lung cancer, and pancreatic adenocarcinoma [10–16]. Phase I clinical trials have demonstrated that curcumin has no dose-limited toxicity, and can be safely used in cancer treatment [17]. However, it remains unclear whether curcumin has anti-cancer activity in GC, and the molecular mechanism also needs to be explored.
Studies have reported that curcumin reduces lung inflammation and diabetic renal fibrosis, and alleviates glucocorticoid-induced osteoporosis by targeting Wnt signaling pathways [18–20]. Curcumin can also inhibit invasion and metastasis of colon cancer cells and proliferation-migration of non-small cell of lung cancer, medulloblastoma, and hepatocellular carcinoma cells through inhibition of the Wnt signaling pathway [21–26]. Curcumin promotes apoptosis of human endometrial carcinoma cells through the Wnt signaling pathway [27], which is closely related to tumorigenesis and plays a central role in tumor cell proliferation, but the mechanism is poorly understood [28]. Modulation of the Wnt/β-catenin signaling is highly correlated with tumor cell metabolism [29], and its activation leads to chemotherapy resistance in several cancers [30,31]. Therefore, therapies targeting the Wnt/β-catenin signaling mat be effective in inhibiting tumor growth.
The goal of this study was to determine whether human GC cells are sensitive to the anti-cancer activity of curcumin, and to evaluate the role of curcumin in modulating a specific signaling pathway. Our results indicate that curcumin inhibits the growth of GC cells and induces apoptosis through down-regulation of Wnt/β-catenin signaling. Curcumin possesses an explicit anti-cancer ability and could be a candidate for use in gastric cancer treatment.
Material and Methods
Reagents
Curcumin (C21H20O6) was obtained from the Zhejiang Institute for Food and Drug Control (Hangzhou, China; batch no. 110823). Curcumin was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) as a stock solution, and then diluted in medium to achieve the final concentration for each experiment. RPMI 1640, Iscove’s Modified Dulbecco’s Medium, F-12K Medium, and fetal bovine serum were obtained from GE Healthcare Life Sciences (Logan, UT, USA). Annexin V Apoptosis Detection kits were obtained from BD Biosciences (Franklin Lakes, NJ, USA). Wnt3a (C64F2) Rabbit mAb #2721, Phospho-LRP6 (Ser1490) Antibody #2568, LRP6 (C47E12) Rabbit mAb #3395, Phospho-β-Catenin (Ser675) (D2F1) Rabbit mAb #4176, β-Catenin (6B3) Rabbit mAb #9582, c-Myc Antibody #9402, survivin (71G4B7) Rabbit mAb #2808, and GAPDH (14C10) Rabbit mAb #2118 at 1: 1000 dilution were obtained from Cell Signaling Technology (Danvers, MA, USA).
Cell lines
The human gastric carcinoma cell lines SNU-1, SNU-5, and AGS were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultured in RPMI 1640(SNU-1), Iscove’s Modified Dulbecco’s Medium (SNU-5), and F-12K Medium (AGS) with 10% fetal bovine serum at 37°C in a 5% CO2 humidified atmosphere.
Cell viability assay
The MTT assay was performed to determine the cell viability. SNU-1, SNU-5, and AGS cells (1×104 cells/well) were seeded into 96-well plates and cultured overnight. Different concentrations of curcumin were added to treat cells for 24 h, 48 h, and 72 h. MTT was added to each well and then dissolved by DMSO. The absorbance value was measured by a multiscanner autoreader (Thermo Fisher Scientific Inc., Waltham, MA, USA). Cell viability curves were generated and 50% inhibition concentration (IC50) values were calculated.
Clonogenic assay
Clonogenic assay was performed to determine the survival of cells treated with curcumin. SNU-1, SNU-5, and AGS cells (1×105 cells/well) were seeded into 6-well plates and incubated overnight. After 48-h exposure to different concentrations of curcumin, the viable cells were seeded at 1000 cells/flask and cultured for 2 weeks. The colonies were then fixed and stained with crystal violet (Sigma-Aldrich, St. Louis, MO, USA), and the colonies containing more than 50 cells were scored under an Olympus inverted light microscope (Olympus Corp., Tokyo, Japan).
Apoptosis assay
Annexin V/PI method was used to detect apoptosis. Cells (3×105 cells/well) were seeded into 6-well plates and incubated overnight. After treatment with different concentrations of curcumin for 48 h, cells were harvested, trypsinized, and washed. Then the cells were stained with Annexin V and PI for 30 min in the dark. Finally, the samples were evaluated by flow cytometry (Beckman Coulter Inc., Brea, CA, USA).
Western blotting analysis
Cells were treated with different concentrations of curcumin for 48 h, and then harvested and lysed in cell lysis buffer containing Tris-HCl and Triton X-100 to extract total protein. An equal amount of denatured protein was decentralized on SDS-polyacrylamide gel transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat milk in TBST. Specific primary antibody was added to the membrane and then incubated at 4°C overnight. Then the samples were incubated with horseradish peroxidase-conjugated second antibody at room temperature for 30 min. Protein bands were visualized using ECL reagents (Pierce, Rockford, IL, USA) and detected by ImageQuant LAS 4000 (Pittsburgh, PA, USA).
Tumor formation assay in vivo
Male BALB/c nude mice (4–6 weeks, weighing 18–20 g) were purchased from the Laboratory Animal Center of Zhejiang University (Hangzhou, China). Mice were housed under pathogen-free conditions. Experimental protocols were performed according to the guidelines provided by the National Cancer Institute (NIH, USA). The protocol was approved by the Hangzhou Cancer Hospital Research Ethics Committee. The mouse model of xenograft tumor was established by subcutaneously injecting AGS cells. For xenografts, 10 mice were randomly divided into 2 groups. The curcumin treatment group received gavage administration of curcumin (1 mg/kg) once a day, and the control group received gavage administration of 1 ml normal saline once a day. Tumor volumes and weights were measured after mice were sacrificed, and tumors were fixed in 4% formalin fixative and embedded in paraffin for TUNEL staining and Western blotting.
TUNEL analysis
Apoptosis in the gastric carcinoma xenograft model was detected by the terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL) method. In situ cell death detection kits were used following the manufacturer’s instructions. Reactions were examined under an Olympus fluorescence microscope (Olympus Corp., Tokyo, Japan).
Statistical analysis
Data are presented as mean ±SD. Statistical analysis was conducted using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Differences between 2 groups were analyzed by the t test, and multiple groups were analyzed by one-way ANOVA. P<0.05 was considered to indicate a statistically significant difference.
Results
The cytotoxic effects of curcumin on gastric carcinoma cells
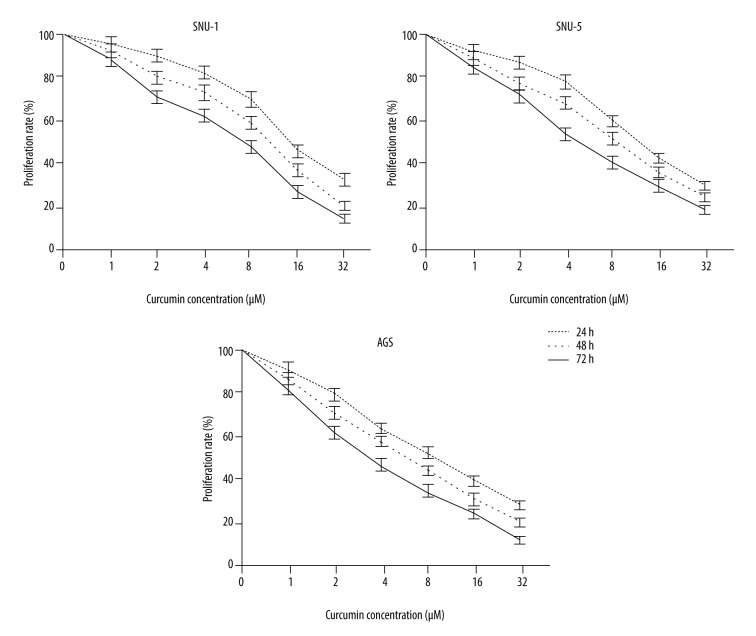
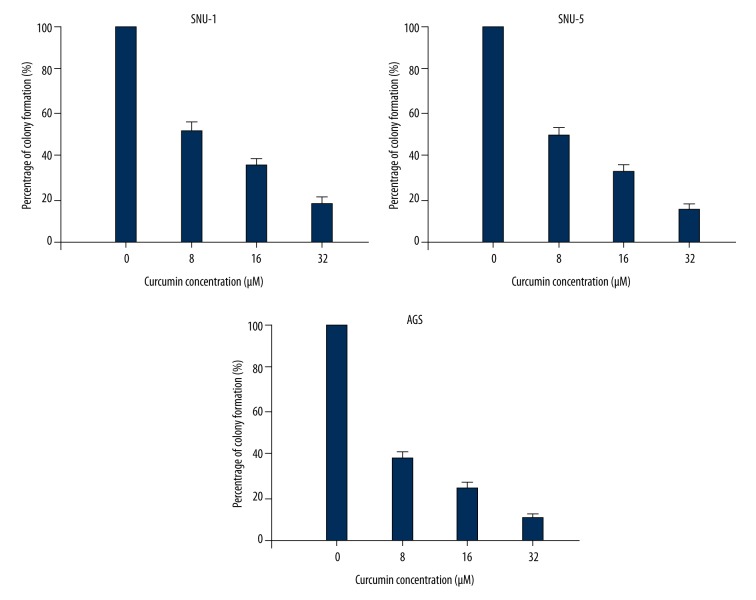
MTT assay and clonogenic assay were performed to determine the potential cytotoxic effect of curcumin on gastric carcinoma cells. SNU-1, SNU-5, and AGS cells were treated with various concentrations (from 1 to 32 μM) of curcumin for 24 h, 48 h, and 72 h, respectively. MTT assay showed that curcumin significantly reduced the cell viability of SNU-1, SNU-5, and AGS cells in dose- and time-dependent manners, respectively (Figure 1). The results from clonogenic assay showed that colony formation significantly decreased after curcumin treatment in a dose-dependent manner (Figure 2). The data indicated that curcumin has an anti-proliferative effect on gastric carcinoma in vitro.
Figure 1.
Anti-proliferative activity of curcumin on gastric cancer cell lines. Curcumin significantly reduced the cell viability of SNU-1, SNU-5, and AGS cells in dose- and time-dependent manners.
Figure 2.
Colony formation of SNU-1, SNU-5, and AGS cells were decreased in a dose-dependent manner after curcumin treatment.
Curcumin induced apoptosis of gastric carcinoma cells
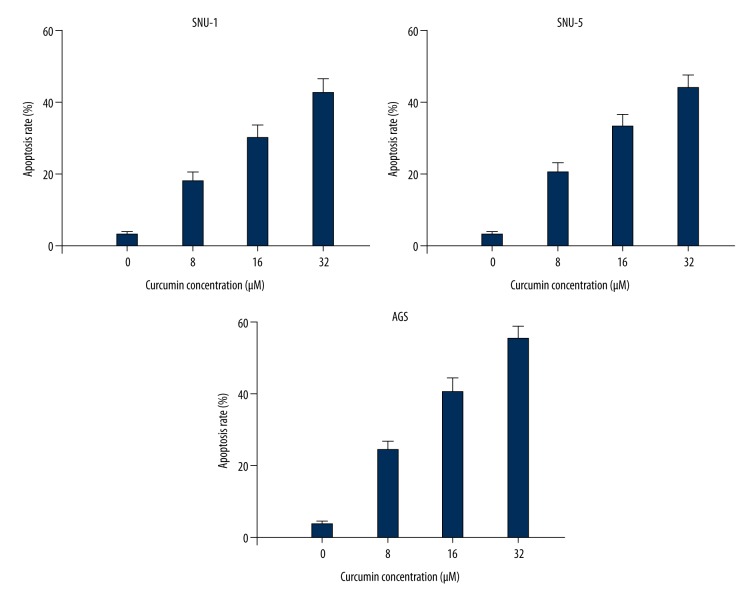
The Annexin V-FITC/PI assay was performed to investigate whether curcumin inhibited gastric carcinoma cell growth by triggering apoptosis. As shown in Figure 3, curcumin significantly triggered cell apoptosis in SNU-1, SNU-5, and AGS cells: apoptosis rates were increased from 3.21% in the control group to 18.1% of 8 μM, 30.2% of 16 μM, and 42.8% of 32 μM in curcumin-treated SNU-1 cells. Similar increased apoptosis rates were observed in SNU-5 and AGS cells. These data indicate that curcumin induced apoptosis of gastric carcinoma cells in a dose-dependent manner.
Figure 3.
Curcumin induced apoptosis of SNU-1, SNU-5, and AGS cells in a dose-dependent manner.
Curcumin down-regulated Wnt/β-catenin signaling
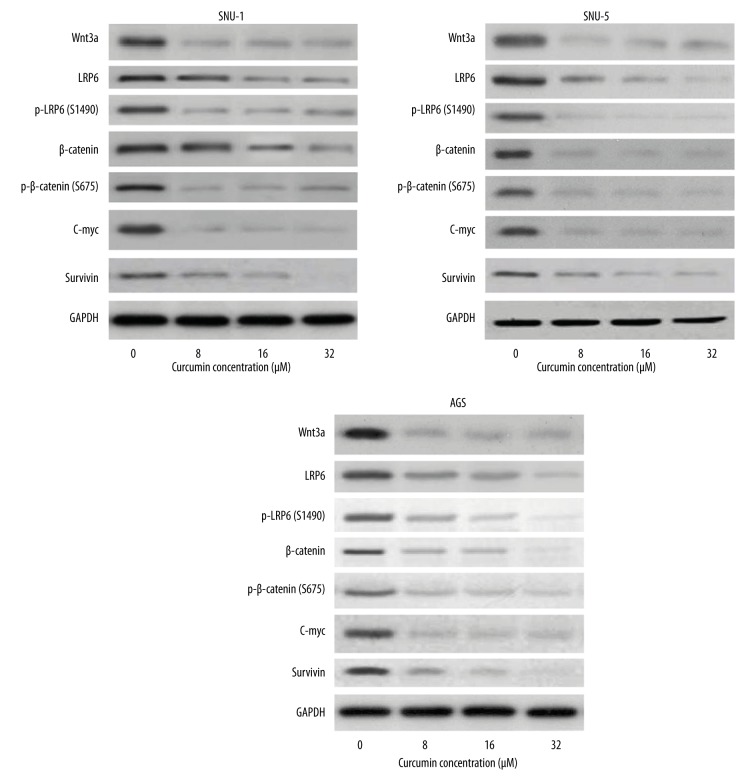
The ability of curcumin to inhibit Wnt/β-catenin signaling in gastric carcinoma cells in vitro was investigated by Western blotting. The results showed that curcumin suppressed the levels of Wnt3a, LRP6, and phospho-LRP6 at Ser1490, β-catenin, phospho-β-catenin at Ser675, and expression of C-myc and Survivin in SNU-1, SNU-5, and AGS cells (Figure 4).
Figure 4.
Curcumin decreased protein levels of the Wnt/β-catenin signaling pathway on SNU-1, SNU-5, and AGS cells in a dose-dependent manner. The expressions of Wnt3a, LRP6, phospho-LRP6 at Ser1490, β-catenin, phospho-β-catenin at Ser675, C-myc, and survivin were decreased in a dose-dependent manner after curcumin treatment.
The anti-tumor and apoptosis-inducing effects of curcumin in the gastric carcinoma xenograft model
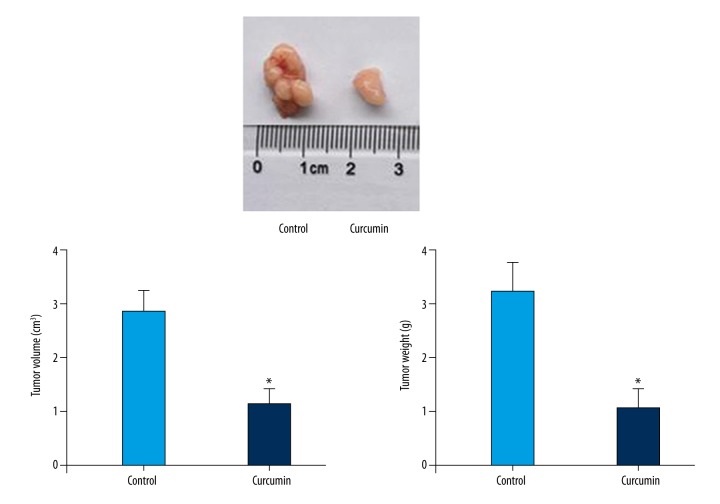
The anti-tumor activity of curcumin was investigated in vivo by using AGS xenografts. The results showed that tumor volumes and weight were reduced significantly after curcumin treatment (Figure 5). TUNEL assay was performed to analyze the apoptosis induced by curcumin by counting positively labeled cells in random microscopic fields. As shown in Figure 6, the apoptotic index of the curcumin treatment group was significantly different from that in the control group. Western blotting analysis of in vivo samples also showed that the expressions of Wnt3a, LRP6, phospho-LRP6 (Ser1490), β-catenin, phospho-β-catenin (Ser675), C-myc, and survivin were decreased significantly after curcumin treatment (Figure 7).
Figure 5.
The anti-tumor effects of curcumin on tumor growth of xenograft model of AGS cells. Tumor volumes and weight of curcumin group reduced significantly in the model mice. * Statistically significant difference (P<0.01) compared to the control group.
Figure 6.
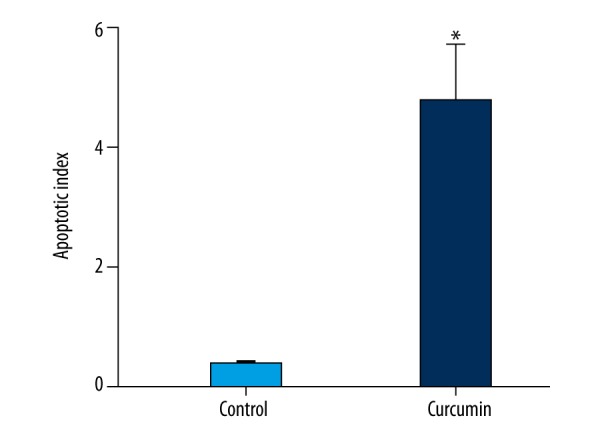
The effect of curcumin on apoptosis in tumor tissues. The apoptosis index significantly increased after curcumin treatment. *Statistically significant difference (P<0.01) compared to the control group.
Figure 7.

The effect of curcumin on Wnt/β-catenin signaling pathway protein expressions in tumor tissues. Western blotting analysis for Wnt3a, LRP6, phospho-LRP6 at Ser1490, β-catenin, phospho-β-catenin at Ser675, C-myc, and survivin proteins from AGS xenografts.
Discussion
GC usually easily transforms to the advanced stage, and the overall survival is poor because GC is difficult to cure. Establishment of molecular therapeutic strategies is required to increase survival of GC patients. The Wnt/β-catenin signaling pathway is important for the initiation, progression, and prognosis of human cancers. The β-catenin protein is multifunctional and plays a vital role in the canonical Wnt/β-catenin pathway [32]. It is activated by Wnt signaling, which prevails in different types of signal transduction. Previous studies reported that silencing of β-catenin inhibited cell growth in colorectal cancer, glioblastoma, lung cancer, and ovarian cancer cells in in vitro studies [33–36], indicating that β-catenin is essential for cancer growth.
Recent evidence indicates that selectively targeting oncogenic proteins via degradation may be an ideal approach for developing anti-tumor agents. Therefore, Wnt/β-catenin signaling could serve as a good biomolecular target for GC treatment. The potential of curcumin to treat GC has been demonstrated in numerous studies [37–42]. However, until now there has been no evidence supporting the value of targeting Wnt/β-catenin signaling in use of curcumin to treat GC. The present study demonstrates that curcumin inhibits tumor growth and apoptosis of GC in vitro and in vivo. Furthermore, evidence shows that the mechanism is associated with inhibition of Wnt/β-catenin signaling and reduced expression of Wnt target genes. First, we demonstrated that curcumin exerts its anti-proliferative and pro-apoptotic effect in 3 GC cell lines in a dose-dependent manner. Results from the in vitro effect of curcumin on GC encouraged us to establish an in vivo xenograft model for further study. Curcumin treatment resulted in inhibition of tumor growth compared to control. Next, we analyzed the molecular mechanism of curcumin on GC in vitro and in vivo. We observed reduced Wnt3a, β-catenin, and LRP6 levels, and blocking of β-catenin and LRP6-phosphorylation by curcumin. LRP6 phosphorylation is critical for the activation of the Wnt/β-catenin signaling pathway [43]. The downstream target genes of the Wnt/β-catenin pathway, such as C-myc and Survivin, are also related to tumor growth, invasion, and metastasis. C-myc is the target oncogene in Wnt/β-catenin signaling and is activated in most human malignancies. C-myc may participate in the regulation of cell proliferation, cell cycle progression, and migration [44]. Survivin, which is another transcriptional target of the Wnt/β-catenin signaling pathway, inhibits caspase activation, thereby leading to negative regulation of apoptosis [45]. Our results showed that the expressions of C-myc and Survivin were significantly decreased in the 3 GC cell lines in a dose-dependent manner, as well as in a xenograft model.
The above findings indicate that inhibition of the Wnt/β-catenin signaling pathway might be responsible for the anti-tumor effect of curcumin in human GC. Although further detailed work is necessary to fully elaborate the mechanism of curcumin in GC treatment through the Wnt/β-catenin signaling pathway, the results of present study may provide valuable insights into the anti-tumor activities of curcumin and its relationship with the Wnt/β-catenin signaling pathway.
Conclusions
Our results demonstrate that curcumin exerts anti-proliferative and pro-apoptotic effects in GC cells and in a xenograft model, and remains a potential candidate for GC treatment. Inhibition of the Wnt/β-catenin signaling pathway and the subsequent reduced expression of Wnt target genes might be a molecular mechanism of curcumin treatment.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Carmona-Bayonas A, Jimenez-Fonseca P, Lorenzo ML, et al. On the effect of triplet or doublet chemotherapy in advanced gastric cancer: Results from a National Cancer Registry. J Natl Compr Canc Netw. 2016;14(11):1379–88. doi: 10.6004/jnccn.2016.0148. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, Zhao P, Feng J, et al. Effect of Paris saponin I on radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett. 2014;7(6):2059–64. doi: 10.3892/ol.2014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang H, Zhao PJ, Su D, et al. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 2014;9(6):2265–72. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 4.Zhao P, Jiang H, Su D, et al. Inhibition of cell proliferation by mild hyperthermia at 43 C with Paris Saponin I in the lung adenocarcinoma cell line PC-9. Mol Med Rep. 2015;11(1):327–32. doi: 10.3892/mmr.2014.2655. [DOI] [PubMed] [Google Scholar]
- 5.Zhao PJ, Song SC, Du LW, et al. Paris Saponins enhance radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line by inducing apoptosis and G2/M cell cycle phase arrest. Mol Med Rep. 2016;13(3):2878–84. doi: 10.3892/mmr.2016.4865. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Jiang H, Li J, et al. Anticancer Effects of Paris saponins by apoptosis and PI3K/AKT pathway in gefitinib-resistant non-small cell lung cancer. Med Sci Monit. 2016;22:1435–41. doi: 10.12659/MSM.898558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song S, Du L, Jiang H, et al. Paris saponin I sensitizes gastric cancer cell lines to cisplatin via cell cycle arrest and apoptosis. Med Sci Monit. 2016;22:3798–803. doi: 10.12659/MSM.898232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng R, Rao Y, Jiang H, et al. Therapeutic potential of Ginsenoside Rg3 via inhibiting Notch/HES1 pathway in lung cancer cells. Transl Cancer Res. 2016;5(4):464–69. [Google Scholar]
- 9.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–99. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin SS, Lai KC, Hsu SC, et al. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF) Cancer Lett. 2009;285(2):127–33. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 11.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zong H, Wang F, Fan Q, Wang LX. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol Biol Rep. 2012;39(4):4803–8. doi: 10.1007/s11033-011-1273-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim JM, Noh EM, Kwon KB, et al. Curcumin suppresses the TPA-induced invasion through inhibition of PKCalpha-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine. 2012;19(12):1085–92. doi: 10.1016/j.phymed.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Hassan ZK, Daghestani MH. Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac J Cancer Prev. 2012;13(7):3259–64. doi: 10.7314/apjcp.2012.13.7.3259. [DOI] [PubMed] [Google Scholar]
- 15.Lee WH, Loo CY, Young PM, et al. Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin Drug Deliv. 2014;11(8):1183–201. doi: 10.1517/17425247.2014.916686. [DOI] [PubMed] [Google Scholar]
- 16.Lee WH, Bebawy M, Loo CY, et al. Fabrication of Curcumin Micellar Nanoparticles with Enhanced Anti-Cancer Activity. J Biomed Nanotechnol. 2015;11(6):1093–105. doi: 10.1166/jbn.2015.2041. [DOI] [PubMed] [Google Scholar]
- 17.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–900. [PubMed] [Google Scholar]
- 18.Chen Z, Xue J, Shen T, et al. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int J Mol Med. 2016;37(2):329–38. doi: 10.3892/ijmm.2015.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Lv JN, Li H, et al. Curcumin reduces lung inflammation via Wnt/beta-catenin signaling in mouse model of asthma. J Asthma. 2016 doi: 10.1080/02770903.2016.1218018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Ho C, Hsu YC, Lei CC, et al. Curcumin rescues diabetic renal fibrosis by targeting superoxide-mediated Wnt signaling pathways. Am J Med Sci. 2016;351(3):286–95. doi: 10.1016/j.amjms.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Chen H, Xu C, et al. Curcumin inhibits tumor epithelialmesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol Rep. 2016;35(5):2615–23. doi: 10.3892/or.2016.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen QY, Jiao DM, Wang LF, et al. Curcumin inhibits proliferation-migration of NSCLC by steering crosstalk between a Wnt signaling pathway and an adherens junction via EGR-1. Mol Biosyst. 2015;11(3):859–68. doi: 10.1039/c4mb00336e. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Wei C, Xi Z. Curcumin suppresses proliferation and invasion in non-small cell lung cancer by modulation of MTA1-mediated Wnt/beta-catenin pathway. In Vitro Cell Dev Biol Anim. 2014;50(9):840–50. doi: 10.1007/s11626-014-9779-5. [DOI] [PubMed] [Google Scholar]
- 24.He M, Li Y, Zhang L, et al. Curcumin suppresses cell proliferation through inhibition of the Wnt/beta-catenin signaling pathway in medulloblastoma. Oncol Rep. 2014;32(1):173–80. doi: 10.3892/or.2014.3206. [DOI] [PubMed] [Google Scholar]
- 25.Xu MX, Zhao L, Deng C, et al. Curcumin suppresses proliferation and induces apoptosis of human hepatocellular carcinoma cells via the wnt signaling pathway. Int J Oncol. 2013;43(6):1951–59. doi: 10.3892/ijo.2013.2107. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Park SY, Park OJ, Kim YM. Curcumin suppresses migration and proliferation of Hep3B hepatocarcinoma cells through inhibition of the Wnt signaling pathway. Mol Med Rep. 2013;8(1):282–86. doi: 10.3892/mmr.2013.1497. [DOI] [PubMed] [Google Scholar]
- 27.Feng W, Yang CX, Zhang L, et al. Curcumin promotes the apoptosis of human endometrial carcinoma cells by downregulating the expression of androgen receptor through Wnt signal pathway. Eur J Gynaecol Oncol. 2014;35(6):718–23. [PubMed] [Google Scholar]
- 28.Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb Perspect Biol. 2012;4(1):a008227. doi: 10.1101/cshperspect.a008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantor JR, Sabatini DM. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012;2(10):881–98. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Campisi J, Higano C, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18(9):1359–68. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaraj AB, Joseph P, Kovalenko O, et al. Critical role of Wnt/beta-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015;6(27):23720–34. doi: 10.18632/oncotarget.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 33.Stein U, Arlt F, Walther W, et al. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131(5):1486–500. doi: 10.1053/j.gastro.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 34.Pu P, Zhang Z, Kang C, et al. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16(4):351–61. doi: 10.1038/cgt.2008.78. [DOI] [PubMed] [Google Scholar]
- 35.Teng Y, Wang X, Wang Y, Ma D. Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392(3):373–79. doi: 10.1016/j.bbrc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Zhou D, He X, et al. Effect of downregulated beta-catenin on cell proliferative activity, the sensitivity to chemotherapy drug and tumorigenicity of ovarian cancer cells. Cell Mol Biol (Noisy-le-grand) 2011;57(Suppl):OL1606–13. [PubMed] [Google Scholar]
- 37.Sun Q, Zhang W, Guo Y, et al. Curcumin inhibits cell growth and induces cell apoptosis through upregulation of miR-33b in gastric cancer. Tumour Biol. 2016;37(10):13177–84. doi: 10.1007/s13277-016-5221-9. [DOI] [PubMed] [Google Scholar]
- 38.Pandey A, Vishnoi K, Mahata S, et al. Berberine and curcumin target survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-Fluorouracil. Nutr Cancer. 2015;67(8):1293–304. doi: 10.1080/01635581.2015.1085581. [DOI] [PubMed] [Google Scholar]
- 39.Cao AL, Tang QF, Zhou WC, et al. Ras/ERK signaling pathway is involved in curcumin-induced cell cycle arrest and apoptosis in human gastric carcinoma AGS cells. J Asian Natr Prod Res. 2015;17(1):56–63. doi: 10.1080/10286020.2014.951923. [DOI] [PubMed] [Google Scholar]
- 40.Uehara Y, Inoue M, Fukuda K, et al. Inhibition of beta-catenin and STAT3 with a curcumin analog suppresses gastric carcinogenesis in vivo. Gastric Cancer. 2015;18(4):774–83. doi: 10.1007/s10120-014-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang T, Zhang X, Xue W, et al. Curcumin induced human gastric cancer BGC-823 cells apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway. Int J Mol Sci. 2014;15(9):15754–65. doi: 10.3390/ijms150915754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Sun K, Song A, et al. Curcumin inhibits proliferation of gastric cancer cells by impairing ATP-sensitive potassium channel opening. World j Surg Oncol. 2014;12:389. doi: 10.1186/1477-7819-12-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu D, Carson DA. Inhibition of Wnt signaling and cancer stem cells. Oncotarget. 2011;2(8):587. doi: 10.18632/oncotarget.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dang CV, O’Donnell KA, Zeller KI, et al. The c-Myc target gene network. Semin Cancer Biol. 2006;16(4):253–64. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24(22):3619–31. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]