Abstract
Aim
To evaluate the efficacy and safety of the glucagon‐like peptide‐1 (GLP‐1) receptor agonist liraglutide in African‐American people with Type 2 diabetes.
Methods
Analyses were performed on patient‐level data from individuals self‐defined as African‐American or non‐African‐American in seven phase III studies. Endpoints included change in HbA1c level, fasting plasma glucose level and body weight from baseline, proportion of patients reaching HbA1c target [< 53 mmol/mol (< 7.0%)], and incidence of hypoglycaemia and nausea. Analyses used data obtained after 26 weeks. Within‐population comparisons of liraglutide were performed vs placebo for African‐American and non‐African‐American patient groups. In addition, between‐population comparisons with non‐African‐American patients were performed for each treatment.
Results
In African‐American patients (n = 225), HbA1c was significantly reduced at 26 weeks with liraglutide 1.2 and 1.8 mg (−11 and −14 mmol/mol, respectively compared with placebo; P < 0.0001). There were also significant reductions in fasting plasma glucose (−2.4 and −3.1 mmol/l, respectively, compared with placebo; P < 0.0001). Statistically significant reductions in body weight were observed with 1.8 mg liraglutide (−2.1 kg compared with placebo; P = 0.0056), but not with 1.2 mg liraglutide (−0.26 kg; P = 0.7307). The P value for interaction between treatment and race was significant for body weight (P = 0.0355). The incidence of non‐severe hypoglycaemia with liraglutide was low (11–15% of patients), and < 25% of patients receiving liraglutide experienced nausea.
Conclusions
This meta‐analysis suggests that liraglutide is well tolerated and efficacious for treatment of Type 2 diabetes in African‐American patients, with an efficacy that was shown not to differ from that observed in non‐African‐American patients over 26 weeks.
What's new?
This study is the first of its kind to assess specifically the efficacy and tolerability of a glucagon‐like peptide‐1 (GLP‐1) receptor agonist in African‐American populations.
We showed that the GLP‐1 receptor agonist liraglutide was efficacious in the treatment of African‐American people with Type 2 diabetes, and had broadly similar efficacy to that seen in non‐African‐American people.
What's new?
This study is the first of its kind to assess specifically the efficacy and tolerability of a glucagon‐like peptide‐1 (GLP‐1) receptor agonist in African‐American populations.
We showed that the GLP‐1 receptor agonist liraglutide was efficacious in the treatment of African‐American people with Type 2 diabetes, and had broadly similar efficacy to that seen in non‐African‐American people.
Introduction
The prevalence of Type 2 diabetes is higher in the US African‐American population than in age‐matched non‐African‐American people 1. Specifically, 13.2% of the non‐Hispanic African‐American population aged ≥ 20 years have diabetes, compared with 7.6% of age‐matched non‐Hispanic white people 1. In addition, a large proportion of African‐American women (58%) and men (38%) are obese 2, a risk factor known to dramatically increase the risk of developing Type 2 diabetes 3, 4. Furthermore, certain genetic variants implicated in diabetes, such as TCF7L2, have been associated with increased risk of diabetes among African‐American patients in particular 5, 6, 7, 8, 9. Evaluation of new diabetes therapies is therefore of particular importance in this population in order to inform clinical options for personalized treatment.
In patients with Type 2 diabetes, glucagon‐like peptide‐1 (GLP‐1) receptor agonists have been shown to improve glycaemia and reduce body weight, with a low risk of hypoglycaemia 10, 11, 12; however, published data from race and ethnic sub‐populations are limited. Since genetic differences can affect native GLP‐1 concentration and influence individual response to pharmaceutical agents 13, it is important to investigate the efficacy and safety of GLP‐1 agonists in a variety of populations, including African‐American patients.
In this analysis, liraglutide data from all seven trials 14, 15, 16, 17, 18, 19, 20 and placebo data from four of these seven trials 16, 17, 19, 20 were pooled and compared.
Methods
Study design and participants
Analyses were carried out using pooled patient data from seven randomized phase III trials: LEAD‐1–6 and 1860‐LIRA‐DPP‐4 (registered at ClinicalTrials.gov: NCT00395746, NCT00614120, NCT00294723, NCT00333151, NCT00331851, NCT00518882 and NCT00700817). Trial designs and results have been published previously for all seven trials 14, 15, 16, 17, 18, 19, 20 and the race/treatment profile of patients involved in these clinical trials is shown in Table 1.
Table 1.
Race/treatment profile of intention‐to‐treat populations in the seven clinical trials (LEAD‐1–6 and 1860‐LIRA‐DPP‐4)
| African‐American patients | Non‐African‐American patients | |||||
|---|---|---|---|---|---|---|
| Liraglutide 1.2 mg, n | Liraglutide 1.8 mg, n | Placebo, n | Liraglutide 1.2 mg, n | Liraglutide 1.8 mg, n | Placebo, n | |
| LEAD‐1 | 7 | 9 | 1 | 221 | 225 | 113 |
| LEAD‐2 | 9 | 5 | 3 | 231 | 237 | 118 |
| LEAD‐3 | 34 | 30 | – | 217 | 216 | – |
| LEAD‐4 | 26 | 18 | 18 | 151 | 160 | 157 |
| LEAD‐5 | – | 9 | 5 | – | 221 | 109 |
| LEAD‐6 | – | 13 | – | – | 220 | – |
| 1860‐LIRA‐DPP‐4 | 22 | 16 | – | 199 | 202 | – |
Participants enrolling in the 7 clinical trials were considered part of the sub population if they identified their race as ‘African‐American/Black.’
Background study medications were different in the seven trials: no medication (LEAD‐3); metformin (LEAD‐2 and 1860‐LIRA‐DPP‐4); glimepiride (LEAD‐1); metformin + rosiglitazone (LEAD‐4); metformin + glimepiride (LEAD‐5); and metformin, sulfonylurea or both (LEAD‐6). The randomized treatment period was 26 weeks for all of the trials except for LEAD‐3, in which it was 52 weeks. In addition to this, each trial contributed differing numbers of patients towards each comparison, i.e. African‐American patients who took placebo ranged from one patient (LEAD‐1) to 18 patients (LEAD‐4), whilst LEAD‐3 (64 patients) and LEAD‐4 (44 patients) contributed the largest numbers of African‐American patients who took liraglutide (Table 1). The data reported in the present study are 26‐week data for all trials.
Outcomes and assessments
The intention‐to‐treat and the safety populations were grouped into African‐American and non‐African‐American populations, based on self‐defined race. Efficacy endpoints assessed in this analysis at 26 weeks were change in HbA1c, proportions of patients reaching the HbA1c target of < 53 mmol/mol, reductions in fasting plasma glucose (FPG) levels and reductions in body weight.
Safety assessments included incidence of nausea and hypoglycaemia, for which the safety population was used (all randomized patients who received at least one dose of study medication). Non‐severe hypoglycaemia was defined as < 3.1 mmol/l (56 mg/dl) and self‐treated; major hypoglycaemia was defined as requiring third‐party assistance.
Data analysis
Least‐squares mean changes from baseline to week 26 were estimated using an analysis of covariance model with trial, previous treatment, treatment, race, interaction between treatment and race as fixed factors, baseline value of outcome of interest as a covariate and country as a random effect. Logistic regression with trial, previous treatment, treatment, race, interaction between treatment and race as fixed factors, and baseline as a covariate, was used to estimate the proportion of patients reaching the HbA1c target of < 53 mmol/mol (< 7.0%). For all efficacy analyses, within‐population comparisons of liraglutide were performed vs placebo; between‐population comparisons were also performed for each treatment. Missing data were imputed using last observation carried forward. No statistical comparisons were performed for analysis of safety outcomes.
Results
Patients
Baseline characteristics were similar between treatment and race groups, with the exceptions of weight and HbA1c (higher in African‐American patients; Table 2). In addition there were more women among the non‐African‐American patients.
Table 2.
Baseline characteristics of the African‐American and non‐African‐American subgroups
| African‐American patients | Non‐African‐American patients | |||||
|---|---|---|---|---|---|---|
| Liraglutide 1.2 mg | Liraglutide 1.8 mg | Placebo | Liraglutide 1.2 mg | Liraglutide 1.8 mg | Placebo | |
| Number of patients | 99 | 100 | 27 | 1024 | 1487 | 501 |
| Mean (sd) age, years | 53.7 (10.5) | 52.4 (11.1) | 53.7 (11.5) | 56.2 (9.9) | 55.7 (9.9) | 55.8 (9.6) |
| Gender: men/women, % | 36/64 | 34/66 | 22/78 | 52/48 | 54/46 | 57/43 |
| Mean (sd) BMI, kg/m2 | 34.1 (5.5) | 33.6 (5.8) | 33.2 (5.9) | 31.9 (5.3) | 31.9 (5.4) | 32.1 (5.1) |
| Mean (sd) duration of diabetes, years | 6.7 (5.5) | 7.6 (5.6) | 8.7 (6.4) | 6.9 (5.4) | 7.7 (5.7) | 8.5 (5.9) |
| Mean (sd) body weight, kg, | 97.1 (18.4) | 95.4 (18.6) | 91.5 (17.0) | 89.6 (17.2) | 90.1 (19.3) | 91.0 (18.8) |
| Mean (sd) HbA1c | ||||||
| mmol/mol | 70 (10.9) | 69 (10.9) | 72 (12.3) | 67 (9.9) | 68 (10.0) | 68 (10.3) |
| % | 8.6 (1.0) | 8.5 (1.0) | 8.7 (1.1) | 8.3 (0.9) | 8.4 (0.9) | 8.4 (0.9) |
| Previous treatment, n (%) | ||||||
| Monotherapy | 53 (54) | 54 (54) | 7 (26) | 523 (51) | 604 (54) | 109 (22) |
| Combination therapy | 34 (34) | 39 (39) | 20 (74) | 422 (41) | 803 (39) | 392 (78) |
| Diet and exercise | 12 (12) | 7 (7) | 0* | 79 (7.7) | 80 (7) | 0* |
Outcomes and assessments
HbA1c reductions
Mean HbA1c reductions in African‐American patients after 26 weeks were significantly greater with both liraglutide doses than with placebo: estimated placebo‐adjusted treatment effect sizes were −11 and −14 mmol/mol (−1.0 and −1.3%) with liraglutide 1.2 mg and liraglutide 1.8 mg, respectively; P < 0.0001 for both doses (Fig. 1). HbA1c reductions in non‐African‐American patients were similar to those observed in African‐American patients: estimated placebo‐adjusted treatment effect sizes were −11 and −12 mmol/mol (−1.0 and −1.1%) with liraglutide 1.2 mg and liraglutide 1.8 mg, respectively; P < 0.0001 for both doses in non‐African‐American patients (Fig. 1). The HbA1c reductions observed with liraglutide did not significantly differ between African‐American and non‐African‐American groups (P value for interaction 0.4439).
Figure 1.
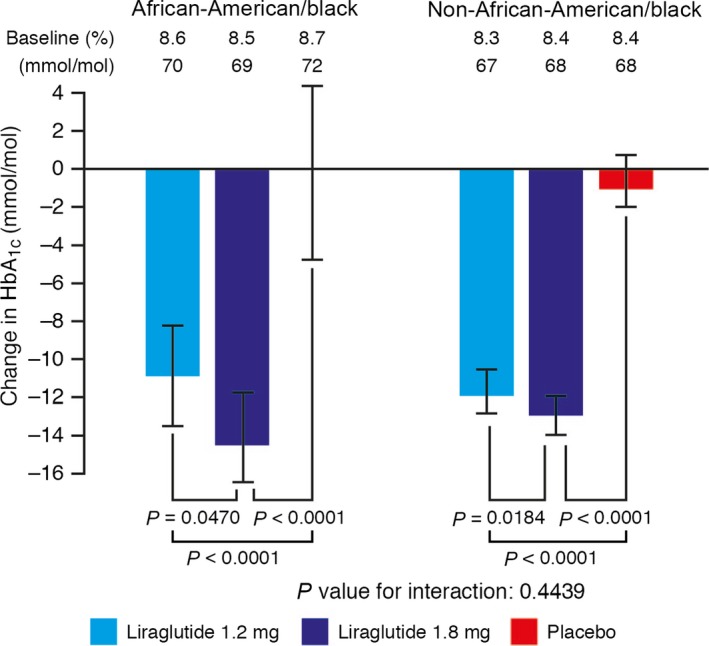
Change in HbA1c (mmol/mol) from baseline to week 26 of treatment. Last observation carried forward, intention‐to‐treat analysis set. Error bars represent 95% CIs for the estimated means. P values are for the estimated treatment difference relative to placebo.
HbA1c target
A significantly higher proportion of African‐American patients reached the American Diabetes Association‐recommended HbA1c target of < 53 mmol/mol (< 7.0%) with liraglutide 1.2 mg (35%) and liraglutide 1.8 mg (62%) than with placebo (11%; Fig. 2). Odds ratios for achieving HbA1c target for liraglutide 1.2 mg and 1.8 mg relative to placebo were 4.38 (95% CI 1.29–14.8; P = 0.0175) and 13.73 (95% CI 4.08–46.2; P < 0.0001), respectively. In the non‐African‐American population, the proportion of patients reaching the HbA1c target with liraglutide 1.2 mg (41%) and liraglutide 1.8 mg (50%) was significantly higher than that with placebo (10%): odds ratios 6.0 (95% CI 4.33–8.33) and 8.41 (95% CI 6.14–11.53) for liraglutide 1.2 mg and 1.8 mg, respectively; P < 0.0001 for both doses of liraglutide. No significant differences in the HbA1c‐lowering effect of liraglutide attributable to race were observed. The P value for test of interaction between treatment and race (African‐American and non‐African‐American) was 0.0898.
Figure 2.
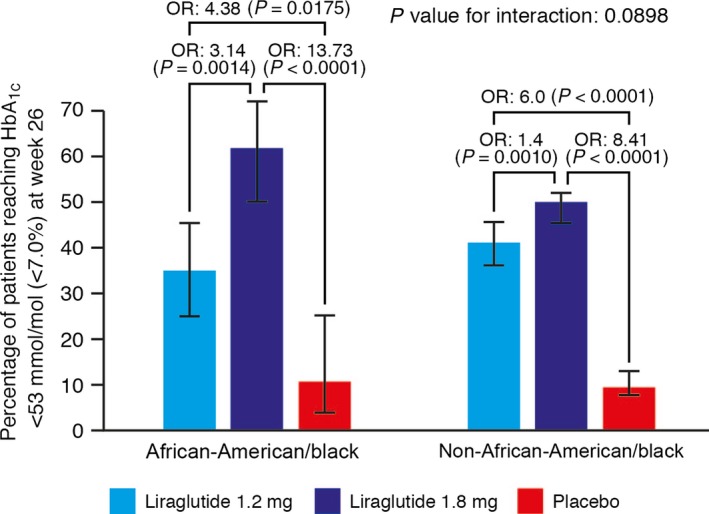
Percentage of patients achieving HbA1c target < 53 mmol/mol at week 26 (estimated using logistic regression analysis, intention‐to‐treat analysis set, last observation carried forward). Error bars represent 95% CIs for the estimated means. P values are for the odds ratio (OR) of treatment relative to placebo.
Fasting plasma glucose reductions
Significant reductions in mean FPG level were seen with both doses of liraglutide compared with placebo. In the African‐American population, the estimated treatment effect sizes were −2.4 and −3.1 mmol/l for liraglutide 1.2 mg and 1.8 mg, respectively (P < 0.0001 compared with placebo for both comparisons; Fig. 3). In the non‐African‐American population, FPG reduction treatment effect estimates were −2.0 and −2.2 mmol/l for liraglutide 1.2 and 1.8 mg, respectively, compared with placebo (P < 0.0001 for both comparisons; Fig. 3).
Figure 3.
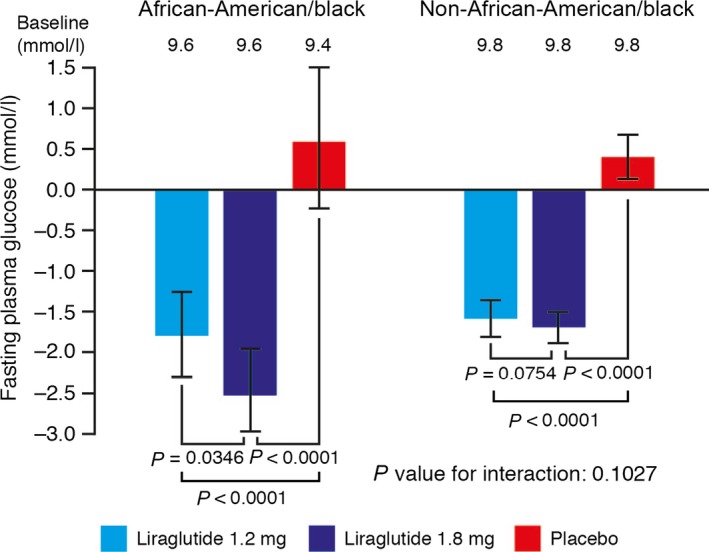
Change in fasting plasma glucose (mmol/l) from baseline to week 26 of treatment. Last observation carried forward, intention‐to‐treat analysis set. Error bars represent 95% CIs for the estimated means. P values are for the estimated treatment difference relative to placebo.
The FPG reductions observed with liraglutide did not significantly differ between African‐American and non‐African‐American groups (P value for interaction 0.1027).
Change in body weight
Significant reductions in mean body weight were observed with 1.8 mg liraglutide compared with placebo in both African‐American and non‐African‐American individuals after 26 weeks of treatment.
In the African‐American population, the estimated treatment effect size with liraglutide 1.8 mg was −2.1 kg (P = 0.0056); however, the estimated treatment effect size with liraglutide 1.2 mg (−0.3 kg) was not statistically significant relative to placebo in the African‐American subgroup (P = 0.7307).
In the non‐African‐American population, significant reductions in body weight were observed with both doses of liraglutide compared with placebo: treatment effect estimates were −0.9 and −1.4 kg for liraglutide 1.2 mg and 1.8 mg, respectively, compared with placebo (P < 0.0001 for both comparisons; Fig. 4).
Figure 4.
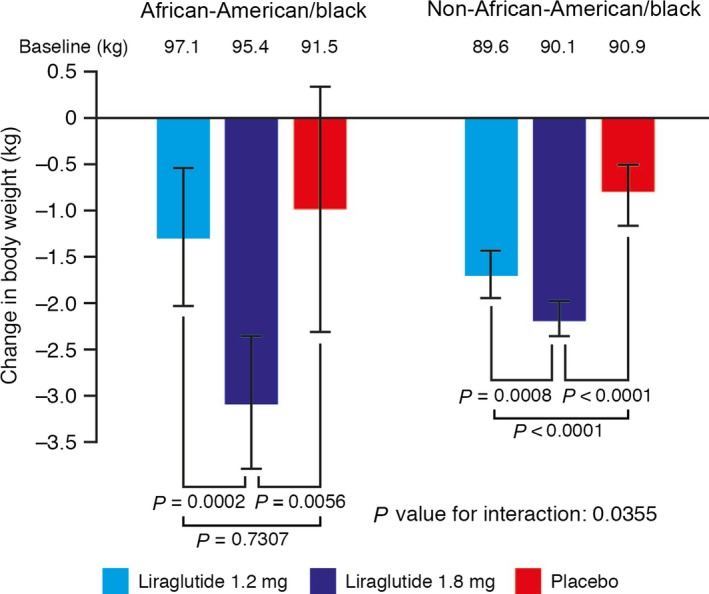
Change in body weight (kg) from baseline to week 26 of treatment. Last observation carried forward, intention‐to‐treat analysis set. Error bars represent 95% CIs for the estimated means. P values are for the estimated treatment difference relative to placebo.
Interestingly, a significant difference in body weight change between African‐American and non‐African‐American groups with liraglutide was observed. The P value for test of interaction between treatment and race (African‐American and non‐African‐American) was 0.0355.
Safety endpoints
After 26 weeks, among the African‐American population, non‐severe hypoglycaemia occurred in 11.2% of patients with liraglutide 1.2 mg, 15.0% with liraglutide 1.8 mg and 7.4% with placebo. One major hypoglycaemic episode occurred in a patient receiving liraglutide 1.2 mg in combination with metformin, and one major hypoglycaemic episode occurred in a patient receiving liraglutide 1.8 mg in combination with metformin and glimepiride. Among the non‐African‐American population, 5.7% of patients experienced non‐severe hypoglycaemia with liraglutide 1.2 mg, 11.7% with liraglutide 1.8 mg, and 6.4% with placebo.
Within the African‐American population after 26 weeks, the proportion of patients experiencing gastrointestinal adverse events was higher with liraglutide 1.2 mg (43.9%) and 1.8 mg (41.0%) than with placebo (18.5%; no statistical comparisons conducted). These proportions appeared similar to those in the non‐African‐American population: 39.9, 43.0 and 17.7% with liraglutide 1.2 mg, liraglutide 1.8 mg and placebo, respectively. Nausea was the most common gastrointestinal event among African‐American patients (experienced by 22.4, 21.0 and 11.1% of patients with liraglutide 1.2 mg, liraglutide 1.8 mg and placebo, respectively). The proportions of non‐African‐American patients experiencing nausea were 20.1, 22.0 and 4.4% with liraglutide 1.2 mg, liraglutide 1.8 mg and placebo, respectively. Nausea was primarily experienced at initiation, and was mild and transient in nature.
Discussion
The present study was a meta‐analysis of a sub‐population, and so the findings should be interpreted with caution because of possible biases; however, some interesting and clinically relevant insights were obtained. In this analysis, African‐American patients treated with either liraglutide 1.2 mg or liraglutide 1.8 mg achieved significant HbA1c and FPG reductions compared with placebo. Additionally, with 1.8 mg liraglutide, body weight reductions were also significant compared with placebo. This shows that liraglutide was efficacious in the present African‐American population. Furthermore, there was no overall difference in effect of glycaemic control between African‐American and non‐African‐American patients.
Among African‐American patients, significant reductions in HbA1c compared with placebo were observed with both 1.2 mg and 1.8 mg liraglutide. These reductions were also apparent in the significantly greater proportions of patients reaching the American Diabetes Association‐recommended HbA1c target of < 53 mmol/mol with both doses of liraglutide. Similarly, both doses of liraglutide were associated with significantly greater FPG reductions compared with placebo, and significant differences in weight change compared with placebo were observed with 1.8 mg liraglutide.
The improvements in glycaemia observed in African‐American patients treated with liraglutide were accompanied by a low risk of hypoglycaemia. Additionally, nausea—a known side effect of GLP‐1 receptor agonists—was reported by < 25% of African‐American patients, a similar proportion to non‐African‐American patients.
In this secondary analysis, the first of its kind to assess the efficacy and tolerability of GLP‐1 receptor agonists in an African‐American population, liraglutide was shown to have broadly similar safety and efficacy in African‐American and non‐African‐American patients.
In terms of differences between African‐American and non‐African‐American patients, the P‐value for interaction (African‐American and non‐African‐American) between treatment and race was significant for weight change, implying greater weight loss with liraglutide attributable to race (P = 0.0355), but not for any other endpoint evaluated. This finding should be interpreted with caution, however, given that baseline weight and BMI was originally greater among the African‐American patients compared with the non‐African‐American patients.
When compared with non‐African‐American patients, there appears to be a more pronounced difference in the dose response between the 1.2 mg and 1.8 mg treatment doses for African‐American patients; however, based on the present analysis, it cannot be concluded that this is illustrative of a real dose–response effect, as potential mechanisms for an altered dose response are not clear. There have been suggestions that genetic differences may confer increased responsiveness to GLP‐1‐based therapies in some populations 21. African‐American individuals generally carry a significantly higher burden of risk alleles compared with European‐American people 6. Most notably there are variants of the gene TCF7L2, which have been shown to negatively modify the effects of incretins on insulin secretion 22, 23, 24, raising the possibility of an effect on the efficacy of GLP‐1 receptor agonists 25. Further studies will be required to determine the effect of these variants on treatment with GLP‐1 receptor agonists.
Overall, personalized medicine and race‐based prescription are viewed by African‐American individuals as positive advances 26; however, these are also associated with a degree of mistrust towards the medical community, with concerns about prescription of inferior medicines and inequity of access to treatment 26. These factors need to be taken into account when considering treatment of different racial sub‐populations.
A limitation of this analysis is that comparison with all separate individual race groups was not possible, and it may be the case that unique responses are observed in other sub‐populations; however, a sensitivity analysis comparing all clinical efficacy outcomes for African‐American patients versus white patients (the largest constituent racial group within the non‐African‐American category) supports the findings of the main analysis of this study (Table S1). Another limitation was that a significant proportion of African‐American patients included were from trials in which there was no placebo arm, and a large proportion of these African‐American subjects came from LEAD‐3, a monotherapy trial, in which subjects were likely to be healthier than those on multiple therapies.
In summary, the reductions in HbA1c levels, FPG levels and body weight observed in the present study were similar to those reported for the overall populations of the LEAD trials and the 1860‐LIRA‐DPP‐4 trial. This, allied to the similar safety profile between race groups, shows that liraglutide is efficacious for the treatment of African‐American patients with Type 2 diabetes, and has similar efficacy to that seen in non‐African‐American patients over 26 weeks. This work supports the use of liraglutide in African‐American populations.
Funding sources
This analysis was funded by Novo Nordisk. Writing assistance was provided by Watermeadow Medical, supported by Novo Nordisk.
Competing interests
M.E.S. has no competing interests to declare. D.D.Ø. is employed by Novo Nordisk and holds Novo Nordisk stock. A.J.C. is a member of the speakers’ bureau for Novo Nordisk, Merck, GSK and Janssen Pharmaceuticals.
Supporting information
Table S1. Baseline values and outcome measures for all clinical endpoints presented as a sensitivity analysis comparing African‐American patients versus white patients.
Acknowledgements
The authors would like to thank Anne Louise Svendsen for assistance with the statistical analyses.
Diabet. Med. 34, 197–203 (2017)
Participants enrolling in the 7 clinical trials were considered part of the sub population if they identified their race as ’African‐American/Black.’
References
- 1. (CDC) CfDCaP . National Diabetes Statistics Report. 2014. Available at https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf Last accessed 26 July 2016.
- 2. (CDC) CfDCaP . Health of Black or African American non‐Hispanic Population. 2014. Available at http://www.cdc.gov/nchs/fastats/black-health.htm Last accessed 26 July 2016.
- 3. Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW et al Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab 2011; 96: 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988‐1994 and 1999‐2010. Ann Intern Med 2014; 160: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooke JN, Ng MC, Palmer ND, An SS, Hester JM, Freedman BI et al Genetic risk assessment of type 2 diabetes‐associated polymorphisms in African Americans. Diabetes Care 2012; 35: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keaton JM, Cooke Bailey JN, Palmer ND, Freedman BI, Langefeld CD, Ng MC et al A comparison of type 2 diabetes risk allele load between African Americans and European Americans. Hum Genet 2014; 133: 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng MC, Saxena R, Li J, Palmer ND, Dimitrov L, Xu J et al Transferability and fine mapping of type 2 diabetes loci in African Americans: the Candidate Gene Association Resource Plus Study. Diabetes 2013; 62: 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ng MC, Shriner D, Chen BH, Li J, Chen WM, Guo X et al Meta‐analysis of genome‐wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS Genet 2014; 10: e1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saxena R, Elbers CC, Guo Y, Peter I, Gaunt TR, Mega JL et al Large‐scale gene‐centric meta‐analysis across 39 studies identifies type 2 diabetes loci. Am J Hum Genet 2012; 90: 410–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnett AH. New treatments in type 2 diabetes: a focus on the incretin‐based therapies. Clin Endocrinol (Oxf) 2009; 70: 343–353. [DOI] [PubMed] [Google Scholar]
- 11. Drab SR. Incretin‐based therapies for type 2 diabetes mellitus: current status and future prospects. Pharmacotherapy 2010; 30: 609–624. [DOI] [PubMed] [Google Scholar]
- 12. Russell S. Incretin‐based therapies for type 2 diabetes mellitus: a review of direct comparisons of efficacy, safety and patient satisfaction. Int J Clin Pharm 2013; 35: 159–172. [DOI] [PubMed] [Google Scholar]
- 13. Velasquez‐Mieyer PA, Cowan PA, Perez‐Faustinelli S, Nieto‐Martinez R, Villegas‐Barreto C, Tolley EA et al Racial disparity in glucagon‐like peptide 1 and inflammation markers among severely obese adolescents. Diabetes Care 2008; 31: 770–775. [DOI] [PubMed] [Google Scholar]
- 14. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH et al Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 15. Garber A, Henry R, Ratner R, Garcia‐Hernandez PA, Rodriguez‐Pattzi H, Olvera‐Alvarez I et al Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481. [DOI] [PubMed] [Google Scholar]
- 16. Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J et al Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH et al Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)‐2 study. Diabetes Care 2009; 32: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S et al Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet 2010; 375: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 19. Russell‐Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S et al Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P et al Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care 2009; 32: 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin CH, Lee YS, Huang YY, Hsieh SH, Chen ZS, Tsai CN. Polymorphisms of GLP‐1 receptor gene and response to GLP‐1 analogue in patients with poorly controlled type 2 diabetes. J Diabetes Res 2015; 2015: 176949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner R, Staiger H, Ullrich S, Stefan N, Fritsche A, Haring HU. Untangling the interplay of genetic and metabolic influences on beta‐cell function: examples of potential therapeutic implications involving TCF7L2 and FFAR1. Mol Metab 2014; 3: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shao W, Wang D, Chiang YT, Ip W, Zhu L, Xu F et al The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes 2013; 62: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Villareal DT, Robertson H, Bell GI, Patterson BW, Tran H, Wice B et al TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes 2010; 59: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zimdahl H, Ittrich C, Graefe‐Mody U, Boehm BO, Mark M, Woerle HJ et al Influence of TCF7L2 gene variants on the therapeutic response to the dipeptidylpeptidase‐4 inhibitor linagliptin. Diabetologia 2014; 57: 1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Marco M, Cykert S, Coad N, Doost K, Schaal J, White B et al Views on personalized medicine: do the attitudes of African American and white prescription drug consumers differ? Public Health Genomics 2010; 13: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline values and outcome measures for all clinical endpoints presented as a sensitivity analysis comparing African‐American patients versus white patients.


