Abstract
A major objective of biobehavioral research is defining the mechanisms that underlie linkages among behavior, biology, health, and disease. The genomic revolution has demonstrated the importance of studying the role of the environment in (epi)genetic mechanisms. The idea that interactions between environment and genetics influence health outcomes is a central concept of the exposome, a measure of environmental exposures throughout a lifetime. Research suggests that telomere length (TL) and biologic factors involved in telomere stability may provide an understanding of the effects of gene–environment interaction on disease risk. Telomeres, thus, have become important biomarkers for aging as well as for stress-related disease. However, incorporating telomeres into biobehavioral research requires consideration of several aspects of the exposome. Internal and external modifiable and nonmodifiable exposures have the potential to influence TL. Future research utilizing the concept of the exposome will provide meaningful findings related to exposure sources as well as dosage and duration across the life span that influence telomere biology and disease occurrence. Such findings can be translated into clinical practice and may provide a basis for personalized disease prevention and treatment approaches.
Keywords: exposome, biobehavioral, telomere
Defining the mechanisms that underlie linkages among behavior, biology, health, and disease is a major objective of biobehavioral research. This body of research has grown substantially following the genomic revolution, which has provided an unparalleled view of the genetic and epigenetic mechanisms that contribute to health outcomes. However, our expanding knowledge of genomics has also heightened awareness of the seminal role of the environment in (epi)genetic mechanisms—a central concept of the exposome (Wild, 2012). The exposome is a measure of all environmental exposures acquired across the life span, which, in conjunction with the (epi)genetic infrastructure, influence health outcomes. With the recent innovations in genetic, epigenetic, and exposome measures and a more in-depth understanding of these phenomena, nursing scientists are challenged to extend their research foci to include genomic measures that have broad pathoetiologic implications for health and disease risk.
One of the most novel and potentially useful genomic measures is telomere length (TL). At a molecular level, TL is a “mitotic clock” that regulates the number of times a cell can divide. Research over the past decade suggests that TL may help to reveal the effects of gene–environment interactions on disease risk and could have clinical and scientific utility as a global biomarker of health. Environmental exposures, including psychological stress as well as lifestyle and behaviors, have also been shown to modify TL, rate of telomere attrition, and telomerase activity. For this reason, telomeres and the biological factors involved in telomere stability have become important biomarkers for multiple conditions, including aging as well as stress-related diseases.
Although the basis for incorporating telomeres into biobehavioral research is expanding, strategies for defining the implications of telomeric measures on health and disease risk are essential in order to reach meaningful findings that can be translated into clinical practice. In the present article, we use a biobehavioral framework to discuss aspects of the exposome to consider when integrating measures of TL, rate of attrition, and telomerase activity in research.
Overview of Telomeric Structure and Processes
Telomeres are noncoding segments of DNA at the end of chromosomes which prevent the loss of genetic material and protect the chromosomes from fusing together during cell division (Blackburn, 2011). The heritability estimates of TL range from 40% to 80% and are highly variable between individuals and within different organs and cell types (Shammas, 2011). At birth, the length of telomeres in blood cells is about 8,000 base pairs (Zanni & Wick, 2011). However, during each cell division a cell loses 30 to 150 base pairs, resulting in a gradual decline in TL over time (Zhu, Belcher, & van der Harst, 2011).
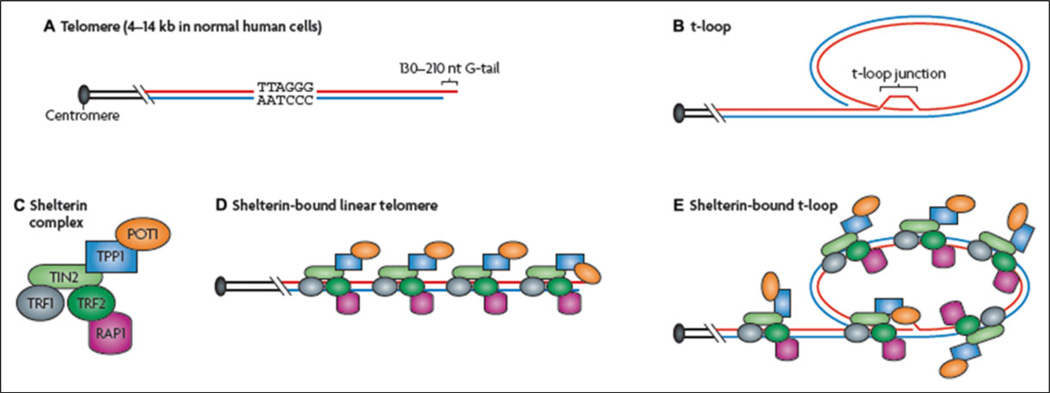
Forming a telomere–shelterin complex with specialized proteins, the telomere protects adjacent chromosomes by creating a cap at the end (Oeseburg, de Boer, van Gilst, & van der Harst, 2010). Unlike coding DNA, telomeres are composed of double-stranded repeating sequences of six base pairs, with TTAGGG on one strand bound to AATCCC on the other strand. At the end of the telomere is a G-rich single-strand extension called the G-tail. When the cell is not undergoing division, the G-tail folds back to encase the telomere by forming two internal loops, the D-loop and T-loop. The stability of the T-loop is dependent on the binding of other telomere-specific proteins with the telomere–shelterin complex. Proteins involved in the shelterin complex include telomeric repeat binding factor 1 (TRF1) and 2 (TRF2), protein protection of telomeres 1, repressor activator protein 1, tripeptidyl peptidase 1, and TRF1-interacting nuclear factor 2 (Figure 1). With telomere shortening, fewer shelterin complexes are able to bind to the telomere and the T-loop is not formed; thus, chromosome ends are uncapped and vulnerable to erosion and fusion.
Figure 1.
Structure of telomeres. A, Vertebrate telomeres contain repetitive DNA with the sequence (5′-TTAGGG-3′). Most of this DNA is double stranded, apart from the terminus, which consists only of the TTAGGG (or “G-rich”) strand. B, The telomere can fold back on itself, and the single-stranded terminus can invade duplex telomeric DNA. This results in the formation of a telomeric loop (t-loop). C, Telomeric DNA is bound by the 6-unit shelterin complex. D and E, Two shelterin proteins (telomeric repeat-binding factor 1 [TRF1], also known as TERF1 and TRF2, also known as TERF2) bind directly to double-stranded telomeric DNA, and one (protection of telomeres 1 [POT1]) binds to single-stranded telomeric DNA directly. POT1, TPP1 (also known as ACD), TRF1-interacting nuclear protein 2 (TIN2, also known as TINF2), and repressor activator protein 1 (RAP1; also known as TERF2IP) also interact indirectly with double-stranded telomeric DNA through their interactions with other shelterin proteins. Reprinted by permission from Macmillan Publishers Ltd.. Cesare, A. J., & Reddel, R. R. (2010). Alternative lengthening of telomeres: Models, mechanisms, and implications. Nature Reviews Genetics, 11, Fig. 1, p. 320.
Depending on the cell type, cell division may occur up to 50 times before cell cycle checkpoints activate genetic programs of replicative senescence (Calado & Young, 2012). When telomeres become shortened to a critical length that can compromise genomic stability, chromosome ends activate DNA damage-response pathways that induce apoptosis. However, disruption of the shelterin proteins and total loss of telomeric DNA can promote the formation of end-to-end chromosome fusions, genetic mutations, neoplastic transformation, and altered gene expression (Bisoffi, Heaphy, & Griffith, 2006). Thus, TL is a critical variable in aging as well as susceptibility to disease.
Different chromosomes have varied rates of telomere attrition. Leukocyte telomere length (LTL) and attrition have been the most extensively studied, and research has shown them to vary greatly among individuals (Shammas, 2011). There are strong associations among telomere length in hematopoietic progenitor cells, T lymphocytes, and granulocytes (Kimura et al., 2010). The relationship between telomere length in leukocytes and granulocytes throughout the life span suggests a role for hematopoietic stem cell telomere dynamics in the biology of human aging. However, the rate of telomere shortening is dependent on oxidation-induced strand breaks in telomeric DNA, cellular oxidant balance, and the activity of the reverse transcriptase telomerase and other telomere maintenance proteins.
The telomere sequence can be elongated by telomerase as well as through nontelomerase-dependent processes termed alternative lengthening of telomeres. Telomerase is an enzyme that adds bases to the ends of telomeres by binding to the open end of the G-strand. The major components of the active telomerase complex are telomerase (TERT), a reverse transcriptase that uses an RNA molecule (TERC) as a template to extend telomeres in cells, and dyskerin, a protein that binds to both TERT and TERC to increase the stability of the telomere–shelterin complex (Calado & Young, 2012). Highly proliferative cells, such as stem cells, progenitor cells, lymphocytes, and skin keratinocytes, can maintain levels of telomerase over long periods of time. However, most tissues do not express, or express very low levels of, telomerase. In contrast, telomerase activity is elevated in many types of cancer. Along with alterations that initiate tumorigenesis including failure of the DNA damage-response pathway, inhibition of tumor suppressor genes, and activation of oncogenes, telomerase activity can promote cancer progression by enabling continuous cell division.
Telomerase can be measured over a short duration (hours) to demonstrate immediate and short-term changes, as opposed to telomere length, changes which take months to years to be detected. Acute psychological stress is positively associated with telomerase activity, suggesting an intrinsic cytoprotective response that reduces oxidative stress and telomere attrition (Epel et al., 2010). In contrast, Epel et al. (2004) found that TL and telomerase activity were reduced in chronically stressed caregivers, which may indicate an association with the duration or intensity of stress and exhaustion of protective coping mechanisms. Although our understanding of the telomere maintenance system continues to increase, it is probably premature for most studies to rely on measurement of telomerase activity, alone, until the dynamic changes in this enzyme have been better characterized (Beery et al., 2012).
Incorporating Telomeric Measures Within the Biobehavioral Framework
Biobehavioral research is focused on understanding the biological and behavioral interactions that influence health and disease. This body of research has revealed significant relationships among the external environment, the internal cellular environment, and the health outcomes. Innovations in epigenetic and genetic measurement have accelerated this line of inquiry by providing a means for researchers to probe deeper into the physiologic mechanisms that contribute to disease. However, the characterization and measurement of environmental (both internal and external) exposures that influence (epi)genetic mechanisms has lagged behind (Buck Louis & Sundaram, 2012). Given the significant impact of early stressful exposures (chronic or serious childhood illness) on adult health and the contributory role of the environment in 70–90% of chronic diseases, it is equally, if not more, important to characterize and measure exposures across the life span (Lin, Epel, & Blackburn, 2012). This imperative has led to the development of the exposome paradigm, which posits that modifiable and nonmodifiable internal and external (nongenetic) exposures acquired across the life span (see Table 1), in conjunction with the (epi)genetic infrastructure, contribute to health outcomes (Wild, 2012). The exposome complements biobehavioral genomic research by recognizing the importance of gene–environment interactions and the potential for environmental exposures to affect epigenetic expression. Identification of the environmental exposures that contribute to alterations in (epi)genetic mechanisms of disease could guide the development of prevention strategies to reduce disease risk as well as new treatments for chronic diseases (Olden, Freudenberg, Dowd, & Shields, 2011). The exposome incorporates a life span approach, recognizing that there are periods of growth and development during which individuals are particularly vulnerable to the effects of environmental exposures on health.
Table 1.
Internal and External Exposures That Influence Telomere Length.
| Modifiable | Nonmodifiable | |
|---|---|---|
| Internal exposures |
Oxidative stress Inflammation Body morphology Physiologic comorbidities Physical activity Psychological and mental health Perceived stress |
Chronological age Endogenous circulating hormones Genetic predisposition |
| External exposures |
Lifestyle factors Dietary intake Smoking Alcohol consumption Occupation Sleep deprivation |
Environmental pollutants Chemical contaminants Radiation Infectious agents Climate |
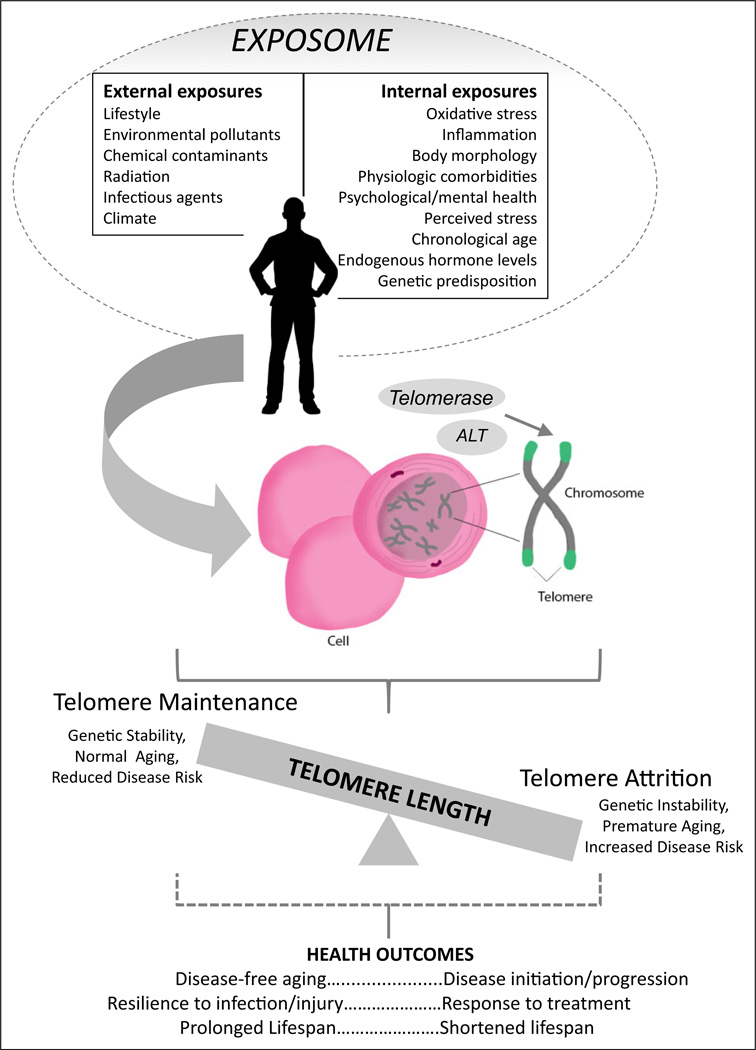
Although the intention of the exposome is to address the totality of environmental exposures from conception onward, there are fundamental internal and external exposures to consider, many of which directly influence telomere biology (Wild, 2011). Internal exposures include aging, inflammation, oxidative stress, psychological conditions, and perceived stress as well as endogenous circulating hormones, body morphology, and physical activity (Wild, 2012). External environmental exposures may include pollutants and chemical contaminants, lifestyle factors such as smoking and alcohol consumption, diet, and psychosocial/economic influences on the individual. Oxidative stress, inflammation, and aging are mechanisms that directly affect TL, rate of attrition, and telomerase activity. These measures thereby represent appropriate biomarkers for these gene–environment interactions. However, each of the other exposure types has also been shown to positively or negatively influence telomere maintenance and therefore must be considered when incorporating telomeric measures in biobehavioral research. Figure 2 illustrates a proposed biological cascade in which internal and external exposures affect the chromosomes, potentially disrupting the telomerase/telomere balance and resulting in adverse health outcomes.
Figure 2.
Model of biobehavioral relationships. Biobehavioral aspects of the exposome and health outcomes to be considered when integrating measures of telomeres into biobehavioral research.
Internal Modifiable Exposures
Multiple internal exposures can mediate TL, rate of attrition, and telomerase activity. Oxidative stress and inflammation directly affect telomere biology and also serve as mechanisms through which other modifiable factors exert their effects.
Oxidative stress
Oxidative stress is an imbalance between oxidants, or reactive oxygen species (ROS), and antioxidants that can result in DNA damage and disruption of normal cell function. It is a common putative etiologic mechanism across a wide range of chronic diseases. Telomeres are especially sensitive to damage from oxidative stress because of the high content of guanine residues (Zhu et al., 2011). Prolonged oxidative stress accelerates telomere shortening and decreases telomerase activity (Matthews et al., 2006). The challenge for clinical research is the dynamic nature of oxidative stress and the lack of clarity regarding the threshold at which telomeres are affected.
Inflammation
Cells of the immune system continue to divide throughout the life span. However, inflammatory conditions may accelerate telomere shortening due to a higher rate of cell turnover (Zhu et al., 2011). Higher levels of C-reactive protein, an index of inflammation, as well as of some proinflammatory cytokines are associated with shorter telomeres (Aviv et al., 2006). As an underlying mechanism of numerous disease states, inflammation contributes to the associations among environment, lifestyle factors, and TL (Lin et al., 2012). However, as with oxidative stress, inflammation presents challenges to researchers because of its dynamic nature and the need for more information regarding thresholds at which adverse effects on telomeres and telomerase begin to occur.
Body morphology
Aspects of body morphology such as body mass index, total and abdominal adiposity, and waist circumference are negatively associated with TL (Lee, Martin, Firpo, & Demerath, 2011; Valdes et al., 2005). Valdes et al. (2005) estimated that excessive loss of telomeres due to obesity was equivalent to 8.8 years of life. ROS and the associated DNA damage, which is strongly associated with body mass index, is a significant moderator of this relationship (Shammas, 2011).
Physiologic comorbidities
As discussed earlier, TL reflects the cumulative burden of oxidative stress and inflammation, which are predominant mechanisms in a host of chronic disease states including cardiovascular disease, diabetes, heart failure, and cancer (Zhu et al., 2011). Of importance to clinical research is the ability to account for and quantify the contributory role of physiological comorbidities on TL. However, as Mainous et al. (2010) demonstrated in a study in which they found a negative association between TL and the presence of atherosclerosis in a low-risk, middle-aged cohort free of previously diagnosed cardiovascular disease, determining the precise role of physiological comorbidities on TL can be challenging.
Psychological and mental health conditions
Psychological characteristics, including personality traits and psychological comorbidities, can also moderate telomere attrition and risk of chronic disease. Accelerated telomere attrition is associated with pessimism (O’Donovan et al., 2009), psychological distress (Huzen et al., 2010), and mood disturbances (Simon et al., 2006). Authors have also reported increased telomere shortening in individuals with major depressive disorder and schizophrenia (Kao et al., 2008; Yu, Chang, Lin, & Cho, 2008), but the duration of illness appears to be a moderator of this relationship (Elvsåshagen et al., 2011; Wolkowitz et al., 2011).
Perceived stress
Although some psychological characteristics may have a relatively high rate of heritability, it is widely accepted that perceptions of stress, more than the level of stress, are more likely to affect TL. The initial study that linked psychological stress with telomere shortening helped to cast telomeres as a potential biomarker of the mind–body connection that is central to the biobehavioral framework (Epel et al., 2004). In this seminal study, investigators found associations among perceived stress, duration of caring for a chronically ill child, short TL, and decreased telomerase activity among premenopausal women. An emerging body of literature also supports a relationship among early childhood adversity and shorter TL in adulthood (Kananen et al., 2010; Tyrka et al., 2010). In another study, perceived stress associated with poverty and waist–hip ratio accounted for shorter telomeres in Black women compared to White women in the United States (Geronimus et al., 2010).
Physical activity
Cherkas et al. (2008) found a positive association between the amount of physical activity and TL in a national sample of twins, as did Werner et al. (2009) in a study comparing trained athletes with sedentary controls. Exercise appears to protect against the effects of perceived stress on telomere shortening. Puterman et al. (2010) found that a 1-unit increase in perceived stress was related to a 15-fold increase in the odds of having short telomeres among nonexercisers, whereas perceived stress was unrelated to TL in exercisers.
Internal Nonmodifiable Exposures
Genetic predisposition
Reported heritability of telomere length ranges from 40% to 80%, and investigators have found evidence of linkage to autosomal regions in genome-wide studies (Andrew et al., 2006). Although there is no gender difference in TL at birth in full-term infants, in some cohorts, adult females have longer telomeres than adult males, possibly due to enhanced repair/recombination processes (Moller et al., 2008). In addition, researchers have noted differences in telomere length among different racial groups, with African Americans having significantly longer telomeres compared with European Americans, Latinos, and Asians (Schaefer et al., 2012). Heterozygous TERT or TERC mutations that result in telomerase deficiency produce offspring with short telomeres, while homozygous mutations appear to express normal telomerase levels (Zhu et al., 2011).
Chronological age
TL decreases with age as a normal cellular process at a rate of approximately 24.8–27.7 base pairs per year (Valdes et al., 2005). Consequently, healthy older adults can have a normal reduction in telomere length to 1,500 base pairs (Shammas, 2011). The average TL for a specific age-group is used to classify shortened TL, which is associated with increased incidence of age-related disease and decreased life span (Schaefer et al., 2012).
Endogenous circulating hormones
Research has shown that endogenous circulating hormones affect telomerase activity and TL. Estrogen upregulates hTERT gene expression and increases telomerase activity (Kyo et al., 1999), exerts anti-inflammatory effects (Knowlton & Lee, 2012), and reduces ROS production as well as oxidative stress (Fuster & Andres, 2006)—all of which offer protection against telomere shortening. Androgen therapy stimulates TERT gene transcription and increases telomerase activity in normal and TERT-mutant lymphocytes and in bone marrow CD34+ cells (Calado et al., 2009). Level of aldosterone, a pro-oxidant hormone, has an inverse relationship with TL (Benetos et al., 2005). Fasting insulin level, a measure of insulin resistance, also has a significant inverse association with TL (Al-Attas et al., 2010; Demissie et al., 2006). In contrast, hormones that improve insulin sensitivity or decrease inflammation may protect against telomere shortening.
External Modifiable Exposures
Lifestyle factors
Human beings exhibit lifestyle patterns across their life span, which are relevant to telomere maintenance. Although lifestyle factors are dynamic, there are particular exposures, doses of exposure, and exposure timing during the life span that can significantly affect TL, rate of attrition, and telomerase activity.
Dietary intake
TL has been positively associated with dietary intake of fiber and negatively associated with intake of polyunsaturated fatty acids (Cassidy et al., 2010). Micronutrients such as vitamins C and E, folic acid, and omega-3 fatty acids are associated with antioxidative function and longer telomeres. Farzaneh-Far et al. (2010) found that increased dietary intake of antioxidant omega-3 fatty acids was associated with a reduced rate of telomere shortening, whereas an increased rate of telomere attrition was associated with a lack of dietary antioxidants. Similarly, researchers reported that multivitamin use in women aged 35–74 years was associated with longer telomeres compared to nonusers after adjusting for age and other confounders (Xu et al., 2009). In addition, Shammas (2011) found that a low-protein diet and reduced caloric intake decrease oxidative burden, DNA damage, and telomere attrition.
Smoking, alcohol consumption, and sleep deprivation
Smoking is associated with shorter TL and accelerated telomere shortening, a relationship that is dosage dependent (Kanenen et al., 2010; McGrath, Wong, Michaud, Hunter, & De Vivo, 2007; Morla et al., 2006). For example, smoking one pack of cigarette each day causes an additional loss of five base pairs (Valdes et al., 2005), and telomere attrition due to smoking one pack daily for a period of 40 years is equivalent to 7.4 years of life (Shammas, 2011). Excessive alcohol consumption is associated with oxidative stress and shorter telomeres; however, even low levels of alcohol consumption in midlife have been associated with shorter telomeres in old age (Strandberg et al., 2012). Research has shown sleep duration and quality to be positively associated with telomere length, particularly in women aged <50 years (Liang et al., 2011; Prather et al., 2011). Lin, Epel, and Blackburn (2012) found that women who had 6 or less hours of sleep per night had shorter telomeres after adjusting for age, body mass index, and cigarette smoking when compared to women who reported 9 or more hours of sleep nightly. The authors suggest that melatonin production, which increases during sleep, may play a protective role in reducing oxidative stress. Research has also demonstrated relationships between work patterns (full time, part time, overtime, unemployment) and TL. For example, Parks et al. (2011) found that shorter TL was associated with a history of and current long-term, full-time work in women, although retired women had a significantly longer TL than homemakers of the same age. Although investigators have studied the relationships between TL and telomere attrition and numerous other lifestyle factors including education level, socioeconomic status, and social standing, the findings remain mixed (Lin et al., 2012).
External Nonmodifiable Exposures
Chemical contaminants and radiation
Levels of toluene and benzene, components of traffic pollution, have been associated with shorter TL. Hoxha et al. (2009) compared TL in leukocytes of traffic police officers and office workers and found that it was shorter in traffic police officers within each age-group. Pavanello et al. (2010) found that reduction in TL is associated with the duration of exposure to polycyclic aromatic hydrocarbons in male coke-oven workers not currently smoking and matched controls. In addition, the authors found that hypomethylation of the p53 promoter, which enhances induction of apoptosis, was associated with reduced TL in lymphocytes. Although Maeda et al. (2013) found that TL and distribution did not change in cancer patients exposed to radiation treatment, they did find a significant proportional decrease in the short telomere fraction per day and per Gy or radiation dose.
Conclusion and Nursing Implications
The philosophical and scientific basis of the exposome is congruent with Florence Nightingale’s vision that “nursing is achieved through environmental alteration” (Selanders, 1998, p. 252). Enhanced understanding of environmental influences on health outcomes at the molecular level provides a link from Nightingale’s 19th-century vision of nursing science to a 21st-century scientific paradigm that encompasses the person (genetic factors) and environment in a new conceptualization. Although there are challenges to measuring the exposome, improved characterization of exposure source, dosage, and duration across the life span will enhance the ability to identify pathoetiologic mechanisms of disease initiation and occurrence and, in the future, to develop targeted interventions to address exposures and measure progress toward diminishing risks.
A biobehavioral framework that incorporates the exposome paradigm lends itself to utilizing TL, rate of attrition, and telomerase activity as biomarkers of gene–environment interactions and provides a foundation to deepen understanding of the associations between TL and health and disease risk. There are overlaps between internal and external as well as modifiable and nonmodifiable exposures due to the interplay between the environment and the biological responses. Although genetic predisposition is a nonmodifiable internal factor, there is increasing evidence that epigenetic factors are also important regulatory mechanisms that may heighten or ameliorate genetic predisposition. Future findings pertaining to the effects of exposure source, dose, and duration on telomere biology; the threshold at which telomere shortening increases risk of disease; and dose/duration of exposure modifications that prevent or reduce telomere attrition will likely be translated into clinical practice, increasing the ability to assess and manage risk and providing a basis for personalized disease prevention and treatment approaches.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Grant #R01 NR012667 (Lyon & Jackson-Cook, MPI) 10/01/2010-8/30/2015, received from Epigenetics and Psychoneurologic Symptoms in Women with Breast Cancer and grant #P30 NR011403 (Pickler/Grap) 08/10/09-05/31/14 received from Center of Excellence in Biobehavioral Approaches to Symptom Management.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Al-Attas O, Al-Daghri NM, Alokail MS, Alfadda A, Bamakhramah A, Sabico S, Chrousos G. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: The influence of circulating adiponectin. European Journal of Endocrinology. 2010;163:601–607. doi: 10.1530/EJE-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, Spector TD. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. American Journal of Human Genetics. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. Journal of Clinical Endocrinology and Metabolism. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- Benetos A, Gardner JP, Kumura M, Labat C, Nzietchueng R, Dousset B, Aviv A. Aldosterone and telomere length in white blood cells. Journal of Gerontology. 2005;60:1593–1596. doi: 10.1093/gerona/60.12.1593. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. Chronic stress elevates telomerase activity in rats. Biological Letters. 2012;8:1063–1066. doi: 10.1098/rsbl.2012.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoffi M, Heaphy CM, Griffith JK. Telomeres: Prognostic markers for solid tumors. International Journal of Cancer. 2006;119:2255–2260. doi: 10.1002/ijc.22120. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Walking the walk from genes through telomere maintenance to cancer risk. Cancer Prevention Research. 2011;4:473–475. doi: 10.1158/1940-6207.CAPR-11-0066. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R. Exposome: Time for transformative research. Statistics in Medicine. 2012;31:2569–2575. doi: 10.1002/sim.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratskis CA, Young NS. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado R, Young N. Telomeres in disease. F1000 Reports Medicine. 2012;4:8–15. doi: 10.3410/M4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy A, De Vivo I, Lui Y, Han J, Prescott J, Hunter DJ, Rimm EB. Associations between diet, lifestyle factors, and telomere length in women. American Journal of Clinical Nutrition. 2010;91:1273–1280. doi: 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Archives of Internal Medicine. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Elvsåshagen T, Vera E, Bøen E, Bratlie J, Andreassen OA, Josefsen D, Boye B. The load of short telomeres increased and associated with lifetime number of depressive episodes in bipolar disorder. Journal of Affective Disorders. 2011;135:43–50. doi: 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Science United States of America. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Lin J, Dhabhar FA, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. Dynamics of telomerase activity in response to acute psychological stress. Brain, Behavior, and Immunity. 2010;24:531–539. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association with marine omega-2-fatty acid levels with telomeric aging in patients with coronary heart disease. Journal of the American Medical Association. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circulation Research. 2006;99:1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Dawson Cruz T. Do US Black women experience stress-related accelerated biological aging? A novel theory and first population-based test of Black–White differences in telomere length. Human Nature. 2010;21:19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxha M, Dioni L, Bonzini M, Pesatori AC, Fustinoni S, Cavallo D, Baccerelli A. Association between leukocyte telomere shortening and exposure to traffic pollution: A cross sectional study on traffic officers and indoor office workers. Environmental Health. 2009;8:41. doi: 10.1186/1476-069X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzen J, van der Harst P, de Boer RA, Lesman-Leegte I, Voors AA, van Gilst WH, van Veldhuisen DJ. Telomere length and psychological well-being in patients with chronic heart failure. Age and Ageing. 2010;39:223–227. doi: 10.1093/ageing/afp256. [DOI] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLOS. 2010;5:1–7. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HT, Cawthon RM, Delisi LE, Bertisch HC, Ji F, Gordon D, Porton B. Rapid telomere erosion in schizophrenia. Molecular Psychiatry. 2008;13:118–119. doi: 10.1038/sj.mp.4002105. [DOI] [PubMed] [Google Scholar]
- Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Experimental Hematology. 2010;38:854–859. doi: 10.1016/j.exphem.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacology & Therapeutics. 2012;135:54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Inoue M. Estrogen activates telomerase. Cancer Research. 1999;59:5917–5921. [PubMed] [Google Scholar]
- Lee M, Martin H, Firpo MA, Demerath EW. Inverse association between adiposity and telomere length: The Fels longitudinal study. American Journal of Human Biology. 2011;23:100–106. doi: 10.1002/ajhb.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Schernhammer E, Qi L, Gao X, De Vivo I, Han J. Associations between rotating night shifts, sleep duration, and telomere length in women. PLoS ONE. 2011;6:e23462. doi: 10.1371/journal.pone.0023462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: Roles in cellular aging. Mutation Research. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mainous AG, III, Codd V, Diaz VA, Schoepf UJ, Everett CJ, Player MS, Samani NJ. Leukocyte telomere length and coronary artery calcification. Atherosclerosis. 2010;210:262–267. doi: 10.1016/j.atherosclerosis.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Maeda T, Nakamura K, Atsumi K, Hirakawa M, Ueda Y, Makino N. Radiation-associated changes in the length of telomeres in peripheral leukocytes from inpatients with cancer. International Journal of Radiation Biology. 2013;89:106–109. doi: 10.3109/09553002.2013.734945. [DOI] [PubMed] [Google Scholar]
- Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circulation Research. 2006;99:156–163. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiology, Biomarkers and Prevention. 2007;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- Moller P, Mayer S, Mattfeldt T, Muller K, Wiegand P, Bruderlein S. Sex-related differences in length and erosion dynamics of human telomeres favor females. Aging. 2008;1:733–739. doi: 10.18632/aging.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. European Respiratory Journal. 2006;27:525–528. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Lin J, Tillie J, Dhabhar FS, Wolkowitz OM, Blackburn EH, Epel E. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in postmenopausal women. Brain, Behavior and Immunity. 2009;23:446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeseburg H, de Boer RA, van Gilst WH, van der Harst P. Telomere biology in healthy aging and disease. European Journal of Physiology. 2010;459:259–268. doi: 10.1007/s00424-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K, Freudenberg N, Dowd J, Shields AE. Discovering how environmental exposures alter genes could lead to new treatments for chronic diseases. Health Affairs. 2011;30:833–840. doi: 10.1377/hlthaff.2011.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CG, DeRoo LA, Miller DB, McCanlies EC, Cawthon RM, Sandler DP. Employment and work schedule are related to telomere length in women. Occupational & Environmental Medicine. 2011;68:582–589. doi: 10.1136/oem.2010.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, Pesatori AC, Dioni L, Hoxha M, Bollati V, Siwinska E, Baccarelli A. Shorter telomere length in peripheral blood lymphocytes of workers exposed to polycyclic aromatic hydrocarbons. Carcinogenesis. 2010;31:216–221. doi: 10.1093/carcin/bgp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Puterman E, Lin J, O’Donovan A, Kraus J, Tomiyama AJ, Blackburn EH. Shorter leukocyte telomere length in midlife women with poor sleep quality. Journal of Aging Research. 2011;2011:721390. doi: 10.4061/2011/721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: Buffering the effect of chronic stress on telomere length. PLOS. 2010;5:1–6. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer C, Sciortino S, Kvale M, Lapham J, Lin D, Ranatunga D, Blackburn E. The Kaiser permanente/UCSF genetic epidemiology research study on adult health and aging: demographic and behavioral influences on telomeres and relationship with all-cause mortality; Paper presented at the American Society of Human Genetics; San Francisco, CA. 2012. Nov, [Google Scholar]
- Selanders LC. The power of environmental adaptation: Florence Nightingale’s original theory for nursing practice. Journal of Holistic Nursing. 1998;16:247–263. doi: 10.1177/089801019801600213. [DOI] [PubMed] [Google Scholar]
- Shammas MA. Telomeres, lifestyle, cancer and aging. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14:28–34. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Wong KK. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biological Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Strandberg AY, Saijonmaa O, Tilvis RS, Pitkala KH, Fyhrquist F. Association between alcohol consumption in healthy midlife and telomere length in older men. The Helsinki businessmen study. European Journal of Epidemiology. 2012;27:815–822. doi: 10.1007/s10654-012-9728-0. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Laufs U. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- Wild CP. Future research perspectives on environment and health: The requirement for a more expansive concept of translational cancer research. Environmental Health. 2011;10:S15. doi: 10.1186/1476-069X-10-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP. The exposome: From concept to utility. International Journal of Epidemiology. 2012;41:24–32. doi: 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Blackburn EH. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress: Preliminary findings. PLoS ONE. 2011;6:e1737. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. Journal of Psychiatry and Neuroscience. 2008;33:244–247. [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Parks CG, DeRoo LA, Cathon RM, Sandler DP, Chen H. Multivitamin use and telomere length in women. American Journal of Clinical Nutrition. 2009;89:1857–1863. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni GR, Wick JY. Telomeres: Unlocking the mystery of cell division and aging. Consultant Pharmacist. 2011;26:78–90. doi: 10.4140/TCP.n.2011.78. [DOI] [PubMed] [Google Scholar]
- Zhu H, Belcher M, van der Harst P. Healthy aging and disease: Role for telomere biology? Clinical Science. 2011;120:427–440. doi: 10.1042/CS20100385. [DOI] [PMC free article] [PubMed] [Google Scholar]