ABSTRACT
BACKGROUND AND PURPOSE
Conventional MRI techniques do not necessarily provide information about multiple sclerosis (MS) disease pathology or progression. Nonconventional MRI techniques, including proton magnetic resonance spectroscopy (1H‐MRS), are increasingly used to improve the qualitative and quantitative specificity of MR images. This study explores potential correlations between MRI measures of disease and disability progression as measured by the Expanded Disability Status Scale (EDSS), Functional Systems (FS), and ambulation index scores in a unique cohort of MS patients treated with glatiramer acetate that has been closely monitored for over 20 years.
METHODS
This was a multicenter, open‐label, cross‐sectional MRI substudy among participants in the GA‐9004 open‐label extension of the 36‐month, double‐blind GA‐9001 study, timed to coincide with the prospectively planned 20‐year clinical exam.
RESULTS
Of 64 patients who participated in the MRI substudy, results are presented for the 39 patients (61%) who had a 1H‐MRS assessment at 20 years of treatment. Both total N‐acetylaspartate relative to total creatinine (tNAA/tCr) concentration ratio and T1 lesion volume were found to be robustly associated with disability levels with different statistical approaches. Gray matter (GM) volume was found to be a more consistent parameter than white matter (WM) volume for disability allocation. The elastic net algorithm showed a trade‐off between WM and GM volumes for disability estimation when different disability definitions were used.
CONCLUSIONS
Among patients with MS receiving long‐term glatiramer acetate therapy, consistent effects on disability levels indicated by EDSS and pyramidal FS score thresholds were found for tNAA/tCr concentration ratio and T1 lesion volume.
Keywords: Multiple sclerosis, glatiramer acetate, MRI, disability correlates, extension study
Introduction
Conventional MRI techniques, such as gadolinium‐enhanced T1‐weighted (T1W) and T2‐weighted (T2W) MRIs, are sensitive indicators of disease activity but do not necessarily provide information about disease pathology or progression.1, 2 Correlations between conventional imaging measures and neurological disability measured by the Expanded Disability Status Scale (EDSS)3 and between imaging and relapse rates4 have also been modest and variable (ie, the “clinico‐radiological paradox”).5, 6 This may reflect the poor pathological specificity of conventional MR images, in that the T2W lesion volume “burden of disease” measure does not differentiate between edema, demyelination, axonal loss, and gliosis within apparently normal‐appearing white matter (WM).6, 7 Additionally, significant neurologic deficits can reflect undetected lesions in the spinal cord, pyramidal tract, or optic nerve, while a large subcortical lesion may be asymptomatic.8
Nonconventional MRI techniques, including magnetization transfer imaging (MT‐MRI), proton magnetic resonance spectroscopy (1H‐MRS), and diffusion tensor imaging (DTI), are increasingly being used to improve the qualitative and quantitative specificity of MR images. These techniques are more sensitive to changes in gray matter (GM), which may be more closely associated with neurologic disability than WM changes,9 and these newer techniques can identify specific aspects of brain tissue injury. However, even with more specific techniques, the current consensus is that a single MRI measure may not completely reflect the disease state and progression in relapsing‐remitting multiple sclerosis (RRMS);10 combining MRI measures of MS‐related tissue damage could better elucidate relationships between clinically evident disability and pathologic changes in the central nervous system.
Glatiramer acetate (GA; Copaxone®, Teva Neuroscience, North Wales, PA) is an immunomodulating drug approved for the treatment of RRMS in several countries.11 This study explores potential correlations between MRI measures of disease and disability progression measured by EDSS and Functional Systems (FS) scores in a unique cohort of patients with MS who have been closely monitored and treated with GA for over 20 years. In addition, the study provides a real‐world perspective on outcomes for patients who are receiving long‐term treatment. The placebo‐controlled US GA trial (GA‐9001)12 and its open‐label extension (GA‐9004)13 constitute the longest prospective study of continuous disease‐modifying monotherapy in RRMS.
In July 2012, patients who continued to receive subcutaneous (s.c.) GA 20 mg/mL daily (GA20) on study as their only disease‐modifying therapy (DMT) were invited to participate in a one‐time, cross‐sectional MRI substudy. We report the results for the 39 patients who had a 1H‐MRS assessment at 20 years of treatment. MRS data, specifically tNAA/tCr concentration ratio, may be used to characterize metabolic injury that accumulates as a result of neuronal/axonal dysfunction or loss and can be reliably used to estimate clinical disability levels. MRS data in patients followed for two decades may provide long‐term metrics of tissue loss, including GM.
Methods
All patients provided written informed consent before participating in the MRI substudy. The study protocol was approved by appropriate local Ethics Committees/Institutional Review Boards and conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization.
Patients
To enter the 36‐month, double‐blind GA‐9001 study, patients must have met Poser criteria14 for MS diagnosis, had an EDSS score between 0 and 5, and had at least two relapses in the 2 years before study randomization. Inclusion in the GA‐9004 open‐label extension study required completion of the GA‐9001 study. In GA‐9004, patients who were originally randomly assigned to receive GA20 continued on treatment, while those randomized to receive placebo switched to GA20 for the duration of the study.
Participants who continued in GA‐9004 in July 2012 were invited to participate in this one‐time, multicenter, US, open‐label, cross‐sectional MRI substudy, timed to coincide with the prospectively planned 20‐year clinical exam.
Disability Assessments
In GA‐9004, EDSS was initially measured every 6 months until year 13, after which EDSS was assessed at 12‐month intervals. For this MRI substudy, EDSS, FS (pyramidal, sensory, mental, cerebellar, brainstem, bladder/bowel, visual), and ambulation index (AI) scores collected nearest to the date of the MRI scan were used for all analyses.
MRI Assessments
No steroid use was permitted in the 30 days before MRI scans. The MRI protocol was developed at the Sastry Foundation Advanced Imaging Laboratory at Wayne State University. The MRI scan algorithm was tailored to 1.5 or 3 T scanners, with sequences modified to accommodate the brand of MRI scanner. Each site was provided image acquisition and quality control feedback in real time.
Imaging Sequences
Imaging sequences obtained included 3D‐T1W spoiled gradient‐recalled echo (GRE); pre‐ and postcontrast T1W spin‐echo (SE) or magnetization‐prepared‐rapid‐gradient‐echo (MPRAGE) contiguous slices; T2W contiguous slices; fluid‐attenuated inversion recovery (FLAIR) axial contiguous slices; 1H‐MRS (multivoxel) to estimate total N‐acetylaspartate relative to total creatinine (tNAA/tCr) concentrations (1H‐MRS assessment was optional); and DTI contiguous slices. Voxel positioning for MRSI acquisition was performed as previously reported.15 GRE with and without MT radiofrequency saturation pulse (RFSP) was used to evaluate magnetization transfer ratio (MTR). MT images were collected using the same acquisition parameters as conventional T1W and T2W images except for the number of slices. Acquisition parameters (standardized imaging parameters with a dummy run) are summarized in Table 1.
Table 1.
MRI Parameters
| Site | Wayne State University Detroit | University of Southern California | University of Texasa | University of New Mexico | University of Utah | University of Rochester | University of Marylandb | University of Wisconsin | University of Pennsylvania |
|---|---|---|---|---|---|---|---|---|---|
| Scanner brand/ field strength | Siemens Verio 3T | GE Signa HDxt 3T | Philips 3T | Siemens TrioTim 3T | Siemens TrioTim 3T | GE Signa HDxt 1.5T | Siemens 1.5T | GE Signa HDxt 1.5T | Siemens 1.5T |
| 3D‐T1W | |||||||||
| Slice thickness (mm) | 1.3 | 1 | 1.0 | 1 | 1 | 1 | 1 | 1 | 1.3 |
| TR (mseconds) | 1,680 | 10.36 | 11.05 | 1,700 | 1,680 | 8.288 | 1,860 | 9.3 | 2,400 |
| TE (mseconds) | 3.52 | 4.36 | 5.31 | 3.73 | 3 | 3.172 | 3.29 | 3.7 | 4.13 |
| Matrix (mm2) | 384 × 384 | 512 × 512 | 384 × 384 | 384 × 384 | 384 × 384 | 512 × 512 | 256 × 256 | 256 × 256 | 256 × 256 |
| In‐plane resolution (mm2) | .67 × .67 | .5 × .5 | .67 × .67 | .67 × .67 | .67 × .67 | .47 × .47 | 1 × 1 | .98 × .98 | .98 × .98 |
| T1W‐Precontrast | |||||||||
| Slice thickness | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| TR (mseconds) | 400 | 8.03 | 425 | 400 | 574 | 500 | 552 | 566.7 | 550 |
| TE (mseconds) | 4.47 | 3.11 | 9.46 | 4.47 | 4.47 | 12 | 17 | 14.4 | 14 |
| Matrix (mm2) | 512 × 512 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 |
| In‐plane resolution (mm2) | .5 × .5 | 1 × 1 | 1 × 1 | 1 × 1 | 1 × 1 | .98 × .98 | .98 × .98 | .98 × .98 | .98 × .98 |
| T1W Postcontrast | |||||||||
| Slice thickness | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| TR (mseconds) | 400 | 8.03 | 425 | 400 | 574 | 500 | 552 | 566.7 | 550 |
| TE (mseconds) | 4.47 | 3.116 | 9.46 | 4.47 | 4.47 | 12 | 17 | 14.4 | 14 |
| Matrix (mm2) | 512 × 512 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 |
| In‐plane resolution (mm2) | .5 × .5 | 1 × 1 | 1 × 1 | 1 × 1 | 1 × 1 | .98 × .98 | .98 × .98 | .98 × .98 | .98 × .98 |
| T2W | |||||||||
| Slice thickness | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| TR (mseconds) | 7,810 | 7,200 | 7,800 | 9,870 | 7,810 | 45,009 | 5,480 | 5,000 | 5,000 |
| TE (mseconds) | 97 | 95.04 | 97 | 95 | 97 | 84.45 | 82 | 84.3 | 82 |
| Matrix (mm2) | 640 × 480 | 512 × 512 | 320 × 320 | 320 × 240 | 320 × 320 | 256 × 256 | 256 × 256 | 512 × 512 | 256 × 256 |
| In‐plane resolution (mm2) | .4 × .4 | .5 × .5 | .8 × .8 | .8 × .8 | .8 × .8 | .98 × .98 | .98 × .98 | .49 × .49 | .98 × .98 |
| FLAIR | |||||||||
| Slice thickness | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| TR (mseconds) | 9,000 | 9,002 | 9,000 | 9,000 | 9,000 | 10,002 | 10,000 | 10,002 | 10,000 |
| TE (mseconds) | 128 | 132 | 125 | 124 | 123 | 122.3 | 101 | 121.8 | 135 |
| Matrix (mm2) | 256 × 192 | 256 × 256 | 256 × 256 | 256 × 192 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 |
| In‐plane resolution (mm2) | 1 × 1 | 1 × 1 | 1 × 1 | 1 × 1 | 1 × 1 | .98 × .98 | .98 × .98 | .98 × .98 | .98 × .98 |
| MTR | |||||||||
| Slice thickness | 3 | 3 | 6 | 3 | 3 | 5 | 5 | 5 | 5 |
| TR (mseconds) | 1,200 | 1,500 | 3,000 (2D) | 1,200 | 1,200 | 700 | 650 | 700 | 650 |
| 65.80 (3D) | |||||||||
| TE (mseconds) | 3.64 | 4 | 6.11 (2D) | 3.64 | 3.64 | 10 | 11.7 | 10 | 10 |
| 5.94 (3D) | |||||||||
| Matrix (mm2) | 512 × 512 | 256 × 256 | 256 × 256 | 256 × 256 | 512 × 512 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 |
| In‐plane resolution (mm2) | .5 × .5 | 1 × 1 | 1 × 1 | 1 × 1 | .5 × .5 | .90 × .90 | .90 × .90 | .90 × .90 | .90 × .90 |
| DTI | |||||||||
| Directions | 20 | 20 | 15 and 32 | 20 | 20 | 6 | 6 | 6 | 6 |
| Slice thickness | 3 | 3 | 3 | 3 | 3 | 4 | 4 (2) | 4 | 4 |
| TR (mseconds) | 10,400 | 12,500 | 8,000 | 10,400 | 10,400 | 5,400 | 8,700 | 8,000 | 5,500 |
| TE (mseconds) | 126 | 88 | 88.42 | 126 | 126 | 97.3 | 97 (83) | 98.6 | 97 |
| Matrix (mm2) | 200 × 200 | 256 × 256 | 224 × 224 | 200 × 200 | 200 × 200 | 256 × 256 | 128 × 128 | 256 × 256 | 128 × 128 |
| In‐plane resolution (mm2) | 1.28 × 1.28 | 1 × 1 | 1.14 × 1.14 | 1.28 × 1.28 | 1.28 × 1.28 | .90 × .90 | 1.72 × 1.72 | 1.09 × 1.09 | 1.8 × 1.8 |
| MRS | |||||||||
| TR (mseconds) | 1,500 | Not | 1,500 | 1,500 | 1,500 | 1,500 | Not | Not | Not |
| TE (mseconds) | 135 | performed | 135 | 135 | 135 | 135 | performed | performed | performed |
| CSI matrix (voxels) | 16 × 16 | 16 × 16 | 16 × 16 | 16 × 16 | 8 × 8 | ||||
| Voxel size (mm) | 10 ×10 × 15 | 10 ×10 × 15 | 10 ×10 × 15 | 10 ×10 × 15 | |||||
2D = 2‐dimensional; 3D = 3‐dimensional; MRS = magnetic resonance spectroscopy; DTI = diffusion tensor imaging; MTR = magnetic transfer ratio; FLAIR = fluid‐attenuated inversion recovery; T1W = T1‐weighted; T2W = T2‐weighted; TE = echo time; TR = repetition time.
The site did not have any dummy scan. 3D‐MT images were acquired for first five patients’ scans. The site acquired 2D‐MT images as recommended for the remaining patients’ scans. The first five scans cannot be repeated.
The first four patients’ scans (in parentheses) were received at the same time. DTI parameters were different from those recommended: mean averages of FA were higher than expected. The site modified MRI parameters as recommended for the remaining patients’ scans.
Image Analysis
Whole brain (WB), GM, and WM volumes were calculated based on tissue segmentation by SPM5 (Wellcome Trust Centre for Neuroimaging, University College London [UCL] Institute of Neurology, London) on 3D‐T1W scans. T1W/T2W lesion volumes were measured using a previously described semiautomated edge detection contouring/thresholding technique.16 Multivoxel 1H‐MRS images were analyzed using LCModel to estimate tNAA/tCr concentration ratio in an 8×8 multivoxel region of interest (ROI) in the central WM. To determine MT ratio (MTR), MT images were processed (Java Image Manipulation version 3.0) to create an MTR map from two MT images (with and without saturation pulse). Cortical surface thickness was estimated in 3D‐T1W images using FreeSurfer (General Hospital Corporation, Boston, MA); tissue segmentation and cortical parcellation were performed with the Desikan‐‐Killiany cortical atlas. Mean diffusivity (MD) and fractional anisotropy (FA) were calculated with DTI Studio (Department of Radiology, Johns Hopkins University, Baltimore, MD). WB DTI maps were created using the B0 image as the reference and obtaining histograms from each map.
Statistical Methods
Descriptive statistics of patient characteristics, EDSS/FS scores, and MRI parameters for the patients with MRS data are included in Table 2. Because of the different natures of the MRI parameters scales, these variables were standardized for further analysis. Correlations among various MRI measures, and between MRI measures and disability (EDSS, FS, and AI) scores, were assessed using Spearman rank correlation coefficient. Actual MRI values, rather than standardized ones, were used for the Spearman rank correlation coefficient analysis because this method uses ranked values. Univariate logistic regression models were used to analyze the influence of individual MRI measures (WB, GM, and WM volumes; tNAA/tCr; and T1W and T2W lesions volumes) on disability levels defined based on EDSS and pyramidal FS thresholds of two points (<2, lower disability subgroup; ≥2, higher disability subgroup), and of three points for pyramidal FS score only. Significance level of 5% was used for the Spearman rank correlation coefficient and univariate logistic regression analyses. Imaging specifications are outlined in Table 1.
Table 2.
Descriptive Statistics for Patient Characteristics, EDSS/FS Status, and MRI Parameters for Patients with 20‐Year MRS Data
| (A) Patient Characteristics | |
|---|---|
| GA‐9004 | N = 39 |
| EDSS score, mean ± SD [median] | 3.6 ± 2.5 [3.0] |
| Age (years), mean ± SD | 56.2 ± 6.4 |
| Disease duration from diagnosis (years), mean ± SD | 27.3 ± 4.7 |
| Exposure to GA (years), mean ± SD | 19.1 ± 1.3 |
| Female, n (%) | 26 (67) |
| (B) EDSS Status at the 20‐Year MRI Scan | |
|---|---|
| EDSS | Patients, n (%) |
| .0 | 4 (10.3) |
| 1.0 | 3 (7.7) |
| 1.5 | 6 (15.4) |
| 2.0 | 1 (2.6) |
| 2.5 | 4 (10.3) |
| 3.0 | 2 (5.1) |
| 3.5 | 1 (2.6) |
| 4.0 | 4 (10.3) |
| 4.5 | 1 (2.6) |
| 5.0 | 1 (2.6) |
| 5.5 | 1 (2.6) |
| 6.0 | 4 (10.3) |
| 6.5 | 3 (7.7) |
| 7.0 | 1 (2.6) |
| 8.0 | 3 (7.7) |
| (C) Pyramidal FS Score at the 20‐Year MRI Scan | |
|---|---|
| Pyramidal FS score | Patients, n (%) |
| 0 | 11 (28.2) |
| 1 | 8 (20.5) |
| 2 | 5 (12.8) |
| 3 | 8 (20.5) |
| 4 | 6 (15.4) |
| 5 | 1 (2.6) |
| (D) MRI Parameters at the 20‐Year MRI Scan | |
|---|---|
| Parameter | Mean ± SD |
| WB volume, cm3 | 1,307 ± 107 |
| WM volume, cm3 | 564 ± 64 |
| GM volume, cm3 | 742 ± 87 |
| T1 lesion volume, cm3 | 15.8 ± 12.9 |
| T2 lesion volume, cm3 | 27.6 ± 20.1 |
| tNAA/tCr | 1.88 ± 0.19 |
EDSS = Expanded Disability Status Scale; FS = Functional Systems; GA = glatiramer acetate.
GM = gray matter; tNAA/tCr = total N‐acetylaspartate relative to total creatinine; WB = whole brain; WM = white matter.
To identify the MRI variables with substantial contributions to the estimation of disability levels using a multivariate analysis, the elastic net variable selection algorithm was applied using a logistic regression setting, which allows covariate selection and model estimation within the same procedure. Moreover, this procedure is designed to accommodate problems in which there is high correlation among covariates and in cases where the dimension of the independent variables surpasses that of the sample size.17 In addition to the MRI variables, the following covariates were added to this analysis: age at the MRI scan date, MS duration at the MRI scan date, GA exposure at the MRI scan date, and gender. These additional covariates were standardized for the elastic net analysis.
The scaling parameter α of the elastic net algorithm was calibrated between .5 and 1 to minimize simultaneous selection of the extremely correlated variables. To find the best model for the disability outcome estimation, the minimal binomial deviance was assessed using the cross‐validation technique with fivefolds. A simulation of the elastic net was run for 1,000 iterations to account for the variability of the estimated coefficient size and the persistency of the variables being selected. Boxplots for the coefficient estimates from these 1,000 simulations are presented for each covariate for each model (Fig 1). Variable selection persistency was calculated as percentage of cases when a variable was selected by the elastic net. Median values of the coefficient estimates were derived from the simulation, and then used in the further analysis. Percentage contribution for disability level estimation was calculated for each variable as absolute value of a coefficient estimate divided by the total sum of the absolute values of all coefficient estimates.
Figure 1.
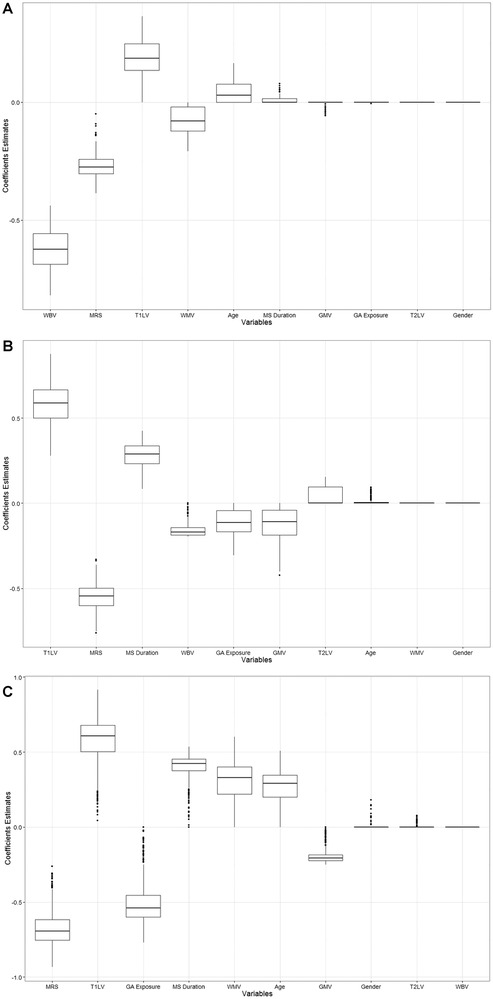
Box plots of coefficient estimates based on 1,000 simulations for estimation of disability levels defined as EDSS score ≥ 2, pyramidal FS score ≥ 2, or pyramidal FS score ≥ 3 disability subgroup using the elastic net model.
(A) Elastic net simulation results for estimating EDSS ≥ 2 versus EDSS ≤ 2. (B) Elastic net simulation results for estimating pyramidal FS score ≥ 2 versus pyramidal FS score ≤ 2. (C) Elastic net simulation results for estimating pyramidal FS score ≥ 3 versus pyramidal FS score ≤ 3. EDSS = Expanded Disability Status Scale; FS = Functional Systems; GA = glatiramer acetate; GMV = gray matter volume; MRS = magnetic resonance spectroscopy (ie, total N‐acetylaspartate relative to total creatinine); T1LV = T1 lesion volume; T2LV = T2 lesion volume; WBV = whole brain volume; WMV = white matter volume.
Area under the curve (with 95% confidence intervals) for receiver operating characteristic curves was calculated for goodness of fit.
Results
Patients
Of the 74 patients remaining in the GA‐9004 trial in November 2012, 64 patients (86%) agreed to participate in this MRI substudy, which was conducted at nine clinical sites (Wayne State University, Detroit, MI; University of Pennsylvania, Philadelphia, PA; University of Maryland, Baltimore, MD; University of Utah, Salt Lake City, UT; University of Texas, Austin, TX; University of Wisconsin, Madison, WI; University of Rochester, Rochester, NY; University of New Mexico, Albuquerque, NM; University of Southern California, Los Angeles, CA). Of the 64 patients who participated in the MRI substudy, 1H‐MRS was assessed in 39 patients (61%) at 20 years of treatment; results are presented for these 39 patients. Mean age at MRI scan date was 56.2 years, and mean disease duration was 27.3 years (Table 2A). Mean exposure to daily GA treatment was 19.1 years for MRI substudy participants.
EDSS examinations and MRI scans were performed within 1 month of each other for 56 patients (88%) and were conducted >1 month apart for 8 patients. The maximum time interval between EDSS assessment and MRI evaluation was <6 months (171 days). Mean (± SD) EDSS score for all participants was 3.6 ± 2.5. Eighteen patients (46%) had total EDSS scores <3 points, including 13 patients (33%) with EDSS scores <2 points (Table 2B). Only 11 patients (28%) had reached an EDSS score ≥6 points at 20 years on study.
Safety
Safety findings for the 74 patients remaining in the GA‐9004 open‐label study were consistent with the established safety profile of GA and were mainly related to injection‐site reactions. Serious adverse events (SAEs) were infrequent and included chest pain (n = 6, 8%) and back pain (n = 4, 5%).
Correlations among MRI Outcomes
Spearman correlation coefficients determined among the various MRI measures were all in the expected direction (Table 3). For example, T1W and T2W lesion volumes were inversely correlated with WB, GM, and WM volumes (Fig 1). Similarly, tNAA/tCr measures were positively correlated with WB volume.
Table 3.
Spearman Correlation Coefficients (rs) for MRI Variables
| WBV | WMV | GMV | MRS | T1LV | T2LV | |
|---|---|---|---|---|---|---|
| WBV | 1.0000 | .5559 | .7294 | .4631 | –.5275 | –.4437 |
| .0002 | <.0001 | .0030 | .0006 | .0047 | ||
| WMV | 1.0000 | –.0231 | .2212 | –.2650 | –.1569 | |
| .8891 | .1760 | .1030 | .3402 | |||
| GMV | 1.0000 | .2511 | –.3988 | –.3887 | ||
| .1230 | .0119 | .0145 | ||||
| MRS | 1.0000 | –.3506 | –.3792 | |||
| .0286 | .0173 | |||||
| T1LV | 1.0000 | .9492 | ||||
| <.0001 | ||||||
| T2LV | 1.0000 | |||||
For each correlation, the top number represents the coefficient estimate and the bottom number represents the P value.
GMV = gray matter volume; MRS = magnetic resonance spectroscopy (ie, total N‐acetylaspartate relative to total creatinine); T1LV = T1 lesion volume; T2LV = T2 lesion volume; WBV = whole brain volume; WMV = white matter volume.
MRI and Disability
The tNAA/tCr concentration ratio showed consistently strong, statistically significant, inverse correlations with total EDSS score, and individual FS and AI scores (Table 4). This was the strongest correlation between MRI measure and disability scores. WB volume was also significantly correlated with total EDSS score, most individual FS scores, and AI score. In addition, GM volume was significantly correlated with total EDSS score, pyramidal FS score, and sensory FS scores (all P < .05; Table 4), whereas borderline correlation was found for cerebellar FS and AI scores. In contrast, WM volume was not significantly correlated with any disability outcome except for cerebellar FS score.
Table 4.
Spearman Correlation Coefficients (rs) for FS Scores and Ambulation Index Scores at Cross‐Section and MRI Outcomes
| Total EDSS | Pyramidal FS | Cerebellar FS | Brainstem FS | Mental FS | Sensory FS | Bladder/ Bowel FS | Visual FS | AI score | |
|---|---|---|---|---|---|---|---|---|---|
| tNAA/tCr | –.540* | –.510* | –.540* | –.527* | –.249 | –.459* | –.315 | –.337* | –.526* |
| WB volume | –.514* | –.466* | –.631* | –.379* | –.276 | –.403* | –.134 | –.197 | –.481* |
| GM volume | –.346* | –.331* | –.309 | –.252 | –.221 | –.338* | –.190 | –.132 | –.306 |
| WM volume | –.146 | –.135 | –.463* | –.090 | .110 | –.057 | .217 | –.022 | –.175 |
| T1W lesion volume | .486* | .452* | .398* | .389* | .172 | .280 | .293 | .424* | .343* |
| T2W lesion volume | .433* | .394* | .307 | .400* | .111 | .182 | .296 | .453* | .295 |
AI = ambulation index; CT = cortical thickness; EDSS = Expanded Disability Status Scale; FS = Functional Systems; GM = gray matter; T1W = T1‐weighted; T2W = T2‐weighted; tNAA/tCr = total N‐acetylaspartate relative to total creatinine; WB = whole brain; WM = white matter.
*P < .05.
T1W lesion volume also showed significant correlations with almost all disability outcomes (Table 4). In addition, T2W lesion volume had similar correlations with disability outcomes to those observed with T1W lesion volume, aside from borderline results for cerebellar FS and AI.
Estimation of Disability Level Determined by Univariate Analysis
Univariate logistic regression analysis showed that most of the MRI parameters have significant influence on the disability levels (Table 5). The magnitude of the tNAA/tCr effect was consistent across the models and showed 61%, 67%, and 68% significant reductions in the odds of higher disability defined by EDSS score ≥2, pyramidal FS score ≥2, and pyramidal FS score ≥3, respectively, following an increase of one standard deviation in this MRI parameter. Whereas WB volume had a greater effect on disability defined by EDSS score ≥2 (81%; P = .0075) when compared to tNAA/tCr, its effect on disability was decreased for pyramidal FS score ≥2 (67%; P = .0171) and pyramidal FS score ≥3 (46%; P = .1039). Similar to WB volume, the effect of WM volume was dependent on the disability definition and was significant only for estimation of disability defined by EDSS ≥ 2 (66%; P = .0272). In contrast, GM volume had a significant effect for disability defined by EDSS score ≥2 (65%; P = .0278) and by pyramidal FS score ≥2 (58%; P = .0404), while borderline effect was seen for pyramidal FS score ≥3 (53%; P = .0603). An increase of one standard deviation in T1 lesion volume significantly increased the odds of higher disability by 228% (P = .0284), 242% (P = .0089), and 122% (P = .0345) for EDSS score ≥2, pyramidal FS score ≥2, and pyramidal FS score ≥3, respectively. The effect of the T2 lesion volume was borderline for EDSS score ≥2 (136%; P = .0579) and significant for estimation of both pyramidal FS score ≥2 (237%; P = .0088) and pyramidal FS score ≥3 (132%; P = .0328).
Table 5.
Univariate Analysis: Effect of Each MRI Parameter on a Disability Level Defined by EDSS or Pyramidal FS Score
| MRI Parameter | EDSS ≥2 (n = 26) versus <2 (n = 13) Odds Ratio (95% Wald Confidence Limits), P Value | Pyramidal FS Score ≥2 (n = 20) versus <2 (n = 19) Odds Ratio (95% Wald Confidence Limits), P Value | Pyramidal FS Score ≥3 (n = 15) versus <3 (n = 24) Odds Ratio (95% Wald Confidence Limits), P Value |
|---|---|---|---|
| WB volume | .19 (.06, .64), .0075 | .33 (.14, .82), .0171 | .54 (.25, 1.14), .1039 |
| WM volume | .34 (.13, .89), .0272 | .60 (.30, 1.21), 01535 | .98 (.51, 1.88), .9525 |
| GM volume | .35 (.13, .89), .0278 | .42 (.18, .96), .0404 | .47 (.21, 1.03), .0603 |
| T1W lesion volume | 3.28 (1.13, 9.47), .0284 | 3.42 (1.36, 8.58), .0089 | 2.22 (1.06, 4.63), .0345 |
| T2W lesion volume | 2.36 (.97, 5.71), .0579 | 3.37 (1.36, 8.37), .0088 | 2.32 (1.07, 5.04), .0328 |
| tNAA/tCr | .39 (.18, .86), .0189 | .33 (.15, .76), .0089 | .32 (.13, .78), .0125 |
EDSS = Expanded Disability Status Scale; FS = Functional Systems; GM = gray matter; tNAA/tCr = total N‐acetylaspartate relative to total creatinine; WB = whole brain; WM = white matter.
Estimation of Disability Levels Based on Elastic Net Variable Selection Algorithm
The results of the elastic net variable selection algorithm show that the tNAA/tCr concentration ratio has a consistent contribution of 23%, 30%, and 22% to the estimation of the disability levels defined by EDSS score ≥2, pyramidal FS score ≥2, and pyramidal FS score ≥3, respectively (Table 6). This MRI parameter was selected in 100% of the cases for each of three models. T1 lesion volume has also shown relatively robust disability estimations with relative contributions of 16%, 32%, and 20% for disability as defined by EDSS score ≥2, pyramidal FS score ≥2, and pyramidal FS score ≥3, respectively. The T1 lesion volume was also selected at high rates of 98% for EDSS score ≥2 and 100% for both pyramidal FS scores. Although WB volume had a considerable contribution of 52% to the estimation of disability levels based on the EDSS≥2 definition, its weight was substantially decreased for pyramidal FS score ≥2 (9%), and it was not associated at all with pyramidal FS score ≥3.
Table 6.
Elastic Net Simulation: Estimation of Disability Level Defined by EDSS or Pyramidal FS Score
| EDSS ≥2 (n = 26) versus <2 (n = 17) | Pyramidal FS score ≥2 (n = 20) versus <2 (n = 19) | Pyramidal FS score ≥3 (n = 15) versus <3 (n = 24) | ||||
|---|---|---|---|---|---|---|
| % contribution | Selection persistency (%) | % contribution | Selection persistency (%) | % contribution | Selection persistency (%) | |
| WBV | 52 | 100 | 9 | 99 | 0 | 0 |
| tNAA/tCr | 23 | 100 | 30 | 100 | 22 | 100 |
| T1LV | 16 | 98 | 32 | 100 | 20 | 100 |
| WMV | 7 | 82 | 0 | 0 | 11 | 93 |
| Age | 2 | 66 | 0 | 30 | 9 | 95 |
| MS duration | 0 | 41 | 16 | 100 | 14 | 100 |
| GMV | 0 | 16 | 6 | 86 | 7 | 99 |
| GA exposure | 0 | 1 | 6 | 87 | 17 | 99 |
| T2LV | 0 | 0 | 0 | 49 | 0 | 9 |
| Gender | 0 | 0 | 0 | 0 | 0 | 3 |
| AUC (95% CI) .8787 | AUC (95% CI) .8474 | AUC (95% CI) .8139 | ||||
| (.7669, .9905) | (.7201, .9746) | (.6752, .9525) | ||||
AUC = area under the curve; CI = confidence interval; EDSS = Expanded Disability Status Scale; FS = Functional Systems; GA = glatiramer acetate; GMV = gray matter volume; OR = odds ratio; T1LV = T1 lesion volume; T2LV = T2 lesion volume; tNAA/tCr = total N‐acetylaspartate relative to total creatinine; WBV = whole brain volume; WMV = white matter volume.
T1 lesion volume, WB volume, WM volume, and age were selected for estimation of EDSS score ≥2 with contributions of 7% and 2%, respectively. Although some other variables had nonzero selection persistency in this model, such as MS duration (41%), GM volume (16%), and GA exposure (1%), the coefficient estimates for these parameters were close to zero with zero medians (Fig 1A).
For estimation of the disability levels defined by pyramidal FS score ≥2, in addition to tNAA/tCr concentration ratio, T1 lesion volume, and WB volume, MS duration, GM volume, and GA exposure showed relative contributions of 16%, 6%, and 6%, respectively. In this model, there were some variables with nonzero persistency selection that did not have any weight in the disability estimation: T2 lesion volume (49%) and age (30%) (Fig 1B).
The analytical model of disability levels based on pyramidal FS score ≥3 had the highest number of selected variables. In addition to tNAA/tCr concentration ratio and T1 lesion volume, both WM volume and GM volume were chosen with relative contributions of 11% and 7%, respectively. WM and GM volumes had opposite effects on the estimation of the disability levels, ie, coefficient estimates of WM volume are positive, suggesting positive correlation with the disability levels, whereas coefficient estimates of GM volume are negative, suggesting negative correlation with the disability. GA exposure, MS duration, and age have shown relative contributions of 17%, 14%, and 9%, respectively. Although the persistency selection was nonzero for T2 lesion volume (9%) and gender (3%), their coefficient estimates were around zero with median zero values (Fig 1C). The area under the curve (95% confidence interval) values based on the elastic net algorithm were found to be .88 (.77, .99), .85 (.72, .97), and .81 (.68, .95) when defining disability levels based on EDSS score ≥2, pyramidal FS score ≥2, and pyramidal FS score ≥3, respectively.
Discussion
In this study, we showed that 1H‐MRS data obtained from patients with RRMS who have been followed for two decades can characterize tissue loss that may be associated with clinical disability. Different statistical approaches used in this study have identified tNAA/tCr concentration ratio and T1 lesion volume as robustly associated with disability indicated by EDSS and pyramidal FS score thresholds among MS patients receiving long‐term GA therapy. These findings are consistent with previous reports of reduced NAA concentrations correlating with neuronal/axonal dysfunction or loss, consistent with the strong inverse relationships between disability scores and tNAA/tCr concentration in this study. A reduced tNAA/tCr concentration ratio has been found in normal‐appearing WM in MS patients versus normal controls.18 Similarly, a reduced tNAA/tCr concentration ratio has been reported in patients presenting with clinically isolated syndromes (CIS) suggestive of MS and patients with RRMS.19 In this study, T1W lesion volume was also significantly correlated with disability levels, as shown by EDSS and pyramidal FS scores. This is consistent with previous reports that have shown that patients with active disease and/or greater accumulated disease burden show greater brain volume loss.20
A relationship between EDSS score and tNAA/tCr concentration ratio or T1 lesion volume has also been reported in other studies in patients with relapsing MS21, 22 or progressive MS.22 In patients with RRMS, composite MR scores were strongly correlated with EDSS scores, indicating that multiparametric MR models are potential measures of MS progression.21
The present study indicates that although WB volume shows a substantial effect on disability measured by EDSS, this effect decreases when using pyramidal FS. In addition, GM volume was more robustly correlated with disability than WM volume, as shown by Spearman's rank coefficient correlation and by univariate logistic models. The elastic net algorithm shows a trade‐off between WM and GM volumes when different definitions of the disability are used; for EDSS score ≥2 only, WM volume was selected, and for pyramidal FS score ≥2, GM volume was chosen. Although both volumes were selected for pyramidal FS score ≥3, GM volume had a negative correlation to disability, suggesting that GM volume has greater influence than WB and WM volumes on disability when a more progressed definition is used.
Our findings are consistent with growing evidence that GM atrophy may be an important indicator of long‐term progression of neurologic disability. GM atrophy appears to worsen over the clinical course of MS, increasing from CIS to RRMS to secondary‐progressive stages of the disease.9, 23 In the present study, EDSS scores were surprisingly low (33% of patients had an EDSS score <2; Table 2B), considering their mean disease duration of 27.3 years. Potential explanations for this may reflect putative neuroprotective effects of GA treatment, such as secretion of brain‐derived neurotrophic factor (BDNF) by GA‐reactive T cells,24, 25 or insensitivity of the EDSS (and other quantitative scales) to impairment caused by subtle abnormalities,26 or some combination of the two. Attrition of patients over time is another key factor that may have contributed to this observation.
There are limitations associated with the use of EDSS score alone to demonstrate progression, including nonlinearity, artificial reduction of variance in cross‐sectional studies, and the short duration of longitudinal studies.6
T2 lesion volume was determined to not be highly associated with disability levels based on the elastic net variable selection algorithm. This is not surprising given that the range of the scaling parameter .5 ≤ α ≤ 1 was chosen to exclude extremely correlated variables within the same model estimation and because T2 lesion volume had a strong correlation with T1W lesion volume (rs = .95), which was selected in all the models.
A key strength of this study is the availability of a unique cohort of patients with MS of long duration using a single DMT, who have been closely monitored for over two decades. This patient cohort constitutes the group of MS patients with the longest prospective follow‐up that is currently still being studied. Other strengths include the study's multicenter design and multivoxel imaging.
A surprising finding of our study was the lack of correlations between disability and DTI and WB MTR assessments. WM volume was inversely correlated with MD‐DTI, but not with FA‐DTI. WB MTR was not significantly correlated with any disability measure. These findings may reflect a limitation of our analysis, namely, the relatively small patient sample, which could influence results of the Spearman correlation analyses.
There are other potential limitations of this study that warrant careful interpretation of the data. It was an open‐label prospective cohort, and no baseline scans were performed except in 27 patients at one center when the study initiated in 1991. MRI scans obtained at years 6 and 10 of the study were not available due to lost data,27, 28 and current imaging sequences included several advanced techniques that were never used previously (including MRS, MTR, 3D‐T1W, and DTI). Thus, comparison to prior scans was not possible, preventing longitudinal analysis. Furthermore, there was attrition of patients over time through the loss of poor responders who discontinued, potentially leading to self‐selection of patients who did well clinically.
Further research using a combination of functional and structural MRI measures may better elucidate GA effects on pathologic mechanisms responsible for clinical manifestations of MS. Future inclusion of cognitive and spinal cord assessments could potentially provide a more complete picture of the long‐term natural history of MS, thereby improving the understanding of disease progression and informing treatment strategies.
Acknowledgments and Disclosures: We thank the patients and site personnel involved with this study; site investigators Amy Pruitt (University of Pennsylvania), Robert Lisak (Wayne State University), Horea Rus (Maryland Center for MS), John Rose (George E. Whalen VA Medical Center), Andrew D. Goodman (University of Rochester), Leslie Weiner (University of Southern California), J. William Lindsey (University of Texas Health Science Center‐Houston), and Christopher Luzzio (University of Washington); Robin Everts (Teva Pharmaceutical Industries) for assistance with study conduct and statistical analyses; and Peter Feldman (Teva Pharmaceutical Industries) and Bryan Sepulveda (Chameleon Communications International with funding from Teva Pharmaceutical Industries) for editorial assistance in the preparation of this report.
Omar Khan has received compensation for consulting from Biogen Idec, Genzyme, and Novartis and for serving on speaker bureaus from Teva Pharmaceutical Industries, Novartis, and Biogen, and he has received research support from the NIH, NINDS, NMSS, Teva Pharmaceutical Industries, Biogen, Genzyme, Roche, and Novartis.
This study was funded by Teva Pharmaceutical Industries, Petach Tikva, Israel. Natalia Ashtamker, Scott Kolodny, and Yulia Sidi are employees of Teva Pharmaceutical Industries.
This report is dedicated to the memory of Professor Omar Khan.
References
- 1. Rovira A, Auger C, Alonso J. Magnetic resonance monitoring of lesion evolution in multiple sclerosis. Ther Adv Neurol Disord 2013;6:298‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zivadinov R, Bakshi R. Role of MRI in multiple sclerosis I: inflammation and lesions. Front Biosci 2004;9:665‐83. [DOI] [PubMed] [Google Scholar]
- 3. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444‐52. [DOI] [PubMed] [Google Scholar]
- 4. Petkau J, Reingold SC, Held U, et al. Magnetic resonance imaging as a surrogate outcome for multiple sclerosis relapses. Mult Scler 2008;14:770‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daumer M, Neuhaus A, Morrissey S, et al. MRI as an outcome in multiple sclerosis clinical trials. Neurology 2009;72:705‐11. [DOI] [PubMed] [Google Scholar]
- 6. Poloni G, Minagar A, Haacke EM, et al. Recent developments in imaging of multiple sclerosis. Neurologist 2011;17:185‐204. [DOI] [PubMed] [Google Scholar]
- 7. van Walderveen MA, Barkhof F, Hommes OR, et al. Correlating MRI and clinical disease activity in multiple sclerosis: relevance of hypointense lesions on short‐TR/short‐TE (T1‐weighted) spin‐echo images. Neurology 1995;45:1684‐90. [DOI] [PubMed] [Google Scholar]
- 8. Wilson M, Tench CR, Morgan PS, et al. Pyramidal tract mapping by diffusion tensor magnetic resonance imaging in multiple sclerosis: improving correlations with disability. J Neurol Neurosurg Psychiatry 2003;74:203‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobsen CO, Farbu E. MRI evaluation of grey matter atrophy and disease course in multiple sclerosis: an overview of current knowledge. Acta Neurol Scand Suppl 2014;129(s198):32‐6. [DOI] [PubMed] [Google Scholar]
- 10. Poonawalla AH, Datta S, Juneja V, et al. Composite MRI scores improve correlation with EDSS in multiple sclerosis. Mult Scler 2010;16:1117‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neuroscience Teva. Copaxone (glatiramer acetate) full prescribing information. Teva Neuroscience, North Wales, PA, USA: 2009. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020622s057lbl.pdf. [Google Scholar]
- 12. Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing‐remitting multiple sclerosis: results of a phase III multicenter, double‐blind placebo‐controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45:1268‐76. [DOI] [PubMed] [Google Scholar]
- 13. Johnson KP, Brooks BR, Cohen JA, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology 1998;50:701‐8. [DOI] [PubMed] [Google Scholar]
- 14. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227‐31. [DOI] [PubMed] [Google Scholar]
- 15. Khan O, Shen Y, Caon C, et al. Axonal metabolic recovery and potential neuroprotective effect of glatiramer acetate in relapsing‐remitting multiple sclerosis. Mult Scler 2005;11:646‐51. [DOI] [PubMed] [Google Scholar]
- 16. Khan O, Rieckmann P, Boyko A, et al. Three times weekly glatiramer acetate in relapsing–remitting multiple sclerosis. Ann Neurol 2013;73:705‐13. [DOI] [PubMed] [Google Scholar]
- 17. Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Statist Soc B 2005;67:301‐20. [Google Scholar]
- 18. Vrenken H, Barkhof F, Uitdehaag BM, et al. MR spectroscopic evidence for glial increase but not for neuro‐axonal damage in MS normal‐appearing white matter. Magn Reson Med 2005;53:256‐66. [DOI] [PubMed] [Google Scholar]
- 19. Wattjes MP, Harzheim M, Lutterbey GG, et al. Axonal damage but no increased glial cell activity in the normal‐appearing white matter of patients with clinically isolated syndromes suggestive of multiple sclerosis using high‐field magnetic resonance spectroscopy. AJNR Am J Neuroradiol 2007;28:1517‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 2006;5:158‐70. [DOI] [PubMed] [Google Scholar]
- 21. Mainero C, De Stefano N, Iannucci G, et al. Correlates of MS disability assessed in vivo using aggregates of MR quantities. Neurology 2001;56:1331‐4. [DOI] [PubMed] [Google Scholar]
- 22. Caramanos Z, DiMaio S, Narayanan S, et al. 1H‐MRSI evidence for cortical gray matter pathology that is independent of cerebral white matter lesion load in patients with secondary progressive multiple sclerosis. J Neurol Sci 2009;282:72‐9. [DOI] [PubMed] [Google Scholar]
- 23. Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long‐term disability in multiple sclerosis. Ann Neurol 2008;64:247‐54. [DOI] [PubMed] [Google Scholar]
- 24. Chen M, Valenzuela RM, Dhib‐Jalbut S. Glatiramer acetate‐reactive T cells produce brain‐derived neurotrophic factor. J Neurol Sci 2003;215:37‐44. [DOI] [PubMed] [Google Scholar]
- 25. Ziemssen T, Kumpfel T, Klinkert WE, et al. Glatiramer acetate‐specific T‐helper 1‐ and 2‐type cell lines produce BDNF: implications for multiple sclerosis therapy. Brain‐derived neurotrophic factor. Brain 2002;125:2381‐91. [DOI] [PubMed] [Google Scholar]
- 26. Newsome SD, Wang JI, Kang JY, et al. Quantitative measures detect sensory and motor impairments in multiple sclerosis. J Neurol Sci 2011;305:103‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Mult Scler 2000;6:255‐66. [DOI] [PubMed] [Google Scholar]
- 28. Ford CC, Johnson KP, Lisak RP, et al. A prospective open‐label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler 2006;12:309‐20. [DOI] [PubMed] [Google Scholar]


