Abstract
Study Objective
Our aim was to assess incidence and risk factors for pelvic pain after pelvic mesh implantation.
Design
Retrospective study (Canadian Task Force classification II-2).
Setting
Single university hospital.
Patients
Women who have undergone surgery with pelvic mesh implant for treatment of pelvic floor disorders including prolapse and incontinence.
Interventions
Telephone interviews to assess pain, sexual function, and general health.
Measurements and Main Results
Pain was measured by the McGill Short-Form Pain Questionnaire for somatic pain, Neuropathic Pain Symptom Inventory for neuropathic pain, Pennebaker Inventory of Limbic Languidness for somatization, and Female Sexual Function Index (FSFI) for sexual health and dyspareunia. General health was assessed with the 12-item Short-Form Health Survey. Among 160 enrolled women, mean time since surgery was 20.8 ± 10.5 months, mean age was 62.1 ± 11.2 years, 93.8% were white, 86.3% were postmenopausal, and 3.1% were tobacco users. Types of mesh included midurethral sling for stress incontinence (78.8%), abdominal/robotic sacrocolpopexy (35.7%), transvaginal for prolapse (6.3%), and perirectal for fecal incontinence (1.9%), with 23.8% concomitant mesh implants for both prolapse and incontinence. Our main outcome, self-reported pelvic pain at least 1 year after surgery, was 15.6%. Women reporting pain were younger, with fibromyalgia, worse physical health, higher somatization, and lower surgery satisfaction (all p < .05). Current pelvic pain correlated with early postoperative pelvic pain (p < .001), fibromyalgia (p = .002), worse physical health (p = .003), and somatization (p = .003). Sexual function was suboptimal (mean FSFI, 16.2 ± 12.1). Only 54.0% were sexually active, with 19.0% of those reporting dyspareunia.
Conclusion
One in 6 women reported de novo pelvic pain after pelvic mesh implant surgery, with decreased sexual function. Risk factors included younger age, fibromyalgia, early postoperative pain, poorer physical health, and somatization. Understanding risk factors for pelvic pain after mesh implantation may improve patient selectionq.
Keywords: Mesh, Pelvic pain, Prolapse, Stress urinary incontinence
Pelvic mesh implant surgery is commonly used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and less commonly used for fecal incontinence (FI). It is estimated that by age 80, 20% of US women will have undergone surgery for the treatment of POP and/or SUI [1]. There is no consensus as to whether surgical repair should be augmented with mesh and what type of mesh to use. In 2008 the US Food and Drug Administration (FDA) released a Safety Notification for the use of pelvic mesh, based on over 1000 reports of complications reported to the Manufacturer and User Device Experience database [2]. Complications with mesh implant surgery reported in the database include pelvic pain, vaginal scarring, mesh erosion or exposure, dyspareunia, infection, urinary problems, bowel/bladder/blood vessel perforation, and POP/SUI recurrence. In 2011 the FDA released an updated Safety Communication based on a growing number of reported complications associated with mesh implant surgery [2]. These reports have led to considerable controversy regarding the use of mesh implants in pelvic surgery for POP and SUI.
A systemic review published in 2008 reported outcomes with mesh use in transvaginal POP repair [3]. The authors found weak evidence supporting mesh augmentation in the anterior vaginal compartment in terms of POP repair, but with higher rates of mesh-related complications. Mesh complications included bleeding (0–3%), visceral injury (1–4%), urinary infection (0–19%), graft erosion (0–30%), and fistula (1%). Data were insufficient regarding pelvic pain and sexual dysfunction. In 2015 another systematic review compared the use of abdominal mesh for sacrocolpopexy with native tissue vaginal repair [4]. Moderate quality evidence favored sacrocolpopexy over native tissue repair for successful surgical treatment of POP. However, complications were higher in the sacrocolpopexy group, including ileus or small bowel obstruction (2.7% vs .2%), mesh or suture complications (4.2% vs .4%), and thromboembolism (.6% vs .1%).
Although mesh augmentation can improve anatomic outcomes, it is associated with higher complication rates such as erosion, pain, and sexual dysfunction that can lead to reoperation [5–9]. The risk of reoperation for mesh complications in the mesh group must be weighed against the risk of reoperation for POP recurrence in the native tissue group [10–12]. Unfortunately, it is difficult to remove a mesh implant in its entirety. Although reoperation for mesh complications can usually address mesh erosion, it is less successful in treating pelvic pain related to mesh implants [13]. This may be due to underlying peripheral neuralgia as well as centrally mediated hypersensitization of neural pathways.
The incidence of persistent pain after pelvic surgery with mesh is not well understood. It is hypothesized that women with other pain syndromes may be at higher risk of developing chronic pelvic pain after mesh implant surgery, but this has not been directly reported for this type of surgery. Literature in other types of gynecologic surgery has shown that baseline preoperative pain is a predictor of chronic postoperative pain after hysterectomy [14]. Similarly, a meta-analysis of patients undergoing total knee arthroplasty found that pain at other sites, catastrophizing, and depression were all predictors of chronic postoperative pain [15]. Thus, our primary objective was to determine the incidence of chronic pelvic pain after mesh implant surgery for the treatment of POP and/or SUI at least 1 year after surgery. Our secondary objective was to identify patient and surgical factors associated with the development of postoperative pelvic pain after pelvic mesh implant surgery.
Methods
After institutional review board approval, women were identified from a surgical databased who had undergone pelvic mesh implant surgery between July 2011 and April 2014 with an attending surgeon in the University of North Carolina at Chapel Hill Division of Female Pelvic Medicine and Reconstructive Surgery. STROBE guidelines were followed [16]. These women were contacted via telephone and offered study enrollment. Assessments were conducted over the telephone. Exclusion criteria were prior pelvic mesh surgery, repeat pelvic surgery after the index surgery (with or without mesh), baseline self-reported pelvic pain, or less than 1 year since index surgery. Concomitant native tissue repair was allowed. Although pre-existing pelvic pain was an exclusion, some subjects did report other baseline chronic pain states, including fibromyalgia, temporomandibular joint pain, arthritis, and chronic back pain. These diagnoses were elicited during subject interview and confirmed in the medical record. Mesh erosion was assessed by querying the medical record based for International Classification of Diseases, Ninth Revision (ICD-9) codes 629.31 and 629.32 for the diagnosis of “erosion of mesh into pelvic and non-pelvic organs” and for cases of surgical mesh revision and also during subject interview.
Eligible women were those who had undergone mesh implant surgery with any of the following procedures: transvaginal midurethral mesh sling for SUI (designated by Current Procedural Terminology [CPT] code 57288), abdominal or robotic sacrocolpopexy mesh for POP (CPT codes 57280 and 57425), transvaginal mesh for POP (CPT code 57267), or transperineal perirectal mesh for FI (CPT code 57267). Subjects who underwent more than 1 mesh implant (i.e., mesh for SUI and POP) were included. Multiple mesh implants involved 2 subgroups: (1) transvaginal mesh implant for POP and transvaginal mesh sling for SUI or (2) sacrocolpopexy mesh implant for POP and transvaginal mesh sling for SUI. Specific mesh brands used for POP repair included Prolene (Ethicon, Somerville, NJ) mesh for abdominal sacrocolpopexy, IntePro (American Medical Systems, Minnetonka, MN) and Alyte (Bard Medical, Covington, GA) mesh for robotic sacrocolpopexy, Uphold (Boston Scientific, Marlborough, MA) mesh for transvaginal POP repair, and TOPAS (American Medical Systems) perirectal mesh for FI. Specific mesh brands used for transvaginal SUI repair included SPARC (American Medical Systems) pubovaginal sling, TVT-Exact (Ethicon) pubovaginal sling, and Monarch (American Medical Systems) transobturator sling. The electronic medical record was used to review the operative report for data including surgical technique, estimated blood loss, and any surgical complications.
For our main outcome, current pelvic pain was defined by self-report of any level of pain between the umbilicus and groin present for ≥6 months and occurring on at least a weekly basis. Pain quality was measured using the McGill Short-Form Pain Questionnaire (MPQ) for somatic pain, the Neuropathic Pain Symptom Inventory (NPSI) for neuropathic pain, and the Pennebaker Inventory of Limbic Languidness (PILL) for somatization. The MPQ contains 11 verbal descriptors assessing sensory components of pain, 5 verbal descriptors assessing affective components of pain, and 1 verbal descriptor describing pain intensity [17]. The MPQ has been successfully used to quantify chronic pelvic pain after surgery [18]. Maximum score is 45 (33 on the Somatic subscale and 12 on the Affective subscale), with higher scores indicating worse somatic pain. The NPSI has been used to characterize neuropathic pain and to identify treatment responders and nonresponders [17–19]. It allows differentiation of subtypes of neuropathic pain, including burning pain, pressing pain, paroxysmal pain, evoked pain, and parasthesia pain. The highest possible score is 100, with higher scores indicating worse neuropathic pain. The PILL assesses somatization, which is the expression of psychological distress with physical symptoms [19]. Elevated PILL scores in pain patients are highly correlated with the number of tender muscle sites, pain sensitivity, and progression to chronicity. The maximum possible score is 216, with higher scores indicating higher levels of somatization. A score > 66 is considered to be above the average range, whereas a score > 84 is considered highly elevated.
Sexual health and dyspareunia were measured with the Female Sexual Function Index (FSFI) [20], a 19-item questionnaire that measures physiologic and affective aspects related to sexual arousal and sexual activity. It contains 6 subscales, including Desire, Arousal, Lubrication, Orgasm, Satisfaction, and Pain. Subjects who are not sexually active can still be fully evaluated with this tool. The FSFI has been used to reliably assess sexual dysfunction associated with pelvic pain [21], including chronic pelvic pain after pelvic mesh implant surgery [22]. The highest possible score is 36, with higher score indicating better sexual health. Dyspareunia was defined as a score of 2.8 or less on the FSFI Pain subscale (describing “moderate” or “severe” pain “most of the time” with intercourse).
General health was assessed with the 12-item Short-Form Health Survey (SF-12) Physical and Mental subscales. The SF-12 is a valid and reliable measure of mental and physical health status reflecting the values and preferences for health from the person’s perspective [23]. The SF-12 has been used to evaluate the impact of therapeutic strategies on quality of life in women with chronic pelvic pain [24]. The maximum possible score is 100 for each subscale, with scores greater than 50 indicating above-average health status and scores less than 50 indicating below-average health status. Finally, surgical satisfaction was assessed on a 10-point scale, with 0 being completely dissatisfied and 10 being completely satisfied. Demographic, medical, and surgical details were abstracted from electronic medical records. Postoperative pain was extracted from the medical record, specifically based on the documentation of continued pain at the 6-week postoperative visit, which is part of the standard assessment.
Statistical analysis was performed with IBM SPSS version 22.0 (IBM SPSS Inc., Chicago, IL). Pearson-χ2 test, Fisher’s exact test, and Student’s t test and Spearman correlation were performed where appropriate. A p < .05 was considered statistically significant.
Results
Of 558 eligible women who underwent mesh implant surgery between July 2011 and April 2014, 398 either could not be reached (n = 342), were ineligible (n = 42), or declined (n = 14), leaving 160 women, who were enrolled. No women reported repeat pelvic mesh surgery since the index surgery. Mean time since surgery was 21 months (Table 1). Most study participants were over age 60, white, and postmenopausal. Tobacco use was rare (1.9%), with an average of half a pack per day for current smokers. Mean stage of prolapse before surgery was stage II for anterior (mean POP Quantification [POP-Q] System point Ba, .4 ± 2.3), stage II for posterior (mean POP-Q System point Bp, −1.0 ± 2.1), and stage I for apical/uterine (mean POP-Q System point C, −3.3 ± 4.4). Concurrent hysterectomy was performed in 23.8% of women, with the majority as total vaginal (50.0%), followed by total robotic (34.2%), robotic supracervical (10.5%), total laparoscopic (2.6%), and total abdominal (2.6%).
Table 1.
Clinical and surgical characteristics for all subjects, women reporting current pelvic pain, and women not reporting current pelvic pain at least 1 year after mesh implant surgery
| Demographic | All subjects (n = 160) |
Current pain (n = 24) |
No current pain (n = 136) |
p |
|---|---|---|---|---|
| Age, yr | 62.1 ± 11.2 | 57.2 ± 10.6 | 63.0 ± 11.1 | .02* |
| Body mass index | 27.8 ± 5.3 | 28.8 ± 4.8 | 27.7 ± 5.4 | .36* |
| Race | ||||
| White | 150 (94.9) | 23 (95.8) | 127 (94.8) | .99† |
| Black | 8 (5.1) | 1 (4.2) | 7 (5.2) | |
| Postmenopausal | 138 (90.2) | 18 (81.8) | 120 (91.6) | .23† |
| Parity | 2.3 ± 1.1 | 2.3 ± .8 | 2.3 ± 1.2 | .81* |
| Tobacco use (at time of surgery) | 3 (1.9) | 1 (4.2) | 2 (1.5) | .39† |
| Packs per day | .5 ± .0 | .0 ± .1 | .0 ± .0 | .43* |
| Fibromyalgia | 10 (6.5) | 5 (21.7) | 5 (3.8) | .007† |
| Temporomandibular joint pain | 5 (3.2) | 0 (.0) | 5 (3.8) | .99† |
| Arthritis | 41 (26.8) | 5 (21.7) | 36 (27.7) | .55‡ |
| Chronic back pain | 13 (8.5) | 1 (4.3) | 12 (9.2) | .69† |
| Months since surgery | 20.8 ± 10.5 | 19.1 ± 11.0 | 21.1 ± 10.4 | .37* |
| Number of implants | 1.2 ± .4 | 1.2 ± .4 | 1.2 ± .4 | .40* |
| Mesh location | ||||
| Vaginal prolapse mesh | 3 (1.9) | 0 (0) | 3 (2.2) | .11† |
| Vaginal SUI sling | 90 (56.3) | 15 (62.5) | 75 (55.1) | |
| SCP mesh | 26 (16.3) | 5 (20.8) | 21 (15.4) | |
| Rectal mesh | 3 (1.9) | 1 (4.2) | 2 (1.5) | |
| SCP mesh and SUI sling | 32 (20.0) | 1 (4.2) | 31 (22.8) | |
| Vaginal prolapse mesh and SUI sling | 6 (3.8) | 2 (8.3) | 4 (2.9) | |
| Mesh erosion | 1 (.6) | 0 (0) | 1 (.7) | .99† |
| Currently sexually active | 75 (46.9) | 12 (54.5) | 63 (53.8) | .95‡ |
Values are mean ± standard deviation or n (%).
Student’s t test.
Fisher’s exact test.
χ2 test.
Types of mesh implant included mesh sling for SUI (78.8%), abdominal or robotic sacrocolpopexy (35.7%), vaginal mesh for POP (6.3%), and perirectal mesh (1.9%). Types of slings used for the treatment of SUI included TVT-Exact (61.9%), Monarch TOT (18.3%), and SPARC (16.7%), with 2% not documented. Types of mesh used for sacrocolpopexy included Alyte (70.6%) and IntePro (11.8%) for robotic cases and Prolene (15.7%) for abdominal cases, with 12% not documented. All transvaginal mesh implants for the treatment of POP were performed with Uphold. All perirectal mesh implants for the treatment of FI were performed with Topas. Among all subjects, 23.8% had concomitant mesh implants for both POP and SUI. Table 1 displays the distribution of mesh by type for 2 study groups, those reporting current pain and those not reporting current pain, distinguishing single implants for POP or SUI from double implants for both POP and SUI. The rate of reported mesh erosion was .6%.
Surgery satisfaction was high at 8.1 for the entire cohort. Physical health was slightly below average, with a mean SF-12 Physical subscale score of 48.5 ± 10.5. Mental health was slightly above average, with a mean SF-12 Mental subscale score of 53.7 ± 9.3. Somatization was within normal range, based on a PILL mean score of 46.1 ± 25.5. For all subjects sexual function was moderately poor, based on a mean FSFI score of 16.2 ± 12.1; 54.0% of women were sexually active at the time of assessment, with 19.0% of those reporting dyspareunia.
Our primary outcome, the rate of current self-reported postoperative pelvic pain (as defined in Methods) at least 1 year after surgery, was 15.6%. We compared women reporting current postoperative pain with those denying current postoperative pelvic pain (Table 1). Women with current pelvic pain were younger (p = .02) and had a higher rate of fibromyalgia (p = .007). There were no differences in other demographics, including mean time since surgery, body mass index, smoking status, concurrent hysterectomy, other chronic pain diagnoses, mesh location, number of mesh implants, or mesh erosion. Women who reported current pelvic pain also demonstrated differences in general health and pain perception compared with those without pelvic pain. Specifically, women reporting pelvic pain demonstrated poorer physical health (SF-12 Physical score), more somatization (PILL score), and lower surgery satisfaction compared with women not reporting pelvic pain. Notably, there was no difference in the rate of sexual activity for women with and without pelvic pain (56.5% vs 53.4%) with no difference in sexual function based on FSFI score (Table 2).
Table 2.
General health, sexual health, and pain quality scores for women reporting current pelvic pain compared with women not reporting current pelvic pain at least 1 year after pelvic mesh implant
| Pain questionnaire | Current pain (n = 25) |
No current pain (n = 135) |
p |
|---|---|---|---|
| SF-12 Physical | 42.4 ± 12.2 | 49.8 ± 9.6 | .002* |
| SF-12 Mental | 51.4 ± 8.6 | 54.2 ± 9.4 | .19* |
| FSFI (all women) | 15.4 ± 11.5 | 16.4 ± 12.3 | .75* |
| FSFI (sexually active only) |
23.4 ± 7.7 | 26.1 ± 7.1 | .27* |
| NPSI† | 17.9 ± 12.8 | N/A | N/A |
| McGill† | 11.6 ± 8.2 | N/A | N/A |
| PILL | 62.1 ± 28.3 | 42.8 ± 23.8 | .001* |
N/A 5 not applicable.
Values are mean ± standard deviation or n (%).
Student’s t test.
Only measured in subjects reporting current pain.
Our secondary objective was to identify specific patient characteristics and surgical factors associated with pelvic pain after pelvic mesh implant surgery. Current pelvic pain at least 1 year after surgery was positively correlated with postoperative pelvic pain at the 6-week postoperative visit (rho = .8, p < .001) and with the presence of fibromyalgia (rho = .3, p = .002). Pain was inversely correlated with age (rho = −.2, p = .02), SF-12 Physical score (rho = −.3, p = .003), and PILL score (rho = −.3, p = .003), that is, women reporting pelvic pain were younger, with poorer physical health and increased somatization. Binomial regression was also performed. When assessing the effects of age, fibromyalgia, physical health (SF-12), and somatization (PILL), only age retained a significant association with current pelvic pain (p = .034; odds ratio, .95).
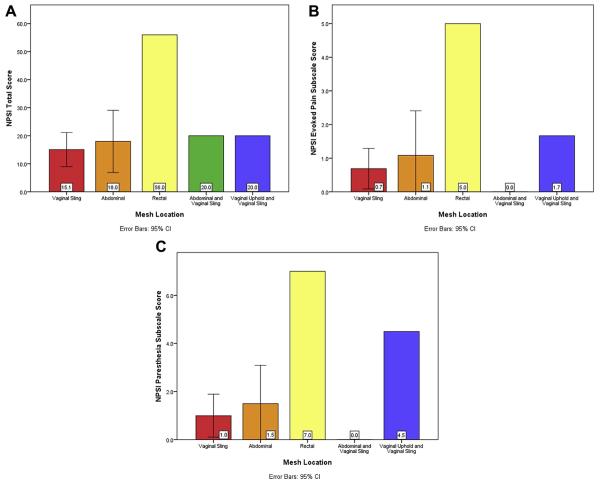
Women who reported current pelvic pain completed 2 additional pain questionnaires: the MPQ to assess somatic pain and the NPSI to assess neuropathic pain (Table 2). The level of somatic pain was mild, based on mean MPQ score. The level of neuropathic pain was relatively low based on mean NPSI score. Mesh location did not predict who would develop postoperative pain. However, in women reporting pelvic pain, there were differences in pain quality based on mesh location. When assessing pain scores based on mesh location, the following scores were significantly different: NPSI total (p = .026), NPSI Evoked Pain subscale (p = .014), and NPSI Parasthesia subscale (p = .008) (Fig). The mesh location with the highest rates of neuropathic pain based on NPSI scores was perirectal, followed by double-mesh implant of transvaginal mesh for POP and SUI. Sacrocolpopexy alone and sacrocolpopexy in combination with the midurethral sling for SUI had comparatively lower levels of neuropathic pain. Use of the midurethral sling for SUI with no other mesh had the lowest level of neuropathic pain. Tukey post-hoc analysis did not identify a specific mesh location as having statistically different scores from the others. There were no subjects in the vaginal Uphold-alone group who reported current pelvic pain, and thus they did not complete the NPSI questionnaire. Therefore, this group was not included in this analysis.
Fig.
Type of pain based on mesh location.
To account for nonresponders, a post-hoc analysis was performed, which showed no differences in the types of surgeries performed in this group compared with the responder group. Although both groups had a mean age in the postmenopausal range, the nonresponders were slightly younger at 58.7 years compared with 62.1 years for the responders (p = .003). No other demographic differences were found between the 2 groups.
Discussion
One in 6 women reported de novo pelvic pain at least 1 year after pelvic mesh implant surgery, with decreased sexual function. Associated factors included younger age, fibromyalgia, persistent early postoperative pain, poorer physical health, and increased somatization. Mesh location did not predict postoperative pelvic pain. Rather, all types of mesh implants were associated with some degree of somatic and neuropathic pain in the group reporting pain. In terms of pain quality, perirectal and double vaginal mesh implant had the highest rates of neuropathic pain, whereas sacrocolpopexy alone and sacrocolpopexy in combination with the midurethral sling for SUI had comparatively lower levels of neuropathic pain and vaginal sling for SUI had the lowest rates of neuropathic pain. Because subjects did not undergo a physical examination, we could not report on the nature of the reported pain in comparison with mesh location. However, in our clinical experience, pelvic pain after mesh implant is variable in presentation, is not typically limited to the actual site of mesh implant, but can be present throughout various sites within and beyond the pelvis. In addition, patients who experience hypersensitization and centralization of their pain often have generalized or paradoxical pain on exam. Thus, although the physical exam is 1 element of the evaluation, a multimodal assessment is needed, including validated questionnaires and a thorough history of the timing of the onset of pelvic pain after mesh implant to diagnose, determine severity and causality, and create a tailored treatment plan.
The process by which pelvic pain develops after mesh implant surgery is likely multifactorial. The challenge lies not only in the lack of understanding of the etiology of pain development and predisposing risk factors but also in the lack of published data that directly measure pelvic pain after mesh implant surgery. Although some studies report on the incidence of dyspareunia, very few directly measure pain with validated instruments, making it difficult to fully grasp the scope and nature of the problem. Dyspareunia does not equate with pelvic pain, because many women with pelvic pain are not sexually active, and thus dyspareunia is not directly assessed. In our study the rate of pelvic pain was 15.6%, whereas the rate of dyspareunia was 19% among sexually active women. Interestingly, most women with dyspareunia did not report current pelvic pain (66.7% denied current pain vs 33.3% reported current pain, p = .11). This may be because 46% of women were not sexually active and thus would not report dyspareunia. Another explanation is that women with dyspareunia did not consider it a criterion for pelvic pain. This highlights the importance of directly measuring pelvic pain rather than using dyspareunia as a proxy.
Miller et al [25] evaluated 5-year outcomes after transvaginal mesh placement using a Prolene mesh implant for the treatment of POP. They reported 3 patients (3.5%) with dyspareunia but did not directly measure pelvic pain. A Cochrane Systematic Review from 2013 also had little data regarding pain after mesh implant surgery [26]. However, their finding that sacrocolpopexy had a lower rate of dyspareunia than vaginal sacrospinous ligament fixation is in line with our findings.
Foon et al [27] performed a systematic review of graft materials in anterior vaginal wall POP repair but found insufficient evidence regarding dyspareunia rates and did not comment on pelvic pain. Feiner et al [28] performed a systematic review of transvaginal mesh kits for the treatment of apical POP and found dyspareunia rates of 2% to 3%. This review is 1 of the few to directly report on pain, with rates of perineal, pelvic, or buttock pain ranging from 1% to 2%. Pain was not a measured with a validated instrument in the included studies, so the reported rates of pain may not be inclusive of all cases. In contrast, Weber et al [29] reported a 19% dyspareunia rate after posterior native tissue repair. Similarly, Pauls et al [30] reported a 25% rate of sexual dysfunction related to vaginal pain after vaginal surgery for POP and SUI The rates of dyspareunia and pain reported by Weber et al and Pauls et al are more similar to our reported rate of 15.6% for pain and 19.0% for dyspareunia.
Mesh erosion can also be a source of pelvic pain, and this has been more directly reported in the literature [9]. However, revision of mesh erosion may be performed for a variety of reasons, including dyspareunia, bleeding, and infection, and it is difficult to make any direct inferences as to the presence or absence of pain. Unfortunately, mesh erosion rates cannot be used as a proxy to identify the presence of pelvic pain after mesh implant surgery. The mesh erosion rate in our study was very low. This information was obtained by querying the medical record based on ICD-9 codes 629.31 and 629.32 for erosion of mesh into pelvic and nonpelvic organs and by checking for any cases of surgical mesh revision and interviewing the subjects. Based on the retrospective nature of the study, it is possible that not all cases were reported and thus not identified.
Strengths of the present study include the use of validated instruments to directly measure pain quality after mesh implant, general health, and sexual health, which allows for very specific assessments of different types of pain responses, including somatic and neuropathic pain, and somatization. In addition, inclusion of a wide variety mesh implant types allows for subanalysis of outcomes by mesh location. Finally, the study period spanned the time directly after the FDA Safety Warning of 2011, allowing for better generalizability of the findings based on current mesh use.
Limitations of the study include the retrospective design, which cannot account for recall and selection bias. However, the use of validated instruments minimizes the bias regarding current prevalence and nature of pain. In addition, although the index surgery occurred in the past, pain and health outcomes were measured based on current symptomatology. This limits our ability to measure change in pain from baseline, although women with baseline chronic pelvic pain were excluded. Another limitation is the high number of patients who could not be reached. Although we cannot draw any conclusions about this group, a post-hoc analysis showed were no differences in the types of surgeries performed in this group compared with the responder group. Although both groups had a mean age in the postmenopausal range, the nonresponders were slightly younger at 58.7 years compared with 62.1 years for the responders (p = .003). There were no other demographic differences between groups. Another limitation is the variety in location of mesh implants used. Whereas stratified analysis showed no difference in the rate of pain based on mesh type, this could be due to lack of power, as this was not the main objective of the study. Based on this study, clinical factors were more likely to affect the development of chronic pain rather than mesh location. However, larger studies powered for mesh location are needed to further assess this.
In summary, mesh augmentation improves the strength of pelvic floor repair but is accompanied by certain risks that must be balanced against these benefits. The ideal mesh material should be durable, noninflammatory, chemically inert, and demonstrate better in vivo performance than native tissue. That ideal material has not yet been developed. In the meantime, surgeons and patients will need to maintain vigilance when opting to supplement surgical repair with mesh and weigh the risks and benefits of each mesh-augmented procedure on an individual level based on patient characteristics. Our findings regarding the quality and nature of pain at least 1 year after pelvic mesh implant surgery may help aid surgeons and patients when considering mesh implant surgery for the treatment of POP and SUI.
Acknowledgments
This work was supported by grant number T35-DK007386 from the National Institutes of Health.
Footnotes
The authors declare that they have no conflict of interest.
Presented in part at the 41st annual scientific meeting of the Society of Gynecologic Surgeons, Orlando, Florida, March 22–25, 2015, and at the 40th annual meeting of the International Urogynecological Association, Nice, France, June 9–13, 2015.
References
- 1.Wu JM, Kawasaki A, Hundley AF, Dieter AA, Myers ER, Sung VW. Predicting the number of women whowill undergo incontinenceand prolapse surgery, 2010 to 2050. Am J Obstet Gynecol. 2011;205:230.e1–230.e5. doi: 10.1016/j.ajog.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Drug Administration [Accessed November 3, 2016];FDA Public Health Notification: Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence. 2008 Oct 20; doi: 10.1016/j.eururo.2009.01.055. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Renumber following references. [DOI] [PubMed]
- 3.Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131–1142. doi: 10.1097/AOG.0b013e3181898ba9. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui NY, Grimes CL, Casiano ER, et al. Mesh sacrocolpopexy compared with native tissue vaginal repair: a systematic review and meta-analysis. Obstet Gynecol. 2015;125:44–55. doi: 10.1097/AOG.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C, Nordic Transvaginal Mesh Group Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med. 2011;364:1826–1836. doi: 10.1056/NEJMoa1009521. [DOI] [PubMed] [Google Scholar]
- 6.Carey M, Higgs P, Goh J, et al. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. Br J Obstet Gynaecol. 2009;116:1380–1386. doi: 10.1111/j.1471-0528.2009.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891–898. doi: 10.1097/AOG.0b013e31816a2489. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol. 2010;203:235.e1–235.e8. doi: 10.1016/j.ajog.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Abbott S, Unger CA, Evans JM, et al. Evaluation and management of complications from synthetic mesh after pelvic reconstructive surgery: a multicenter study. Am J Obstet Gynecol. 2014;210:163.e1–163.e8. doi: 10.1016/j.ajog.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303. doi: 10.1097/AOG.0b013e3181e7d7f8. [DOI] [PubMed] [Google Scholar]
- 11.Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2011;118:1337–1344. doi: 10.1097/AOG.0b013e318237edc4. [DOI] [PubMed] [Google Scholar]
- 12.Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME. Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol. 2011;117(2 Pt 1):242–250. doi: 10.1097/AOG.0b013e318203e6a5. [DOI] [PubMed] [Google Scholar]
- 13.Parden AM, Tang Y, Szychowski J, Richter HE. Characterization of lower urinary tract symptoms before and after midurethral sling revision. J Minim Invasive Gynecol. 2016;23:979–985. doi: 10.1016/j.jmig.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theunissen M, Peters ML, Schepers J, et al. Recovery 3 and 12 months after hysterectomy: epidemiology and predictors of chronic pain, physical functioning, and global surgical recovery. Medicine. 2016;95:e3980. doi: 10.1097/MD.0000000000003980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114:551–561. doi: 10.1093/bja/aeu441. [DOI] [PubMed] [Google Scholar]
- 16.STROBE statement—checklist of items that should be included in reports of observational studies (STROBE initiative) Int J Public Health. 2008;53:3–4. doi: 10.1007/s00038-007-0239-9. [DOI] [PubMed] [Google Scholar]
- 17.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 18.Fabbri E, Villa G, Mabrouk M, et al. McGill Pain Questionnaire: a multi-dimensional verbal scale assessing postoperative changes in pain symptoms associated with severe endometriosis. J Obstet Gynaecol Res. 2009;35:753–760. doi: 10.1111/j.1447-0756.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 19.Pennebaker JW, Gonder-Frederick L, Stewart H, Elfman L, Skelton JA. Physical symptoms associated with blood pressure. Psychophysiology. 1982;19:201–210. doi: 10.1111/j.1469-8986.1982.tb02547.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 21.Verit FF, Verit A. Validation of the female sexual function index in women with chronic pelvic pain. J Sex Med. 2007;4:1635–1641. doi: 10.1111/j.1743-6109.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- 22.Vollebregt A, Fischer K, Gietelink D, van der Vaart CH. Effects of vaginal prolapse surgery on sexuality in women and men; results from a RCTon repair with and without mesh. J Sex Med. 2012;9:1200–1211. doi: 10.1111/j.1743-6109.2011.02647.x. [DOI] [PubMed] [Google Scholar]
- 23.Ware J, Jr, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862–865. doi: 10.1016/j.urology.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 25.Miller D, Lucente V, Babin E, Beach P, Jones P, Robinson D. Prospective clinical assessment of the transvaginal mesh technique for treatment of pelvic organ prolapse—5-year results. Female Pelvic Med Reconstr Surg. 2011;17:139–143. doi: 10.1097/SPV.0b013e3182175da6. [DOI] [PubMed] [Google Scholar]
- 26.Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database System Rev. 2013;CD004014 doi: 10.1002/14651858.CD004014.pub5. [DOI] [PubMed] [Google Scholar]
- 27.Foon R, Toozs-Hobson P, Latthe PM. Adjuvant materials in anterior vaginal wall prolapse surgery: a systematic review of effectiveness and complications. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1697–1706. doi: 10.1007/s00192-008-0668-x. [DOI] [PubMed] [Google Scholar]
- 28.Feiner B, Jelovsek JE, Maher C. Efficacy and safety of transvaginal mesh kits in the treatment of prolapse of the vaginal apex: a systematic review. Br J Obstet Gynaecol. 2009;116:15–24. doi: 10.1111/j.1471-0528.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 29.Weber AM, Walters MD, Piedmonte MR. Sexual function and vaginal anatomy in women before and after surgery for pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2000;182:1610–1615. doi: 10.1067/mob.2000.107436. [DOI] [PubMed] [Google Scholar]
- 30.Pauls RN, Silva WA, Rooney CM, et al. Sexual function after vaginal surgery for pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2007;197:622.e1–622.e7. doi: 10.1016/j.ajog.2007.08.014. [DOI] [PubMed] [Google Scholar]