Abstract
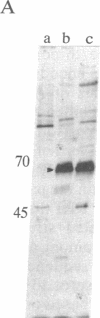
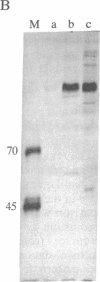
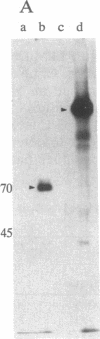
The 1.3-kilobase (kb) Pst I DNA fragment C (Pst I-C) of herpes simplex virus type 2 (HSV-2) morphological transforming region III (mtrIII; map unit 0.562-0.570) encodes part of the N-terminal half of the large subunit of ribonucleotide reductase (RR1; amino acid residues 71-502) and induces the neoplastic transformation of immortalized cell lines. To assess directly the role of these RR1 protein sequences in cell transformation, the Pst I-C fragment was cloned in an expression vector (p91023) containing an adenovirus-simian virus 40 promoter-enhancer to generate recombinant plasmid p9-C. Expression of a protein domain (approximately 65 kDa) was observed in p9-C-transfected COS-7 and Rat2 cells but not in those transfected with plasmid pHC-14 (Pst I-C in a promoterless vector). In Rat2 cells, p9-C induced highly transformed foci at an elevated frequency compared with that of pHC-14. Introduction of translation termination (TAG) condons within the RR1 coding sequence and within all three reading frames inactivated RR1 protein expression from p9-C and reduced its transforming activity to the level seen with the standard pHC-14 construct. Wild-type p9-C specified a protein kinase capable of autophosphorylation. Computer-assisted analysis further revealed significant similarity between regions of mtrIII-specific RR1 and amino acid patterns conserved within the proinsulin precursor family and DNA transposition proteins. These results identify a distinct domain of the HSV-2 RR1 protein involved in the induction of enhanced malignant transformation. In addition, the data indicate that the mtrIII DNA itself can induce basal-level transformation in the absence of protein expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M. A., Butcher M., Ghosh H. P. Expression and nuclear envelope localization of biologically active fusion glycoprotein gB of herpes simplex virus in mammalian cells using cloned DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5675–5679. doi: 10.1073/pnas.84.16.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. A. Oligomerization of herpes simplex virus glycoprotein B occurs in the endoplasmic reticulum and a 102 amino acid cytosolic domain is dispensable for dimer assembly. Virology. 1990 Oct;178(2):588–592. doi: 10.1016/0042-6822(90)90359-y. [DOI] [PubMed] [Google Scholar]
- Camacho A., Spear G. Transformation of hamster embryo fibroblasts by a specific fragment of the herpes simplex virus genome. Cell. 1978 Nov;15(3):993–1002. doi: 10.1016/0092-8674(78)90283-0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T. D., Wymer J. P., Smith C. C., Kulka M., Aurelian L. Protein kinase activity associated with the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10). J Virol. 1989 Aug;63(8):3389–3398. doi: 10.1128/jvi.63.8.3389-3398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner D. V., Jurka J. Multiple aligned sequence editor (MASE). Trends Biochem Sci. 1988 Aug;13(8):321–322. doi: 10.1016/0968-0004(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Iwasaka T., Smith C. C., Aurelian L., Lewis G. K., Ts'o P. O. Multistep transformation by defined fragments of herpes simplex virus type 2 DNA: oncogenic region and its gene product. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8493–8497. doi: 10.1073/pnas.82.24.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Tanczos B., Jones C., Ortiz J., Salimi-Lopez S. DNA amplification and neoplastic transformation mediated by a herpes simplex DNA fragment containing cell-related sequences. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1738–1742. doi: 10.1073/pnas.83.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C., Ortiz J., Jariwalla R. J. Localization and comparative nucleotide sequence analysis of the transforming domain in herpes simplex virus DNA containing repetitive genetic elements. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7855–7859. doi: 10.1073/pnas.83.20.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. The minimal transforming fragment of HSV-2 mtrIII can function as a complex promoter element. Virology. 1989 Apr;169(2):346–353. doi: 10.1016/0042-6822(89)90160-8. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):689–693. doi: 10.1073/pnas.82.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankinen H., Telford E., MacDonald D., Marsden H. The unique N-terminal domain of the large subunit of herpes simplex virus ribonucleotide reductase is preferentially sensitive to proteolysis. J Gen Virol. 1989 Dec;70(Pt 12):3159–3169. doi: 10.1099/0022-1317-70-12-3159. [DOI] [PubMed] [Google Scholar]
- Nikas I., McLauchlan J., Davison A. J., Taylor W. R., Clements J. B. Structural features of ribonucleotide reductase. Proteins. 1986 Dec;1(4):376–384. doi: 10.1002/prot.340010411. [DOI] [PubMed] [Google Scholar]
- Paradis H., Gaudreau P., Massie B., Lamarche N., Guilbault C., Gravel S., Langelier Y. Affinity purification of active subunit 1 of herpes simplex virus type 1 ribonucleotide reductase exhibiting a protein kinase activity. J Biol Chem. 1991 May 25;266(15):9647–9651. [PubMed] [Google Scholar]
- Peterson E., Schmidt O. W., Goldstein L. C., Nowinski R. C., Corey L. Typing of clinical herpes simplex virus isolates with mouse monoclonal antibodies to herpes simplex virus types 1 and 2: comparison with type-specific rabbit antisera and restriction endonuclease analysis of viral DNA. J Clin Microbiol. 1983 Jan;17(1):92–96. doi: 10.1128/jcm.17.1.92-96.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes G. R., LaFemina R., Hayward S. D., Hayward G. S. Morphological transformation by DNA fragments of human herpesviruses: evidence for two distinct transforming regions in herpes simplex virus types 1 and 2 and lack of correlation with biochemical transfer of the thymidine kinase gene. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):629–641. doi: 10.1101/sqb.1980.044.01.066. [DOI] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Automatic generation of primary sequence patterns from sets of related protein sequences. Proc Natl Acad Sci U S A. 1990 Jan;87(1):118–122. doi: 10.1073/pnas.87.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain M. A., Galloway D. A. Herpes simplex virus specifies two subunits of ribonucleotide reductase encoded by 3'-coterminal transcripts. J Virol. 1986 Mar;57(3):802–808. doi: 10.1128/jvi.57.3.802-808.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner B. T., Wade T., Pato M. L. Identification of the bacteriophage D108 kil gene and of the second region of sequence nonhomology with bacteriophage Mu. Gene. 1988;62(1):111–119. doi: 10.1016/0378-1119(88)90584-7. [DOI] [PubMed] [Google Scholar]